Recent Progress and Challenges of Flexible Zn-Based Batteries with Polymer Electrolyte
Abstract
:1. Introduction
2. Fundamentals of Flexible Zn-Based Batteries
2.1. Aqueous Rechargeable Zn-Based Batteries
2.2. Polymer Electrolytes
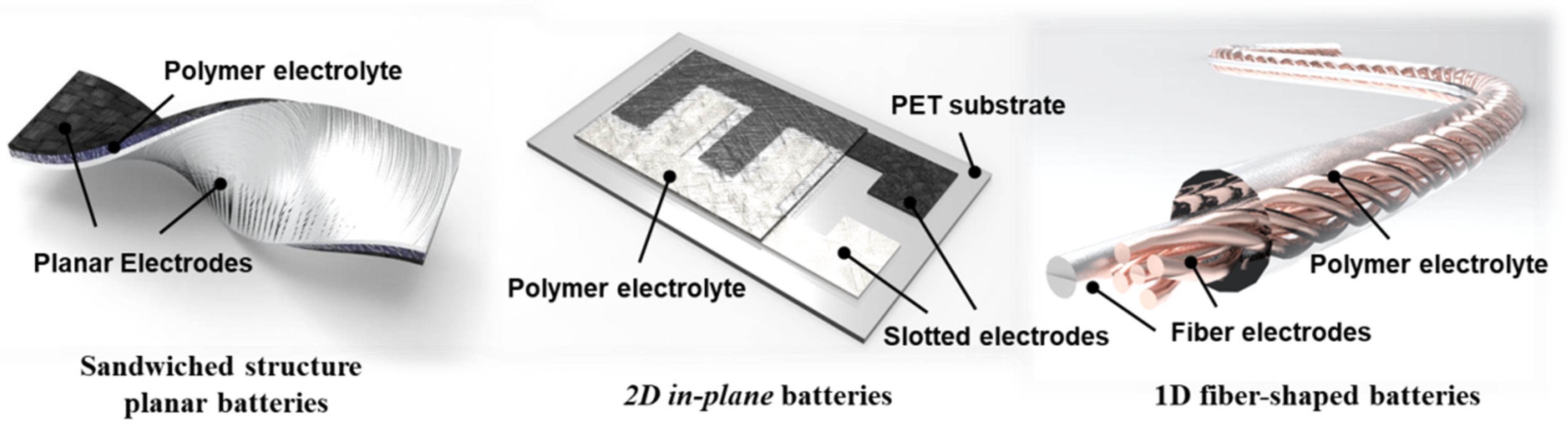
2.3. Configuration
3. Advanced Flexible Zn-Based Batteries with Functional Polymer Electrolytes
3.1. Low-Temperature Tolerant Zn-Based Batteries
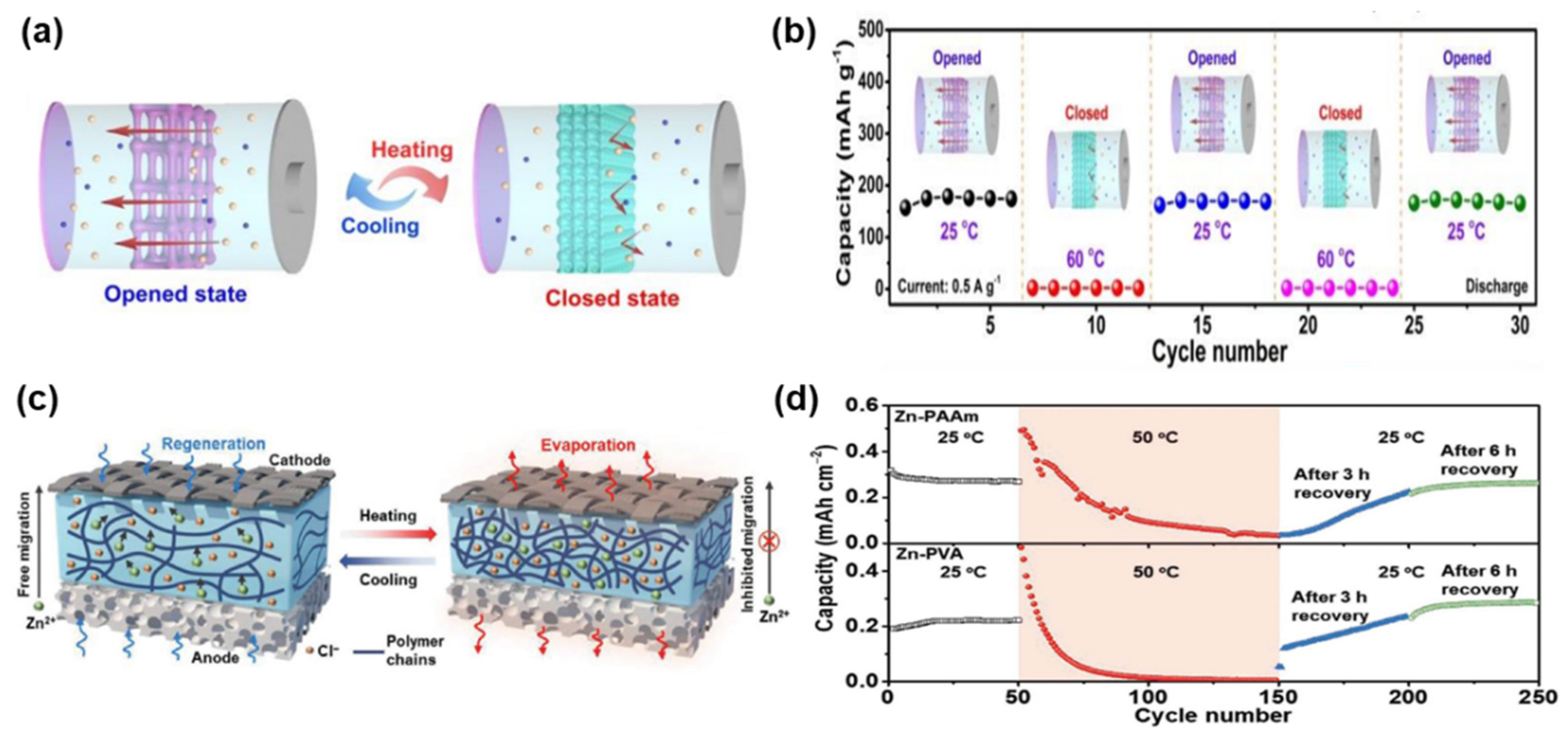
3.2. Thermoresponsive Zn-Based Batteries
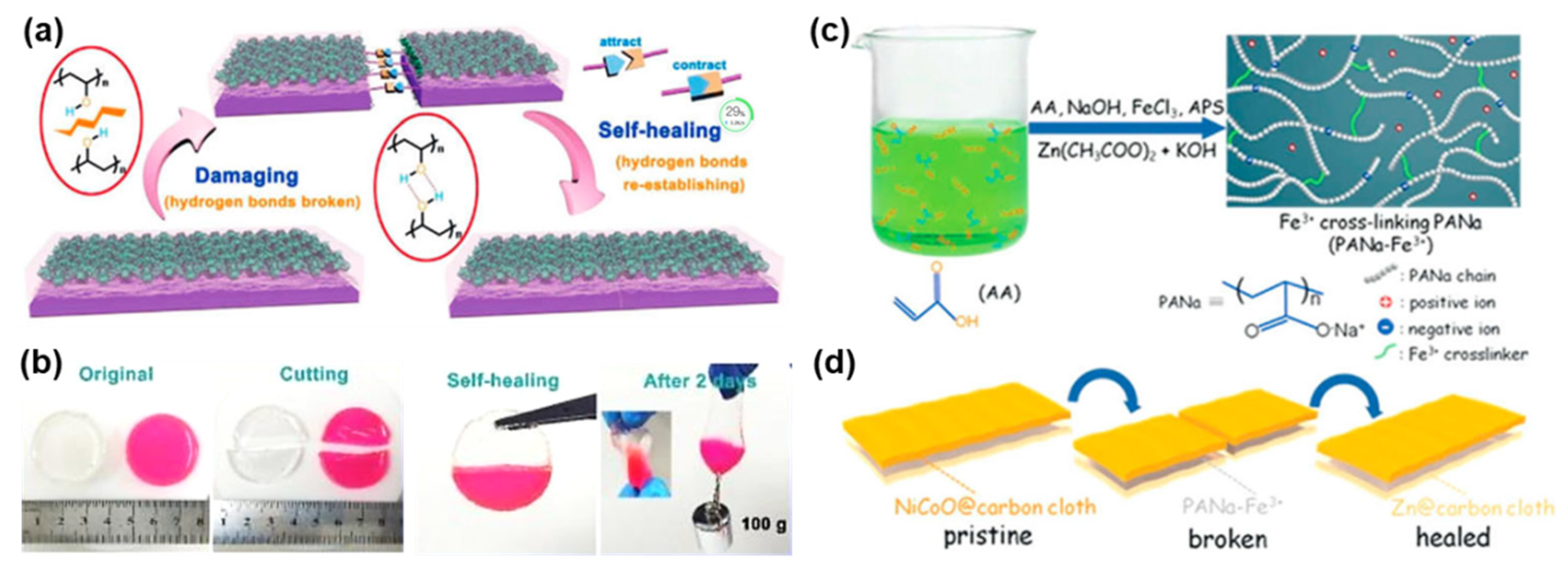
3.3. Self-Healing Zn-Based Batteries
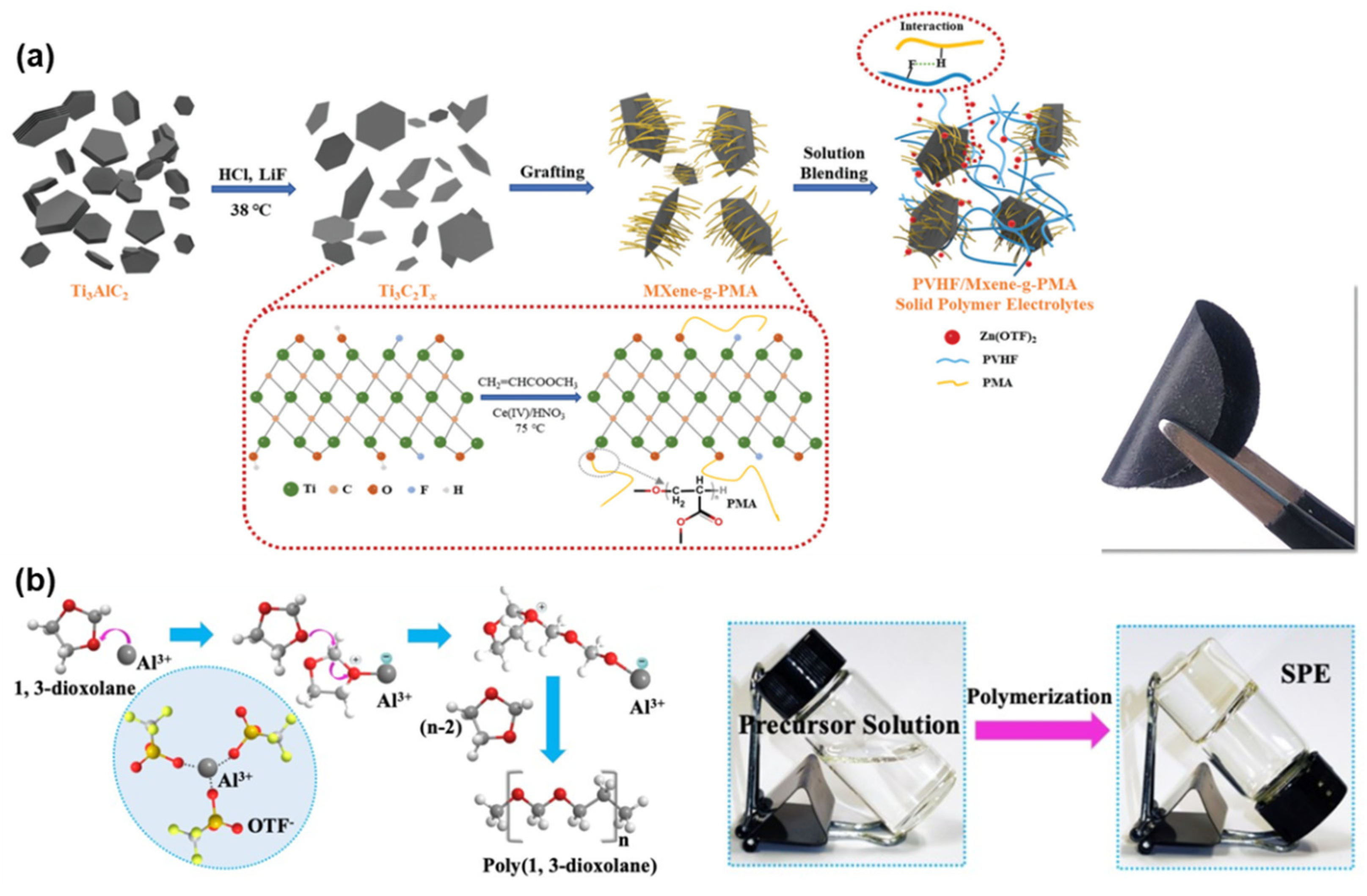
3.4. All-Solid-State Zn-Based Batteries
4. Interaction between Electrodes and Polymer Electrolytes
4.1. Stabilization of Zn Anodes for Aqueous Zn-Based Batteries
4.2. Improvement of Electrochemical Peformance of Cathodes
5. Summary and Perspective
- (1)
- It is noteworthy that, with the increase in demand for flexible batteries, the demand for solid-state and quasi-solid-state batteries is also increasing. In particular, zinc metal anodes are often used directly in zinc-based batteries, but it is difficult to solve the problem of dendrite growth associated with the use of a liquid electrolyte which may cause short-circuiting. Using polymer electrolytes with enhanced mechanical properties is an effective solution for the alleviation of dendritic growth and side-reactions, such as HER and corrosion. In general, the use of polymer electrolytes does not change the intrinsic electrochemical reactions of the battery electrodes. However, relatively larger thickness polymer electrolytes may degrade the battery performance, especially by reducing the energy density, which is a specific problem that needs to be solved through future research.
- (2)
- The mechanical properties of a battery include its intrinsic flexibility, layer cohesion (self-strength), interlayer adhesion, etc. Its flexibility is determined by the Young’s modulus of the battery. When polymer electrolytes are used for the fabrication of battery devices, the Young’s modulus of the electrolyte will largely determine the Young’s modulus of the battery. It should be noted that the electrode materials and the current collectors also need to be considered. The softness of polymer electrolytes (such as the comfort of wearable applications of flexible batteries) and their mechanical strength (tensile limit and impact resistance) represent a trade-off. Therefore, numerous researchers have focused on the design of advanced materials and electrolyte configurations to enhance their mechanical strength. For example, a double network structure can be used to enhance mechanical strength, such as in terms of tensile and compressive properties. The flexibility of a physical/chemical crosslinked single network is also satisfactory, but, at present, attention is focused on the whole device (especially the collector). Li et al. have discussed the softness from a device-level perspective [69]. Yang et al. also discussed the softness of the entire Zn-based battery, in which the softness of the electrolyte is often ignored [70]. These concerns indicate that the softness of the electrolyte needs to be considered further. In addition, for polymer-based flexible battery devices, the adhesion between the electrolyte and the layers should be considered to ensure the structural integrity of devices by increasing the adhesion between layers.
- (3)
- When working at extremely cold temperatures, the performance of the electrode materials, especially the electrochemical activity and the ion diffusion rate, will inevitably deteriorate, but, to date, this has not received much attention compared to hydrogel electrolytes. Moreover, due to the water splitting that may occur under an electric field, most aqueous Zn-based batteries containing a mild electrolyte only exhibited a narrow voltage window of <2.0 V. Recently developed “water-in-salt” electrolytes could significantly increase the voltage window of aqueous ZIBs to 2.1 V. However, the high cost is hindering implementation at scale. Therefore, the development of hydrogel electrolytes with a wider electrochemical potential window is to be encouraged for high-voltage aqueous flexible batteries.
- (4)
- The ultimate purpose of the development of flexible Zn-based batteries is commercialization. However, the persistent issues of zinc dendrite formation and side-reactions, such as HER and corrosion, continue to adversely affect the stability of the Zn anode. In addition, due to the complicated synthesis of electrode materials and the involved chemical modification of hydrogel electrolytes, the fabrication and assembly processes of flexible batteries are not sufficiently simplified with production efficiency needing improvement. Advanced manufacturing technologies, such as 3D printing and screen printing can be integrated and applied into the fabrication of high-performance batteries for enhanced efficiency.
- (5)
- During daily use as a portable power accessory for wearable electronics, flexible batteries inevitably undergo frequent extreme deformations. The mechanical stability of polymer electrolytes is thus of great significance for the long-term usability of flexible batteries. However, most hydrogel electrolytes are insufficiently durable to endure frequent deformations and external impacts. New technologies for the synthesis of super-tough or fast self-healing polymer electrolytes should be further explored and disseminated. Moreover, due to unavoidable direct contact of wearable batteries with the human body in realistic use, guaranteeing the biocompatibility of polymer electrolytes is also critical for wearable applications.
- (6)
- A well-formed SEI can not only improve the compatibility between electrodes and electrolyte to facilitate the rate of ion flow, but can also prevent the cathodes from pulverization and inhibit side-reactions. Additionally, building an artificial SEI with larger specific active surface area could effectively alleviate concentration polarization, which is instrumental in increasing the electronic conductivity and suppression of Zn dendrites. However, the fundamental mechanism of interfacial reaction between the polymer electrolytes and electrodes is still insufficient. Furthermore, the SEI is usually affected and destroyed by the surface topography changes of the Zn anode due to dendrite growth. Further research efforts should be dedicated to the stabilization of the SEI.
- (7)
- Multifunctional polymer electrolytes can effectively broaden the application range of flexible Zn-based batteries to satisfy diverse application requirements. Some intriguing functionalities, such as extreme temperature tolerance, thermoresponsive properties, and self-healing ability have been summarized in the preceding sections. Many other potential functionalities, including photodetection, electrochromism, and shape memory are also worthy of exploration for the development of advanced flexible batteries. In addition, the integration of flexible Zn-based batteries with other wearable device systems, such as sensors, self-powering generators, and energy conversion devices has been identified as a promising direction for next-generation wearable electronics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Fu, J.; Zhong, C.; Wu, T.; Chen, Z.; Hu, W.; Amine, K.; Lu, J. Recent Advances in Flexible Zinc-Based Rechargeable Batteries. Adv. Energy Mater. 2019, 9, 1802605. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Zeng, Y.; Zhang, H.; Yu, M.; Tong, Y.; Lu, X. Flexible Zn-Ion Batteries: Recent Progresses and Challenges. Small 2019, 15, 1804760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, J. Development of Flexible Li-Ion Batteries for Flexible Electronics. InfoMat 2020, 2, 866–878. [Google Scholar] [CrossRef] [Green Version]
- Qian, G.; Liao, X.; Zhu, Y.; Pan, F.; Chen, X.; Yang, Y. Designing Flexible Lithium-Ion Batteries by Structural Engineering. ACS Energy Lett. 2019, 4, 690–701. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-Ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-Ion Battery Aging Mechanisms and Diagnosis Method for Automotive Applications: Recent Advances and Perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A Review on the Key Issues of the Lithium Ion Battery Degradation among the Whole Life Cycle. ETransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and Opportunities Facing Aqueous Zinc-Ion Batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Yu, M.; Niu, Z.; Cheng, F.; Chen, J. Materials Chemistry for Rechargeable Zinc-Ion Batteries. Chem. Soc. Rev. 2020, 49, 4203–4219. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, J.; Park, Y.; Choi, J.W. Aqueous Zinc Ion Batteries: Focus on Zinc Metal Anodes. Chem. Sci. 2020, 11, 2028–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Li, C.; Xie, X.; Lu, B.; He, Z.; Liang, S.; Zhou, J. Anode Materials for Aqueous Zinc Ion Batteries: Mechanisms, Properties, and Perspectives. Acs Nano 2020, 14, 16321–16347. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the Material and Structural Design of Zinc Anode Towards High-Performance Aqueous Zinc-Ion Batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Q.; Liu, Z.; Wang, D.; Guo, Y.; Li, X.; Tang, Y.; Li, H.; Dong, B.; Zhi, C. Dendrites in Zn-Based Batteries. Adv. Mater. 2020, 32, 2001854. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fu, J.; Miao, G.; Wang, S.; Zhao, W.; Wu, Z.; Zhang, Y.; Yang, X. Toward Planar and Dendrite-Free Zn Electrodepositions by Regulating Sn-Crystal Textured Surface. Adv. Mater. 2021, 33, 2008424. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Chen, G.; Hou, F.; Wang, L.; Liang, J. Strategies for the Stabilization of Zn Metal Anodes for Zn-Ion Batteries. Adv. Energy Mater. 2021, 11, 2003065. [Google Scholar] [CrossRef]
- Xie, C.; Li, Y.; Wang, Q.; Sun, D.; Tang, Y.; Wang, H. Issues and Solutions toward Zinc Anode in Aqueous Zinc-Ion Batteries: A Mini Review. Carbon Energy. 2020, 2, 540–560. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.; Ahmed, H.M. A Conceptual Review on Polymer Electrolytes and Ion Transport Models. J. Sci Adv. Mater. Dev. 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Bocharova, V.; Sokolov, A.P. Perspectives for Polymer Electrolytes: A View from Fundamentals of Ionic Conductivity. Macromolecules 2020, 53, 4141–4157. [Google Scholar] [CrossRef]
- Wu, K.; Huang, J.; Yi, J.; Liu, X.; Liu, Y.; Wang, Y.; Zhang, J.; Xia, Y. Recent Advances in Polymer Electrolytes for Zinc Ion Batteries: Mechanisms, Properties, and Perspectives. Adv. Energy Mater. 2020, 10, 1903977. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Tang, Z.; Liu, Z.; Ruan, Z.; Ma, L.; Yang, Q.; Wang, D.; Zhi, C. Hydrogel Electrolytes for Flexible Aqueous Energy Storage Devices. Adv. Funct Mater. 2018, 28, 1804560. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, J.; Jiang, J.; Qiu, Y.; Wang, J. Flexible Quasi-Solid-State Aqueous Zn-Based Batteries: Rational Electrode Designs for High-Performance and Mechanical Flexibility. Mater. Today Energy 2020, 18, 100523. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced Rechargeable Zinc-Based Batteries: Recent Progress and Future Perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Selvakumaran, D.; Pan, A.; Liang, S.; Cao, G. A Review on Recent Developments and Challenges of Cathode Materials for Rechargeable Aqueous Zn-Ion Batteries. J. Mater. Chem. A 2019, 7, 18209–18236. [Google Scholar] [CrossRef]
- Deng, Y.-P.; Liang, R.; Jiang, G.; Jiang, Y.; Yu, A.; Chen, Z. The Current State of Aqueous Zn-Based Rechargeable Batteries. ACS Energy Lett. 2020, 5, 1665–1675. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Y.; Shi, L.; Wang, K.; Wang, B.; Li, L.; Ma, Y.; Li, Y.; Sun, Z.; Ali, W. An Overview and Future Perspectives of Rechargeable Zinc Batteries. Small 2020, 16, 2000730. [Google Scholar] [CrossRef]
- Naveed, A.; Yang, H.; Shao, Y.; Yang, J.; Yanna, N.; Liu, J.; Shi, S.; Zhang, L.; Ye, A.; He, B. A Highly Reversible Zn Anode with Intrinsically Safe Organic Electrolyte for Long-Cycle-Life Batteries. Adv. Mater. 2019, 31, 1900668. [Google Scholar] [CrossRef]
- Naveed, A.; Yang, H.; Yang, J.; Nuli, Y.; Wang, J. Highly Reversible and Rechargeable Safe Zn Batteries Based on a Triethyl Phosphate Electrolyte. Angew. Chem. Int. Ed. 2019, 58, 2760–2764. [Google Scholar] [CrossRef]
- Shi, B.; Li, Z.; Fan, Y. Implantable Energy-Harvesting Devices. Adv. Mater. 2018, 30, 1801511. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Guo, S.; He, P.; Zhou, H. Status and Prospects of Polymer Electrolytes for Solid-State Li–O 2 (Air) Batteries. Energy Environ. Sci. 2017, 10, 860–884. [Google Scholar] [CrossRef]
- Jost, K.; Durkin, D.P.; Haverhals, L.M.; Brown, E.K.; Langenstein, M.; De Long, H.C.; Trulove, P.C.; Gogotsi, Y.; Dion, G. Natural Fiber Welded Electrode Yarns for Knittable Textile Supercapacitors. Adv. Energy Mater. 2015, 5, 1401286. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Pei, Z.; Liu, Z.; Li, H.; Zhu, M.; Fan, J.; Dai, Q.; Zhang, M.; Dai, L. Solid-State Rechargeable Zn//Nico and Zn–Air Batteries with Ultralong Lifetime and High Capacity: The Role of a Sodium Polyacrylate Hydrogel Electrolyte. Adv. Energy Mater. 2018, 8, 1802288. [Google Scholar] [CrossRef]
- Huang, S.; Wan, F.; Bi, S.; Zhu, J.; Niu, Z.; Chen, J. A Self-Healing Integrated All-in-One Zinc-Ion Battery. Angew. Chem. 2019, 131, 4357–4361. [Google Scholar] [CrossRef]
- Karan, S.; Sahu, T.B.; Sahu, M.; Mahipal, Y.; Agrawal, R. Characterization of Ion Transport Property in Hot-Press Cast Solid Polymer Electrolyte (Spe) Films: [Peo: Zn (Cf3so3) 2]. Ionics 2017, 23, 2721–2726. [Google Scholar] [CrossRef]
- Hiralal, P.; Imaizumi, S.; Unalan, H.E.; Matsumoto, H.; Minagawa, M.; Rouvala, M.; Tanioka, A.; Amaratunga, G.A. Nanomaterial-Enhanced All-Solid Flexible Zinc− Carbon Batteries. ACS Nano 2010, 4, 2730–2734. [Google Scholar] [CrossRef]
- Chan, C.Y.; Wang, Z.; Li, Y.; Yu, H.; Fei, B.; Xin, J.H. Single-Ion Conducting Double-Network Hydrogel Electrolytes for Long Cycling Zinc-Ion Batteries. ACS Appl. Mat. Interfaces 2021, 13, 30594–30602. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, H.; Cao, Y.; Ai, X. Preparation and Electrochemical Characterization of the Alkaline Polymer Gel Electrolyte Polymerized from Acrylic Acid and Koh Solution. Electtrochim. Acta 2004, 49, 2533–2539. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Whiting, G.L.; Steingart, D.A.; Arias, A.C. Highly Flexible, Printed Alkaline Batteries Based on Mesh-Embedded Electrodes. Adv. Mater. 2011, 23, 3251–3255. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, N.; Zeng, S.; Zhou, S.; Chen, M.; Di, J.; Li, Q. An Adaptive and Stable Bio-Electrolyte for Rechargeable Zn-Ion Batteries. J. Mater. Chem. A 2018, 6, 12237–12243. [Google Scholar] [CrossRef]
- Han, Q.; Chi, X.; Zhang, S.; Liu, Y.; Zhou, B.; Yang, J.; Liu, Y. Durable, Flexible Self-Standing Hydrogel Electrolytes Enabling High-Safety Rechargeable Solid-State Zinc Metal Batteries. J. Mater. Chem. A 2018, 6, 23046–23054. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Arias, A.C.; Steingart, D.A. Recent Progress on Printed Flexible Batteries: Mechanical Challenges, Printing Technologies, and Future Prospects. Energy Technol. 2015, 3, 305–328. [Google Scholar] [CrossRef]
- Sun, C.-F.; Zhu, H.; Baker III, E.B.; Okada, M.; Wan, J.; Ghemes, A.; Inoue, Y.; Hu, L.; Wang, Y. Weavable High-Capacity Electrodes. Nano Energy 2013, 2, 987–994. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Ren, J.; Weng, W.; Peng, H. Advances in Wearable Fiber-Shaped Lithium-Ion Batteries. Adv. Mater. 2016, 28, 4524–4531. [Google Scholar] [CrossRef]
- Pei, Z.; Ding, L.; Wang, C.; Meng, Q.; Yuan, Z.; Zhou, Z.; Zhao, S.; Chen, Y. Make It Stereoscopic: Interfacial Design for Full-Temperature Adaptive Flexible Zinc–Air Batteries. Energy Environ. Sci. 2021, 14, 4926–4935. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Wang, J.; Hu, M.; Feng, Y.; Wang, P.; Wang, Y.; Nie, N.; Zhang, J.; Chen, H. Concentrated Hydrogel Electrolyte-Enabled Aqueous Rechargeable Nico//Zn Battery Working from −20 to 50 °C. ACS Appl. Mat. Interfaces 2018, 11, 49–55. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, W.; Wang, A.; Huang, A.; Chen, J.; Xu, J.; Wong, C.-P. Anti-Freezing Flexible Aqueous Zn–Mno 2 Batteries Working at −35 °C Enabled by a Borax-Crosslinked Polyvinyl Alcohol/Glycerol Gel Electrolyte. J. Mater. Chem. A 2020, 8, 6828–6841. [Google Scholar] [CrossRef]
- Pei, Z.; Yuan, Z.; Wang, C.; Zhao, S.; Fei, J.; Wei, L.; Chen, J.; Wang, C.; Qi, R.; Liu, Z. A Flexible Rechargeable Zinc–Air Battery with Excellent Low-Temperature Adaptability. Angew. Chem. Int. Ed. 2020, 59, 4793–4799. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Z.; Chandran, B.K.; Deng, J.; Yu, J.; Qi, D.; Li, W.; Tang, Y.; Zhang, C.; Chen, X. Self-Protection of Electrochemical Storage Devices Via a Thermal Reversible Sol–Gel Transition. Adv. Mater. 2015, 27, 5593–5598. [Google Scholar] [CrossRef]
- Zhu, J.; Yao, M.; Huang, S.; Tian, J.; Niu, Z. Thermal-Gated Polymer Electrolytes for Smart Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 16480–16484. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Feng, C.; Liu, Y.; Cheng, T.; Yang, X.; Liu, H.; Liu, K.; Fan, H.J. Thermal Self-Protection of Zinc-Ion Batteries Enabled by Smart Hygroscopic Hydrogel Electrolytes. Adv. Energy Mater. 2020, 10, 2002898. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Wang, J.; Hu, M.; Mo, F.; Liang, G.; Zhi, C. An Intrinsically Self-Healing Nico|| Zn Rechargeable Battery with a Self-Healable Ferric-Ion-Crosslinking Sodium Polyacrylate Hydrogel Electrolyte. Angew. Chem. Int. Ed. 2018, 57, 9810–9813. [Google Scholar] [CrossRef]
- Amamoto, Y.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Self-Healing of Covalently Cross-Linked Polymers by Reshuffling Thiuram Disulfide Moieties in Air under Visible Light. Adv. Mater. 2012, 24, 3975–3980. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, S.; Li, N.; Liu, Z.; Tang, Z.; Zapien, J.A.; Chen, S.; Fan, J.; Zhi, C. Hydrogen-Free and Dendrite-Free All-Solid-State Zn-Ion Batteries. Adv. Mater. 2020, 32, 1908121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, X.; Qin, L.; Fang, G.; Liang, S. Progress and Prospect of Low-Temperature Zinc Metal Batteries. Adv. Powder Mater. 2022, 1, 100011. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Sun, J.; Hsu, P.-C.; Li, Y.; Lee, H.-W.; Cui, Y. Ionic Conductivity Enhancement of Polymer Electrolytes with Ceramic Nanowire Fillers. Nano Lett. 2015, 15, 2740–2745. [Google Scholar] [CrossRef]
- Zhai, H.; Xu, P.; Ning, M.; Cheng, Q.; Mandal, J.; Yang, Y. A Flexible Solid Composite Electrolyte with Vertically Aligned and Connected Ion-Conducting Nanoparticles for Lithium Batteries. Nano Lett. 2017, 17, 3182–3187. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Wang, D.; Yang, Q.; Ma, L.; Huang, Z.; Liang, G.; Chen, A.; Guo, Y.; Dong, B. Grafted Mxene/Polymer Electrolyte for High Performance Solid Zinc Batteries with Enhanced Shelf Life at Low/High Temperatures. Energy Environ. Sci. 2021, 14, 3492–3501. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Li, X.; Chen, A.; Dong, B.; Zhi, C. Liquid-Free All-Solid-State Zinc Batteries and Encapsulation-Free Flexible Batteries Enabled by in Situ Constructed Polymer Electrolyte. Angew. Chem. 2020, 132, 24044–24052. [Google Scholar] [CrossRef]
- Bommier, C.; Ji, X. Electrolytes, Sei Formation, and Binders: A Review of Nonelectrode Factors for Sodium-Ion Battery Anodes. Small 2018, 14, 1703576. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood III, D.L. The State of Understanding of the Lithium-Ion-Battery Graphite Solid Electrolyte Interphase (Sei) and Its Relationship to Formation Cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.M.; Su, W.-N.; Hwang, B.J. In Situ Analytical Techniques for Battery Interface Analysis. Chem. Soc. Rev. 2018, 47, 736–851. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Mo, F.; Wang, J.; Liu, Q.; Liu, Y.; Yang, Q.; Qiu, Y.; Huang, Y. Self-Healable Hydrogel Electrolyte for Dendrite-Free and Self-Healable Zinc-Based Aqueous Batteries. Mater. Today Phys. 2021, 20, 100458. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, X.; Tang, H.; Wang, J.; Hao, Q.; Liu, L.; Li, Y.; Zhang, K.; Schmidt, O.G. Antifreezing Hydrogel with High Zinc Reversibility for Flexible and Durable Aqueous Batteries by Cooperative Hydrated Cations. Adv. Funct. Mater. 2020, 30, 1907218. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Chen, Z.; Wang, Y.; Li, H.; Zhang, J. In-Situ Growth of Zno Nanoplates on Graphene for the Application of High Rate Flexible Quasi-Solid-State Ni-Zn Secondary Battery. J. Power Source 2018, 407, 137–146. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, L.; Zhu, Y.; Qin, P.; Li, H.; Mo, F.; Wang, D.; Liang, G.; Yang, Q.; Liu, W. Inhibiting Grain Pulverization and Sulfur Dissolution of Bismuth Sulfide by Ionic Liquid Enhanced Poly (3, 4-Ethylenedioxythiophene): Poly (Styrenesulfonate) for High-Performance Zinc-Ion Batteries. ACS Nano 2019, 13, 7270–7280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, B.; Hu, J.; Liu, J.; Miao, L.; Jiang, J. An in Situ Artificial Cathode Electrolyte Interphase Strategy for Suppressing Cathode Dissolution in Aqueous Zinc Ion Batteries. Small Meth. 2021, 5, 2100094. [Google Scholar] [CrossRef]
- Li, H.; Tang, Z.; Liu, Z.; Zhi, C. Evaluating Flexibility and Wearability of Flexible Energy Storage Devices. Joule 2019, 3, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Chen, A.; Li, C.; Zou, G.; Li, H.; Zhi, C. Categorizing Wearable Batteries: Unidirectional and Omnidirectional Deformable Batteries. Matter 2021, 4, 3146–3160. [Google Scholar] [CrossRef]




| Names of Hydrogel | Molecular Structures | Functional Groups | Features | Ionic Conductivity (S cm−1) | Cathode Material | Mechanical Properties | Refs. |
|---|---|---|---|---|---|---|---|
| Polyvinyl alcohol (PVA) | 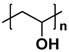 | Hydroxyl | Self-healable | 0.05–12.6 | Polyaniline nanorods 123 mAh g−1 0.1 A g−1 | Physical crosslinking Stretchable | [35] |
| Poly(ethylene oxide) (PEO) | 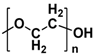 | Hydroxyl | Reusable | 1.09 × 10−6–6.33 × 10−3 | MnO2 140 mAh g−1 0.5 mA cm−2 | Physical crosslinking Flexible | [36,37] |
| Polyacrylamide (PAM) |  | Amide | Reusable | 2.15 × 10−3 | V2O5 271 mA h g−1 2 C | Chemical crosslinking Stretchable | [38] |
| Polyacrylic acid (PAA) |  | Carboxyl | Self-healable | 0.288 | MnO2 5.6 mAh cm−2 0.5 mA cm−2 | Physical/ chemical crosslinking Stretchable | [39,40] |
| Sodium polyacrylate (PANa) | 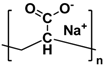 | Sodium carboxylate | Self-healable Alkali resistant | 0.17 | NiCo 259 mAh g−1 5.8 C | Physical crosslinking Stretchable | [34] |
| Xanthan gum | 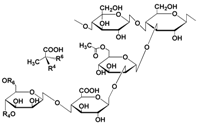 | Polysaccharide | Biodegradable | 1.46 × 10−2 | MnO2 260 mA h g−1 1 C | Physical crosslinking Fragile | [41] |
| Gelatin | 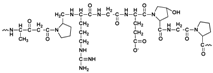 | Peptides | Biodegradable | 6.15 × 10−3–2.0 × 10−2 | LiMn2O4 110 mAh g−1 at 25 mA g−1 | Physical crosslinking Fragile | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, F.; Guo, B.; Ling, W.; Wei, J.; Chen, L.; Yu, S.; Liang, G. Recent Progress and Challenges of Flexible Zn-Based Batteries with Polymer Electrolyte. Batteries 2022, 8, 59. https://doi.org/10.3390/batteries8060059
Mo F, Guo B, Ling W, Wei J, Chen L, Yu S, Liang G. Recent Progress and Challenges of Flexible Zn-Based Batteries with Polymer Electrolyte. Batteries. 2022; 8(6):59. https://doi.org/10.3390/batteries8060059
Chicago/Turabian StyleMo, Funian, Binbin Guo, Wei Ling, Jun Wei, Lina Chen, Suzhu Yu, and Guojin Liang. 2022. "Recent Progress and Challenges of Flexible Zn-Based Batteries with Polymer Electrolyte" Batteries 8, no. 6: 59. https://doi.org/10.3390/batteries8060059






