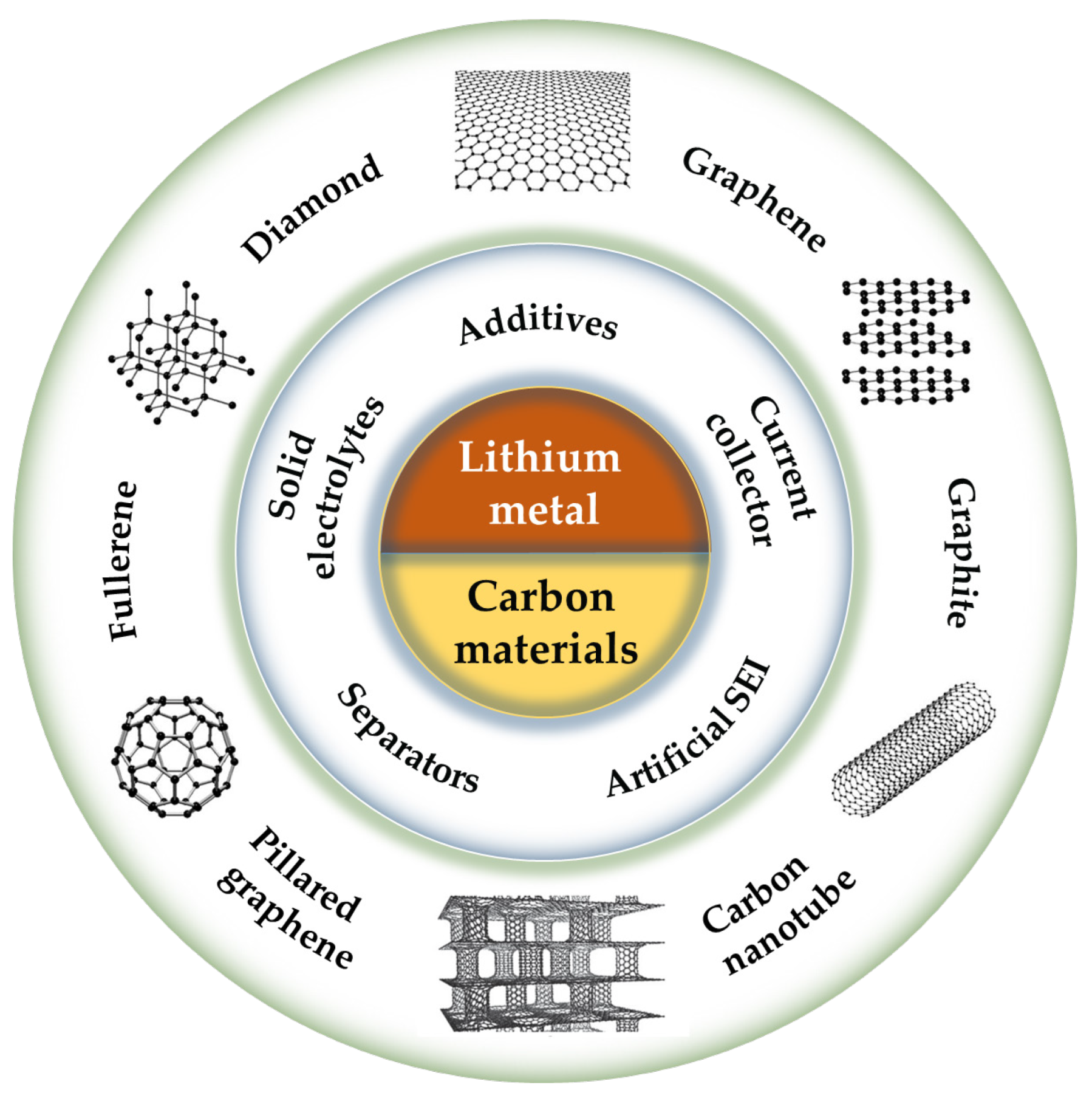
A Review of the Application of Carbon Materials for Lithium Metal Batteries
Abstract
:1. Introduction
2. Issues and Challenges of Lithium Metal Anodes
2.1. Nucleation of Lithium Metal
2.2. Growth of Dendrites and Formation of “Dead Lithium”
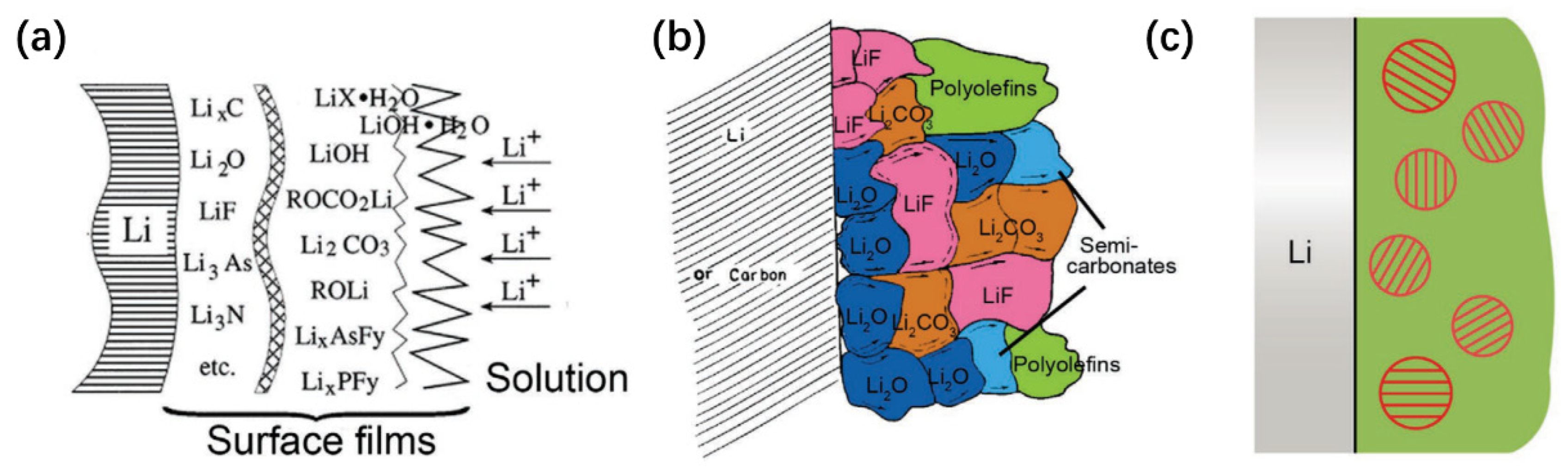
2.3. Solid Electrolyte Interphase of Lithium Metal Anode
3. Recent Progress
3.1. Electrolyte Additives
3.2. Separator Modification

3.3. Artificial SEI

3.4. Current Collector Design
3.4.1. Lithium Metal Anode Using Carbon Material as Current Collector
| Current Collector | Half Cell Performance (Cycle Number/h, CE) | Operating Conditions (Current Density/mA·cm−2, Areal Capacity/mA h·cm−2) | Reference | |
|---|---|---|---|---|
| 0D | Au@hollow carbon sphere | 300, 98% | 0.5, 1 | [52] |
| S-doped carbon nanospheres | 220, −97.5 % | 0.5, 1 | [99] | |
| Nitrogen-doped hollow porous carbon spheres | 270, 98.5% | 1, 1 | [100] | |
| hollow carbon spheres modified with evenly dispersed Ni2P nanoparticles | 400, 98.4% | 1, 1 | [101] | |
| 1D | graphitic carbon tubes | 350, 99.3% | 0.5, 1 | [102] |
| hollow carbon fiber | 350, 99.5% | 0.5, 2 | [103] | |
| Lotus-root-like Ni–Co hollow prisms@carbon fibers | 250, 98% | 3, 1 | [104] | |
| Li/carbon nanotube hybrid | 150, 95% | 1, 0.5 | [105] | |
| hollow carbon fiber | 350, 99.5 | 0.5, 2 | [106] | |
| oxygen-rich carbon nanotube | 200, 99% | 2, 1 | [107] | |
| 2D | layered reduced graphene oxide | - | - | [108] |
| oxygen-codoped vertical carbon nanosheet arrays | 325, 98% | 0.5, 1 | [109] | |
| S-doped graphene | 180, 98.23% | 1, 0.5 | [110] | |
| 3D fluorine-doped graphene | 150, 98% | 2, 1 | [111] | |
| 3D | Nitrogen-doped amorphous Zn–carbon multichannel fibers decorated with carbon cages | 800, 98% | 1, 1 | [112] |
| Au nanoparticles@graphene hybrid aerogel | 175, 91.2% | 1, 1 | [113] | |
| Carbon nanofiber-stabilized graphene aerogel film | 100, 98.5% | 3, 1 | [114] |
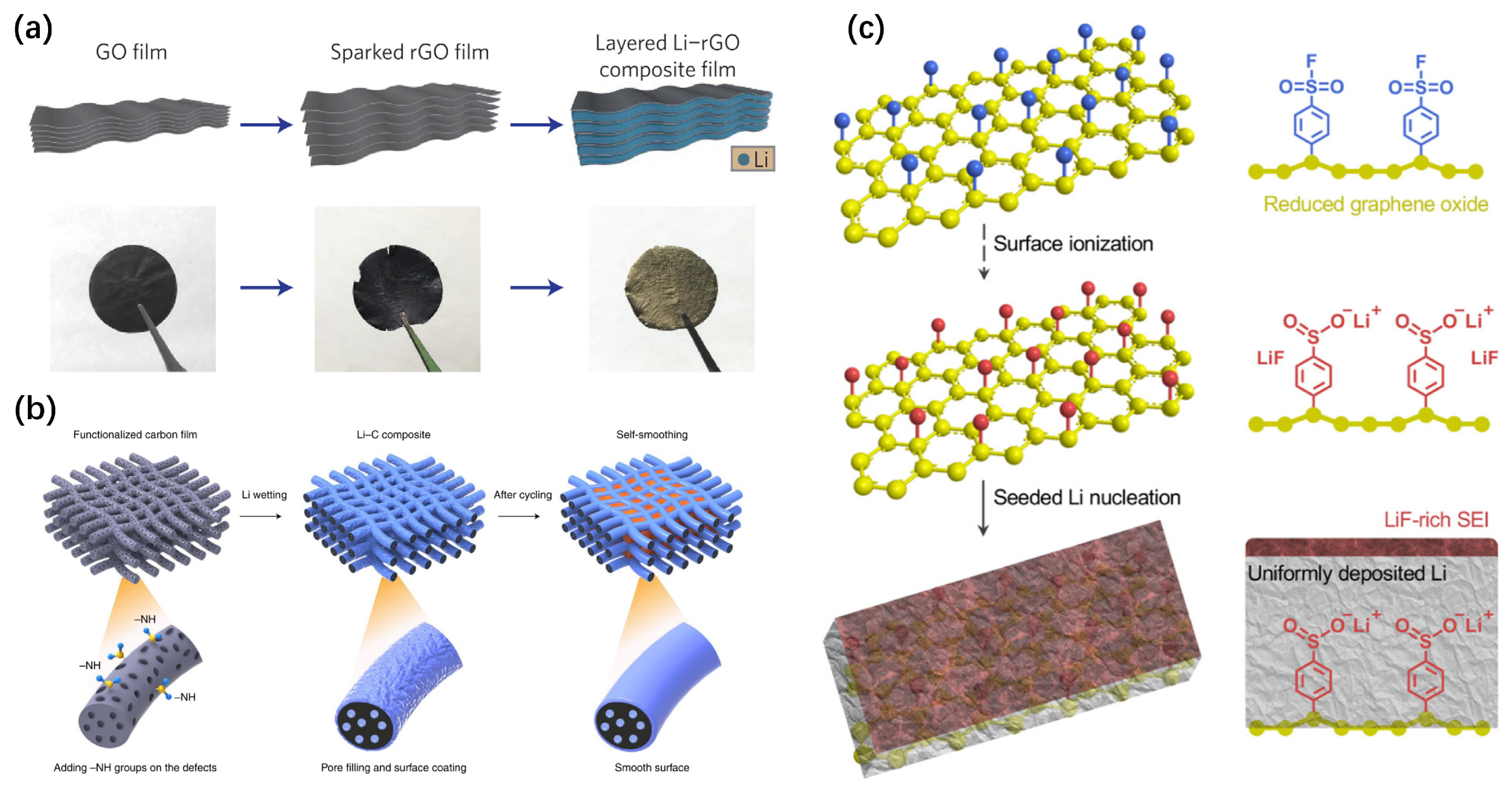
3.4.2. Graphite–Lithium Metal Composite Electrode
3.5. Carbon Materials in Solid-State Batteries
4. Summary and Outlook
- When introducing carbon materials into the design of lithium metal batteries, the negative effects of carbon materials, such as chemical/electrochemical stability, structural stability, etc., should be considered at the same time.
- When designing carbon-based three-dimensional current collectors, the effects of porosity and specific surface area should be considered at the same time. The size of porosity directly affects the mass transfer process of lithium ions: too large porosity will weaken the advantages brought by the 3D structure, while too small porosity will affect the mass transfer process of lithium ions in it. A large specific surface area can achieve more uniform deposition by dispersing the local current density, but at the same time, it will also increase the SEI film area and reduce the first effect and Coulomb efficiency of the battery.
- The lithium metal foil used in the laboratory test is generally thick. The excessive lithium metal and electrolyte greatly prolong the failure time of the battery. When conducting battery tests, the experimental conditions should be scientifically controlled in order to truly reflect the role of materials in the battery.
- Pay attention to the overall specific capacity of the battery. Excess lithium metal will reduce the actual specific capacity of the battery. The use of carbon materials can improve the cycle stability and battery life of lithium metal batteries to a certain extent. However, the mass and volume of carbon materials themselves are often overlooked. Controlling the lithium–carbon ratio is particularly important to ensure the specific capacity of the battery.
- The experiment is established on the basis of the full cell, and its feasibility is verified with a pouch cell or a cylindrical cell.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [Green Version]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.-J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, H.; Zhai, T. Reviving Lithium-Metal Anodes for Next-Generation High-Energy Batteries. Adv. Mater. 2017, 29, 1700007. [Google Scholar] [CrossRef] [PubMed]
- Zu, C.-X.; Li, H. Thermodynamic analysis on energy densities of batteries. Energy Environ. Sci. 2011, 4, 2614–2624. [Google Scholar] [CrossRef]
- Albertus, P.; Babinec, S.; Litzelman, S.; Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 2018, 3, 16–21. [Google Scholar] [CrossRef]
- Zhang, H.; Eshetu, G.G.; Judez, X.; Li, C.M.; Rodriguez-Martinez, L.M.; Armand, M. Electrolyte Additives for Lithium Metal Anodes and Rechargeable Lithium Metal Batteries: Progress and Perspectives. Angew. Chem. Int. Ed. 2018, 57, 15002–15027. [Google Scholar] [CrossRef]
- Chen, S.R.; Zheng, J.M.; Mei, D.H.; Han, K.S.; Engelhard, M.H.; Zhao, W.G.; Xu, W.; Liu, J.; Zhang, J.G. High-Voltage Lithium-Metal Batteries Enabled by Localized High-Concentration Electrolytes. Adv. Mater. 2018, 30, 1706102. [Google Scholar] [CrossRef]
- Ren, X.D.; Zou, L.F.; Cao, X.; Engelhard, M.H.; Liu, W.; Burton, S.D.; Lee, H.; Niu, C.J.; Matthews, B.E.; Zhu, Z.H.; et al. Enabling High-Voltage Lithium-Metal Batteries under Practical Conditions. Joule 2019, 3, 1662–1676. [Google Scholar] [CrossRef]
- Qian, J.F.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.L.; Chen, L.; Borodin, O.; Ji, X.; Chen, J.; Hou, S.; Deng, T.; Zheng, J.; Yang, C.Y.; Liou, S.C.; et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 2018, 13, 715–722. [Google Scholar] [CrossRef]
- Suo, L.M.; Xue, W.J.; Gobet, M.; Greenbaum, S.G.; Wang, C.; Chen, Y.M.; Yang, W.L.; Li, Y.X.; Li, J. Fluorine-donating electrolytes enable highly reversible 5-V-class Li metal batteries. Proc. Natl. Acad. Sci. USA 2018, 115, 1156–1161. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, J.; Sushko, M.L.; Chen, X.L.; Shao, Y.Y.; Engelhard, M.H.; Nie, Z.M.; Xiao, J.; et al. Dendrite-Free Lithium Deposition via Self-Healing Electrostatic Shield Mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, B.; Fang, Y.; Dang, D.; Shen, X.; Li, Z.; Wu, M.; Hong, Y.; Liu, Q. Inhibition of lithium dendrites and dead lithium by an ionic liquid additive toward safe and stable lithium metal anodes. Chin. Chem. Lett. 2022, 33, 3951–3954. [Google Scholar] [CrossRef]
- Zhang, W.D.; Tu, Z.Y.; Qian, J.W.; Choudhury, S.; Archer, L.A.; Lu, Y.Y. Design Principles of Functional Polymer Separators for High-Energy, Metal-Based Batteries. Small 2018, 14, 1703001. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.M.; Zhu, J.; Jiang, X.; Wu, H.T.; Qiao, J.S.; Sun, W.; Wang, Z.H.; Sun, K.N. Ultrastrong Polyoxyzole Nanofiber Membranes for Dendrite-Proof and Heat-Resistant Battery Separators. Nano Lett. 2016, 16, 2981–2987. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Liu, S.H.; Shi, C.G.; Liang, G.H.; Lu, Z.T.; Fu, R.W.; Wu, D.C. Two-dimensional molecular brush-functionalized porous bilayer composite separators toward ultrastable high-current density lithium metal anodes. Nat. Commun. 2019, 10, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, R.; Fu, L.X.; Zhou, H.L.; Wang, Z.Y.; Qiu, Z.F.; Shi, L.Y.; Zhu, J.F.; Yuan, S. High Li+ Ionic Flux Separator Enhancing Cycling Stability of Lithium Metal Anode. ACS Sustain. Chem. Eng. 2018, 6, 2961–2968. [Google Scholar] [CrossRef]
- Woo, S.G.; Hwang, E.K.; Kang, H.K.; Lee, H.; Lee, J.N.; Kim, H.S.; Jeong, G.; Yoo, D.J.; Lee, J.; Kim, S.; et al. High transference number enabled by sulfated zirconia superacid for lithium metal batteries with carbonate electrolytes. Energy Environ. Sci. 2021, 14, 1420–1428. [Google Scholar] [CrossRef]
- Liu, Y.J.; Tao, X.Y.; Wang, Y.; Jiang, C.; Ma, C.; Sheng, O.W.; Lu, G.X.; Lou, X.W. Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium metal batteries. Science 2022, 375, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, S.Y.; Liang, Z.W.; Wen, Y.C.; Liu, Z.B.; Wu, Y.W.; Liu, J.; Zhu, M. A Self-Supporting Covalent Organic Framework Separator with Desolvation Effect for High Energy Density Lithium Metal Batteries. ACS Energy Lett. 2022, 7, 885–896. [Google Scholar] [CrossRef]
- Wu, H.; Jia, H.; Wang, C.; Zhang, J.G.; Xu, W. Recent Progress in Understanding Solid Electrolyte Interphase on Lithium Metal Anodes. Adv. Energy Mater. 2020, 11, 2003092. [Google Scholar] [CrossRef]
- Meyerson, M.L.; Papa, P.E.; Heller, A.; Mullins, C.B. Recent Developments in Dendrite-Free Lithium-Metal Deposition through Tailoring of Micro- and Nanoscale Artificial Coatings. ACS Nano 2021, 15, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, R.G.; Maletti, S.; Heubner, C.; Michaelis, A.; Ein-Eli, Y. Molecular Engineering Approaches to Fabricate Artificial Solid-Electrolyte Interphases on Anodes for Li-Ion Batteries: A Critical Review. Adv. Energy Mater. 2021, 11, 2101173. [Google Scholar] [CrossRef]
- Liu, K.; Pei, A.; Lee, H.R.; Kong, B.; Liu, N.; Lin, D.C.; Liu, Y.Y.; Liu, C.; Hsu, P.C.; Bao, Z.A.; et al. Lithium Metal Anodes with an Adaptive "Solid-Liquid" Interfacial Protective Layer. J. Am. Chem. Soc. 2017, 139, 4815–4820. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.Y.; Choudhury, S.; Zachman, M.J.; Wei, S.Y.; Zhang, K.H.; Kourkoutis, L.F.; Archer, L.A. Designing Artificial Solid-Electrolyte Interphases for Single-Ion and High-Efficiency Transport in Batteries. Joule 2017, 1, 394–406. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Yan, Z.F.; Gray, J.L.; He, X.; Wang, D.W.; Chen, T.H.; Huang, Q.Q.; Li, Y.G.C.; Wang, H.Y.; Kim, S.H.; et al. Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 2019, 18, 384–389. [Google Scholar] [CrossRef]
- Cha, E.; Patel, M.D.; Park, J.; Hwang, J.; Prasad, V.; Cho, K.; Choi, W. 2D MoS2 as an efficient protective layer for lithium metal anodes in high-performance Li-S batteries. Nat. Nanotechnol. 2018, 13, 337–344. [Google Scholar] [CrossRef]
- Zheng, G.; Lee, S.W.; Liang, Z.; Lee, H.-W.; Yan, K.; Yao, H.; Wang, H.; Li, W.; Chu, S.; Cui, Y. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 2014, 9, 618–623. [Google Scholar] [CrossRef]
- Lin, Z.; Ma, Y.; Wang, W.; He, Y.; Wang, M.; Tang, J.; Fan, C.; Sun, K. A homogeneous and mechanically stable artificial diffusion layer using rigid-flexible hybrid polymer for high-performance lithium metal batteries. J. Energy Chem. 2022, 76, 631–638. [Google Scholar] [CrossRef]
- Han, Y.H.; Jie, Y.L.; Huang, F.Y.; Chen, Y.W.; Lei, Z.W.; Zhang, G.Q.; Ren, X.D.; Qin, L.J.; Cao, R.G.; Jiao, S.H. Enabling Stable Lithium Metal Anode through Electrochemical Kinetics Manipulation. Adv. Funct. Mater. 2019, 29, 1904629. [Google Scholar] [CrossRef]
- Zhou, B.X.; Bonakdarpour, A.; Stosevski, I.; Fang, B.Z.; Wilkinson, D.P. Modification of Cu current collectors for lithium metal batteries-A review. Prog. Mater. Sci. 2022, 130, 100996. [Google Scholar] [CrossRef]
- Zhao, H.; Lei, D.N.; He, Y.B.; Yuan, Y.F.; Yun, Q.B.; Ni, B.; Lv, W.; Li, B.H.; Yang, Q.H.; Kang, F.Y.; et al. Compact 3D Copper with Uniform Porous Structure Derived by Electrochemical Dealloying as Dendrite-Free Lithium Metal Anode Current Collector. Adv. Energy Mater. 2018, 8, 1800266. [Google Scholar] [CrossRef]
- Ke, X.; Liang, Y.H.; Ou, L.H.; Liu, H.D.; Chen, Y.M.; Wu, W.L.; Cheng, Y.F.; Guo, Z.P.; Lai, Y.Q.; Liu, P.; et al. Surface engineering of commercial Ni foams for stable Li metal anodes. Energy Stor. Mater. 2019, 23, 547–555. [Google Scholar] [CrossRef]
- Fan, L.; Wei, S.Y.; Li, S.Y.; Li, Q.; Lu, Y.Y. Recent Progress of the Solid-State Electrolytes for High-Energy Metal-Based Batteries. Adv. Energy Mater. 2018, 8, 1702657. [Google Scholar] [CrossRef]
- Yang, C.P.; Fu, K.; Zhang, Y.; Hitz, E.; Hu, L.B. Protected Lithium-Metal Anodes in Batteries: From Liquid to Solid. Adv. Mater. 2017, 29, 1701169. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G.X. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Chen, R.S.; Li, Q.H.; Yu, X.Q.; Chen, L.Q.; Li, H. Approaching Practically Accessible Solid-State Batteries: Stability Issues Related to Solid Electrolytes and Interfaces. Chem. Rev. 2020, 120, 6820–6877. [Google Scholar] [CrossRef]
- Yu, Q.J.; Jiang, K.C.; Yu, C.L.; Chen, X.J.; Zhang, C.J.; Yao, Y.; Jiang, B.; Long, H.J. Recent progress of composite solid polymer electrolytes for all-solid-state lithium metal batteries. Chin. Chem. Lett. 2021, 32, 2659–2678. [Google Scholar] [CrossRef]
- Zhao, B.L.; Ma, L.X.; Wu, K.; Cao, M.X.; Xu, M.G.; Zhang, X.X.; Liu, W.; Chen, J.T. Asymmetric double-layer composite electrolyte with enhanced ionic conductivity and interface stability for all-solid-state lithium metal batteries. Chin. Chem. Lett. 2021, 32, 125–131. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.B.; Huang, J.Q.; Yuan, H.; Lu, Y.; Yan, C.; Zhu, G.L.; Xu, R.; Zhao, C.Z.; Hou, L.P.; et al. Controlling Dendrite Growth in Solid-State Electrolytes. ACS Energy Lett. 2020, 5, 833–843. [Google Scholar] [CrossRef]
- Sun, M.H.; Liu, T.F.; Yuan, Y.F.; Ling, M.; Xu, N.; Liu, Y.Y.; Yan, L.J.; Li, H.; Liu, C.Y.; Lu, Y.Y.; et al. Visualizing Lithium Dendrite Formation within Solid-State Electrolytes. ACS Energy Lett. 2021, 6, 451–458. [Google Scholar] [CrossRef]
- Raj, V.; Venturi, V.; Kankanallu, V.R.; Kuiri, B.; Viswanathan, V.; Aetukuri, N.P.B. Direct correlation between void formation and lithium dendrite growth in solid-state electrolytes with interlayers. Nat. Mater. 2022, 21, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Lu, Z.; Lee, H.-W.; Xiong, F.; Hsu, P.-C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Pei, A.; Zheng, G.Y.; Shi, F.F.; Li, Y.Z.; Cui, Y. Nanoscale Nucleation and Growth of Electrodeposited Lithium Metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef]
- Jaeckle, M.; Gross, A. Microscopic properties of lithium, sodium, and magnesium battery anode materials related to possible dendrite growth. J. Chem. Phys. 2014, 141, 174710. [Google Scholar] [CrossRef]
- Xiao, J. How lithium dendrites form in liquid batteries. Science 2019, 366, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Fleury, V.; Chazalviel, J.N.; Rosso, M.; Sapoval, B. The role of the anions in the growth speed of fractal electrodeposits. J. Electroanal. Chem. Interfacial Electrochem. 1990, 290, 249–255. [Google Scholar] [CrossRef]
- Chazalviel, J. Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys. Rev. A 1990, 42, 7355–7367. [Google Scholar] [CrossRef]
- Seong, I.W.; Hong, C.H.; Kim, B.K.; Yoon, W.Y. The effects of current density and amount of discharge on dendrite formation in the lithium powder anode electrode. J. Power Sources 2008, 178, 769–773. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 2005, 152, A396–A404. [Google Scholar] [CrossRef]
- Ota, H.; Shima, K.; Ue, M.; Yamaki, J. Effect of vinylene carbonate as additive to electrolyte for lithium metal anode. Electrochim. Acta 2004, 49, 565–572. [Google Scholar] [CrossRef]
- Fang, C.C.; Li, J.X.; Zhang, M.H.; Zhang, Y.H.; Yang, F.; Lee, J.Z.; Lee, M.H.; Alvarado, J.; Schroeder, M.A.; Yang, Y.Y.C.; et al. Quantifying inactive lithium in lithium metal batteries. Nature 2019, 572, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, Y.; Tao, M.; Zhong, G.; Liang, Z.; Zheng, G.; Huang, X.; Liu, X.; Jin, Y.; Xu, N.; Armand, M.; et al. Quantitatively analyzing the failure processes of rechargeable Li metal batteries. Sci. Adv. 2021, 7, eabj3423. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Cohen, Y.S.; Cohen, Y.; Aurbach, D. Micromorphological studies of lithium electrodes in alkyl carbonate solutions using in situ atomic force microscopy. J. Phys. Chem. B 2000, 104, 12282–12291. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L., III. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef] [Green Version]
- Aurbach, D.; Daroux, M.L.; Faguy, P.W.; Yeager, E. IDENTIFICATION OF SURFACE-FILMS FORMED ON LITHIUM IN PROPYLENE CARBONATE SOLUTIONS. J. Electrochem. Soc. 1987, 134, 1611–1620. [Google Scholar] [CrossRef]
- Aurbach, D.; Zaban, A.; Ein-Eli, Y.; Weissman, I.; Chusid, O.; Markovsky, B.; Levi, M.; Levi, E.; Schechter, A.; Granot, E. Recent studies on the correlation between surface chemistry, morphology, three-dimensional structures and performance of Li and Li-C intercalation anodes in several important electrolyte systems. J. Power Sources 1997, 68, 91–98. [Google Scholar] [CrossRef]
- Aurbach, D.; Zaban, A.; Gofer, Y.; Ely, Y.E.; Weissman, I.; Chusid, O.; Abramson, O. Recent studies of the lithium liquid electrolyte interface-electrochemical, morphological and spectral studies of a few important systems. J. Power Sources 1995, 54, 76–84. [Google Scholar] [CrossRef]
- Aurbach, D. Review of selected electrode-solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 2000, 89, 206–218. [Google Scholar] [CrossRef]
- Peled, E.; Ardel, G. Advanced Model for Solid Electrolyte Interphase Electrodes in Liquid and Polymer Electrolytes. J. Electrochem. Soc. 1997, 144, L208–L210. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Pei, A.; Yan, K.; Sun, Y.; Wu, C.-L.; Joubert, L.-M.; Chin, R.; Koh, A.L.; Yu, Y.; et al. Atomic structure of sensitive battery materials and Interfaces revealed by cryo-electron microscopy. Science 2017, 358, 506–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasubramanian, A.; Yurkiv, V.; Foroozan, T.; Ragone, M.; Shahbazian-Yassar, R.; Mashayek, F.l. Lithium Diffusion Mechanism through Solid-Electrolyte Interphase in Rechargeable Lithium Batteries. J. Phys. Chem. C 2019, 123, 10237–10245. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Cheng, X.B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene Carbonate Additives to Render Uniform Li Deposits in Lithium Metal Batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Mogi, R.; Inaba, M.; Jeong, S.K.; Iriyama, Y.; Abe, T.; Ogumi, Z. Effects of some organic additives on lithium deposition in propylene carbonate. J. Electrochem. Soc. 2002, 149, A1578–A1583. [Google Scholar] [CrossRef]
- Mukra, T.; Peled, E. Elucidation of the Losses in Cycling Lithium-Metal Anodes in Carbonate-Based Electrolytes. J. Electrochem. Soc. 2020, 167, 100520. [Google Scholar] [CrossRef]
- Zhang, M.S.; Liu, R.J.; Wang, Z.K.; Xing, X.Y.; Liu, Y.G.; Deng, B.B.; Yang, T. Electrolyte additive maintains high performance for dendrite-free lithium metal anode. Chin. Chem. Lett. 2020, 31, 1217–1220. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhao, M.Q.; Chen, C.; Pentecost, A.; Maleski, K.; Mathis, T.; Zhang, X.Q.; Zhang, Q.; Jiang, J.J.; Gogotsi, Y. Nanodiamonds suppress the growth of lithium dendrites. Nat. Commun. 2017, 8, 336. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Chen, W.; Lei, T.; Jiao, Y.; Wang, H.; Wang, X.; Rao, G.; Wang, X.; Chen, B.; Xiong, J. Graphene Quantum Dots as the Nucleation Sites and Interfacial Regulator to Suppress Lithium Dendrites for High-Loading Lithium-Sulfur Battery. Nano Energy 2019, 68, 104373. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, T.; Ye, Y.; Yang, T.; Luo, R.; Li, L.; Wu, F.; Chen, R. A Designed Lithiophilic Carbon Channel on Separator to Regulate Lithium Deposition Behavior. Small 2022, 18, 2104390. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, M.; Zhou, X.; Shin, K.; Tunmee, S.; Zhang, X.; Xie, C.; Saitoh, H.; Zheng, Y.; Zhou, Z.; et al. In Situ Chemical Lithiation Transforms Diamond-Like Carbon into an Ultrastrong Ion Conductor for Dendrite-Free Lithium-Metal Anodes. Adv. Mater. 2021, 33, 2100793. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Wang, X.D.; Sun, W.; Sun, K.N. Dendrite-Free Lithium Metal Anodes in High Performance Lithium-Sulfur Batteries with Bifunctional Carbon Nanofiber Interlayers. Electrochim. Acta 2017, 252, 127–137. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Xin, L.; Liu, Y.; Yang, F.; Stach, E.A.; Xie, J. Making Li-metal electrodes rechargeable by controlling the dendrite growth direction. Nat. Energy 2017, 2, 17083. [Google Scholar] [CrossRef]
- Wondimkun, Z.T.; Beyene, T.T.; Weret, M.A.; Sahalie, N.A.; Huang, C.-J.; Thirumalraj, B.; Jote, B.A.; Wang, D.; Su, W.-N.; Wang, C.-H.; et al. Binder-free ultra-thin graphene oxide as an artificial solid electrolyte interphase for anode-free rechargeable lithium metal batteries. J. Power Sources 2020, 450, 227589. [Google Scholar] [CrossRef]
- Liu, W.; Xia, Y.; Wang, W.; Wang, Y.; Jin, J.; Chen, Y.; Paek, E.; Mitlin, D. Pristine or Highly Defective? Understanding the Role of Graphene Structure for Stable Lithium Metal Plating. Adv. Energy Mater. 2019, 9, 1802918. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, P.; Ma, J.; Wei, L.; Zhao, L.; Cheng, J.; Su, Y.; Gu, Y.; Lian, Y.; Peng, Y.; et al. Wax-Transferred Hydrophobic CVD Graphene Enables Water-Resistant and Dendrite-Free Lithium Anode toward Long Cycle Li-Air Battery. Adv. Sci. 2021, 8, 2100488. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.; Ding, Y.; Zhang, L.; Yu, G. Reversible Deposition of Lithium Particles Enabled by Ultraconformal and Stretchable Graphene Film for Lithium Metal Batteries. Adv. Mater. 2020, 32, 2005763. [Google Scholar] [CrossRef]
- Xie, K.; Wei, W.; Yuan, K.; Lu, W.; Guo, M.; Li, Z.; Song, Q.; Liu, X.; Wang, J.-G.; Shen, C. Toward Dendrite-Free Lithium Deposition via Structural and Interfacial Synergistic Effects of 3D Graphene@Ni Scaffold. ACS Appl. Mater. Interfaces 2016, 8, 26091–26097. [Google Scholar] [CrossRef]
- Fan, L.; Sun, B.; Yan, K.; Xiong, P.; Guo, X.; Guo, Z.; Zhang, N.; Feng, Y.; Sun, K.; Wang, G. A Dual-Protective Artificial Interface for Stable Lithium Metal Anodes. Adv. Energy Mater. 2021, 11, 2102242. [Google Scholar] [CrossRef]
- Zhu, J.; Li, P.; Chen, X.; Legut, D.; Fan, Y.; Zhang, R.; Lu, Y.; Cheng, X.; Zhang, Q. Rational design of graphitic-inorganic Bi-layer artificial SEI for stable lithium metal anode. Energy Stor. Mater. 2019, 16, 426–433. [Google Scholar] [CrossRef]
- Tang, K.; Xiao, J.; Li, X.; Wang, D.; Long, M.; Chen, J.; Gao, H.; Chen, W.; Liu, C.; Liu, H. Advances of Carbon-Based Materials for Lithium Metal Anodes. Front. Chem. 2020, 8, 595972. [Google Scholar] [CrossRef] [PubMed]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, 969–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, W.; Chen, W.; Lu, M.; Zhang, C.; Tian, D.; Wang, L.; Yu, F. In situ generation of Li3N concentration gradient in 3D carbon-based lithium anodes towards highly-stable lithium metal batteries. J. Energy Chem. 2022, 76, 648–656. [Google Scholar] [CrossRef]
- Liu, J.; Ma, H.; Wen, Z.; Li, H.; Yang, J.; Pei, N.; Zhang, P.; Zhao, J. Layered Ag-graphene films synthesized by Gamma ray irradiation for stable lithium metal anodes in carbonate-based electrolytes. J. Energy Chem. 2022, 64, 354–363. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Li, C.; Li, X.; Wu, Y.; Chen, X.; Wu, J.; Li, X.; Chen, Y. Electrospun carbon nanofibers for lithium metal anodes: Progress and perspectives. Chin. Chem. Lett. 2022, 33, 141–152. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, P.; Li, C.; Yu, J.; Cai, J.; Yang, Z. Needle-like cobalt phosphide arrays grown on carbon fiber cloth as a binder-free electrode with enhanced lithium storage performance. Chin. Chem. Lett. 2021, 32, 154–157. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Liu, Y.; Wei, Y.; Ren, S.; Pan, L.; Su, Y.; Xiao, J.; Fan, H.; Lin, Y.; et al. A flexible artificial solid-electrolyte interlayer supported by compactness-tailored carbon nanotube network for dendrite-free lithium metal anode. J. Energy Chem. 2022, 69, 421–427. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, X.Y.; Yang, M.H.; Cao, X.Q.; Huang, X.Y.; Tian, Y.; Zhang, F.; Li, H.X. Novel S-doped ordered mesoporous carbon nanospheres toward advanced lithium metal anodes. Nano Energy 2020, 69, 104443. [Google Scholar] [CrossRef]
- Qi, J.; Li, Y.; Wei, G.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Nitrogen doped porous hollow carbon spheres for enhanced benzene removal. Sep. Purif. Technol. 2017, 188, 112–118. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, Y.; Zhu, H.; Qin, F.; Han, Z.; Bai, M.; Yang, J.; Li, J.; Hong, B.; Lai, Y. Interconnected stacked hollow carbon spheres uniformly embedded with Ni2P nanoparticles as scalable host for practical Li metal anode. Chem. Eng. J. 2022, 428, 132648. [Google Scholar] [CrossRef]
- Chen, W.; Hong, Y.; Zhao, Z.; Zhang, Y.; Pan, L.; Wan, J.; Nazir, M.; Wang, J.; He, H. Directing the deposition of lithium metal to the inner concave surface of graphitic carbon tubes to enable lithium-metal batteries. J. Mater. Chem. A 2021, 9, 16936–16942. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Y.-X.; Li, J.-Y.; Li, N.-W.; Zeng, X.-X.; Ye, H.; Guo, Y.-G.; Wan, L.-J. Free-Standing Hollow Carbon Fibers as High-Capacity Containers for Stable Lithium Metal Anodes. Joule 2017, 1, 563–575. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Guan, J.; Li, N.W.; Lu, Y.; Luan, D.; Zhang, C.H.; Cheng, G.; Yu, L.; Lou, X.W.D. Lotus-Root-Like Carbon Fibers Embedded with Ni-Co Nanoparticles for Dendrite-Free Lithium Metal Anodes. Adv. Mater. 2021, 33, 2100608. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Lu, Z.X.; Guo, W.; Luo, Q.; Yin, Y.H.; Liu, X.B.; Li, Y.S.; Xia, B.Y.; Wu, Z.P. A Dendrite-Free Lithium/Carbon Nanotube Hybrid for Lithium-Metal Batteries. Adv. Mater. 2021, 33, 2006702. [Google Scholar] [CrossRef]
- Niu, C.J.; Pan, H.L.; Xu, W.; Xiao, J.; Zhang, J.G.; Luo, L.L.; Wang, C.M.; Mei, D.H.; Meng, J.S.; Wang, X.P.; et al. Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions. Nat. Nanotechnol. 2019, 14, 594–601. [Google Scholar] [CrossRef]
- Liu, K.; Li, Z.; Xie, W.; Li, J.; Rao, D.; Shao, M.; Zhang, B.; Wei, M. Oxygen-rich carbon nanotube networks for enhanced lithium metal anode. Energy Stor. Mater. 2018, 15, 308–314. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Liang, Z.; Lee, H.-W.; Sun, J.; Wang, H.; Yan, K.; Xie, J.; Cui, Y. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 2016, 11, 626–632. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, L.; Xu, Z.; Deng, Z.; Wang, X. N, O-Codoped Carbon Nanosheet Array Enabling Stable Lithium Metal Anode. Adv. Funct. Mater. 2021, 31, 2102354. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, P.; Legut, D.; Wang, L.; Liu, X.; Li, B.; Dong, C.; Fan, Y.; Gong, Y.; Zhang, Q. S-Doped Graphene-Regional Nucleation Mechanism for Dendrite-Free Lithium Metal Anodes. Adv. Energy Mater. 2019, 9, 1804000. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zhou, L.; Xiao, Z.; Zhou, S.; Zhang, X.; Li, L.; Zhi, L. A synergistic strategy for stable lithium metal anodes using 3D fluorine-doped graphene shuttle-implanted porous carbon networks. Nano Energy 2018, 49, 179–185. [Google Scholar] [CrossRef]
- Fang, Y.; Zeng, Y.; Jin, Q.; Lu, X.F.; Luan, D.; Zhang, X.; Lou, X.W.D. Nitrogen-Doped Amorphous Zn-Carbon Multichannel Fibers for Stable Lithium Metal Anodes. Angew. Chem. Int. Ed. 2021, 60, 8515–8520. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, L.; Wu, F.; Chen, R. A Soft Lithiophilic Graphene Aerogel for Stable Lithium Metal Anode. Adv. Funct. Mater. 2020, 30, 2002013. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, C.; Li, S.; Guo, W.; Zhao, Y.; Dong, Q.; Lin, X.; Song, Z.; Tan, X.; Wang, C.; et al. Ultrahigh-Capacity and Long-Life Lithium-Metal Batteries Enabled by Engineering Carbon Nanofiber-Stabilized Graphene Aerogel Film Host. Small 2018, 14, 1803310. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, L.; Tao, H.; Li, Q.; Zhang, J.; Yang, X. Lithiophilicity: The key to efficient lithium metal anodes for lithium batteries. J. Energy Chem. 2022, in press. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, P.; Tian, Y.; Wu, J.; Wang, X.; Li, H.; Liu, X. Uniform lithium nucleation/deposition regulated by N/S co-doped carbon nanospheres towards ultra-stable lithium metal anodes. J. Mater. Chem. A 2022, 10, 1463–1472. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Yin, X.J.; Guo, Z.K.; Zhao, D.; Yang, G.Y.; Chen, A.S.; Fan, L.S.; Zhang, Y.; Zhang, N.Q. High lithiophilic nitrogen-doped carbon nanotube arrays prepared by in-situ catalyze for lithium metal anode. Chin. Chem. Lett. 2021, 32, 2254–2258. [Google Scholar] [CrossRef]
- Chu, Y.T.; Xi, B.J.; Xiong, S.L. One-step construction of MoO2 uniform nanoparticles on graphene with enhanced lithium storage. Chin. Chem. Lett. 2021, 32, 1983–1987. [Google Scholar] [CrossRef]
- Li, D.; Gao, Y.; Xie, C.; Zheng, Z. Au-coated carbon fabric as Janus current collector for dendrite-free flexible lithium metal anode and battery. Appl. Phys. Rev. 2022, 9, 011424. [Google Scholar] [CrossRef]
- Tian, R.; Wan, S.L.; Guan, L.; Duan, H.N.; Guo, Y.P.; Li, H.; Liu, H.Z. Oriented growth of Li metal for stable Li/carbon composite negative electrode. Electrochim. Acta 2018, 292, 227–233. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, D.; Shin, Y.K.; Yan, Z.; Han, Z.; Wang, K.; Hossain, M.J.; Shen, S.; AlZahrani, A.; van Duin, A.C.T.; et al. Stable metal anodes enabled by a labile organic molecule bonded to a reduced graphene oxide aerogel. Proc. Natl. Acad. Sci. USA 2020, 117, 30135–30141. [Google Scholar] [CrossRef]
- Martin, C.; Genovese, M.; Louli, A.J.; Weber, R.; Dahn, J.R. Cycling Lithium Metal on Graphite to Form Hybrid Lithium-Ion/Lithium Metal Cells. Joule 2020, 4, 1296–1310. [Google Scholar] [CrossRef]
- Duan, J.; Zheng, Y.; Luo, W.; Wu, W.; Wang, T.; Xie, Y.; Li, S.; Li, J.; Huang, Y. Is graphite lithiophobic or lithiophilic? Natl. Sci. Rev. 2020, 7, 1208–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Zhang, S.; Weng, S.; Li, X.; Wang, X.; Wang, Z.; Chen, L. Anionic Effect on Enhancing the Stability of a Solid Electrolyte Interphase Film for Lithium Deposition on Graphite. Nano Lett. 2021, 21, 5316–5323. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Xin, S.; Yin, Y.-X.; Li, J.-Y.; Guo, Y.-G.; Wan, L.-J. Stable Li Plating/Stripping Electrochemistry Realized by a Hybrid Li Reservoir in Spherical Carbon Granules with 3D Conducting Skeletons. J. Am. Chem. Soc. 2017, 139, 5916–5922. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, G.; Seh, Z.W.; Liu, N.; Wang, S.; Sun, J.; Lee, H.R.; Cui, Y. Graphite-Encapsulated Li-Metal Hybrid Anodes for High-Capacity Li Batteries. Chem 2016, 1, 287–297. [Google Scholar] [CrossRef]
- Zuo, T.T.; Wu, X.W.; Yang, C.P.; Yin, Y.X.; Ye, H.; Li, N.W.; Guo, Y.G. Graphitized Carbon Fibers as Multifunctional 3D Current Collectors for High Areal Capacity Li Anodes. Adv. Mater. 2017, 29, 1700389. [Google Scholar] [CrossRef]
- Cai, W.; Yan, C.; Yao, Y.X.; Xu, L.; Chen, X.R.; Huang, J.Q.; Zhang, Q. The Boundary of Lithium Plating in Graphite Electrode for Safe Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 13007–13012. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, S.; Tong, Y.; Li, X.; Wang, Z.; Chen, L. Minimizing carbon particle size to improve lithium deposition on natural graphite. Carbon 2019, 155, 9–15. [Google Scholar] [CrossRef]
- Ho, A.S.; Parkinson, D.Y.; Finegan, D.P.; Trask, S.E.; Jansen, A.N.; Tong, W.; Balsara, N.P. 3D Detection of Lithiation and Lithium Plating in Graphite Anodes during Fast Charging. ACS Nano 2021, 15, 10480–10487. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.E.; Su, L.Y.; Huang, S.S.; Fang, C.C.; Wu, Y.S.; Chou, J.; Wu, N.L. A proof-of-concept graphite anode with a lithium dendrite suppressing polymer coating. J. Power Sources 2018, 406, 63–69. [Google Scholar] [CrossRef]
- Cui, C.; Yang, C.; Eidson, N.; Chen, J.; Han, F.; Chen, L.; Luo, C.; Wang, P.F.; Fan, X.; Wang, C. A Highly Reversible, Dendrite-Free Lithium Metal Anode Enabled by a Lithium-Fluoride-Enriched Interphase. Adv. Mater. 2020, 32, 1906427. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, D.; Cai, X.; Zhang, L.; Liu, Y.; Qin, X.; Zhao, R.; Zeng, X.; Han, C.; Zhan, C.; et al. Promoting the reversibility of lithium ion/lithium metal hybrid graphite anode by regulating solid electrolyte interface. Nano Energy 2021, 90, 106510. [Google Scholar] [CrossRef]
- Feng, W.; Dong, X.; Zhang, X.; Lai, Z.; Li, P.; Wang, C.; Wang, Y.; Xia, Y. Li/Garnet Interface Stabilization by Thermal-Decomposition Vapor Deposition of an Amorphous Carbon Layer. Angew. Chem. Int. Ed. 2020, 59, 5346–5349. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, J.; Tong, R.A.; Zhang, J.; Wang, H.; Shao, G.; Wang, C.A. Excellent Li/Garnet Interface Wettability Achieved by Porous Hard Carbon Layer for Solid State Li Metal Battery. Small 2022, 18, 2106142. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Duan, J.; Wu, W.Y.; Nolan, A.M.; Wang, T.R.; Wen, J.Y.; Hu, C.C.; Mo, Y.F.; Luo, W.; Huang, Y.H. Lithium-Graphite Paste: An Interface Compatible Anode for Solid-State Batteries. Adv. Mater. 2019, 31, 1807243. [Google Scholar] [CrossRef]
- Xing, X.; Li, Y.; Wang, S.; Liu, H.; Wu, Z.; Yu, S.; Holoubek, J.; Zhou, H.; Liu, P. YY Graphite-Based Lithium-Free 3D Hybrid Anodes for High Energy Density All-Solid-State Batteries. ACS Energy Lett. 2021, 6, 1831–1838. [Google Scholar] [CrossRef]
- Bao, W.; Hu, Z.Y.; Wang, Y.Y.; Jiang, J.H.; Huo, S.K.; Fan, W.Z.; Chen, W.J.; Jing, X.; Long, X.Y.; Zhang, Y.F. Poly(ionic liquid)-functionalized graphene oxide towards ambient temperature operation of all-solid-state PEO-based polymer electrolyte lithium metal batteries. Chem. Eng. J. 2022, 437, 135420. [Google Scholar] [CrossRef]
- Nicotera, I.; Simari, C.; Agostini, M.; Enotiadis, A.; Brutti, S. A Novel Li+-Nafion-Sulfonated Graphene Oxide Membrane as Single Lithium-Ion Conducting Polymer Electrolyte for Lithium Batteries. J. Phys. Chem. C 2019, 123, 27406–27416. [Google Scholar] [CrossRef]
- Li, Z.Y.; Liu, F.; Chen, S.S.; Zhai, F.; Li, Y.; Feng, Y.Y.; Feng, W. Single Li ion conducting solid-state polymer electrolytes based on carbon quantum dots for Li-metal batteries. Nano Energy 2021, 82, 105698. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Sun, K.; Wang, Z. A Review of the Application of Carbon Materials for Lithium Metal Batteries. Batteries 2022, 8, 246. https://doi.org/10.3390/batteries8110246
Wu Z, Sun K, Wang Z. A Review of the Application of Carbon Materials for Lithium Metal Batteries. Batteries. 2022; 8(11):246. https://doi.org/10.3390/batteries8110246
Chicago/Turabian StyleWu, Zeyu, Kening Sun, and Zhenhua Wang. 2022. "A Review of the Application of Carbon Materials for Lithium Metal Batteries" Batteries 8, no. 11: 246. https://doi.org/10.3390/batteries8110246
APA StyleWu, Z., Sun, K., & Wang, Z. (2022). A Review of the Application of Carbon Materials for Lithium Metal Batteries. Batteries, 8(11), 246. https://doi.org/10.3390/batteries8110246





