Rational A/B Site Ion Doping to Design Efficient and Stable Pr0.5Ba0.4Ca0.1Fe1-xCoxO3-δ Perovskites as Zinc–Air Batteries Cathode
Abstract
:1. Introduction
2. Experimental Section
2.1. The Synthesis of Material
2.2. Characterizations
2.3. Electrochemical Measurements
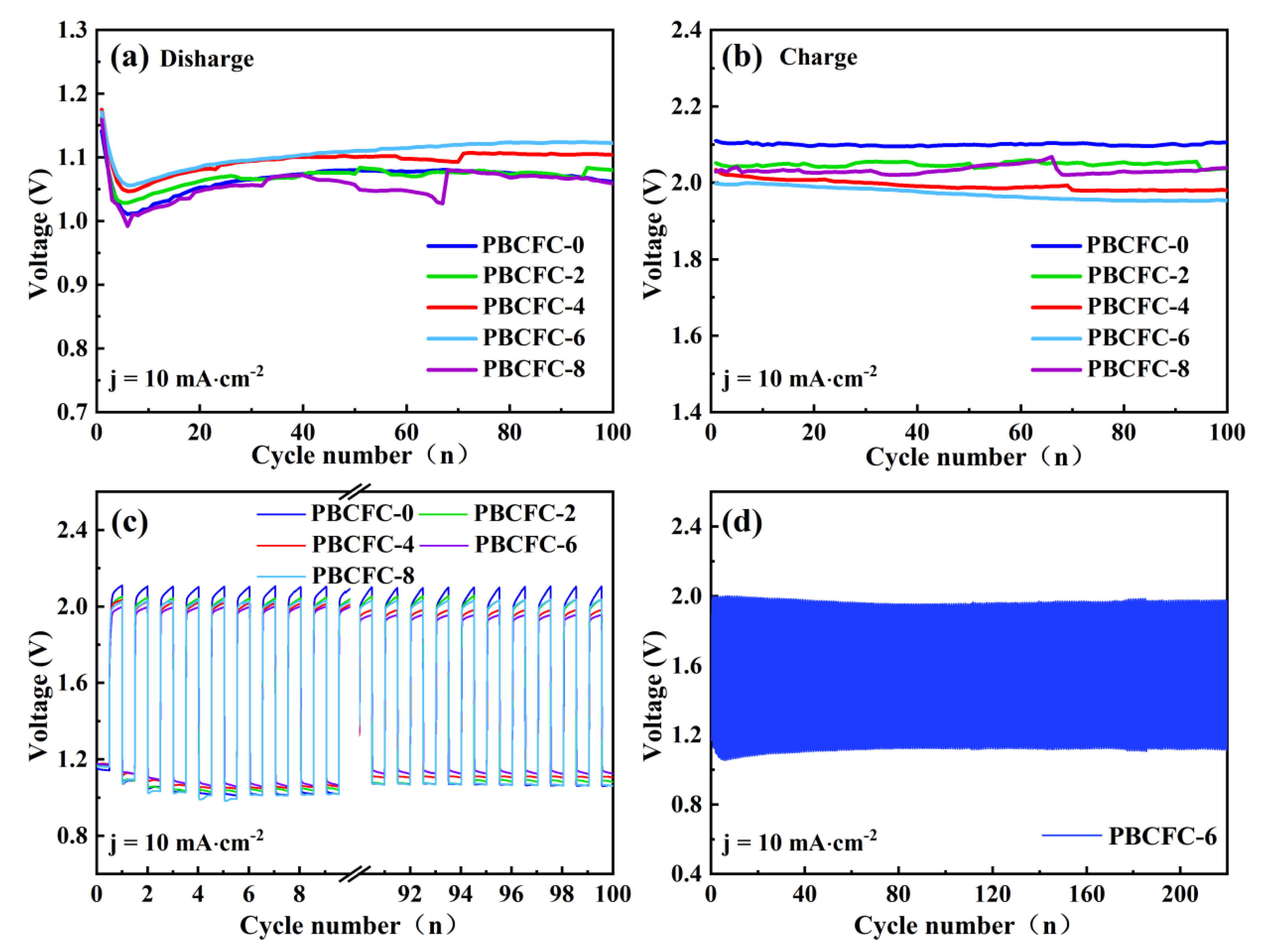
2.4. Characterization and Preparation of Zinc–Air Batteries
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.L.; Zhou, W.; Chen, Y.B.; Yu, J.; Liu, M.L.; Shao, Z.P. A High-Performance Electrocatalyst for Oxygen Evolution Reaction: LiCo0.8Fe0.2O2. Adv. Mater. 2015, 27, 7150–7155. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.J.; Chen, C.; Baiyee, Z.M.; Shao, Z.P.; Ciucci, F. Nonstoichiometric Oxides as Low-Cost and Highly-Efficient Oxygen Reduction/Evolution Catalysts for Low-Temperature Electrochemical Devices. Chem. Rev. 2015, 115, 9869–9921. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.A.; Kumar, K.D.; Kim, H.J. Facile preparation of a highly efficient NiZn2O4-NiO anoflower composite grown on Ni foam as an advanced battery-type electrode material for high-performance electrochemical supercapacitors. Dalton Trans. 2020, 49, 3622–3629. [Google Scholar] [CrossRef] [PubMed]
- Yedluri, A.K.; Kim, H.J. Wearable super-high specific performance supercapacitors using a honeycomb with folded silk-like composite of NiCo2O4 nanoplates decorated with NiMoO4 honeycombs on nickel foam. Dalton Trans. 2018, 47, 15545–15554. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kumar, K.D.; Kim, H.J. Reagents assisted ZnCo2O4 nanomaterial for supercapacitor application. Electrochim. Acta 2020, 330, 135261. [Google Scholar] [CrossRef]
- Mola, B.A.; Pallavolu, M.R.; Al-Asbahi, B.A.; Noh, Y.; Jilcha, S.K.; Kumar, Y.A. Design and construction of hierarchical MnFe2Ce4@MnNiCe4 nanosheets on Ni foam as an advanced electrode for battery-type supercapacitor applications. J. Energy Storage 2022, 51, 104542. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kumar, Y.A.; Sambasivam, S.; Hira, S.A.; Krishna, T.N.V.; Zeb, K.; Uddin, W.; Kumar, K.D.; Obaidat, I.M.; Kim, S.; et al. Highly efficient copper-cobalt sulfide nano-reeds array with simplistic fabrication strategy for battery-type supercapacitors. J. Energy Storage 2020, 32, 101988. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O-2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Khan, Z.; Vagin, M.; Crispin, X. Can Hybrid Na-Air Batteries Outperform Nonaqueous Na-O2 Batteries? Adv. Sci. 2020, 7, 1902866. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.D.; Liu, Q.; Chen, Z.G. Advances in thermoelectric devices for localized cooling. Chem. Eng. J. 2022, 450, 138389. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Xiao, M.L.; Zhu, J.B.; Zeng, T.T.; Li, J.D.; Zhang, W.Y.; Su, D.; Yu, A.P.; Chen, Z.W. A “trimurti” heterostructured hybrid with an intimate CoO/CoxP interface as a robust bifunctional air electrode for rechargeable Zn-air batteries. J. Mater. Chem. A 2020, 8, 9177–9184. [Google Scholar] [CrossRef]
- Wang, M.F.; Qian, T.; Zhou, J.Q.; Yan, C.L. An Efficient Bifunctional Electrocatalyst for a Zinc-Air Battery Derived from Fe/N/C and Bimetallic Metal-Organic Framework Composites. ACS Appl Mater. Inter. 2017, 9, 5213–5221. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.M.; Guo, X.S.; Wang, T.T.; Liu, P.T.; Zhang, H.; Gao, D.Q. Bifunctional porous Co-doped NiO nanoflowers electrocatalysts for rechargeable zinc-air batteries. Appl. Catal. B-Environ. 2019, 250, 71–77. [Google Scholar] [CrossRef]
- Guo, S.J.; Zhang, S.; Sun, S.H. Tuning Nanoparticle Catalysis for the Oxygen Reduction Reaction. Angew Chem. Int Ed. 2013, 52, 8526–8544. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Yu, H.J.; Yin, S.L.; Xu, Y.; Li, X.N.; Yamauchi, Y.; Xue, H.R.; Wang, L. In situ coating of a continuous mesoporous bimetallic PtRu film on Ni foam: A nanoarchitectured self-standing all-metal mesoporous electrode. J. Mater. Chem. A 2018, 6, 12744–12750. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zaman, W.Q.; Sun, W.; Cao, L.M.; Tariq, M.; Yang, J. Cultivating crystal lattice distortion in IrO2 via coupling with MnO2 to boost the oxygen evolution reaction with high intrinsic activity. Chem. Commun. 2018, 54, 4959–4962. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Bick, D.S.; Kindsmuller, A.; Staikov, G.; Gunkel, F.; Muller, D.; Schneller, T.; Waser, R.; Valov, I. Stability and Degradation of Perovskite Electrocatalysts for Oxygen Evolution Reaction. Electrochim. Acta 2016, 218, 156–162. [Google Scholar] [CrossRef]
- Xue, Y.J.; Miao, H.; Sun, S.S.; Wang, Q.; Li, S.H.; Liu, Z.P. (La1-xSrx)(0.98)MnO3 perovskite with A-site deficiencies toward oxygen reduction reaction in aluminum-air batteries. J. Power Sources 2017, 342, 192–201. [Google Scholar] [CrossRef]
- Miao, H.; Wang, Z.H.; Wang, Q.; Sun, S.S.; Xue, Y.J.; Wang, F.; Zhao, J.P.; Liu, Z.P.; Yuan, J.L. A new family of Mn-based perovskite (La1-xYxMnO3) with improved oxygen electrocatalytic activity for metal-air batteries. Energy 2018, 154, 561–570. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.R.; Almomani, F.; Malik, S.S.; Suslov, S.; Tarlochan, F. Combustion synthesis of bifunctional LaMO3 (M = Cr, Mn, Fe, Co, Ni) perovskites for oxygen reduction and oxygen evolution reaction in alkaline media. J. Electroanal. Chem. 2018, 809, 22–30. [Google Scholar] [CrossRef]
- Ge, X.M.; Goh, F.W.T.; Li, B.; Hor, T.S.A.; Zhang, J.; Xiao, P.; Wang, X.; Zong, Y.; Liu, Z.L. Efficient and durable oxygen reduction and evolution of a hydrothermally synthesized La(Co0.55Mn0.45)(0.99)O3-δ nanorod/graphene hybrid in alkaline media. Nanoscale 2015, 7, 9046–9054. [Google Scholar] [CrossRef]
- Prabu, M.; Ramakrishnan, P.; Ganesan, P.; Manthiram, A.; Shanmugam, S. LaTi0.65Fe0.35O3-δ nanoparticle-decorated nitrogen-doped carbon nanorods as an advanced hierarchical air electrode for rechargeable metal-air batteries. Nano Energy 2015, 15, 92–103. [Google Scholar] [CrossRef]
- Gupta, S.; Kellogg, W.; Xu, H.; Liu, X.; Cho, J.; Wu, G. Bifunctional Perovskite Oxide Catalysts for Oxygen Reduction and Evolution in Alkaline Media. Chem.-Asian J. 2016, 11, 10–21. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Zhou, W.; Chen, Z.G.; Chen, Y.B.; Su, C.; Tade, M.O.; Shao, Z.P. SrNb0.1Co0.7Fe0.2O3-δ Perovskite as a Next-Generation Electrocatalyst for Oxygen Evolution in Alkaline Solution. Angew Chem. Int. Ed. 2015, 54, 3897–3901. [Google Scholar] [CrossRef]
- Hua, B.; Zhang, Y.Q.; Yan, N.; Li, M.; Sun, Y.F.; Chen, J.; Li, J.; Luo, J.L. The Excellence of Both Worlds: Developing Effective Double Perovskite Oxide Catalyst of Oxygen Reduction Reaction for Room and Elevated Temperature Applications. Adv. Funct. Mater. 2016, 26, 4106–4112. [Google Scholar] [CrossRef]
- Li, P.Z.; Wei, B.; Lu, Z.; Wu, Y.Y.; Huang, X.Q.; Zhang, Y.H. In-situ reduction synthesis of La2O3/NiM-NCNTs (M = Fe, Co) as efficient bifunctional electrocatalysts for oxygen reduction and evolution reactions. Int. J. Hydrog. Energy 2018, 43, 21959–21968. [Google Scholar] [CrossRef]
- Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.L.; Risch, M.; Hong, W.T.; Zhou, J.G.; Shao-Horn, Y. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun. 2013, 4, 2439. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Dai, Z.Y.; Zhang, Z.B.; Chen, Z.L.; Lin, H.Q.; Gao, Y.; Chen, D.J. Double perovskite PrBaCo2O5.5: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Power Sources 2019, 427, 194–200. [Google Scholar] [CrossRef]
- Bu, Y.F.; Jang, H.; Gwon, O.; Kim, S.H.; Joo, S.H.; Nam, G.; Kim, S.; Qin, Y.; Zhong, Q.; Kwak, S.K.; et al. Synergistic interaction of perovskite oxides and N-doped graphene in versatile electrocatalyst. J. Mater. Chem. A 2019, 7, 2048–2054. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Zhang, L.; Zhao, B.T.; Chen, H.J.; Liu, X.; Zhao, R.; Wang, X.W.; Liu, J.; Chen, Y.; Liu, M.L. Improving the Activity for Oxygen Evolution Reaction by Tailoring Oxygen Defects in Double Perovskite Oxides. Adv. Funct Mater. 2019, 29, 1901783. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Wan, Y.H.; Hua, Z.H.; Tang, K.B.; Xia, C.R. Tungsten-Doped PrBaFe2O5+δ Double Perovskite as a High-Performance Electrode Material for Symmetrical Solid Oxide Fuel Cells. ACS Appl. Energ. Mater. 2021, 4, 8401–8409. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Reimus, L.; Brodersen, P.; Mims, C.A. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered A cations. J. Mater. Chem. 2007, 17, 2500–2505. [Google Scholar] [CrossRef]
- Wu, Z.L.; Sun, L.P.; Xia, T.; Huo, L.H.; Zhao, H.; Rougier, A.; Grenier, J.C. Effect of Sr doping on the electrochemical properties of bi-functional oxygen electrode PrBa1-xSrxCo2O5+δ. J. Power Sources 2016, 334, 86–93. [Google Scholar] [CrossRef]
- Bu, Y.F.; Gwon, O.; Nam, G.; Jang, H.; Kim, S.; Zhong, Q.; Cho, J.; Kim, G. A Highly Efficient and Robust Cation Ordered Perovskite Oxide as a Bifunctional Catalyst for Rechargeable Zinc-Air Batteries. ACS Nano 2017, 11, 11594–11601. [Google Scholar] [CrossRef]
- He, D.D.; He, G.G.; Jiang, H.Q.; Chen, Z.K.; Huang, M.H. Enhanced durability and activity of the perovskite electrocatalyst Pr0.5Ba0.5CoO3-δ by Ca doping for the oxygen evolution reaction at room temperature. Chem. Commun. 2017, 53, 5132–5135. [Google Scholar] [CrossRef]
- Li, X.N.; Zhang, J.; Feng, Q.; Pu, C.Y.; Zhang, L.Z.; Hu, M.M.; Zhou, X.Y.; Zhong, X.W.; Yi, W.D.; Tang, J.; et al. Redox inactive ion meliorated BaCo0.4Fe0.4Zr0.1Y0.1O3-δ perovskite oxides as efficient electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 2018, 6, 17288–17296. [Google Scholar] [CrossRef]
- He, W.; Wu, X.L.; Dong, F.F.; Ni, M. A novel layered perovskite electrode for symmetrical solid oxide fuel cells: PrBa(Fe0.8Sc0.2)(2)O5+δ. J. Power Sources 2017, 363, 16–19. [Google Scholar] [CrossRef]
- Hua, B.; Sun, Y.F.; Li, M.; Yan, N.; Chen, J.; Zhang, Y.Q.; Zeng, Y.M.; Amirkhiz, B.S.; Luo, J.L. Stabilizing Double Perovskite for Effective Bifunctional Oxygen Electrocatalysis in Alkaline Conditions. Chem. Mater. 2017, 29, 6228–6237. [Google Scholar] [CrossRef] [Green Version]
- Li, P.Z.; Wei, B.; Lu, Z.; Wu, Y.Y.; Zhang, Y.H.; Huang, X.Q. La1.7Sr0.3CO0.5Ni0.5O4+δ layered perovskite as an efficient bifunctional electrocatalyst for rechargeable zinc-air batteries. Appl. Surf. Sci. 2019, 464, 494–501. [Google Scholar] [CrossRef]
- Li, K.X.; Dong, Z.H.; Lue, Z. Study of the bifunctional catalytic activity on Sr and Mn co-doped PrFeO3-δ Zinc-Air batteries cathode. Electrochim. Acta 2022, 430, 141123. [Google Scholar] [CrossRef]
- Lim, C.; Sengodan, S.; Jeong, D.; Shin, J.; Kim, G. Investigation of the Fe doping effect on the B-site of the layered perovskite PrBa0.8Ca0.2Co2O5+δ for a promising cathode material of the intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energ. 2019, 44, 1088–1095. [Google Scholar] [CrossRef]
- Lu, C.L.; Niu, B.B.; Yi, W.D.; Ji, Y.; Xu, B.M. Efficient symmetrical electrodes of PrBaFe2-xCoxO5+δ (x=0, 0.2,0.4) for solid oxide fuel cells and solid oxide electrolysis cells. Electrochim. Acta 2020, 358, 136916. [Google Scholar] [CrossRef]
- Ge, X.M.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z.L. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Waidha, A.I.; Ni, L.M.; Ali, J.; Lepple, M.; Donzelli, M.; Dasgupta, S.; Wollstadt, S.; Alff, L.; Kramm, U.I.; Clemens, O. Synthesis of bifunctional BaFe1-xCoxO3-y-δ(OH)(y) catalysts for the oxygen reduction reaction and oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 616–625. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.; Gwon, O.; Kwon, O.; Jeong, H.Y.; Kim, G.; Lee, S.Y. Monolithic heteronanomat paper air cathodes toward origami-foldable/rechargeable Zn-air batteries. J. Mater. Chem. A 2019, 7, 24231–24238. [Google Scholar] [CrossRef]
- Wang, H.C.; Zhang, W.W.; Guan, K.; Wei, Z.Y.; Meng, J.L.; Meng, J.; Liu, X.J. Enhancing Activity and Durability of A-Site-Deficient (La0.6Sr0.4)(0.95)Co0.2Fe0.8O3-δ Cathode by Surface Modification with Pr2-δ Nanoparticles. Acs Sustain. Chem. Eng. 2020, 8, 3367–3380. [Google Scholar] [CrossRef]
- Li, Z.S.; Lv, L.; Wang, J.S.; Ao, X.; Ruan, Y.J.; Zha, D.C.; Hong, G.; Wu, Q.H.; Lan, Y.C.; Wang, C.D.; et al. Engineering phosphorus-doped LaFeO3-δ perovskite oxide as robust bifunctional oxygen electrocatalysts in alkaline solutions. Nano Energy 2018, 47, 199–209. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Lin, Q.; Wang, Z.B.; Qi, D.C.; Yin, Y.C.; Liu, Y.; Zhang, X.W.; Shao, Z.P.; Wang, H.T. Chlorine-anion doping induced multi-factor optimization in perovskties for boosting intrinsic oxygen evolution. J. Energy Chem. 2021, 52, 115–120. [Google Scholar] [CrossRef]
- Zhang, D.W.; Song, Y.F.; Du, Z.Z.; Wang, L.; Li, Y.T.; Goodenough, J.B. Active LaNi1-xFexO3 bifunctional catalysts for air cathodes in alkaline media. J. Mater. Chem. A 2015, 3, 9421–9426. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Zhou, W.; Yu, J.; Chen, Y.B.; Liu, M.L.; Shao, Z.P. Enhancing Electrocatalytic Activity of Perovskite Oxides by Tuning Cation Deficiency for Oxygen Reduction and Evolution Reactions. Chem. Mater. 2016, 28, 1691–1697. [Google Scholar] [CrossRef]
- She, S.X.; Yu, J.; Tang, W.Q.; Zhu, Y.L.; Chen, Y.B.; Sunarso, J.; Zhou, W.; Shao, Z.P. Systematic Study of Oxygen Evolution Activity and Stability on La1-xSrxFeO3-δ Perovskite Electrocatalysts in Alkaline Media. ACS Appl. Mater. Inter. 2018, 10, 11715–11721. [Google Scholar] [CrossRef] [PubMed]
- Mondal, R.; Ratnawat, H.; Mukherjee, S.; Gupta, A.; Singh, P. Investigation of the Role of Sr and Development of Superior Sr-Doped Hexagonal BaCoO3-δ Perovskite Bifunctional OER/ORR Catalysts in Alkaline Media. Energ. Fuel 2022, 36, 3219–3228. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, A.P.; Higgins, D.; Li, H.; Wang, H.J.; Chen, Z.W. Highly Active and Durable Core-Corona Structured Bifunctional Catalyst for Rechargeable Metal-Air Battery Application. Nano Lett. 2012, 12, 1946–1952. [Google Scholar] [CrossRef]
- Lee, D.U.; Choi, J.Y.; Feng, K.; Park, H.W.; Chen, Z.W. Advanced Extremely Durable 3D Bifunctional Air Electrodes for Rechargeable Zinc-Air Batteries. Adv. Energy Mater. 2014, 4, 1301389. [Google Scholar] [CrossRef]
- Wang, Z.; You, Y.; Yuan, J.; Yin, Y.X.; Li, Y.T.; Xin, S.; Zhang, D. Nickel-Doped La0.8Sr0.2Mn(1-x)Ni(x)O3 Nanoparticles Containing Abundant Oxygen Vacancies as an Optimized Bifunctional Catalyst for Oxygen Cathode in Rechargeable Lithium-Air Batteries. ACS Appl. Mater. Interfaces 2016, 8, 6520–6528. [Google Scholar] [CrossRef]
- Yan, L.; Lin, Y.; Yu, X.; Xu, W.; Salas, T.; Smallidge, H.; Zhou, M.; Luo, H. La0.8Sr0.2MnO3-Based Perovskite Nanoparticles with the A-Site Deficiency as High Performance Bifunctional Oxygen Catalyst in Alkaline Solution. ACS Appl. Mater. Interfaces 2017, 9, 23820–23827. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, C.; Sui, J.; Li, C.; Yang, R. Phosphorus-doped SrCo0.5Mo0.5O3 perovskites with enhanced bifunctional oxygen catalytic activities. Int. J. Hydrogen Energy 2018, 43, 20727–20733. [Google Scholar] [CrossRef]
- Bian, J.; Su, R.; Yao, Y.; Wang, J.; Zhou, J.; Li, F.; Wang, Z.L.; Sun, C. Mg Doped Perovskite LaNiO3 Nanofibers as an Efficient Bifunctional Catalyst for Rechargeable Zinc–Air Batteries. Acs. Appl. Energy Mater. 2019, 2, 923–931. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, J.N.; Yao, N.; Wang, J.; Ren, D.; Chen, X.; Li, B.Q.; Zhang, Q. Can Aqueous Zinc-Air Batteries Work at Sub-Zero Temperatures? Angew. Chem. Int. Edit. 2021, 60, 15281–15285. [Google Scholar] [CrossRef]
- Jiao, D.; Ma, Z.; Li, J.S.; Han, Y.J.; Mao, J.; Ling, T.; Qiao, S.Z. Test factors affecting the performance of zinc-air battery. J. Energy Chem. 2020, 44, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Shang, S.S.; Gu, Q.F.; Shang, J.; Li, X.Y. Metal-organic framework-derived carbon nanotubes with multi-active Fe-N/Fe sites as a bifunctional electrocatalyst for zinc-air battery. J. Energy Chem. 2022, 66, 306–313. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Velusamy, D.B.; Sengodan, S.; Nagaraju, G.; Kim, D.H.; Kim, A.R.; Yoo, D.J. Rational design of multifunctional electrocatalyst: An approach towards efficient overall water splitting and rechargeable flexible solid-state zinc-air battery. Appl. Catal. B Environ. 2022, 300, 120752. [Google Scholar] [CrossRef]
- Li, Z.S.; Lv, L.; Ao, X.; Li, J.G.; Sun, H.C.; An, P.D.; Xue, X.Y.; Li, Y.; Liu, M.; Wang, C.D.; et al. An effective method for enhancing oxygen evolution kinetics of LaMO3 (M = Ni, Co, Mn) perovskite catalysts and its application to a rechargeable zinc-air battery. Appl. Catal. B-Environ. 2020, 262, 118291. [Google Scholar] [CrossRef]
- Wang, X.X.; Sunarso, J.; Lu, Q.; Zhou, Z.L.; Dai, J.; Guan, D.Q.; Zhou, W.; Shao, Z.P. High-Performance Platinum-Perovskite Composite Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Battery. Adv. Energy Mater. 2020, 10, 1903271. [Google Scholar] [CrossRef]
- Wu, X.Y.; Miao, H.; Hu, R.G.; Chen, B.; Yin, M.M.; Zhang, H.C.; Xia, L.; Zhang, C.F.; Yuan, J.L. A-site deficient perovskite nanofibers boost oxygen evolution reaction for zinc-air batteries. Appl. Surf. Sci. 2021, 536, 147806. [Google Scholar] [CrossRef]
- Yuan, R.H.; Chen, B.; Zhang, Y.; Tan, F.R.; Liu, T. Boosting the bifunctional electrocatalytic activity of cobalt free perovskite oxide (La0.8Sr0.2)(0.95)MnO3 via iron doping for high-efficiency Zn-air batteries. Sep. Purif. Technol. 2022, 300, 121858. [Google Scholar] [CrossRef]
- Lin, H.Q.; Xie, J.; Zhang, Z.B.; Wang, S.F.; Chen, D.J. Perovskite nanoparticles@N-doped carbon nanofibers as robust and efficient oxygen electrocatalysts for Zn-air batteries. J. Colloid Interf. Sci. 2021, 581, 374–384. [Google Scholar] [CrossRef]
- Nie, R.G.; Deng, Y.Q.; Yang, H.; Tan, Y.; Yuan, H.B.; Sagar, R.U.R.; Liang, T.X. Efficient oxygen evolution reaction in SrCo0.8Fe0.2O3-delta perovskite and surface reconstruction for practical zinc-air batteries. Appl. Surf. Sci. 2021, 552, 149509. [Google Scholar] [CrossRef]
- Shi, X.J.; Deng, Y.Z.; Zhao, L.; Gong, Y.S.; Wang, R.; Wang, H.W.; He, B.B. In-situ photodeposition of CoSx on Pa0.5Ba0.5 Mn-0.25 Fe-0.75 O3-delta perovskite to boost bifunctional oxygen electrocatalysis for rechargeable Zn-air batteries. Electrochim. Acta. 2021, 391, 138951. [Google Scholar] [CrossRef]
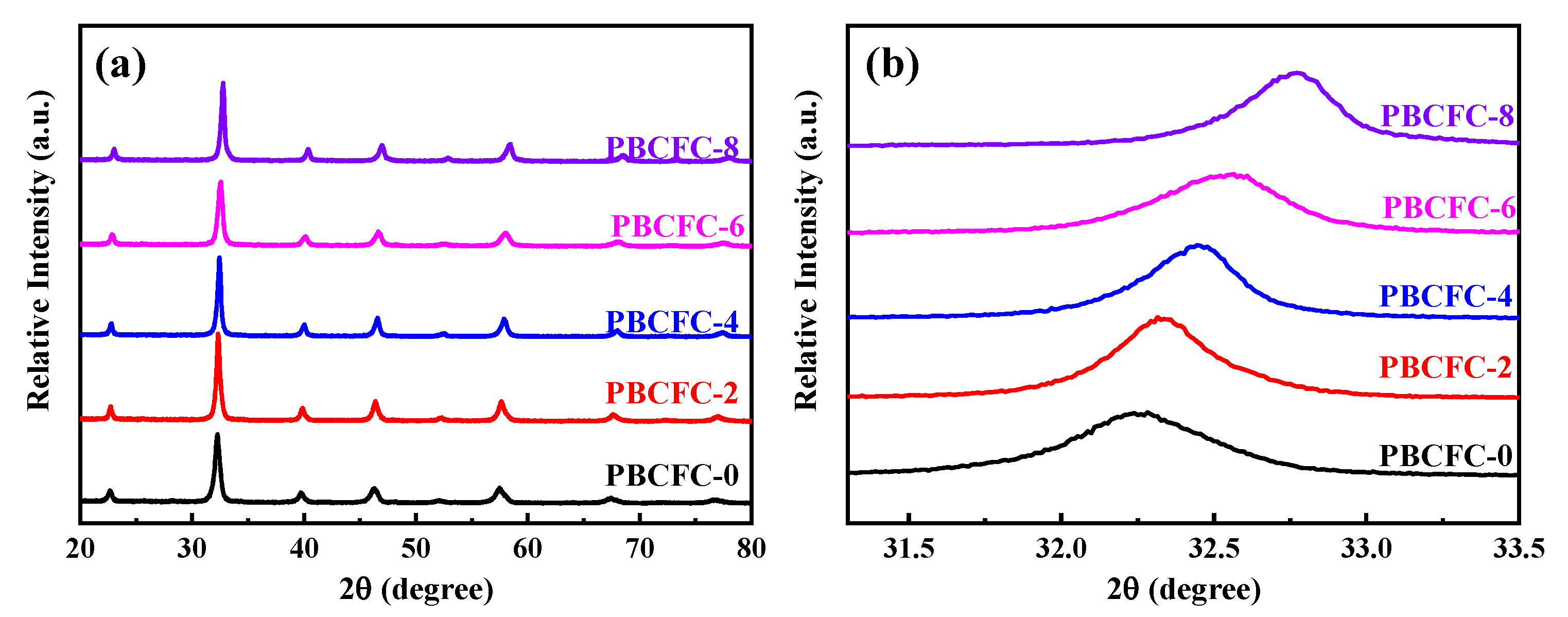

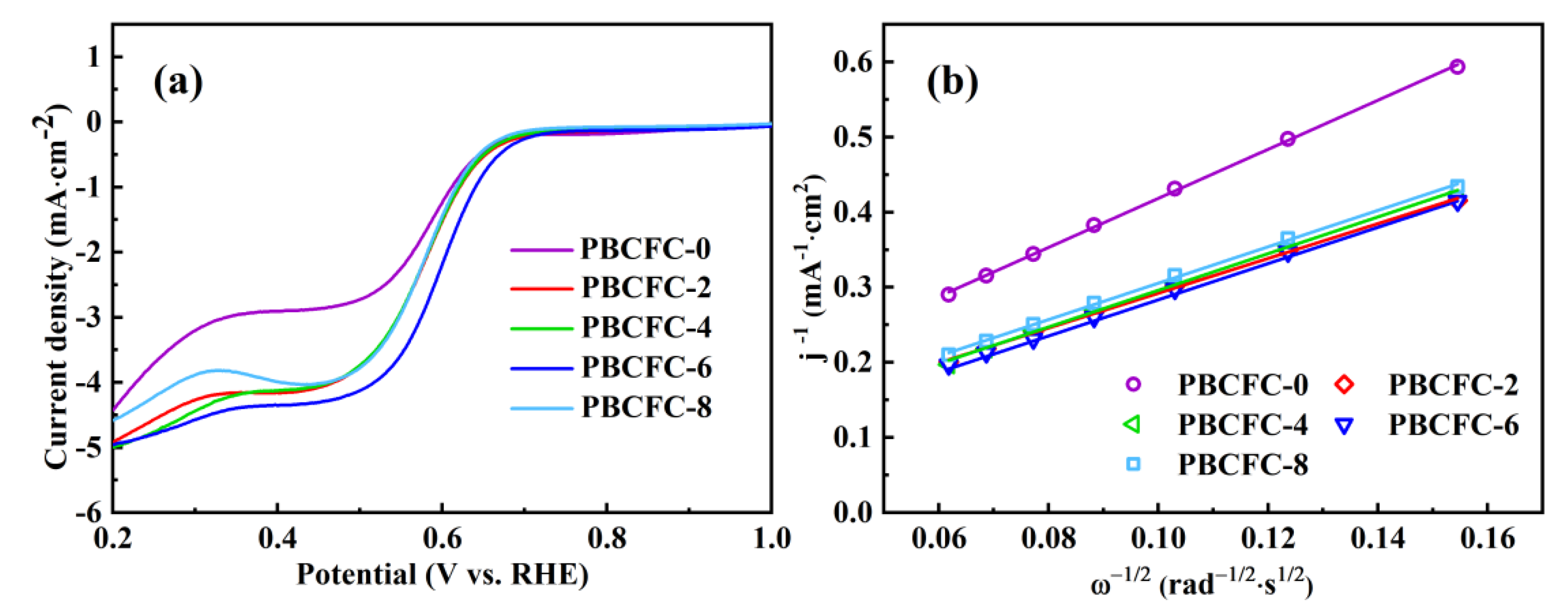
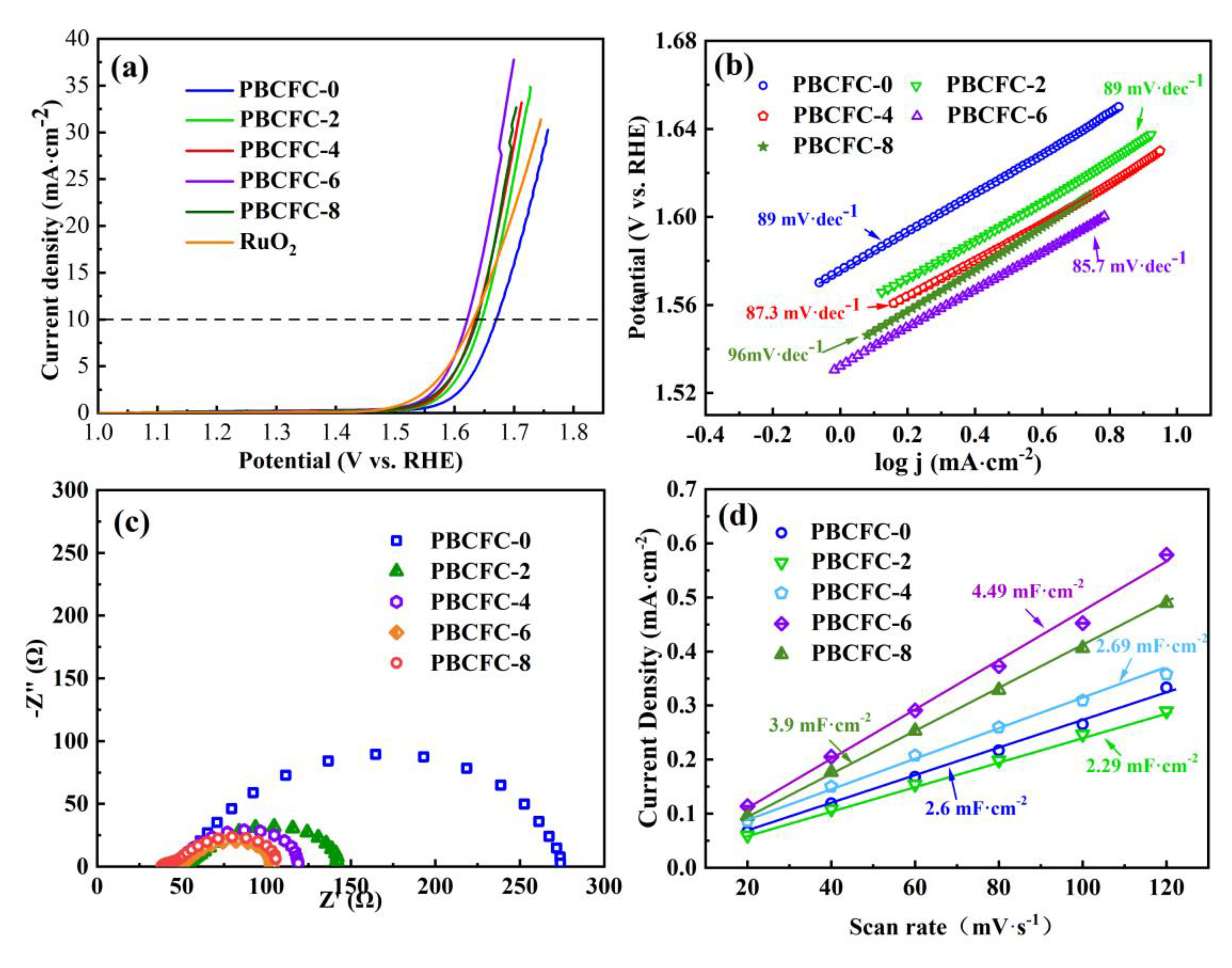

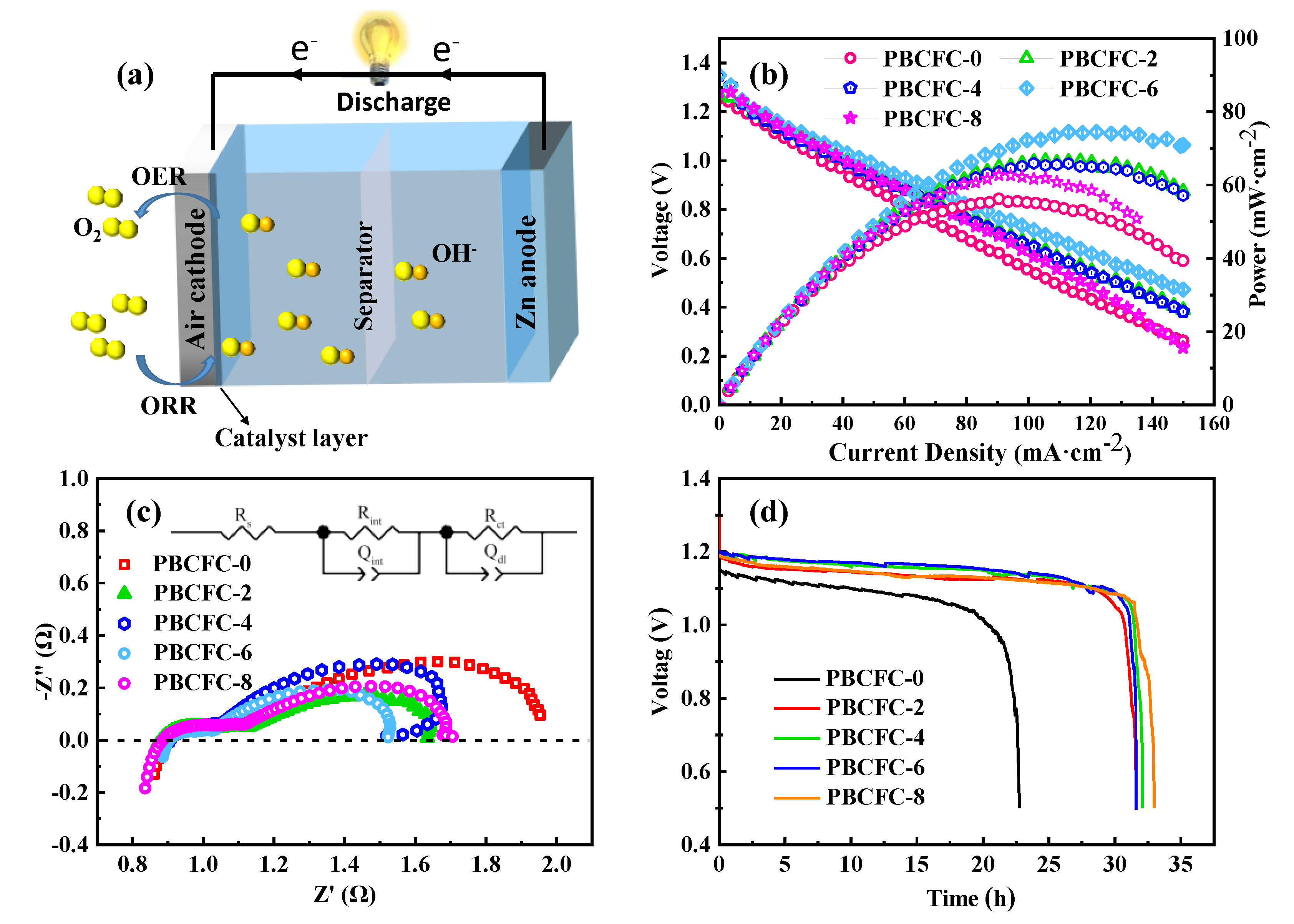
| Sample | RS (Ω) | Rint (Ω) | Rct (Ω) |
|---|---|---|---|
| PBCFC-0 | 0.89 | 0.31 | 0.83 |
| PBCFC-2 | 0.88 | 0.30 | 0.55 |
| PBCFC-4 | 0.88 | 0.27 | 0.69 |
| PBCFC-6 | 0.89 | 0.20 | 0.47 |
| PBCFC-8 | 0.87 | 0.28 | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Dong, Z.; Lü, Z. Rational A/B Site Ion Doping to Design Efficient and Stable Pr0.5Ba0.4Ca0.1Fe1-xCoxO3-δ Perovskites as Zinc–Air Batteries Cathode. Batteries 2022, 8, 259. https://doi.org/10.3390/batteries8120259
Li K, Dong Z, Lü Z. Rational A/B Site Ion Doping to Design Efficient and Stable Pr0.5Ba0.4Ca0.1Fe1-xCoxO3-δ Perovskites as Zinc–Air Batteries Cathode. Batteries. 2022; 8(12):259. https://doi.org/10.3390/batteries8120259
Chicago/Turabian StyleLi, Kaixin, Zhanhua Dong, and Zhe Lü. 2022. "Rational A/B Site Ion Doping to Design Efficient and Stable Pr0.5Ba0.4Ca0.1Fe1-xCoxO3-δ Perovskites as Zinc–Air Batteries Cathode" Batteries 8, no. 12: 259. https://doi.org/10.3390/batteries8120259





