Unveil Overcharge Performances of Activated Carbon Cathode in Various Li-Ion Electrolytes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Stable Upper Potential Limits of AC Electrode
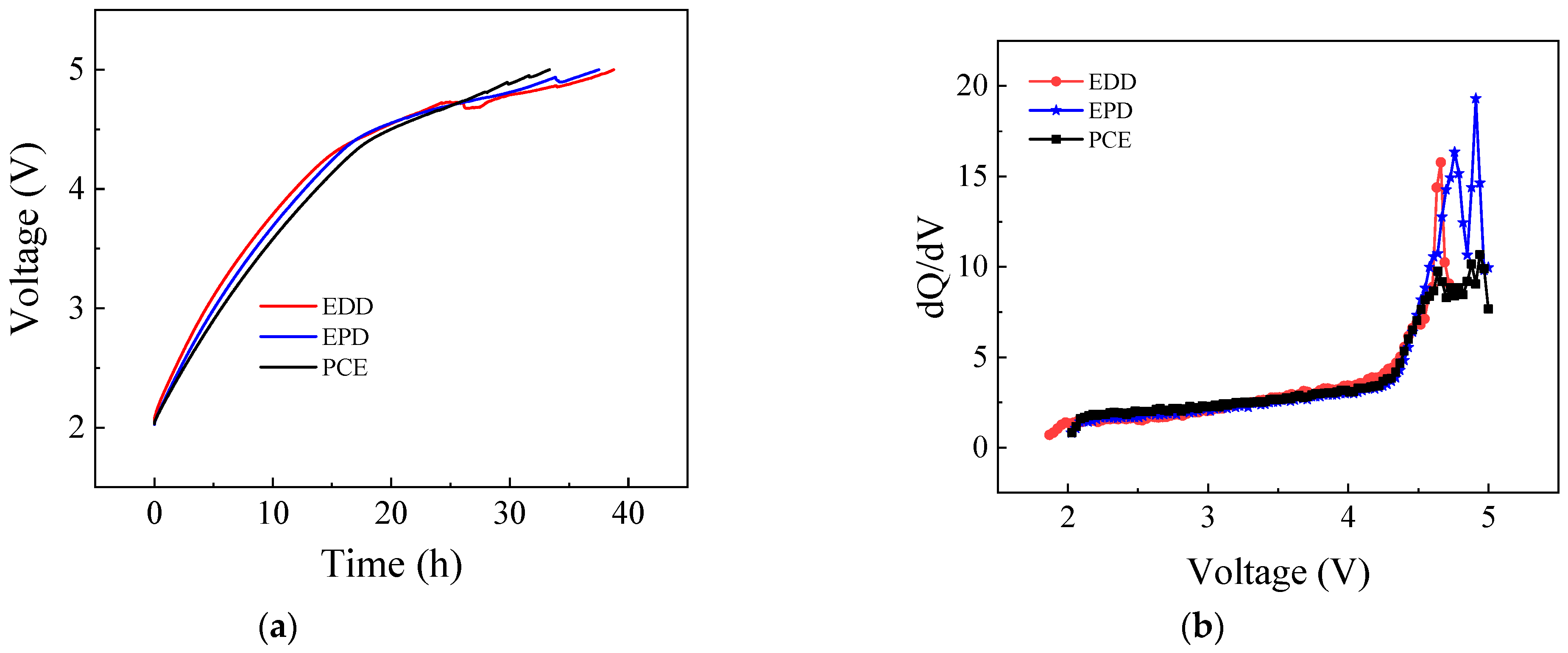
3.2. Overcharge to 5.0 V
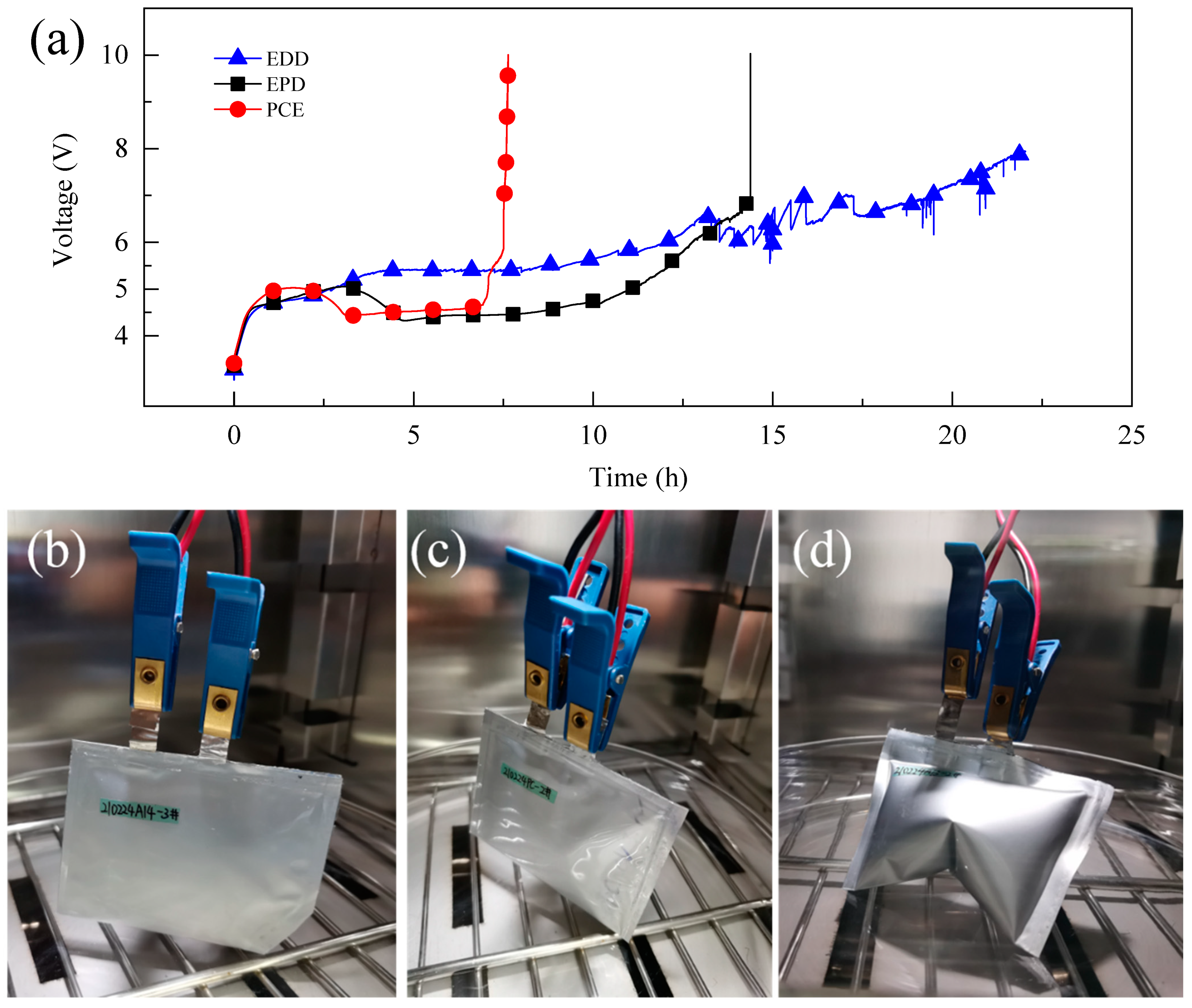
3.3. Overcharge to 10.0 V
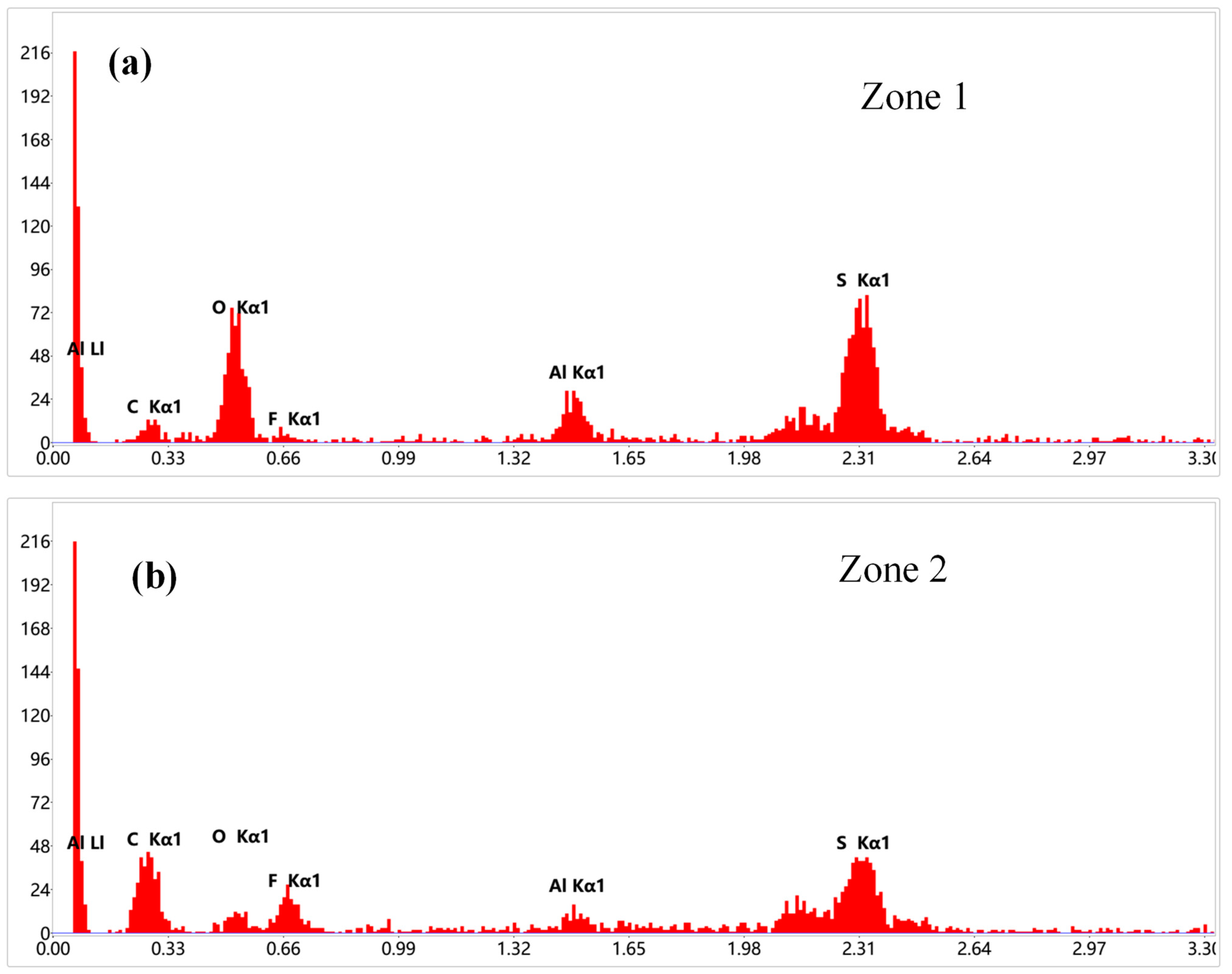
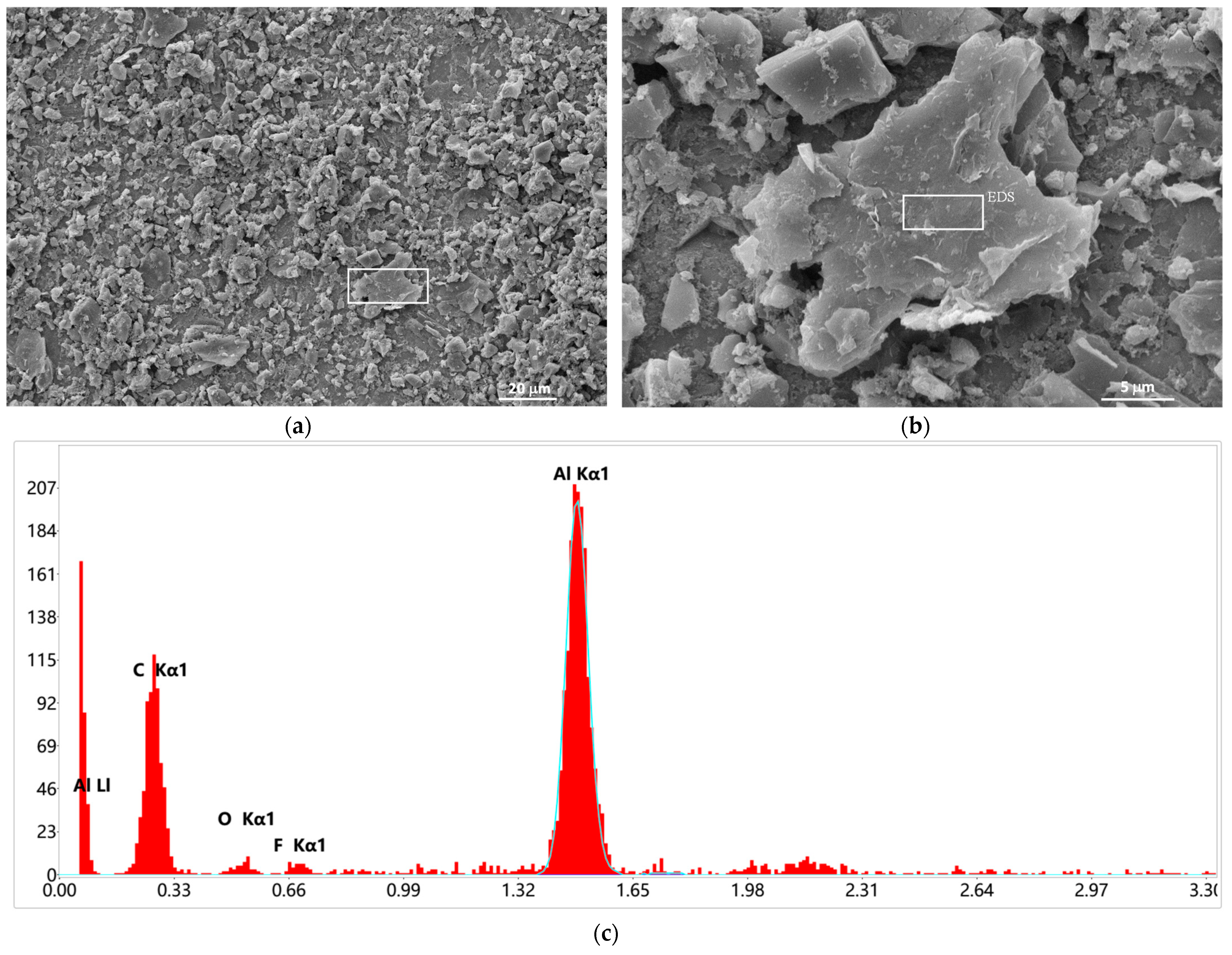
3.4. Post-Mortem Analysis of Half-Cells
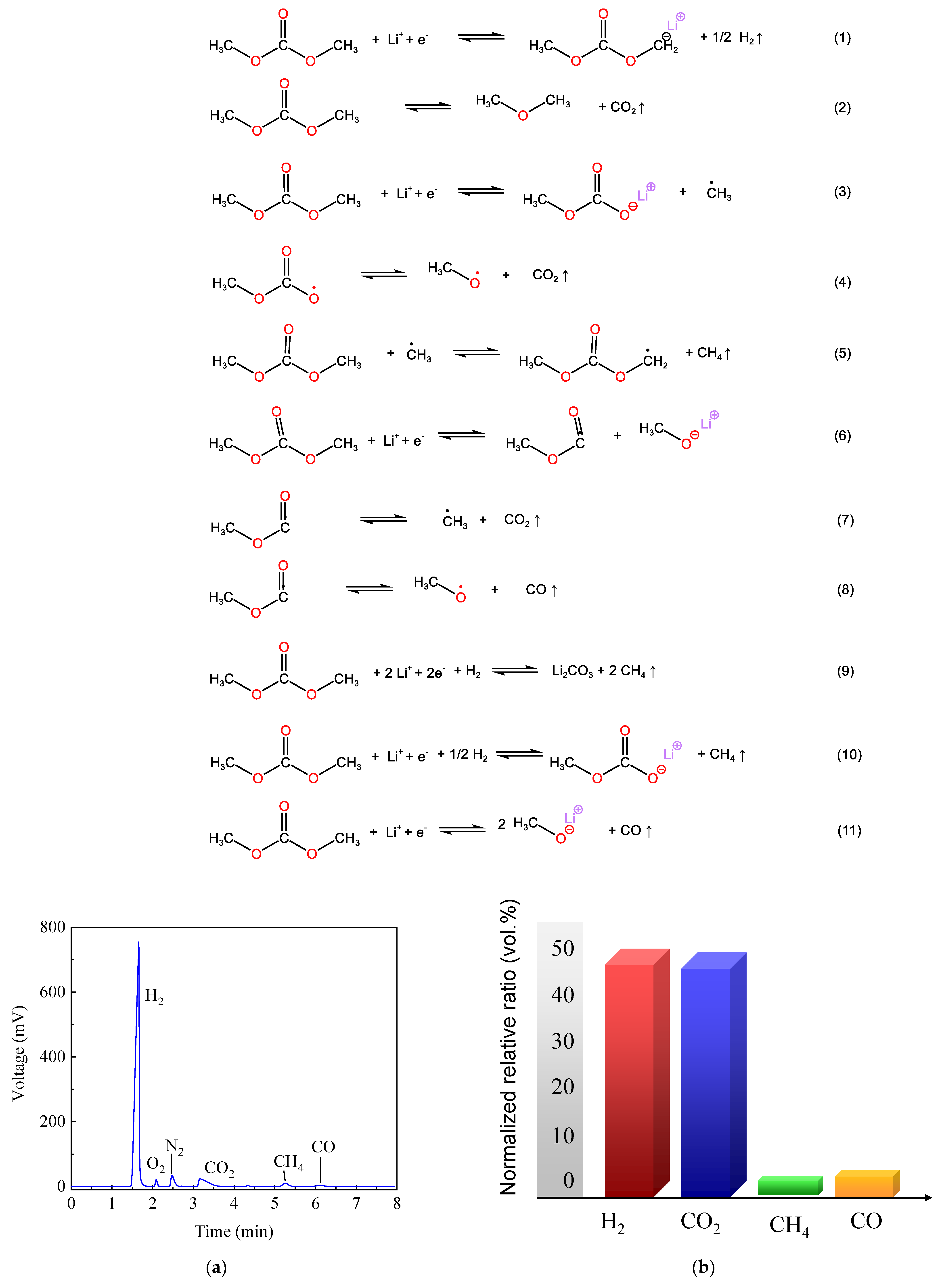
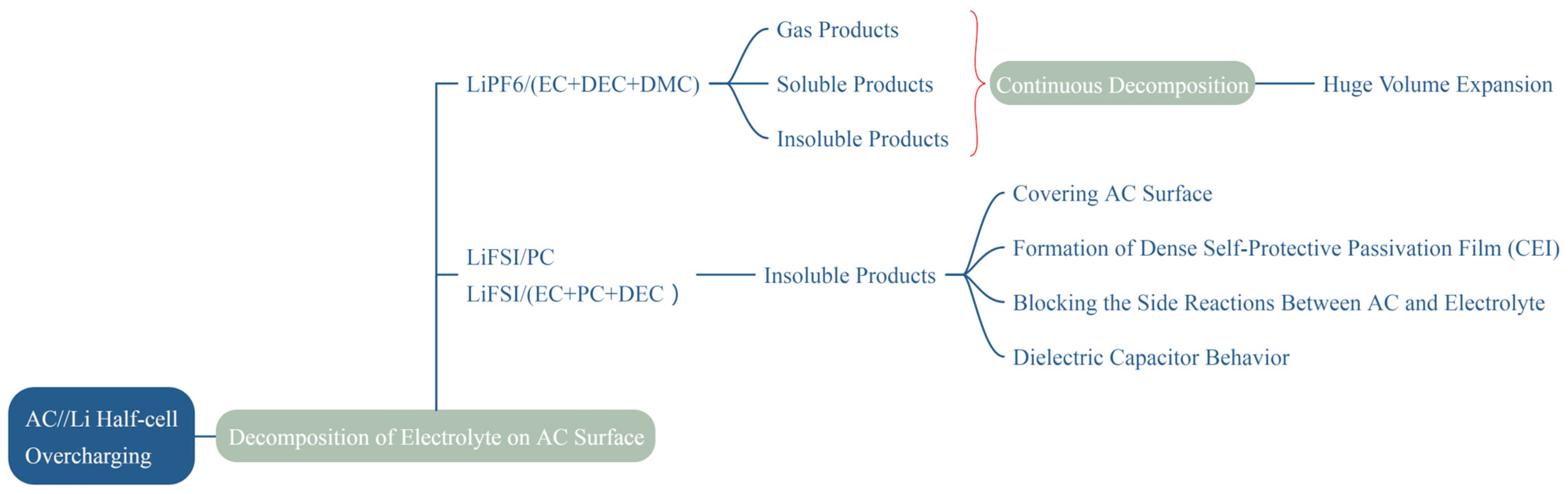
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater 2020, 19, 1151–1163. [Google Scholar] [PubMed]
- Feng, X.; Shi, X.; Ning, J.; Wang, D.; Zhang, J.; Hao, Y.; Wu, Z.-S. Recent advances in micro-supercapacitors for AC line-filtering performance: From fundamental models to emerging applications. eScience 2021, 1, 124–140. [Google Scholar] [CrossRef]
- Karimi, D.; Behi, H.; Van Mierlo, J.; Berecibar, M. A Comprehensive Review of Lithium-Ion Capacitor Technology: Theory, Development, Modeling, Thermal Management Systems, and Applications. Molecules 2022, 27, 3119. [Google Scholar] [CrossRef]
- Eguchi, T.; Sugawara, R.; Abe, Y.; Tomioka, M.; Kumagai, S. Impact of full prelithiation of Si-based anodes on the rate and cycle performance of Li-ion capacitors. Batteries 2022, 8, 49. [Google Scholar] [CrossRef]
- Han, F.; Qian, O.; Meng, G.; Lin, D.; Chen, G.; Zhang, S.; Pan, Q.; Zhang, X.; Zhu, X.; Wei, B. Structurally integrated 3D carbon tube grid-based high-performance filter capacitor. Science 2022, 377, 1004. [Google Scholar]
- Ding, J.; Hu, W.; Paek, E.; Mitlin, D. Review of hybrid ion capacitors: From aqueous to lithium to sodium. Chem. Rev. 2018, 118, 6457–6498. [Google Scholar] [CrossRef]
- Han, P.X.; Xu, G.J.; Han, X.Q.; Zhao, J.W.; Zhou, X.H.; Cui, G.L. Lithium ion capacitors in organic electrolyte system: Scientific problems, material development, and key technologies. Adv. Energy Mater. 2018, 8, 1801243. [Google Scholar] [CrossRef]
- Arnaiz, M.; Canal-Rodríguez, M.; Carriazo, D.; Villaverde, A.; Ajuria, J. Enabling versatile, custom-made lithium-ion capacitor prototypes: Benefits and drawbacks of using hard carbon instead of graphite. Electrochim. Acta 2023, 437, 141456. [Google Scholar] [CrossRef]
- Arnaiz, M.; Ajuria, J. Pre-lithiation strategies for lithium ion capacitors: Past, present, and future. Batter. Supercaps 2021, 4, 733–748. [Google Scholar] [CrossRef]
- Yuan, J.; Qin, N.; Lu, Y.; Jin, L.; Zheng, J.; Zheng, J.P. The effect of electrolyte additives on the rate performance of hard carbon anode at low temperature for lithium-ion capacitor. Chin. Chem. Lett. 2022, 33, 3889–3893. [Google Scholar] [CrossRef]
- Jin, L.M.; Yuan, J.M.; Shellikeri, A.; Naderi, R.; Qin, N.; Lu, Y.Y.; Fan, R.L.; Wu, Q.; Zheng, J.S.; Zhang, C.M.; et al. An overview on design parameters of practical lithium-ion capacitors. Batter. Supercaps 2021, 4, 749. [Google Scholar] [CrossRef]
- Oca, L.; Guillet, N.; Tessard, R.; Iraola, U. Lithium-ion capacitor safety assessment under electrical abuse tests based on ultrasound characterization and cell opening. J. Energy Storage 2019, 23, 29. [Google Scholar] [CrossRef]
- Bolufawi, O.; Shellikeri, A.; Zheng, J.P. Lithium-ion capacitor safety testing for commercial application. Batteries 2019, 5, 74. [Google Scholar] [CrossRef] [Green Version]
- Soltani, M.; Beheshti, S.H. A comprehensive review of lithium ion capacitor: Development, modelling, thermal management and applications. J. Energy Storage 2021, 34, 102019. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Jiang, L.H.; Liang, C.; Zhao, C.P.; Wei, Z.S.; Wang, Q.S. Effects of 3-fluoroanisol as an electrolyte additive on enhancing the overcharge endurance and thermal stability of lithium-ion batteries. J. Electrochem. Soc. 2020, 167, 130517. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Wang, K.; An, Y.; Zhang, X.; Li, C.; Ma, Y. Determination strategy of stable electrochemical operating voltage window for practical lithium-ion capacitors. Electrochim. Acta 2022, 428, 140972. [Google Scholar] [CrossRef]
- Liang, J.; Wang, D.W. Design Rationale and Device Configuration of Lithium-Ion Capacitors. Adv. Energy Mater 2022, 12, 2200920. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Shen, C.; Qin, N.; Zheng, J.; Wu, Q.; Zhang, C.; Zheng, J.P. A universal matching approach for high power-density and high cycling-stability lithium ion capacitor. J. Power Sources 2019, 441, 227211. [Google Scholar] [CrossRef]
- El Ghossein, N.; Sari, A.; Venet, P. Development of a capacitance versus voltage model for lithium-ion capacitors. Batteries 2020, 6, 54. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Z.; Han, G.; Zhao, Q.; Lu, G.; Hu, X.; Sun, J.; Wang, R.; Xu, C. Nitrogen-doped activated porous carbon for 4.5 V lithium-ion capacitor with high energy and power density. J. Energy Storage 2022, 47, 103675. [Google Scholar] [CrossRef]
- Santos, M.C.G.; Silva, G.G.; Santamaria, R.; Ortega, P.F.R.; Lavall, R.L. Discussion on operational voltage and efficiencies of ionic-liquid-based electrochemical capacitors. J. Phys. Chem. C. 2019, 123, 8541. [Google Scholar] [CrossRef]
- Bahdanchyk, M.; Hashempour, M.; Vicenzo, A. Evaluation of the operating potential window of electrochemical capacitors. Electrochim. Acta 2020, 332, 135503. [Google Scholar] [CrossRef] [Green Version]
- Cougnon, C. Exploring the interdependence between the coulombic, voltage and energy efficiencies. Electrochem. Commun. 2020, 120, 106832. [Google Scholar] [CrossRef]
- Levi, M.; Levy, N.; Sigalov, S.; Salitra, G.; Aurbach, D.; Maier, J. Electrochemical quartz crystal microbalance (EQCM) studies of ions and solvents insertion into highly porous activated carbons. J. Am. Chem. Soc. 2010, 132, 13220. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Jin, L.; Lu, Y.; Wu, Q.; Zheng, J.; Zhang, C.; Chen, Z.; Zheng, J.P. Over-potential tailored thin and dense lithium carbonate growth in solid electrolyte interphase for advanced lithium ion batteries. Adv. Energy Mater. 2022, 12, 2103402. [Google Scholar] [CrossRef]
- Sun, X.; An, Y.; Geng, L.; Zhang, X.; Wang, K.; Yin, J.; Huo, Q.; Wei, T.; Zhang, X.; Ma, Y. Leakage current and self-discharge in lithium-ion capacitor. J. Electroanal. Chem. 2019, 850, 113386. [Google Scholar] [CrossRef]
- Ishimoto, S.; Asakawa, Y.; Shinya, M.; Naoi, K. Degradation responses of activated-carbon-based EDLCs for higher voltage operation and their factors. J. Electrochem. Soc. 2009, 156, A563. [Google Scholar] [CrossRef]
- An, Z.; Xu, X.; Fan, L.; Yang, C.; Xu, J. Investigation of Electrochemical Performance and Gas Swelling Behavior on Li4Ti5O12/Activated Carbon Lithium-Ion Capacitor with Acetonitrile-Based and Ester-Based Electrolytes. Electronics 2021, 10, 2623. [Google Scholar] [CrossRef]
- Cao, W.; Li, Q.; Yu, X.; Li, H. Controlling Li deposition below the interface. eScience 2022, 2, 47. [Google Scholar] [CrossRef]
- Fernandes, Y.; Bry, A.; de Persis, S. Thermal degradation analyses of carbonate solvents used in Li-ion batteries. J. Power Sources 2019, 414, 250. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95. [Google Scholar] [CrossRef]
- Fernandes, Y.; Bry, A.; de Persis, S. Identification and quantification of gases emitted during abuse tests by overcharge of a commercial Li-ion battery. J. Power Sources. 2018, 389, 106. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; An, Y.; Zhang, X.; Wang, K.; Yuan, C.; Zhang, X.; Li, C.; Xu, Y.; Ma, Y. Unveil Overcharge Performances of Activated Carbon Cathode in Various Li-Ion Electrolytes. Batteries 2023, 9, 11. https://doi.org/10.3390/batteries9010011
Sun X, An Y, Zhang X, Wang K, Yuan C, Zhang X, Li C, Xu Y, Ma Y. Unveil Overcharge Performances of Activated Carbon Cathode in Various Li-Ion Electrolytes. Batteries. 2023; 9(1):11. https://doi.org/10.3390/batteries9010011
Chicago/Turabian StyleSun, Xianzhong, Yabin An, Xiong Zhang, Kai Wang, Changzhou Yuan, Xiaohu Zhang, Chen Li, Yanan Xu, and Yanwei Ma. 2023. "Unveil Overcharge Performances of Activated Carbon Cathode in Various Li-Ion Electrolytes" Batteries 9, no. 1: 11. https://doi.org/10.3390/batteries9010011
APA StyleSun, X., An, Y., Zhang, X., Wang, K., Yuan, C., Zhang, X., Li, C., Xu, Y., & Ma, Y. (2023). Unveil Overcharge Performances of Activated Carbon Cathode in Various Li-Ion Electrolytes. Batteries, 9(1), 11. https://doi.org/10.3390/batteries9010011








