Recent Health Diagnosis Methods for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Electrical Properties Analysis
2.1. Open-Circuit-Voltage (OCV) Curve-Based Analysis
2.2. Electrochemical Impedance Spectrum (EIS) Analysis
2.3. Electrochemical Parameter-Based Methods
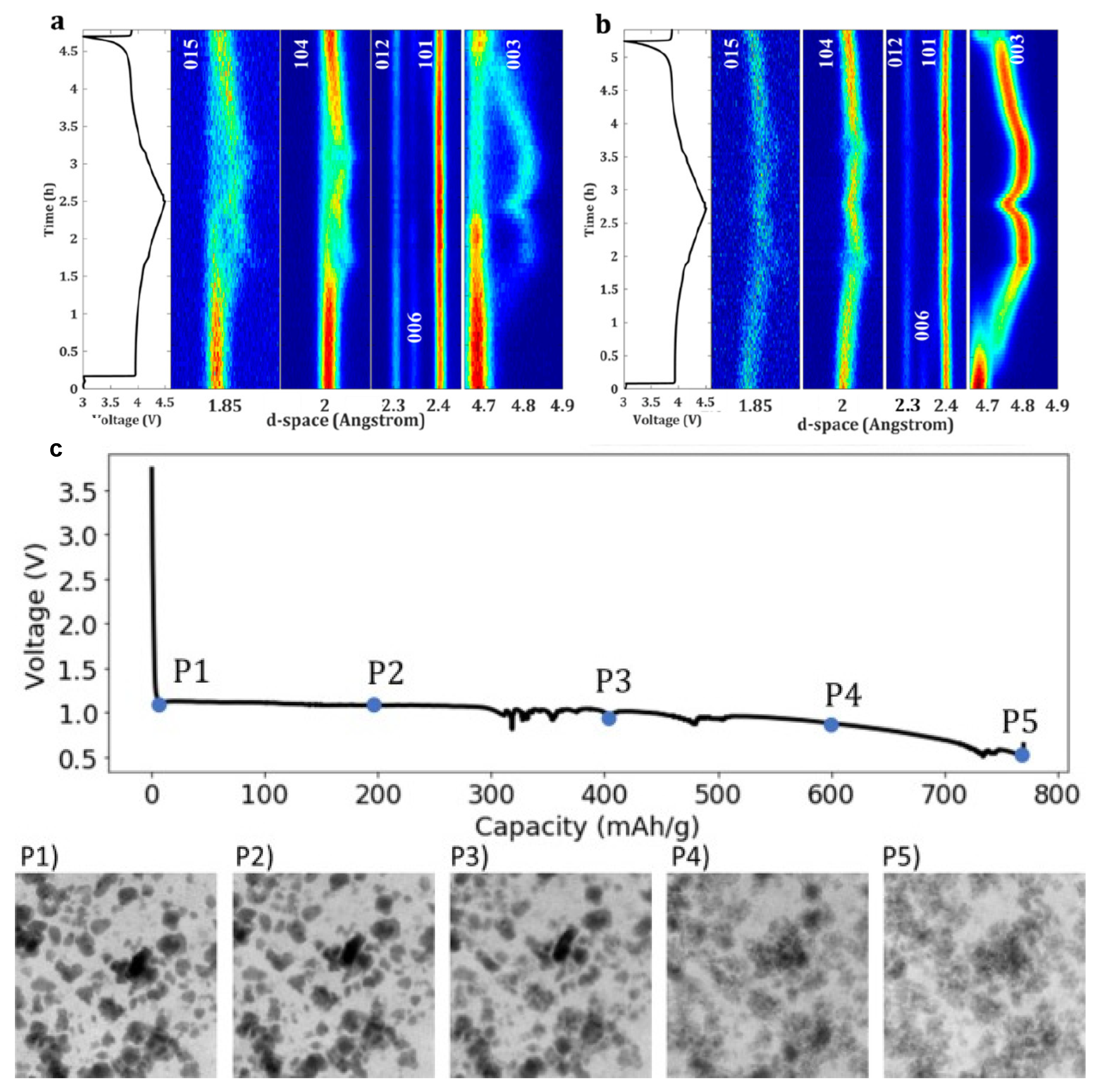
2.4. In Situ Operando Characterization
3. Disassembly-Based Analysis
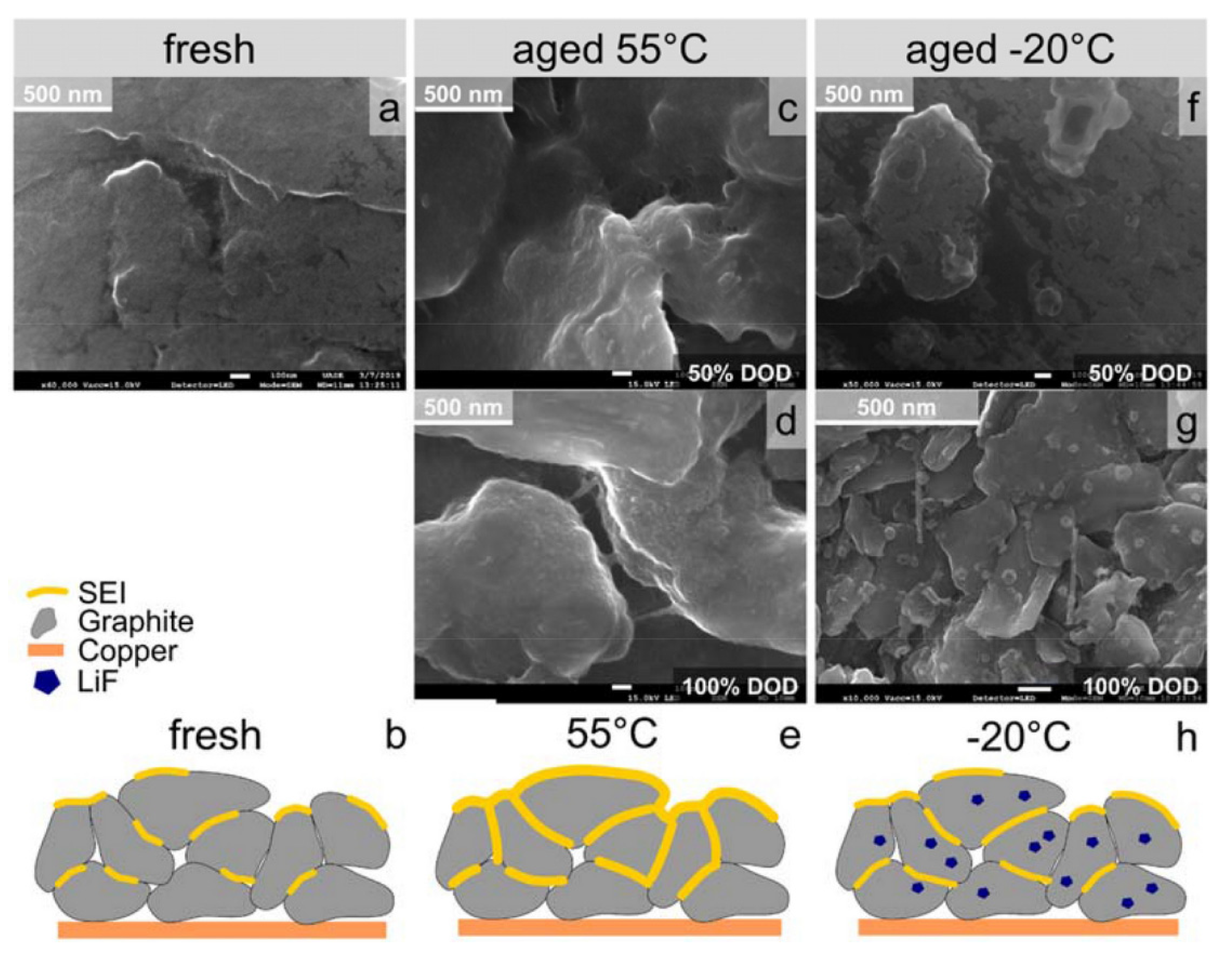
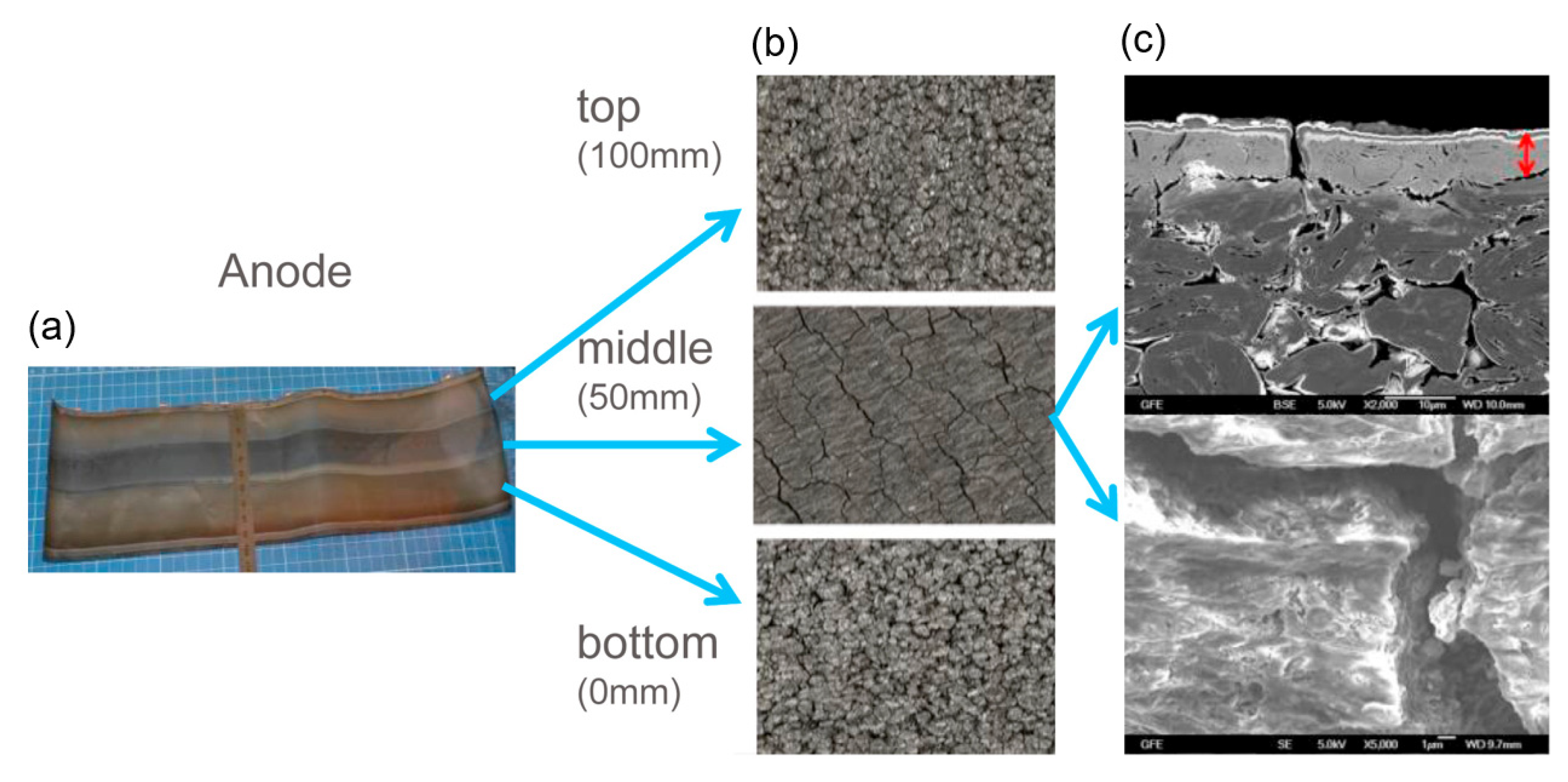
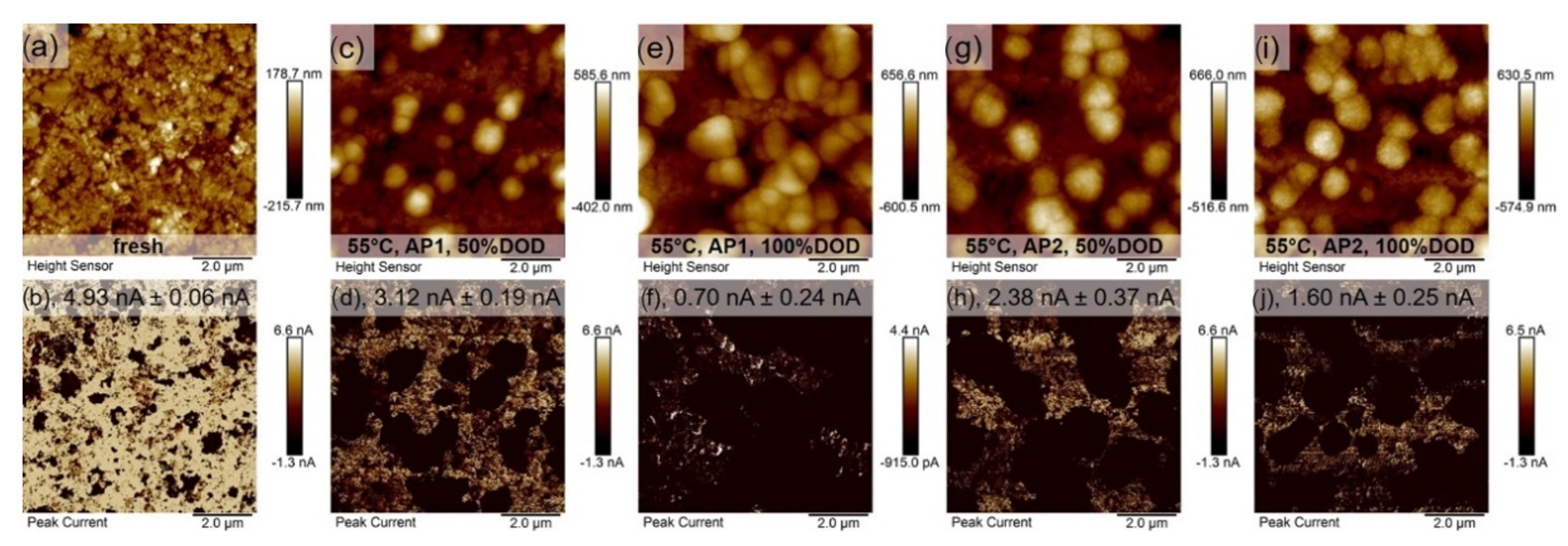
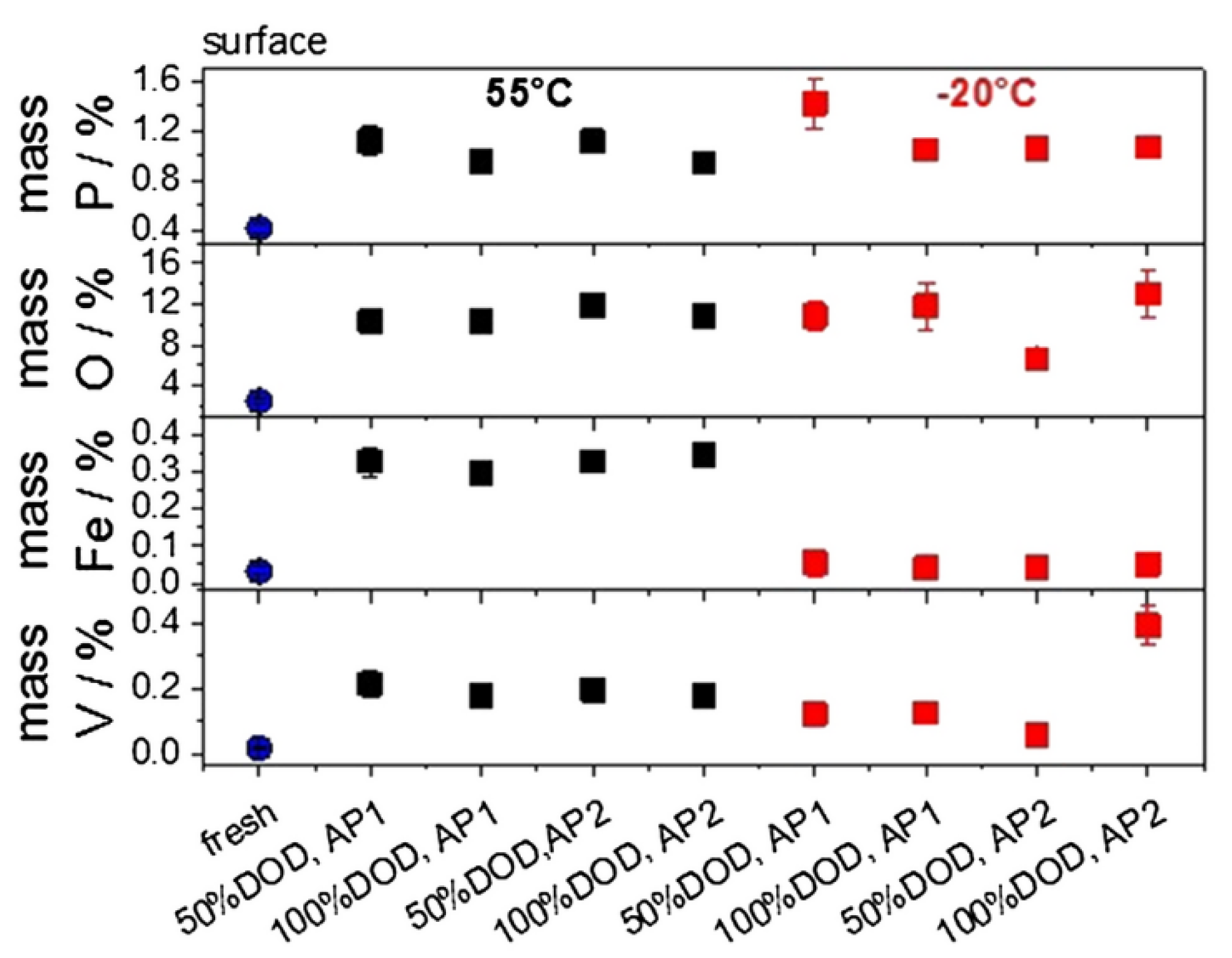
3.1. Morphology Characterization
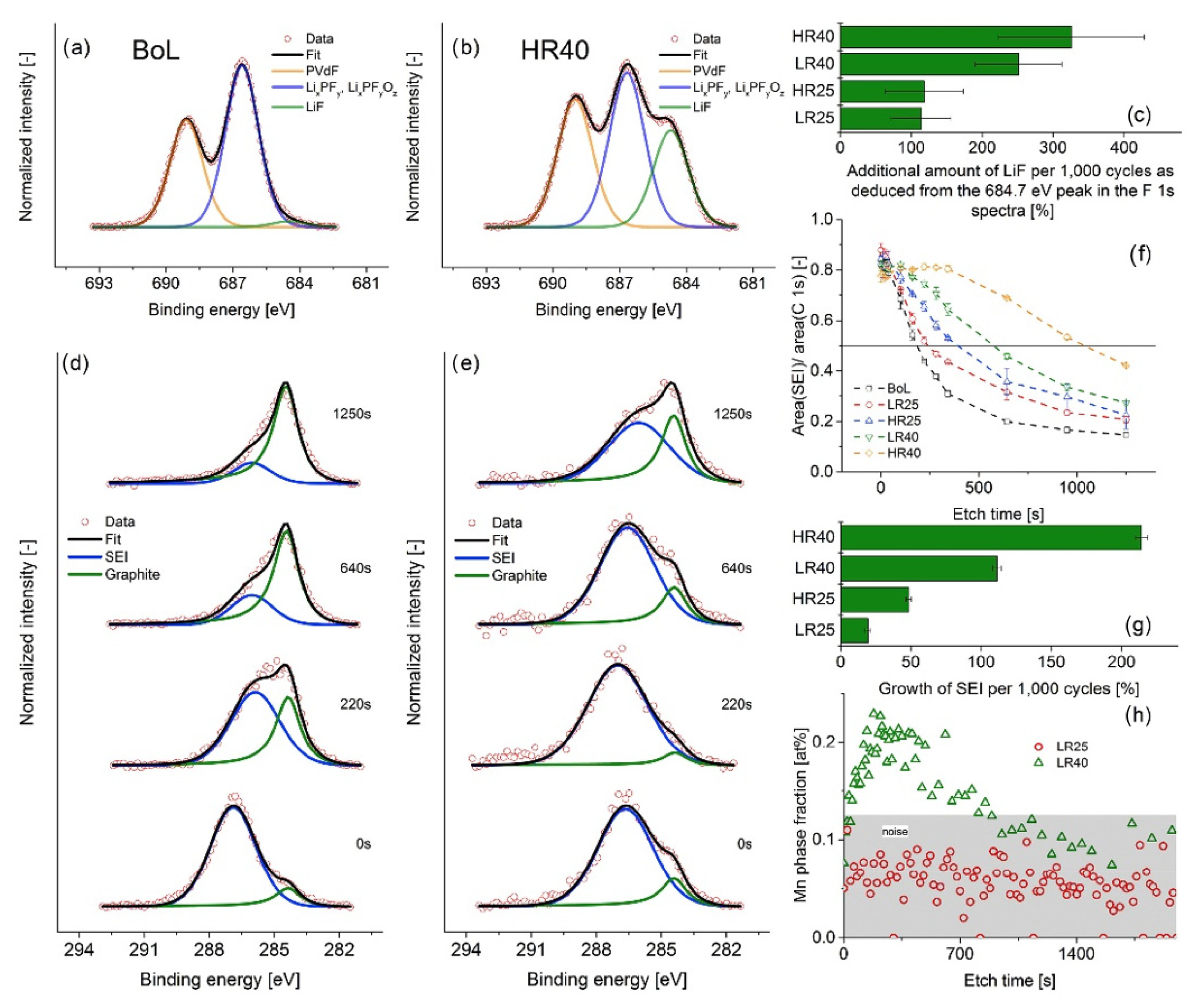
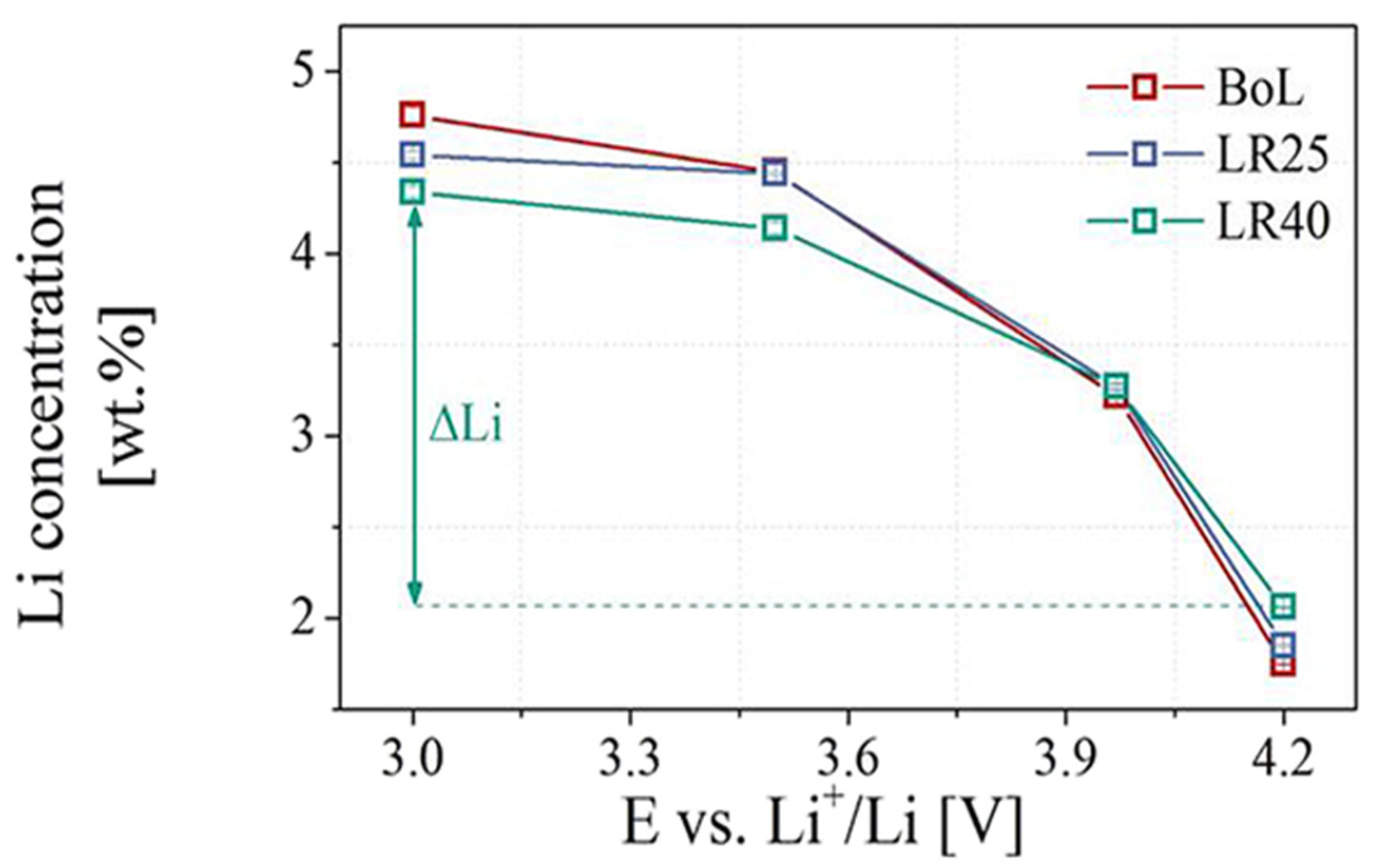
3.2. Composition Characterization
3.3. Structure Characterization
4. Challenges and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.-S.; Nam, K.-W.; Jang, D.; Chung, K.Y.; Cho, Y.-H.; Choi, S.; Hanson, J.C.; Yang, X.-Q. The Kinetic Effect on Structural Behavior of Mixed LiMn2O4–LiNi1/3Co1/3Mn1/3O2 Cathode Materials Studied by in Situ Time-Resolved X-ray Diffraction Technique. Electrochem. Commun. 2012, 15, 74–77. [Google Scholar] [CrossRef]
- Kitao, H.; Fujihara, T.; Takeda, K.; Nakanishi, N.; Nohma, T. High-Temperature Storage Performance of Li-Ion Batteries Using a Mixture of Li-Mn Spinel and Li-Ni-Co-Mn Oxide as a Positive Electrode Material. Electrochem. Solid-State Lett. 2005, 8, A87. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, J.; Kang, S. Investigation on the Dissolution of Mn Ions from LiMn2O4 Cathode in the Application of Lithium Ion Batteries: First Principle Molecular Orbital Method. Int. J. Quantum Chem. 2013, 113, 148–154. [Google Scholar] [CrossRef]
- Dai, Y.; Cai, L.; White, R.E. Capacity Fade Model for Spinel LiMn2O4Electrode. J. Electrochem. Soc. 2012, 160, A182–A190. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Li, Y.; Pedersen, K.; Stroe, D.-I. Lithium-Ion Battery Operation, Degradation, and Aging Mechanism in Electric Vehicles: An Overview. Energy 2021, 14, 5220. [Google Scholar] [CrossRef]
- Daubinger, P.; Schelter, M.; Petersohn, R.; Nagler, F.; Hartmann, S.; Herrmann, M.; Giffin, G.A. Impact of Bracing on Large Format Prismatic Lithium-Ion Battery Cells during Aging. Adv. Energy Mater. 2022, 12, 2102448. [Google Scholar] [CrossRef]
- Cordoba-Arenas, A.; Onori, S.; Guezennec, Y.; Rizzoni, G. Capacity and Power Fade Cycle-Life Model for Plug-in Hybrid Electric Vehicle Lithium-Ion Battery Cells Containing Blended Spinel and Layered-Oxide Positive Electrodes. J. Power Sources 2015, 278, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing Mechanisms in Lithium-Ion Batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Dubarry, M.; Liaw, B.Y.; Chen, M.-S.; Chyan, S.-S.; Han, K.-C.; Sie, W.-T.; Wu, S.-H. Identifying Battery Aging Mechanisms in Large Format Li Ion Cells. J. Power Sources 2011, 196, 3420–3425. [Google Scholar] [CrossRef]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A Review on Lithium-Ion Battery Ageing Mechanisms and Estimations for Automotive Applications. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Schmalstieg, J.; Käbitz, S.; Ecker, M.; Sauer, D.U. A Holistic Aging Model for Li(NiMnCo)O2 Based 18650 Lithium-Ion Batteries. J. Power Sources 2014, 257, 325–334. [Google Scholar] [CrossRef]
- Meng, J.; Cai, L.; Stroe, D.-I.; Ma, J.; Luo, G.; Teodorescu, R. An Optimized Ensemble Learning Framework for Lithium-Ion Battery State of Health Estimation in Energy Storage System. Energy 2020, 206, 118140. [Google Scholar] [CrossRef]
- Rumberg, B.; Epding, B.; Stradtmann, I.; Kwade, A. Identification of Li Ion Battery Cell Aging Mechanisms by Half-Cell and Full-Cell Open-Circuit-Voltage Characteristic Analysis. J. Energy Storage 2019, 25, 100890. [Google Scholar] [CrossRef]
- Rezvanizaniani, S.M.; Liu, Z.; Chen, Y.; Lee, J. Review and Recent Advances in Battery Health Monitoring and Prognostics Technologies for Electric Vehicle (EV) Safety and Mobility. J. Power Sources 2014, 256, 110–124. [Google Scholar] [CrossRef]
- Waag, W.; Fleischer, C.; Sauer, D.U. Critical Review of the Methods for Monitoring of Lithium-Ion Batteries in Electric and Hybrid Vehicles. J. Power Sources 2014, 258, 321–339. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Han, G.; Dai, H.; Zhu, J.; Wang, X.; Tang, X.; Ye, J. Lithium Plating on the Anode for Lithium-Ion Batteries during Long-Term Low Temperature Cycling. J. Power Sources 2021, 484, 229312. [Google Scholar] [CrossRef]
- Zhu, J.; Su, P.; Dewi Darma, M.S.; Hua, W.; Mereacre, L.; Liu-Théato, X.; Heere, M.; Sørensen, D.R.; Dai, H.; Wei, X.; et al. Multiscale Investigation of Discharge Rate Dependence of Capacity Fade for Lithium-Ion Battery. J. Power Sources 2022, 536, 231516. [Google Scholar] [CrossRef]
- Kisters, T.; Kuder, J.; Töpel, A.; Langkemper, R.; Nau, S.; Schopferer, S. Strain-Rate Dependence of the Failure Behavior of Lithium-Ion Pouch Cells under Impact Loading. J. Energy Storage 2021, 41, 102901. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Chen, S.; Han, G.; Zhu, J.; Dai, H. Investigation the Degradation Mechanisms of Lithium-Ion Batteries under Low-Temperature High-Rate Cycling. ACS Appl. Energy Mater. 2022, 5, 6462–6471. [Google Scholar] [CrossRef]
- Han, X.; Ouyang, M.; Lu, L.; Li, J. A Comparative Study of Commercial Lithium Ion Battery Cycle Life in Electric Vehicle: Capacity Loss Estimation. J. Power Sources 2014, 268, 658–669. [Google Scholar] [CrossRef]
- Zhang, Q.; White, R.E. Capacity Fade Analysis of a Lithium Ion Cell. J. Power Sources 2008, 179, 793–798. [Google Scholar] [CrossRef]
- Knap, V.; Stroe, D.-I. Effects of Open-Circuit Voltage Tests and Models on State-of-Charge Estimation for Batteries in Highly Variable Temperature Environments: Study Case Nano-Satellites. J. Power Sources 2021, 498, 229913. [Google Scholar] [CrossRef]
- Han, X.; Ouyang, M.; Lu, L.; Li, J.; Zheng, Y.; Li, Z. A Comparative Study of Commercial Lithium Ion Battery Cycle Life in Electrical Vehicle: Aging Mechanism Identification. J. Power Sources 2014, 251, 38–54. [Google Scholar] [CrossRef]
- Marongiu, A.; Nlandi, N.; Rong, Y.; Sauer, D.U. On-Board Capacity Estimation of Lithium Iron Phosphate Batteries by Means of Half-Cell Curves. J. Power Sources 2016, 324, 158–169. [Google Scholar] [CrossRef]
- Birkl, C.R.; McTurk, E.; Roberts, M.R.; Bruce, P.G.; Howey, D.A. A Parametric Open Circuit Voltage Model for Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A2271–A2280. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Xiong, R.; Shen, W. A Review on State of Health Estimation for Lithium Ion Batteries in Photovoltaic Systems. eTransportation 2019, 2, 100028. [Google Scholar] [CrossRef]
- Bloom, I.; Jansen, A.N.; Abraham, D.P.; Knuth, J.; Jones, S.A.; Battaglia, V.S.; Henriksen, G.L. Differential Voltage Analyses of High-Power, Lithium-Ion Cells: 1. Technique and Application. J. Power Sources 2005, 139, 295–303. [Google Scholar] [CrossRef]
- Safari, M.; Delacourt, C. Aging of a Commercial Graphite/LiFePO4 Cell. J. Electrochem. Soc. 2011, 158, A1123. [Google Scholar] [CrossRef]
- Schmidt, A.P.; Bitzer, M.; Imre, Á.W.; Guzzella, L. Model-Based Distinction and Quantification of Capacity Loss and Rate Capability Fade in Li-Ion Batteries. J. Power Sources 2010, 195, 7634–7638. [Google Scholar] [CrossRef]
- Fu, R.; Choe, S.-Y.; Agubra, V.; Fergus, J. Modeling of Degradation Effects Considering Side Reactions for a Pouch Type Li-Ion Polymer Battery with Carbon Anode. J. Power Sources 2014, 261, 120–135. [Google Scholar] [CrossRef]
- Dubarry, M.; Liaw, B.Y. Identify Capacity Fading Mechanism in a Commercial LiFePO4 Cell. J. Power Sources 2009, 194, 541–549. [Google Scholar] [CrossRef]
- Chen, K.; Zheng, F.; Jiang, J.; Zhang, W.; Jiang, Y.; Chen, K. Practical Failure Recognition Model of Lithium-Ion Batteries Based on Partial Charging Process. Energy 2017, 138, 1199–1208. [Google Scholar] [CrossRef]
- Berecibar, M.; Gandiaga, I.; Villarreal, I.; Omar, N.; van Mierlo, J.; van den Bossche, P. Critical Review of State of Health Estimation Methods of Li-Ion Batteries for Real Applications. Renew. Sustain. Energy Rev. 2016, 56, 572–587. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Uddin, K.; Chouchelamane, G.H.; Widanage, W.D.; Marco, J. A Comparison between Electrochemical Impedance Spectroscopy and Incremental Capacity-Differential Voltage as Li-Ion Diagnostic Techniques to Identify and Quantify the Effects of Degradation Modes within Battery Management Systems. J. Power Sources 2017, 360, 301–318. [Google Scholar] [CrossRef]
- Berecibar, M.; Garmendia, M.; Gandiaga, I.; Crego, J.; Villarreal, I. State of Health Estimation Algorithm of LiFePO4 Battery Packs Based on Differential Voltage Curves for Battery Management System Application. Energy 2016, 103, 784–796. [Google Scholar] [CrossRef]
- Weng, C.; Cui, Y.; Sun, J.; Peng, H. On-Board State of Health Monitoring of Lithium-Ion Batteries Using Incremental Capacity Analysis with Support Vector Regression. J. Power Sources 2013, 235, 36–44. [Google Scholar] [CrossRef]
- Weng, C.; Feng, X.; Sun, J.; Peng, H. State-of-Health Monitoring of Lithium-Ion Battery Modules and Packs via Incremental Capacity Peak Tracking. Appl. Energy 2016, 180, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jiang, J.; Wang, L.Y.; Chen, D.; Zhang, Y.; Zhang, C. A Capacity Model Based on Charging Process for State of Health Estimation of Lithium Ion Batteries. Appl. Energy 2016, 177, 537–543. [Google Scholar] [CrossRef]
- Riviere, E.; Venet, P.; Sari, A.; Meniere, F.; Bultel, Y. LiFePO4 Battery State of Health Online Estimation Using Electric Vehicle Embedded Incremental Capacity Analysis. In Proceedings of the 2015 IEEE Vehicle Power and Propulsion Conference (VPPC), Montreal, QC, Canada, 19–22 October 2015; pp. 1–6. [Google Scholar]
- Gao, Y.; Jiang, J.; Zhang, C.; Zhang, W.; Ma, Z.; Jiang, Y. Lithium-Ion Battery Aging Mechanisms and Life Model under Different Charging Stresses. J. Power Sources 2017, 356, 103–114. [Google Scholar] [CrossRef]
- Abraham, D.P.; Poppen, S.D.; Jansen, A.N.; Liu, J.; Dees, D.W. Application of a Lithium–Tin Reference Electrode to Determine Electrode Contributions to Impedance Rise in High-Power Lithium-Ion Cells. Electrochim. Acta 2004, 49, 4763–4775. [Google Scholar] [CrossRef]
- Schmid, A.U.; Kurka, M.; Birke, K.P. Reproducibility of Li-Ion Cell Reassembling Processes and Their Influence on Coin Cell Aging. J. Energy Storage 2019, 24, 100732. [Google Scholar] [CrossRef]
- Klett, M.; Eriksson, R.; Groot, J.; Svens, P.; Ciosek Högström, K.; Lindström, R.W.; Berg, H.; Gustafson, T.; Lindbergh, G.; Edström, K. Non-Uniform Aging of Cycled Commercial LiFePO4//Graphite Cylindrical Cells Revealed by Post-Mortem Analysis. J. Power Sources 2014, 257, 126–137. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, M.W.; et al. Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
- Waldmann, T.; Kasper, M.; Wohlfahrt-Mehrens, M. Optimization of Charging Strategy by Prevention of Lithium Deposition on Anodes in High-Energy Lithium-Ion Batteries—Electrochemical Experiments. Electrochim. Acta 2015, 178, 525–532. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation Diagnostics for Lithium Ion Cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Synthesize Battery Degradation Modes via a Diagnostic and Prognostic Model. J. Power Sources 2012, 219, 204–216. [Google Scholar] [CrossRef]
- Schmid, A.U.; Ridder, A.; Hahn, M.; Schofer, K.; Birke, K.P. Aging of Extracted and Reassembled Li-Ion Electrode Material in Coin Cells—Capabilities and Limitations. Batteries 2020, 6, 33. [Google Scholar] [CrossRef]
- Berecibar, M.; Dubarry, M.; Omar, N.; Villarreal, I.; van Mierlo, J. Degradation Mechanism Detection for NMC Batteries Based on Incremental Capacity Curves. World Electr. Veh. J. 2016, 8, 350–361. [Google Scholar] [CrossRef] [Green Version]
- Mussa, A.S.; Liivat, A.; Marzano, F.; Klett, M.; Philippe, B.; Tengstedt, C.; Lindbergh, G.; Edström, K.; Lindström, R.W.; Svens, P. Fast-Charging Effects on Ageing for Energy-Optimized Automotive LiNi1/3Mn1/3Co1/3O2/Graphite Prismatic Lithium-Ion Cells. J. Power Sources 2019, 422, 175–184. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Widanage, W.D.; Marco, J.; Gama-Valdez, M.; Chouchelamane, G.H. Identification and Quantification of Ageing Mechanisms in Lithium-Ion Batteries Using the EIS Technique. In Proceedings of the 2016 IEEE Transportation Electrification Conference and Expo (ITEC), Busan, Korea, 1–4 June 2016; pp. 1–6. [Google Scholar]
- Huang, J.; Ge, H.; Li, Z.; Zhang, J. Dynamic Electrochemical Impedance Spectroscopy of a Three-Electrode Lithium-Ion Battery during Pulse Charge and Discharge. Electrochim. Acta 2015, 176, 311–320. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; He, X.; Wan, C.; Jiang, C. Determination of Lithium-Ion Transference Numbers in LiPF[Sub 6]–PC Solutions Based on Electrochemical Polarization and NMR Measurements. J. Electrochem. Soc. 2008, 155, A292. [Google Scholar] [CrossRef]
- Dong, T.K.; Kirchev, A.; Mattera, F.; Kowal, J.; Bultel, Y. Dynamic Modeling of Li-Ion Batteries Using an Equivalent Electrical Circuit. J. Electrochem. Soc. 2011, 158, A326. [Google Scholar] [CrossRef]
- Itagaki, M.; Kobari, N.; Yotsuda, S.; Watanabe, K.; Kinoshita, S.; Ue, M. In Situ Electrochemical Impedance Spectroscopy to Investigate Negative Electrode of Lithium-Ion Rechargeable Batteries. J. Power Sources 2004, 135, 255–261. [Google Scholar] [CrossRef]
- Delacourt, C.; Ridgway, P.L.; Srinivasan, V.; Battaglia, V. Measurements and Simulations of Electrochemical Impedance Spectroscopy of a Three-Electrode Coin Cell Design for Li-Ion Cell Testing. J. Electrochem. Soc. 2014, 161, A1253–A1260. [Google Scholar] [CrossRef] [Green Version]
- Jiménez Gordon, I.; Grugeon, S.; Débart, A.; Pascaly, G.; Laruelle, S. Electrode Contributions to the Impedance of a High-Energy Density Li-Ion Cell Designed for EV Applications. Solid State Ion. 2013, 237, 50–55. [Google Scholar] [CrossRef]
- Thomas-Alyea, K.E.; Newman, J.; Chen, G.; Richardson, T.J. Modeling the Behavior of Electroactive Polymers for Overcharge Protection of Lithium Batteries. J. Electrochem. Soc. 2004, 151, A509. [Google Scholar] [CrossRef]
- Newman, J.; Tiedemann, W. Potential and Current Distribution in Electrochemical Cells: Interpretation of the Half-Cell Voltage Measurements as a Function of Reference-Electrode Location. J. Electrochem. Soc. 1993, 140, 1961–1968. [Google Scholar] [CrossRef]
- Ramadesigan, V.; Chen, K.; Burns, N.A.; Boovaragavan, V.; Braatz, R.D.; Subramanian, V.R. Parameter Estimation and Capacity Fade Analysis of Lithium-Ion Batteries Using Reformulated Models. J. Electrochem. Soc. 2011, 158, A1048. [Google Scholar] [CrossRef]
- Su, L.; Weaver, J.L.; Groenenboom, M.; Nakamura, N.; Rus, E.; Anand, P.; Jha, S.K.; Okasinski, J.S.; Dura, J.A.; Reeja-Jayan, B. Tailoring Electrode–Electrolyte Interfaces in Lithium-Ion Batteries Using Molecularly Engineered Functional Polymers. ACS Appl. Mater. Interfaces 2021, 13, 9919–9931. [Google Scholar] [CrossRef]
- Su, L.; Choi, P.; Nakamura, N.; Charalambous, H.; Litster, S.; Ilavsky, J.; Reeja-Jayan, B. Multiscale Operando X-ray Investigations Provide Insights into Electro-Chemo-Mechanical Behavior of Lithium Intercalation Cathodes. Appl. Energy 2021, 299, 117315. [Google Scholar] [CrossRef]
- Simolka, M.; Heger, J.-F.; Kaess, H.; Biswas, I.; Friedrich, K.A. Influence of Cycling Profile, Depth of Discharge and Temperature on Commercial LFP/C Cell Ageing: Post-Mortem Material Analysis of Structure, Morphology and Chemical Composition. J. Appl. Electrochem. 2020, 50, 1101–1117. [Google Scholar] [CrossRef]
- Lewerenz, M.; Warnecke, A.; Sauer, D.U. Post-Mortem Analysis on LiFePO4|Graphite Cells Describing the Evolution & Composition of Covering Layer on Anode and Their Impact on Cell Performance. J. Power Sources 2017, 369, 122–132. [Google Scholar] [CrossRef]
- Lang, M.; Darma, M.S.D.; Kleiner, K.; Riekehr, L.; Mereacre, L.; Ávila Pérez, M.; Liebau, V.; Ehrenberg, H. Post Mortem Analysis of Fatigue Mechanisms in LiNi0.8Co0.15Al0.05O2—LiNi0.5Co0.2Mn0.3O2—LiMn2O4/Graphite Lithium Ion Batteries. J. Power Sources 2016, 326, 397–409. [Google Scholar] [CrossRef]
- Lu, P.; Li, C.; Schneider, E.W.; Harris, S.J. Chemistry, Impedance, and Morphology Evolution in Solid Electrolyte Interphase Films during Formation in Lithium Ion Batteries. J. Phys. Chem. C 2014, 118, 896–903. [Google Scholar] [CrossRef]
- Anseán, D.; Dubarry, M.; Devie, A.; Liaw, B.Y.; García, V.M.; Viera, J.C.; González, M. Fast Charging Technique for High Power LiFePO4 Batteries: A Mechanistic Analysis of Aging. J. Power Sources 2016, 321, 201–209. [Google Scholar] [CrossRef]
- Leroy, S.; Blanchard, F.; Dedryvère, R.; Martinez, H.; Carré, B.; Lemordant, D.; Gonbeau, D. Surface Film Formation on a Graphite Electrode in Li-Ion Batteries: AFM and XPS Study. Surf. Interface Anal. 2005, 37, 773–781. [Google Scholar] [CrossRef]
- Lang, M.; Dewi Darma, M.S.; Mereacre, L.; Liebau, V.; Ehrenberg, H. Post Mortem Analysis of Ageing Mechanisms in LiNi0.8Co0.15Al0.05O2—LiNi0.5Co0.2Mn0.3O2—LiMn2O4/Graphite Lithium Ion Batteries. J. Power Sources 2020, 453, 227915. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Mansour, A.N.; Yabuuchi, N.; Shao-Horn, Y. Probing the Origin of Enhanced Stability of “AlPO4” Nanoparticle Coated LiCoO2 during Cycling to High Voltages: Combined XRD and XPS Studies. Chem. Mater. 2009, 21, 4408–4424. [Google Scholar] [CrossRef]
- Shin, H.; Park, J.; Sastry, A.M.; Lu, W. Degradation of the Solid Electrolyte Interphase Induced by the Deposition of Manganese Ions. J. Power Sources 2015, 284, 416–427. [Google Scholar] [CrossRef]
- Liu, W.; Huang, X.; Guobao, L.; Wang, Z.; Huang, H.; Zhonghua, L.; Xue, R.; Chen, L. Electrochemical and X-Ray Photospectroscopy Studies of Polytetrafluoroethylene and Polyvinylidene Fluoride in Li/C Batteries. J. Power Sources 1997, 68, 344–347. [Google Scholar] [CrossRef]
- Broussely, M.; Biensan, P.; Bonhomme, F.; Blanchard, P.; Herreyre, S.; Nechev, K.; Staniewicz, R.J. Main Aging Mechanisms in Li Ion Batteries. J. Power Sources 2005, 146, 90–96. [Google Scholar] [CrossRef]
- Agubra, V.A.; Fergus, J.W.; Fu, R.; Choe, S.-Y. Analysis of Effects of the State of Charge on the Formation and Growth of the Deposit Layer on Graphite Electrode of Pouch Type Lithium Ion Polymer Batteries. J. Power Sources 2014, 270, 213–220. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Hicks-Garner, J.; Sherman, E.; Soukiazian, S.; Verbrugge, M.; Tataria, H.; Musser, J.; Finamore, P. Cycle-Life Model for Graphite-LiFePO4 Cells. J. Power Sources 2011, 196, 3942–3948. [Google Scholar] [CrossRef]
- Darma, M.S.D.; Lang, M.; Kleiner, K.; Mereacre, L.; Liebau, V.; Fauth, F.; Bergfeldt, T.; Ehrenberg, H. The Influence of Cycling Temperature and Cycling Rate on the Phase Specific Degradation of a Positive Electrode in Lithium Ion Batteries: A Post Mortem Analysis. J. Power Sources 2016, 327, 714–725. [Google Scholar] [CrossRef]
- Nam, K.-W.; Yoon, W.-S.; Yang, X.-Q. Structural Changes and Thermal Stability of Charged LiNi1/3Co1/3Mn1/3O2 Cathode Material for Li-Ion Batteries Studied by Time-Resolved XRD. J. Power Sources 2009, 189, 515–518. [Google Scholar] [CrossRef]
- Maher, K.; Yazami, R. Effect of Overcharge on Entropy and Enthalpy of Lithium-Ion Batteries. Electrochim. Acta 2013, 101, 71–78. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A. Solid-State Redox Reactions of LiCoO2 (R3m) for 4 Volt Secondary Lithium Cells. J. Electrochem. Soc. 1994, 141, 2972–2977. [Google Scholar] [CrossRef]
- Amatucci, G.G.; Tarascon, J.M.; Klein, L.C. CoO2, The End Member of the Li x CoO2 Solid Solution. J. Electrochem. Soc. 1996, 143, 1114–1123. [Google Scholar] [CrossRef]
- Gabrisch, H.; Yazami, R.; Fultz, B. Hexagonal to Cubic Spinel Transformation in Lithiated Cobalt Oxide. J. Electrochem. Soc. 2004, 151, A891. [Google Scholar] [CrossRef]
- Ozawa, Y.; Yazami, R.; Fultz, B. Self-Discharge Study of LiCoO2 Cathode Materials. J. Power Sources 2003, 119–121, 918–923. [Google Scholar] [CrossRef]
- Yoshio, M.; Tanaka, H.; Tominaga, K.; Noguchi, H. Synthesis of LiCoO2 from Cobalt—Organic Acid Complexes and Its Electrode Behaviour in a Lithium Secondary Battery. J. Power Sources 1992, 40, 347–353. [Google Scholar] [CrossRef]






| Methods | Representative Techniques | Advantages | Disadvantages |
|---|---|---|---|
| Non-destructive analysis | Open-circuit-voltage curves | Testers are easy to be operated and the data are simple to obtain | Difficult to understand the specific electrochemical reactions inside the cells |
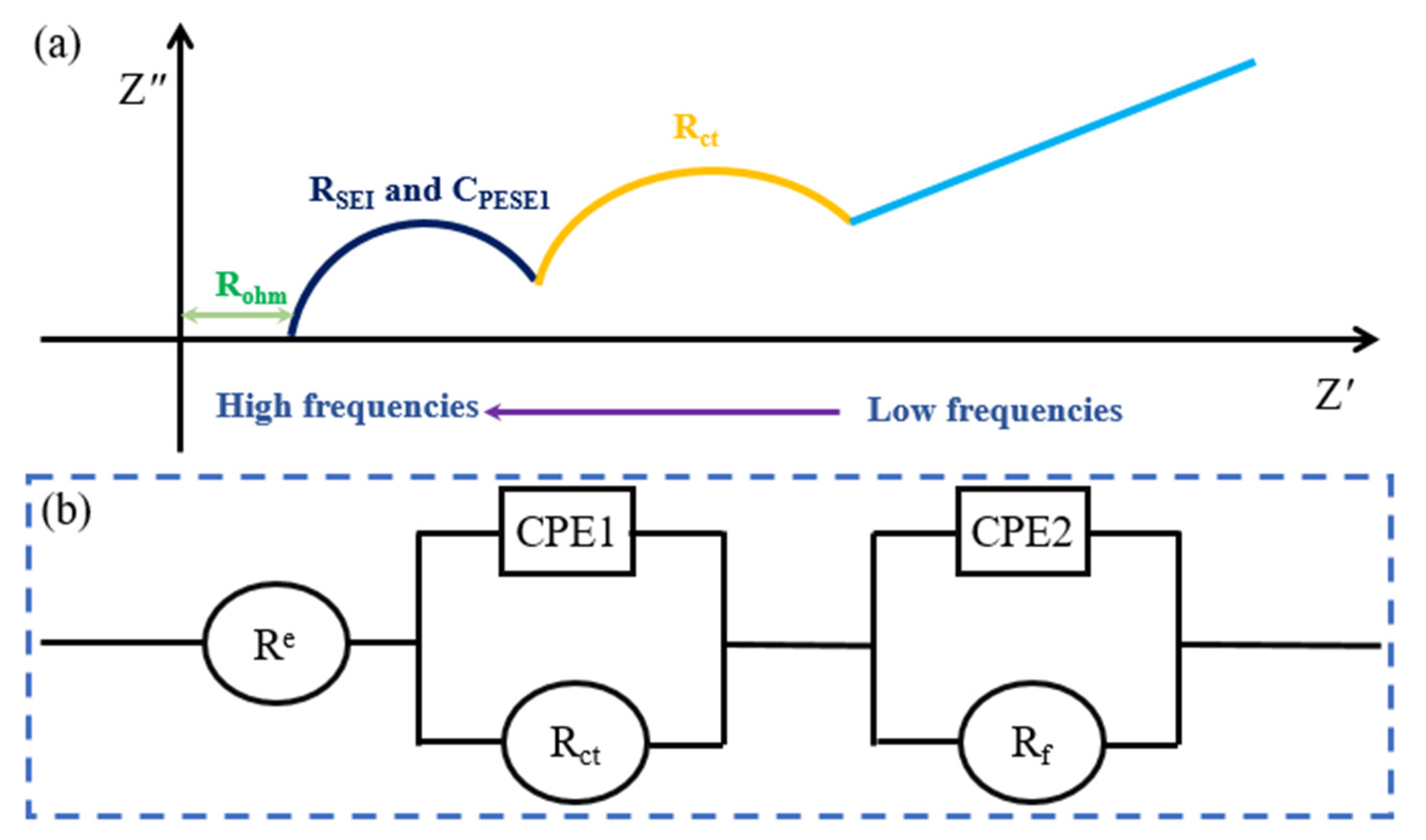
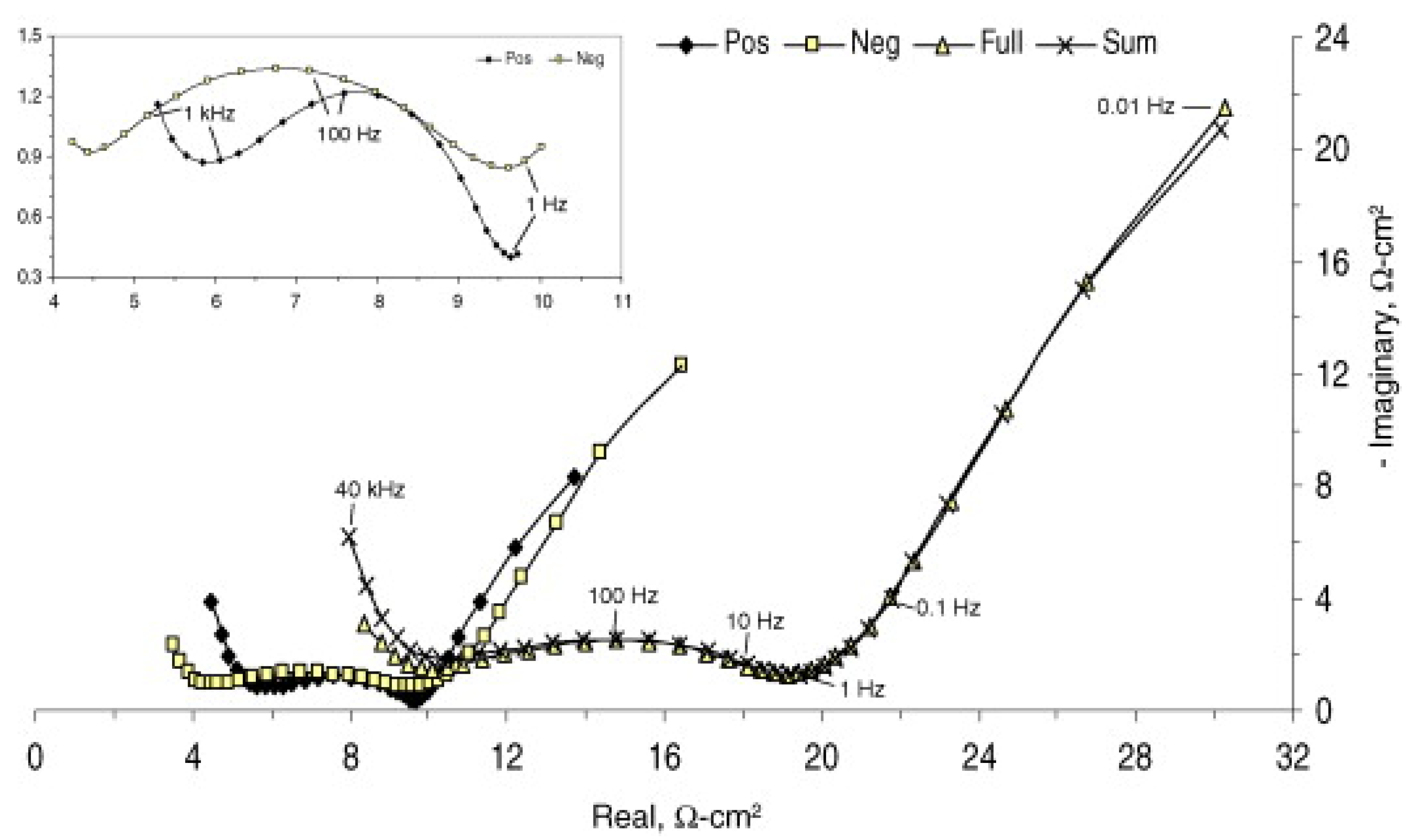
| Electrochemical impedance spectroscopy | The equivalent circuit model can be provided and non-destructive diagnosis | Time-consuming procedures and the estimation of SOH is influenced by the model | |
| In situ operando characterization | Characterizing the electro-chemo-mechanical behavior during cycling without destructing the battery | High instrument costs and high requirements for both experimental environment and users | |
| Destructive analysis | Electron microscopy | Visualized morphology observation of the aging changes inside the cells | Irreversible destruction to cells and only used in vacuum |
| X-ray spectroscopy | Detailed composition results and specific area can be investigated and quantified | High requirements for the experimental environment and specialized skills for user | |
| X-ray diffraction | Structure features can be measured and can be used for SOH diagnostics both in situ and ex situ | High requirements for the samples and the specialized skills for user |
| Methods | Principle | Advantages | Disadvantages |
|---|---|---|---|
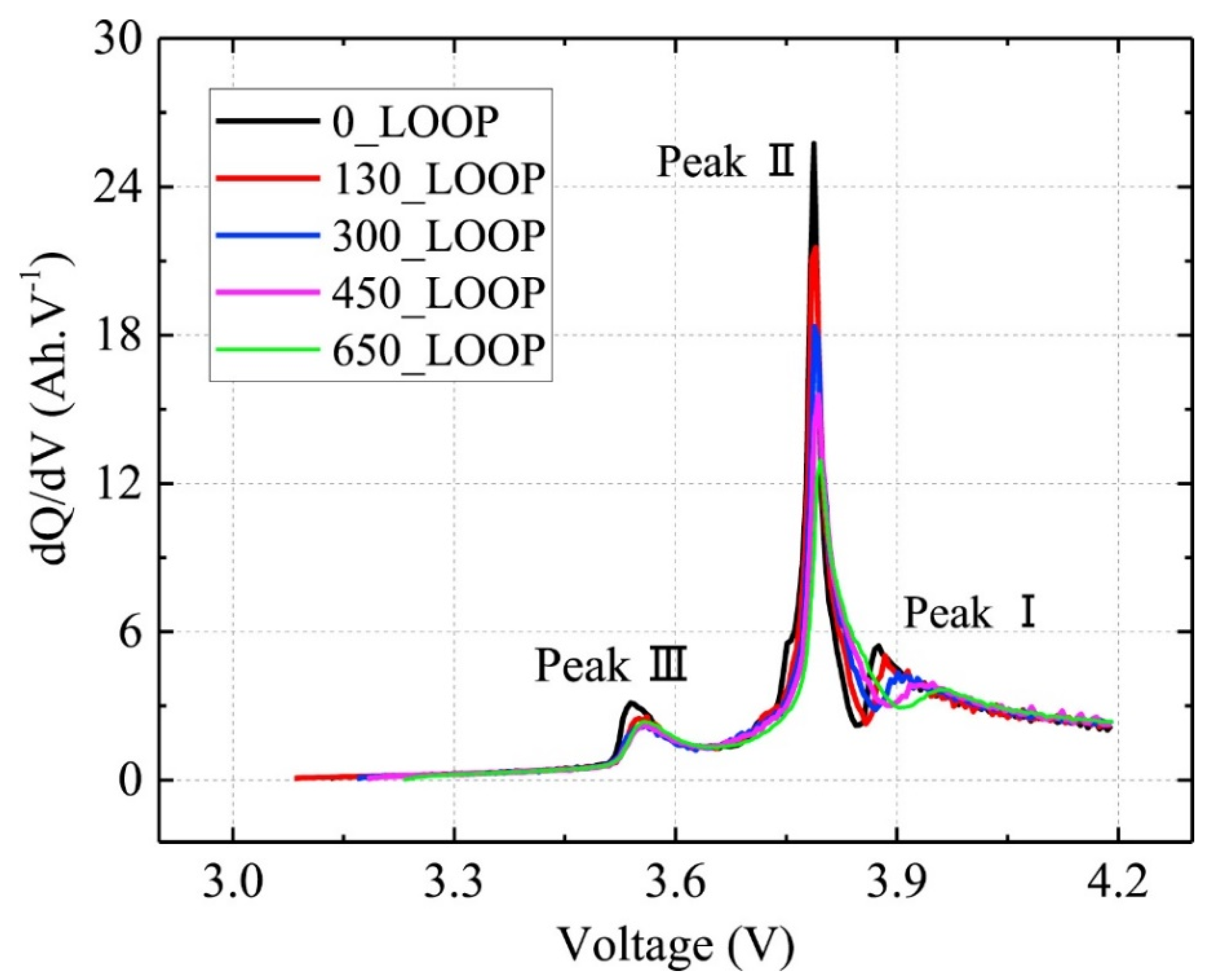
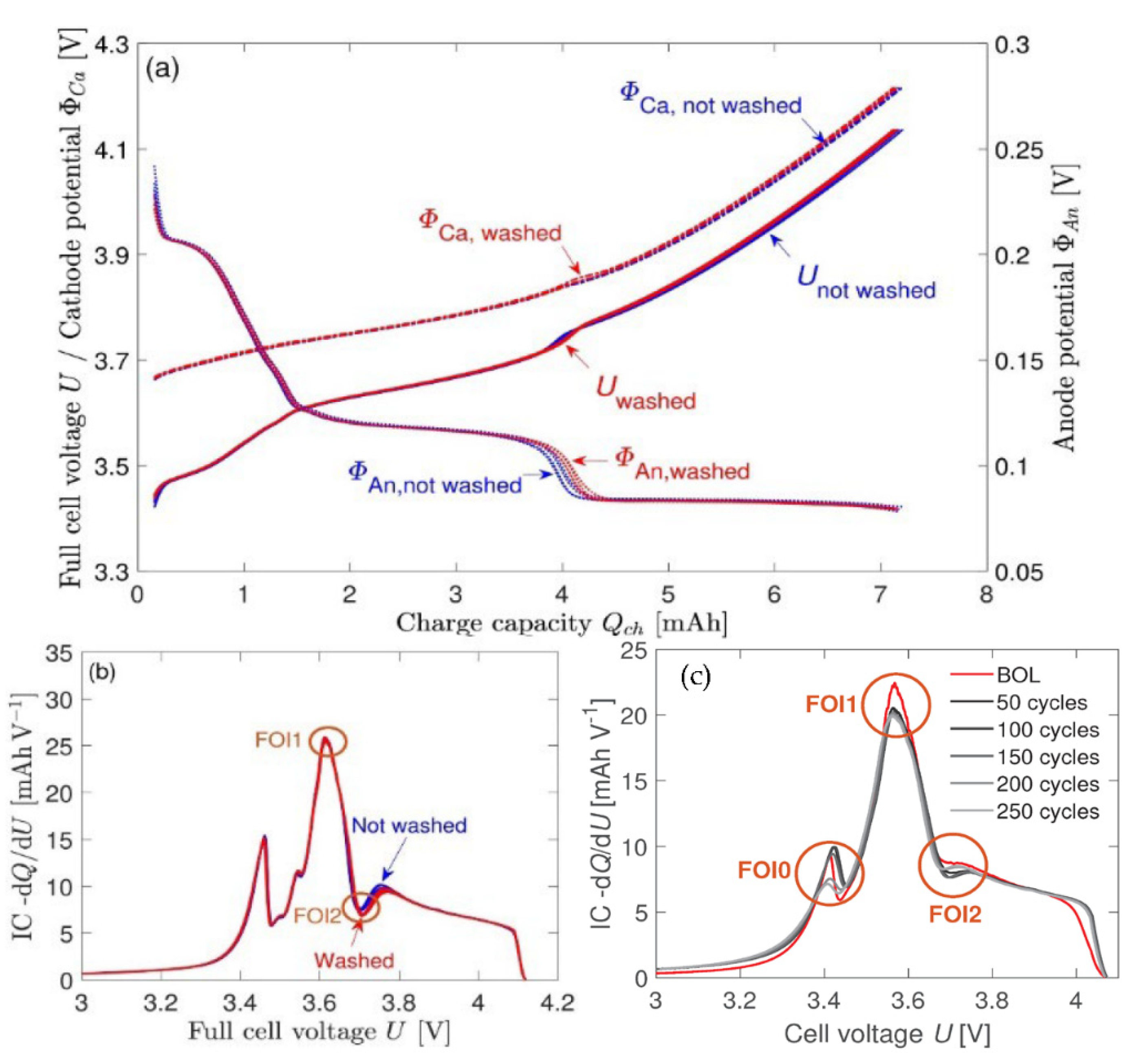
| Incremental capacity analysis (ICA) | IC curve changes with capacity decay | IC (dQ/dV) measuring can be directly obtained | Time-consuming modeling and impractical for implementations with incomplete data |
| Differential voltage analysis (DVA) | By counting the inverse of the IC value, the DV value can be easily obtained | Considering the aging mechanism, the estimation error can be controlled by charging data | DV (dV/dQ) measuring requires the capacity of batteries, which is varied while battery aging and hard to be measured directly |
| FOI | Attribute | LLI | LAMNE | LAMPE |
|---|---|---|---|---|
| 0 | Peak height dQ/dU | ↑ | steady | ↓ |
| 0 | Position U | ↑ | steady | ↑ |
| 1 | Peak height dQ/dU | ↓ | steady | ↓ |
| 1 | Position U | steady | steady | steady |
| 2 | Peak height dQ/dU | ↓ | ↑ | steady |
| 2 | Position U | ↑ | ↓ | ↑ |
| Methods | Advantages | Disadvantages |
|---|---|---|
| SEM | The apparatus is simple and convenient for operation | Only used in vacuum |
| TEM | Higher resolution | Strict cleanliness requirements for the operation and expensive costs |
| AFM | Detailed results and specific area can be investigated | Results can be easily influenced by environment and the situation of instrument |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Guo, J.; Pedersen, K.; Gurevich, L.; Stroe, D.-I. Recent Health Diagnosis Methods for Lithium-Ion Batteries. Batteries 2022, 8, 72. https://doi.org/10.3390/batteries8070072
Li Y, Guo J, Pedersen K, Gurevich L, Stroe D-I. Recent Health Diagnosis Methods for Lithium-Ion Batteries. Batteries. 2022; 8(7):72. https://doi.org/10.3390/batteries8070072
Chicago/Turabian StyleLi, Yaqi, Jia Guo, Kjeld Pedersen, Leonid Gurevich, and Daniel-Ioan Stroe. 2022. "Recent Health Diagnosis Methods for Lithium-Ion Batteries" Batteries 8, no. 7: 72. https://doi.org/10.3390/batteries8070072







