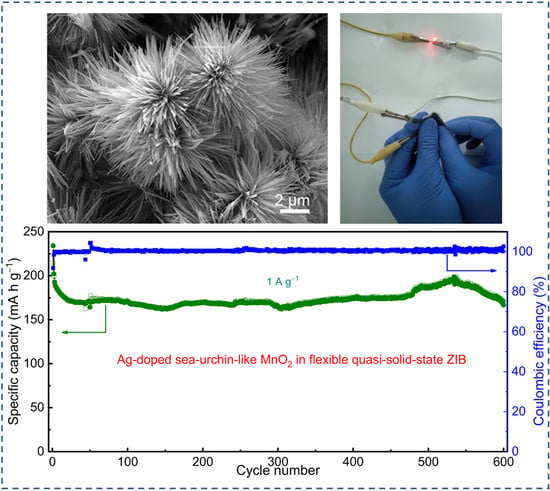
Ag-Doping Effect on MnO2 Cathodes for Flexible Quasi-Solid-State Zinc-Ion Batteries
Abstract
:1. Introduction
2. Experimental
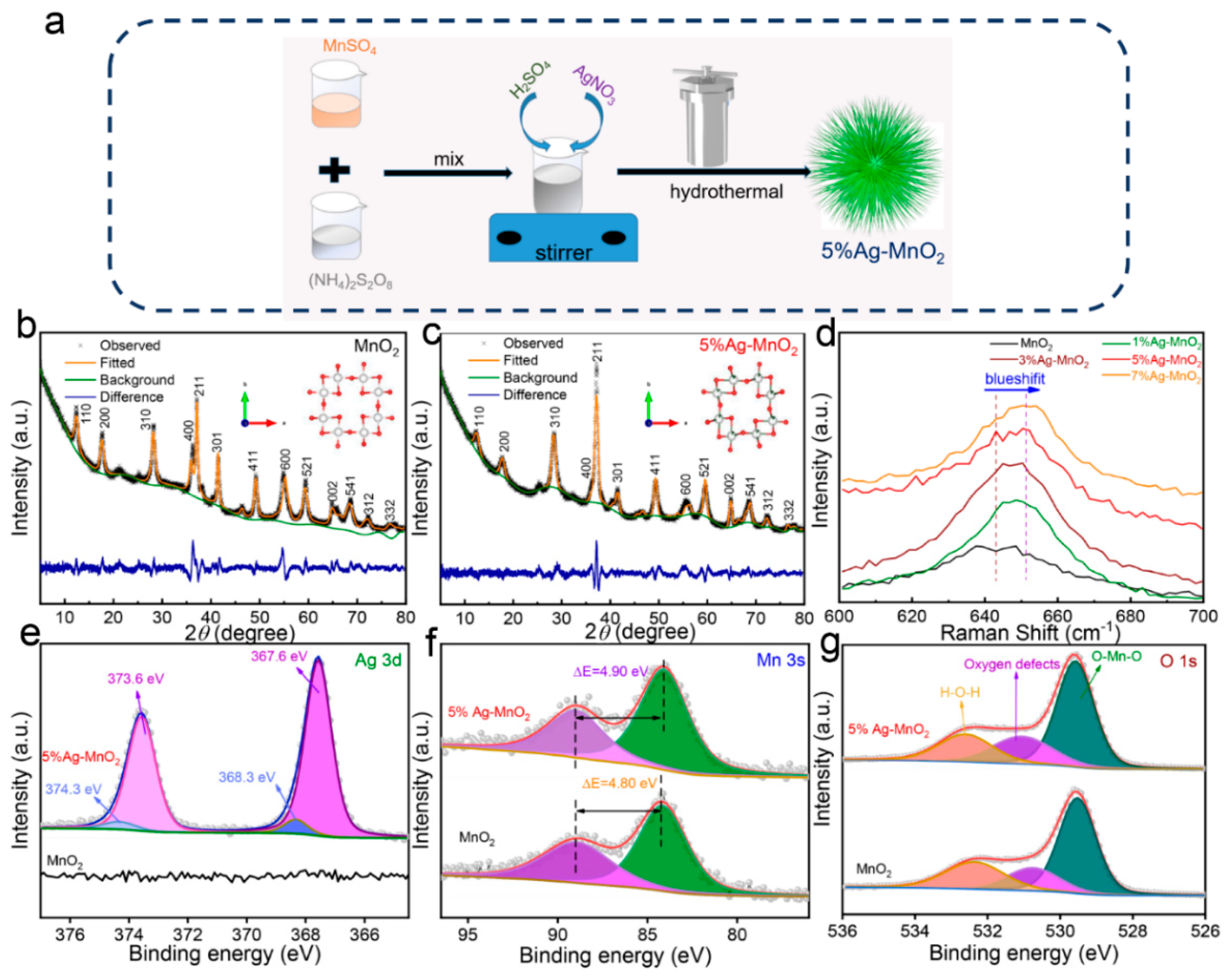
2.1. Synthesis of Ag-Doped Sea-Urchin-like MnO2 and Pure MnO2
2.2. Synthesis of the Polyacrylamide (PAM) Hydrogel Electrolyte
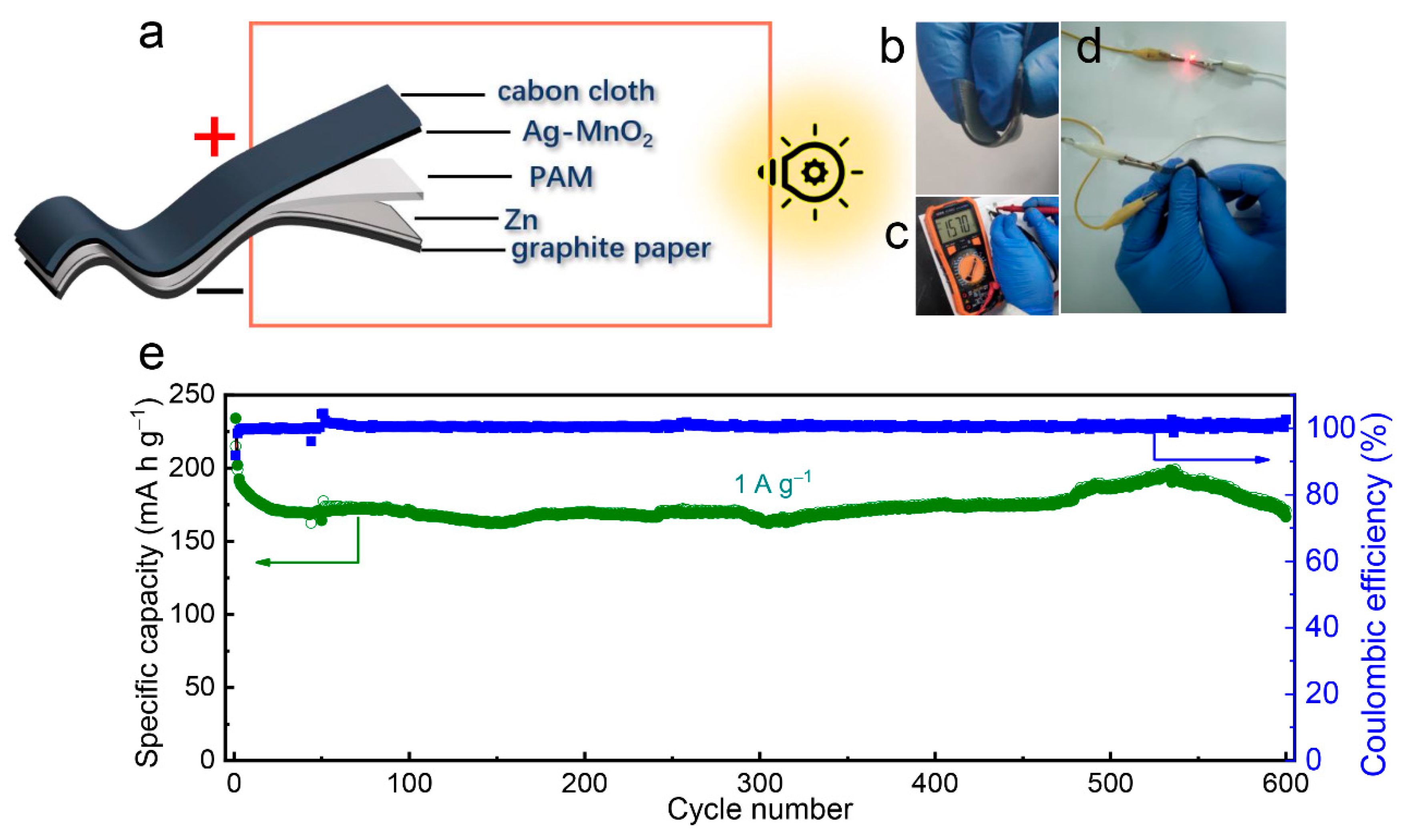
2.3. Synthesis of the Flexible Zn Anode
2.4. Materials Characterizations
2.5. Electrochemical Measurements
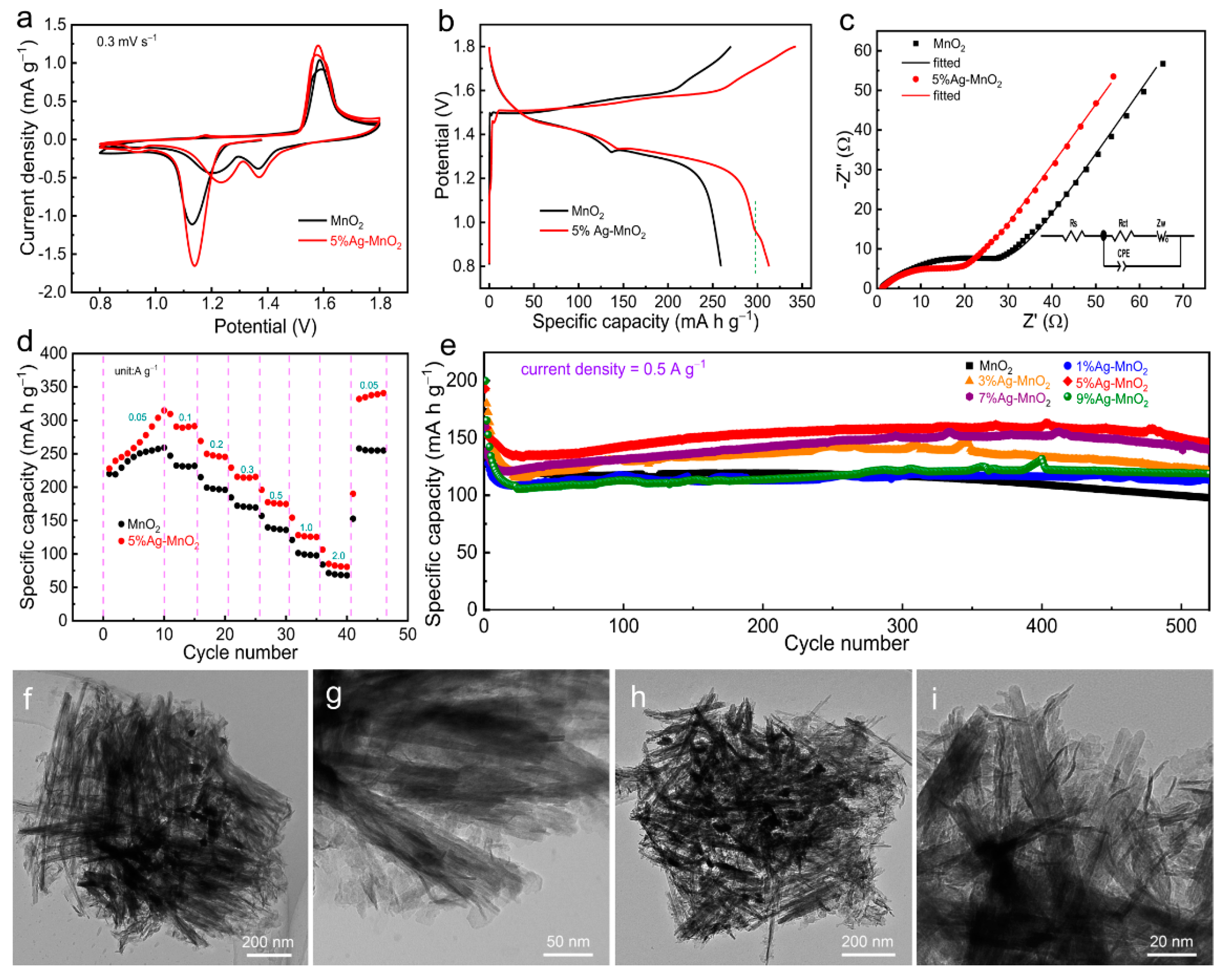
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.S.; Hong, S.-T. Prototype System of Rocking-Chair Zn-Ion Battery Adopting Zinc Chevrel Phase Anode and Rhombohedral Zinc Hexacyanoferrate Cathode. Batteries 2019, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Zhuang, P.; Zhang, X.; Ye, M.; Shen, J.; Ajayan, P.M. Strategies for dendrite-free anode in aqueous rechargeable zinc ion batteries. Adv. Energy Mater. 2020, 10, 2001599. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active Materials for Aqueous Zinc Ion Batteries: Synthesis, Crystal Structure, Morphology, and Electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef] [PubMed]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Liu, B. Transition Metal Dichalcogenides for High−Performance Aqueous Zinc Ion Batteries. Batteries 2022, 8, 62. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Ansari, M.Z.; Ansari, M.O. Manganese oxide as an effective electrode material for energy storage: A review. Environ. Chem. Lett. 2022, 20, 283–309. [Google Scholar] [CrossRef]
- Kang, L.; Cui, M.; Zhang, Z.; Jiang, F. Rechargeable Aqueous Zinc-Ion Batteries with Mild Electrolytes: A Comprehensive Review. Batter. Supercaps 2020, 3, 966–1005. [Google Scholar] [CrossRef]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous Zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Fitz, O.; Ingenhoven, S.; Bischoff, C.; Gentischer, H.; Birke, K.P.; Saracsan, D.; Biro, D. Comparison of Aqueous- and Non-Aqueous-Based Binder Polymers and the Mixing Ratios for Zn//MnO2 Batteries with Mildly Acidic Aqueous Electrolytes. Batteries 2021, 7, 40. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, J.; Wang, L.; Long, X.; Liu, X.; Li, F.; Chen, J. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Chen, H.-C.; Yang, C.; Liu, R.; Peng, Z.; Cao, H.; Wang, K. Unveiling performance evolution mechanisms of MnO2 polymorphs for durable aqueous zinc-ion batteries. Energy Storage Mater. 2022, 44, 508–516. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, G.; Yan, M.; Xiong, T.; He, P.; He, L.; Xu, X.; Mai, L. Graphene Scroll-Coated α-MnO2 Nanowires as High-Performance Cathode Materials for Aqueous Zn-Ion Battery. Small 2018, 14, 1703850. [Google Scholar] [CrossRef]
- Islam, S.; Alfaruqi, M.H.; Song, J.; Kim, S.; Pham, D.T.; Jo, J.; Kim, S.; Mathew, V.; Baboo, J.P.; Xiu, Z.; et al. Carbon-coated manganese dioxide nanoparticles and their enhanced electrochemical properties for zinc-ion battery applications. J. Energy Chem. 2017, 26, 815–819. [Google Scholar] [CrossRef] [Green Version]
- Xiong, T.; Zhang, Y.; Lee, W.S.V.; Xue, J. Defect Engineering in Manganese-Based Oxides for Aqueous Rechargeable Zinc-Ion Batteries: A Review. Adv. Energy Mater. 2020, 10, 2001769. [Google Scholar] [CrossRef]
- Ma, K.; Li, Q.; Hong, C.; Yang, G.; Wang, C. Bi Doping-Enhanced Reversible-Phase Transition of α-MnO2 Raising the Cycle Capability of Aqueous Zn–Mn Batteries. ACS Appl. Mater. Interfaces 2021, 13, 55208–55217. [Google Scholar] [CrossRef]
- Dai, L.; Sun, Q.; Chen, L.; Guo, H.; Nie, X.; Cheng, J.; Guo, J.; Li, J.; Lou, J.; Ci, L. Ag doped urchin-like α-MnO2 toward efficient and bifunctional electrocatalysts for Li-O2 batteries. Nano Res. 2020, 13, 2356–2364. [Google Scholar] [CrossRef]
- Lian, S.; Sun, C.; Xu, W.; Huo, W.; Luo, Y.; Zhao, K.; Yao, G.; Xu, W.; Zhang, Y.; Li, Z.; et al. Built-in oriented electric field facilitating durable Zn-MnO2 battery. Nano Energy 2019, 62, 79–84. [Google Scholar] [CrossRef]
- Han, M.; Qin, L.; Liu, Z.; Zhang, L.; Li, X.; Lu, B.; Huang, J.; Liang, S.; Zhou, J. Reaction mechanisms and optimization strategies of manganese-based materials for aqueous zinc batteries. Mater. Today Energy 2021, 20, 100626. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Islam, S.; Mathew, V.; Song, J.; Kim, S.; Tung, D.P.; Jo, J.; Kim, S.; Baboo, J.P.; Xiu, Z.; et al. Ambient redox synthesis of vanadium-doped manganese dioxide nanoparticles and their enhanced zinc storage properties. Appl. Surf. Sci. 2017, 404, 435–442. [Google Scholar] [CrossRef]
- Zhao, Q.; Song, A.; Zhao, W.; Qin, R.; Ding, S.; Chen, X.; Song, Y.; Yang, L.; Lin, H.; Li, S.; et al. Boosting the Energy Density of Aqueous Batteries via Facile Grotthuss Proton Transport. Angew. Chem. Int. Ed. 2021, 60, 4169–4174. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Koo, B.-R.; Jo, Y.-R.; An, H.-R.; Lee, Y.-G.; Huang, C.; An, G.-H. Defect engineering via the F-doping of β-MnO2 cathode to design hierarchical spheres of interlaced nanosheets for superior high-rate aqueous zinc ion batteries. J. Mater. Chem. A 2021, 9, 17211–17222. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, Z.; Wu, X.; Wen, Y.; Chen, H.; Ni, X.; Liu, G.; Huang, J.; Peng, S. MnO2 cathode materials with the improved stability via nitrogen doping for aqueous zinc-ion batteries. J. Energy Chem. 2022, 64, 23–32. [Google Scholar] [CrossRef]
- Cai, X.; Bai, H.; Liu, Y.; Shi, W. Facile in situ synthesis of Ag and MnO2 anchored on carbon microtubes for high-performance asymmetric supercapacitor applications. Appl. Mater. Today 2018, 11, 193–199. [Google Scholar] [CrossRef]
- Panimalar, S.; Logambal, S.; Thambidurai, R.; Inmozhi, C.; Uthrakumar, R.; Muthukumaran, A.; Rasheed, R.A.; Gatasheh, M.K.; Raja, A.; Kennedy, J.; et al. Effect of Ag doped MnO2 nanostructures suitable for wastewater treatment and other environmental pollutant applications. Environ. Res. 2022, 205, 112560. [Google Scholar] [CrossRef]
- Wang, D.; Wu, J.; Liu, X.; Wu, L.; Ao, J.; Liu, W.; Sun, Y.; Zhang, Y. Formation of the front-gradient bandgap in the Ag doped CZTSe thin films and solar cells. J. Energy Chem. 2019, 35, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Gu, A.; Lou, Z.; Sun, J.; Zhou, Q.; Chan, K.Y. Facile synthesis of iron-doped hollow urchin-like MnO2 for supercapacitors. J. Mater. Sci. 2017, 52, 4852–4865. [Google Scholar] [CrossRef]
- Cao, Q.; Gao, H.; Gao, Y.; Yang, J.; Li, C.; Pu, J.; Du, J.; Yang, J.; Cai, D.; Pan, Z.; et al. Regulating Dendrite-Free Zinc Deposition by 3D Zincopilic Nitrogen-Doped Vertical Graphene for High-Performance Flexible Zn-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2103922. [Google Scholar] [CrossRef]
- Li, J.; Shu, C.; Hu, A.; Ran, Z.; Li, M.; Zheng, R.; Long, J. Tuning oxygen non-stoichiometric surface via defect engineering to promote the catalysis activity of Co3O4 in Li-O2 batteries. Chem. Eng. J. 2020, 381, 122678. [Google Scholar] [CrossRef]
- Xu, J.-W.; Gao, Q.-L.; Xia, Y.-M.; Lin, X.-S.; Liu, W.-L.; Ren, M.-M.; Kong, F.-G.; Wang, S.-J.; Lin, C. High-performance reversible aqueous zinc-ion battery based on iron-doped alpha-manganese dioxide coated by polypyrrole. J. Colloid Interface Sci. 2021, 598, 419–429. [Google Scholar] [CrossRef]
- Rahman, A.U.; Zarshad, N.; Wu, J.; Faiz, F.; Raziq, F.; Ali, A.; Li, G.; Ni, H. Fabrication of Ag-doped MnO2 nanosheets@carbon cloth for energy storage device. Mater. Sci. Eng. B 2021, 269, 115150. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Hou, M.; Dong, X.; Liu, Y.; Wang, Y.; Xia, Y. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.M.; Kim, I.Y.; Adpakpang, K.; Hwang, S.J. The beneficial effect of nanocrystalline and amorphous nature on the anode performance of manganese oxide for lithium ion batteries. Electrochim. Acta 2015, 174, 391–399. [Google Scholar] [CrossRef]
- Gao, R.; Yang, Z.; Zheng, L.; Gu, L.; Liu, L.; Lee, Y.; Hu, Z.; Liu, X. Enhancing the Catalytic Activity of Co3O4 for Li–O2 Batteries through the Synergy of Surface/Interface/Doping Engineering. ACS Catal. 2018, 8, 1955–1963. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, H.; Hong, Q.; Xu, B.; Wang, J.; Deng, Y.; Yang, W.; Cai, W. Synthesis of Ag/Co@CoO NPs anchored within N-doped hierarchical porous hollow carbon nanofibers as a superior free-standing cathode for Li-O2 batteries. Carbon 2019, 144, 280–288. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, B.; Jiang, S.; Jiang, Q.; Huang, C.; Chen, H.C. Strongly anchored MnO nanoparticles on graphene as high-performance anode materials for lithium-ion batteries. Ionics 2020, 26, 3315–3323. [Google Scholar] [CrossRef]
- Tan, Q.; Li, X.; Zhang, B.; Chen, X.; Tian, Y.; Wan, H.; Zhang, L.; Miao, L.; Wang, C.; Gan, Y.; et al. Valence Engineering via In Situ Carbon Reduction on Octahedron Sites Mn3O4 for Ultra-Long Cycle Life Aqueous Zn-Ion Battery. Adv. Energy Mater. 2020, 10, 2001050. [Google Scholar] [CrossRef]
- Fang, G.; Zhu, C.; Chen, M.; Zhou, J.; Tang, B.; Cao, X.; Zheng, X.; Pan, A.; Liang, S. Suppressing Manganese Dissolution in Potassium Manganate with Rich Oxygen Defects Engaged High-Energy-Density and Durable Aqueous Zinc-Ion Battery. Adv. Funct. Mater. 2019, 29, 1808375. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Diemant, T.; Cao, K.; Liu, X.; Kaiser, U.; Behm, R.J.; Varzi, A.; Passerini, S. Unveiling the Intricate Intercalation Mechanism in Manganese Sesquioxide as Positive Electrode in Aqueous Zn-Metal Battery. Adv. Energy Mater. 2021, 11, 2100962. [Google Scholar] [CrossRef]
- Su, S.; Xu, Y.; Wang, Y.; Wang, X.; Shi, L.; Wu, D.; Zou, P.; Nairan, A.; Lin, Z.; Kang, F.; et al. Holey nickel nanotube reticular network scaffold for high-performance flexible rechargeable Zn/MnO2 batteries. Chem. Eng. J. 2019, 370, 330–336. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, A.; Du, L.; Yang, C.; Ye, L.; Wang, X.; Zhao, L.; Jiang, Q. Mn-doped ZnO microspheres as cathode materials for aqueous zinc ion batteries with ultrastability up to 10 000 cycles at a large current density. Chem. Eng. J. 2021, 421, 127770. [Google Scholar] [CrossRef]
- Le, S.; Zhang, L.; Song, X.; He, S.; Yuan, Z.; Liu, F.; Zhang, N.; Sun, K.; Feng, Y. Review—Status of Zinc-Silver Battery. J. Electrochem. Soc. 2019, 166, A2980–A2989. [Google Scholar] [CrossRef]
- Jin, Y.; Zou, L.; Liu, L.; Engelhard, M.H.; Patel, R.L.; Nie, Z.; Han, K.S.; Shao, Y.; Wang, C.; Zhu, J.; et al. Joint Charge Storage for High-Rate Aqueous Zinc–Manganese Dioxide Batteries. Adv. Mater. 2019, 31, 1900567. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Yang, C.; Xu, Q.; Zhao, W.; Zhao, J.; Wang, K.; Chen, H.-C. Ag-Doping Effect on MnO2 Cathodes for Flexible Quasi-Solid-State Zinc-Ion Batteries. Batteries 2022, 8, 267. https://doi.org/10.3390/batteries8120267
Liao Y, Yang C, Xu Q, Zhao W, Zhao J, Wang K, Chen H-C. Ag-Doping Effect on MnO2 Cathodes for Flexible Quasi-Solid-State Zinc-Ion Batteries. Batteries. 2022; 8(12):267. https://doi.org/10.3390/batteries8120267
Chicago/Turabian StyleLiao, Yanxin, Chun Yang, Qimeng Xu, Wenxuan Zhao, Jingwen Zhao, Kuikui Wang, and Hai-Chao Chen. 2022. "Ag-Doping Effect on MnO2 Cathodes for Flexible Quasi-Solid-State Zinc-Ion Batteries" Batteries 8, no. 12: 267. https://doi.org/10.3390/batteries8120267
APA StyleLiao, Y., Yang, C., Xu, Q., Zhao, W., Zhao, J., Wang, K., & Chen, H.-C. (2022). Ag-Doping Effect on MnO2 Cathodes for Flexible Quasi-Solid-State Zinc-Ion Batteries. Batteries, 8(12), 267. https://doi.org/10.3390/batteries8120267