The Effect of Electrode Thickness on the High-Current Discharge and Long-Term Cycle Performance of a Lithium-Ion Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrode
2.3. Preparation of Cell
2.4. Characterization
3. Experimental Results and Discussion
3.1. Energy Density
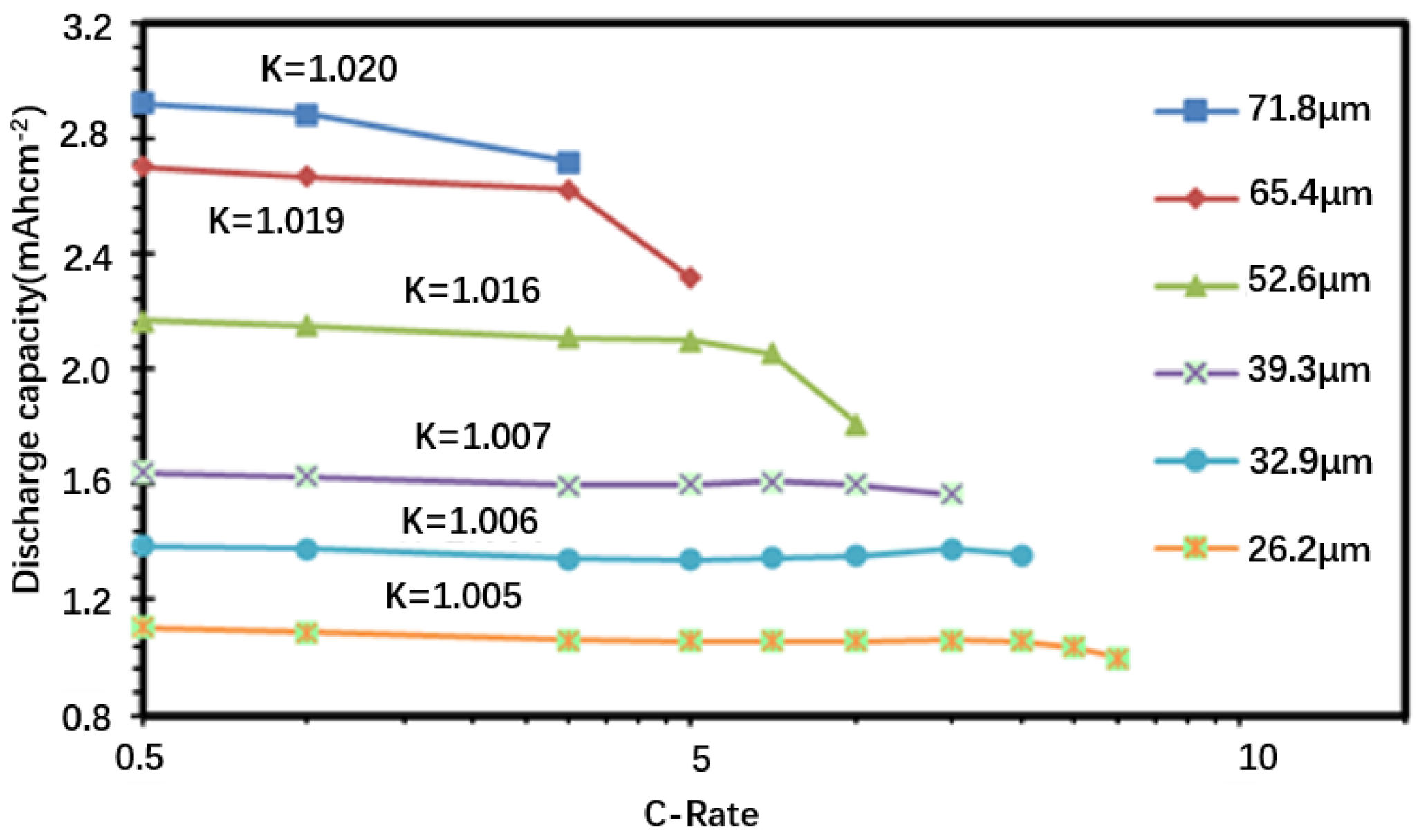
3.2. Discharge Performance (Continuous)
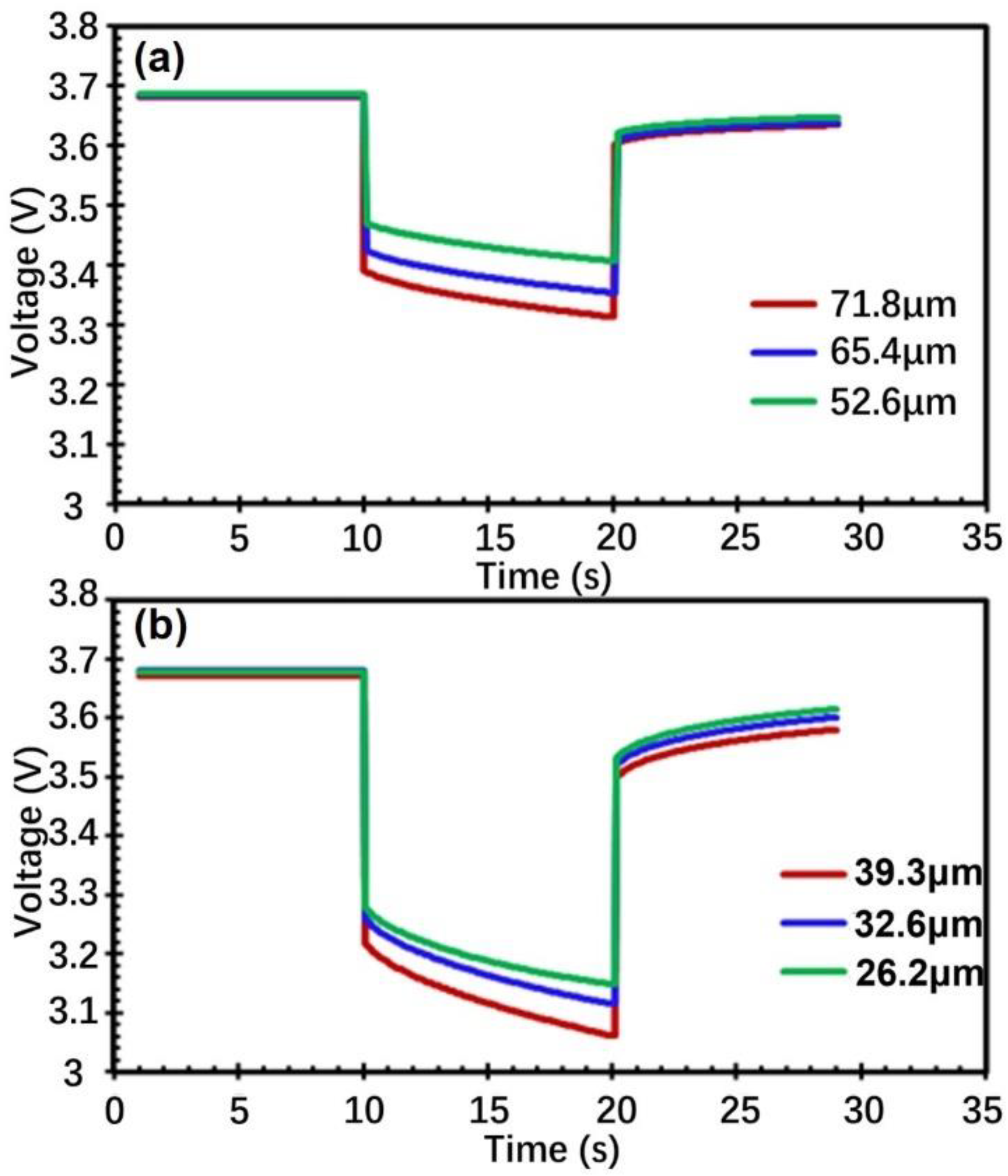
3.3. Discharge Performance (Pulse)
3.4. Long-Term Cycle Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fatima, H.; Zhong, Y.; Wu, H.; Shao, Z. Recent advances in functional oxides for high energy density sodium-ion batteries. Mater. Rep. Energy 2021, 1, 100022. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Feng, L.; Chen, W.; Yang, W.; Dong, Y.; Jiang, S.; Zhang, Q.; He, S. All-cellulose-based high-rate performance solid-state supercapacitor enabled by nitrogen doping and porosity tuning. Diam. Relat. Mater. 2022, 128, 109238. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.; Chi, M.; Guo, C.; Wang, S.; Min, D. Preparation and performance of different carbonized wood electrodes. J. For. Eng. 2022, 7, 127–135. [Google Scholar] [CrossRef]
- Han, Z.; Zhong, W.; Wang, K. Preparation and examination of nitrogen-doped bamboo porous carbon for supercapacitor materials. J. For. Eng. 2020, 5, 76–83. [Google Scholar] [CrossRef]
- Chen, S.; Jeong, S.R.; Tao, S. Key materials and future perspective for aqueous rechargeable lithium-ion batteries. Mater. Rep. Energy 2022, 2, 100096. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.; Duan, G.; Fang, H.; Hou, H. Dense and thin coating of gel polymer electrolyte on sulfur cathode toward high performance Li-sulfur battery. Compos. Commun. 2020, 19, 239–245. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J.; Tillmetz, W. Advances in Battery Technologies for Electric Vehicles; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Yin, H.; Ji, S.; Gu, M.; Zhang, L.; Liu, J. Scalable synthesis of Li1.2 Mn0.54 Ni0.13 Co0.13 O2/LiNi0.5 Mn1.5 O4 sphere composites as stable and high capacity cathodes for Li-ion batteries. RSC Adv. 2015, 5, 84673–84679. [Google Scholar] [CrossRef]
- Liao, C.; Li, F.; Liu, J. Challenges and Modification Strategies of Ni-Rich Cathode Materials Operating at High-Voltage. Nanomaterials 2022, 12, 1888. [Google Scholar] [CrossRef]
- Pires, V.F.; Romero-Cadaval, E.; Vinnikov, D.; Roasto, I.; Martins, J. Power converter interfaces for electrochemical energy storage systems—A review. Energy Convers. Manag. 2014, 86, 453–475. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, B.; Feng, L.; Zheng, J.; You, B.; Chen, J.; Zhao, X.; Zhang, C.; Jiang, S.; He, S. Progress in the use of organic potassium salts for the synthesis of porous carbon nanomaterials: Microstructure engineering for advanced supercapacitors. Nanoscale 2022, 14, 8216–8244. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, B.; Feng, L.; Zhang, Q.; Zhang, C.; Yang, W.; Han, J.; Jiang, S.; He, S. Potassium citrate assisted synthesis of hierarchical porous carbon materials for high performance supercapacitors. Diam. Relat. Mater. 2022, 128, 109247. [Google Scholar] [CrossRef]
- Qin, Y.; Liao, Y.; Liu, J.; Tian, C.; Xu, H.; Wu, Y. Research progress of wood-derived energy storage materials. J. For. Eng. 2021, 6, 100654. [Google Scholar] [CrossRef]
- Guo, W.; Guo, X.; Yang, L.; Wang, T.; Zhang, M.; Duan, G.; Liu, X.; Li, Y. Synthetic melanin facilitates MnO supercapacitors with high specific capacitance and wide operation potential window. Polymer 2021, 235, 124276. [Google Scholar] [CrossRef]
- Hyung-Joo, N.; Sungjune, Y.; Seung, Y.C.; Yang-Kook, S. Comparison of the structural and electrochemical properties of layered Li [NixCoyMnz] O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar]
- Zhang, M.J.; Hu, X.; Li, M.; Duan, Y.; Yang, L.; Yin, C.; Ge, M.; Xiao, X.; Lee, W.K.; Ko, J.Y.P. Cooling Induced Surface Reconstruction during Synthesis of High-Ni Layered Oxides. Adv. Energy Mater. 2019, 9, 1901915. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, K.-J.; Yoon, C.S.; Sun, Y.-K. Capacity fading of Ni-rich Li [NixCoyMn1–x–y] O2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Kim, U.-H.; Myung, S.-T.; Yoon, C.S.; Sun, Y.-K. Extending the battery life using an Al-doped Li [Ni0.76Co0.09Mn0.15]O2 cathode with concentration gradients for lithium ion batteries. ACS Energy Lett. 2017, 2, 1848–1854. [Google Scholar] [CrossRef]
- Araki, K.; Taguchi, N.; Sakaebe, H.; Tatsumi, K.; Ogumi, Z. Electrochemical properties of LiNi1/3Co1/3Mn1/3O2 cathode material modified by coating with Al2O3 nanoparticles. J. Power Sources 2014, 269, 236–243. [Google Scholar] [CrossRef]
- Li, F.; Liu, Z.; Shen, J.; Xu, X.; Zeng, L.; Zhang, B.; Zhu, H.; Liu, Q.; Liu, J.; Zhu, M. A nanorod-like Ni-rich layered cathode with enhanced Li+ diffusion pathways for high-performance lithium-ion batteries. J. Mater. Chem. A 2021, 9, 2830–2839. [Google Scholar] [CrossRef]
- Li, F.; Liu, Z.; Shen, J.; Xu, X.; Zeng, L.; Li, Y.; Zhang, D.; Zuo, S.; Liu, J. Ni-rich layered oxide with preferred orientation (110) plane as a stable cathode material for high-energy lithium-ion batteries. Nanomaterials 2020, 10, 2495. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yan, B.; Zheng, J.; Chen, J.; Wei, R.; Jiang, S.; Yang, W.; Zhang, Q.; He, S. Soybean protein-derived N, O co-doped porous carbon sheets for supercapacitor applications. New J. Chem. 2022, 46, 10844–10853. [Google Scholar] [CrossRef]
- Indrikova, M.; Grunwald, S.; Golks, F.; Netz, A.; Westphal, B.; Kwade, A. The morphology of battery electrodes with the focus of the conductive additives paths. J. Electrochem. Soc. 2015, 162, A2021. [Google Scholar] [CrossRef]
- Jin, Z.; Yang, L.; Shi, S.; Wang, T.; Duan, G.; Liu, X.; Li, Y. Flexible Polydopamine Bioelectronics. Adv. Funct. Mater. 2021, 31, 2103391. [Google Scholar] [CrossRef]
- Yang, L.; Guo, X.; Jin, Z.; Guo, W.; Duan, G.; Liu, X.; Li, Y. Emergence of melanin-inspired supercapacitors. Nano Today 2021, 37, 101075. [Google Scholar] [CrossRef]
- Han, X.; Xiao, G.; Wang, Y.; Chen, X.; Duan, G.; Wu, Y.; Gong, X.; Wang, H. Design and fabrication of conductive polymer hydrogels and their applications in flexible supercapacitors. J. Mater. Chem. A 2020, 8, 23059–23095. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, G.; Duan, G.; Wu, Y.; Zhao, X.; Gong, X. Structural design of carbon dots/porous materials composites and their applications. Chem. Eng. J. 2021, 421, 127743. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A comprehensive understanding of electrode thickness effects on the electrochemical performances of Li-ion battery cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- Denis, Y.; Donoue, K.; Inoue, T.; Fujimoto, M.; Fujitani, S. Effect of electrode parameters on LiFePO4 cathodes. J. Electrochem. Soc. 2006, 153, A835. [Google Scholar]
- Hamankiewicz, B.; Michalska, M.; Krajewski, M.; Ziolkowska, D.; Lipinska, L.; Korona, K.; Kaminska, M.; Czerwinski, A. The effect of electrode thickness on electrochemical performance of LiMn2O4 cathode synthesized by modified sol–gel method. Solid State Ion. 2014, 262, 9–13. [Google Scholar] [CrossRef]
- Thunman, M.; Marquardt, K.; Hahn, R.; Kober, D.; Goerke, O.; Schubert, H. Discharge performance dependence on electrode thickness for li4ti5o12/limn2o4 cells for application in wafer-integrated microbatteries. Ecs Trans. 2012, 41, 147. [Google Scholar] [CrossRef]
- Heubner, C.; Nickol, A.; Seeba, J.; Reuber, S.; Junker, N.; Wolter, M.; Schneider, M.; Michaelis, A. Understanding thickness and porosity effects on the electrochemical performance of LiNi0.6Co0.2Mn0.2O2-based cathodes for high energy Li-ion batteries. J. Power Sources 2019, 419, 119–126. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Conveying Advanced Li-ion Battery Materials into Practice The Impact of Electrode Slurry Preparation Skills. Adv. Energy Mater. 2016, 6, 1600655. [Google Scholar] [CrossRef]
- Parikh, D.; Christensen, T.; Li, J. Correlating the influence of porosity, tortuosity, and mass loading on the energy density of LiNi0.6Mn0.2Co0.2O2 cathodes under extreme fast charging (XFC) conditions. J. Power Sources 2020, 474, 228601. [Google Scholar] [CrossRef]
- Dubarry, M.; Liaw, B.Y. Identify capacity fading mechanism in a commercial LiFePO4 cell. J. Power Sources 2009, 194, 541–549. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Cugnet, M.; Liaw, B.Y.; Gering, K.; Sazhin, S.; Jamison, D.; Michelbacher, C. Evaluation of commercial lithium-ion cells based on composite positive electrode for plug-in hybrid electric vehicle applications. Part I: Initial characterizations. J. Power Sources 2011, 196, 10328–10335. [Google Scholar] [CrossRef]
- Ding, C.; Meng, Q.; Wang, L.; Chen, C. Synthesis, structure, and electrochemical characteristics of LiNi1/3Co1/3Mn1/3O2 prepared by thermal polymerization. Mater. Res. Bull. 2009, 44, 492–498. [Google Scholar] [CrossRef]
- Lee, J.-W.; Lee, J.-H.; Viet, T.T.; Lee, J.-Y.; Kim, J.-S.; Lee, C.-H. Synthesis of LiNi1/3Co1/3Mn1/3O2 cathode materials by using a supercritical water method in a batch reactor. Electrochim. Acta 2010, 55, 3015–3021. [Google Scholar] [CrossRef]
- Tran, T.; Feikert, J.; Pekala, R.; Kinoshita, K. Rate effect on lithium-ion graphite electrode performance. J. Appl. Electrochem. 1996, 26, 1161–1167. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G. Effect of electrode morphology on the diffusion length of the doping process of electronically conducting polypyrrole films. Electrochem. Commun. 2003, 5, 236–240. [Google Scholar] [CrossRef]
- Bisquert, J.; Vikhrenko, V.S. Analysis of the kinetics of ion intercalation. Two state model describing the coupling of solid state ion diffusion and ion binding processes. Electrochim. Acta 2002, 47, 3977–3988. [Google Scholar] [CrossRef]
- Pouraghajan, F.; Thompson, A.I.; Hunter, E.E.; Mazzeo, B.; Christensen, J.; Subbaraman, R.; Wray, M.; Wheeler, D. The effects of cycling on ionic and electronic conductivities of Li–ion battery electrodes. J. Power Sources 2021, 492, 229636. [Google Scholar] [CrossRef]
- Nyman, A.; Zavalis, T.G.; Elger, R.; Behm, M.; Lindbergh, G. Analysis of the polarization in a Li-ion battery cell by numerical simulations. J. Electrochem. Soc. 2010, 157, A1236. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; He, X.; Wu, H.; Wan, C. The effect of local current density on electrode design for lithium-ion batteries. J. Power Sources 2012, 207, 127–133. [Google Scholar] [CrossRef]
- Jiang, F.; Peng, P. Elucidating the performance limitations of lithium-ion batteries due to species and charge transport through five characteristic parameters. Sci. Rep. 2016, 6, 32639. [Google Scholar] [CrossRef] [Green Version]
- Lain, M.J.; Kendrick, E. Understanding the limitations of lithium ion batteries at high rates. J. Power Sources 2021, 493, 229690. [Google Scholar] [CrossRef]
- Park, J.; Lu, W.; Sastry, A.M. Numerical simulation of stress evolution in lithium manganese dioxide particles due to coupled phase transition and intercalation. J. Electrochem. Soc. 2010, 158, A201. [Google Scholar] [CrossRef]
- Remmlinger, J.; Buchholz, M.; Meiler, M.; Bernreuter, P.; Dietmayer, K. State-of-health monitoring of lithium-ion batteries in electric vehicles by on-board internal resistance estimation. J. Power Sources 2011, 196, 5357–5363. [Google Scholar] [CrossRef]
- Kim, H.; Oh, S.K.; Lee, J.; Doo, S.W.; Kim, Y.; Lee, K.T. Failure mode of thick cathodes for li-ion batteries: Variation of state-of-charge along the electrode thickness direction. Electrochim. Acta 2021, 370, 137743. [Google Scholar] [CrossRef]
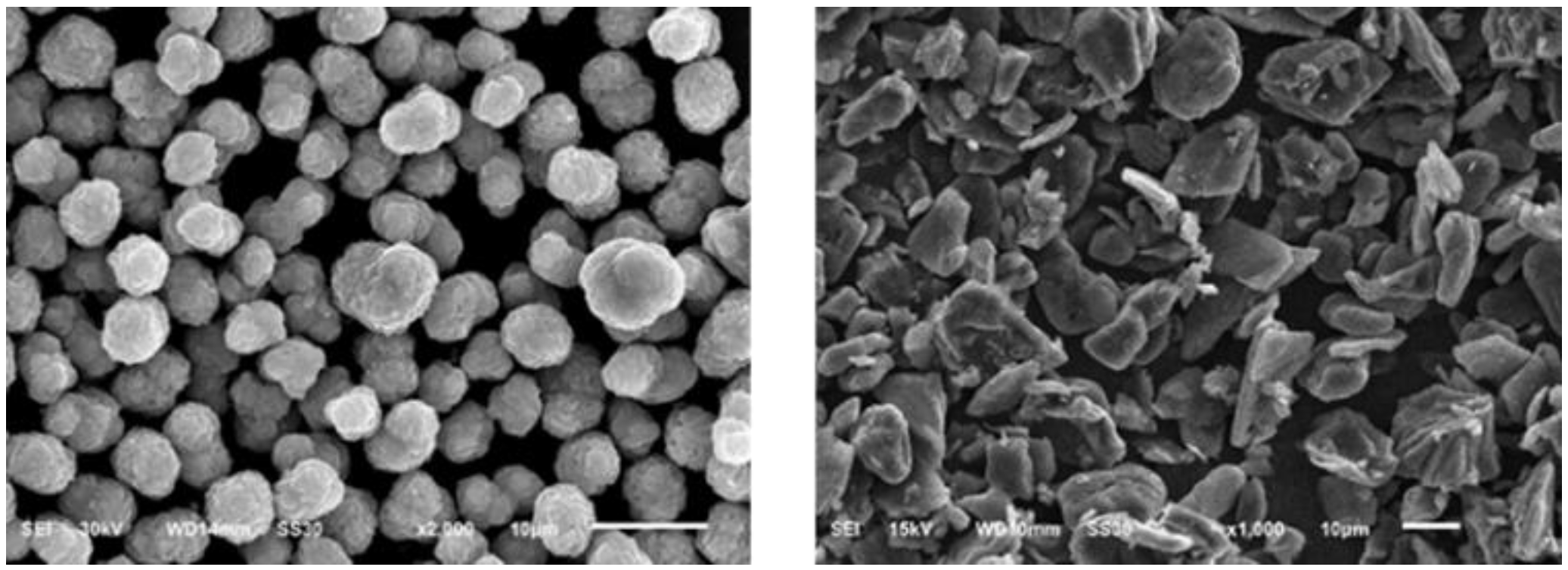






| Components | True Density (kg/m3) |
|---|---|
| Al | 2700 |
| Li[Ni1/3Co1/3Mn1/3]O2 | 4651 |
| Carbon black | 1800 |
| CNT | 2100 |
| PVDF | 1710 |
| Electrode Thickness (μm) | Coated Weight (mg/cm2) | Compaction Density (g/cm3) | Porosity |
|---|---|---|---|
| 71.8 | 21.9 | 3.05 | 27.1% |
| 65.4 | 20.0 | 3.05 | 27.1% |
| 52.6 | 16.0 | 3.04 | 27.3% |
| 39.3 | 11.9 | 3.04 | 27.4% |
| 32.9 | 10.0 | 3.05 | 27.1% |
| 26.2 | 8.0 | 3.04 | 27.4% |
| Electrode thickness (μm) | 71.8 | 65.4 | 52.6 | 39.3 | 32.9 | 26.2 |
| Cell thickness (mm) | 5.5 | 5.6 | 5.8 | 6.2 | 6.5 | 7.1 |
| Positive\negative electrode layers | 11\12 | 12\13 | 15\14 | 20\21 | 24\25 | 30\31 |
| capacity@1C, 25 °C (Ah) | 8.7 | 8.8 | 8.8 | 8.9 | 9.0 | 9.0 |
| Internal resistance (mΩ) | 3.3 | 2.7 | 2.1 | 1.6 | 1.4 | 1.2 |
| Weight of the cell (g) | 181.9 | 183.3 | 192.9 | 205.7 | 215.4 | 231.1 |
| Energy density (Wh/kg) | 174.6 | 175.2 | 166.5 | 157.9 | 152.5 | 142.1 |
| Energy density (Wh/L) | 345.4 | 343 | 331.2 | 313.4 | 302.3 | 276.7 |
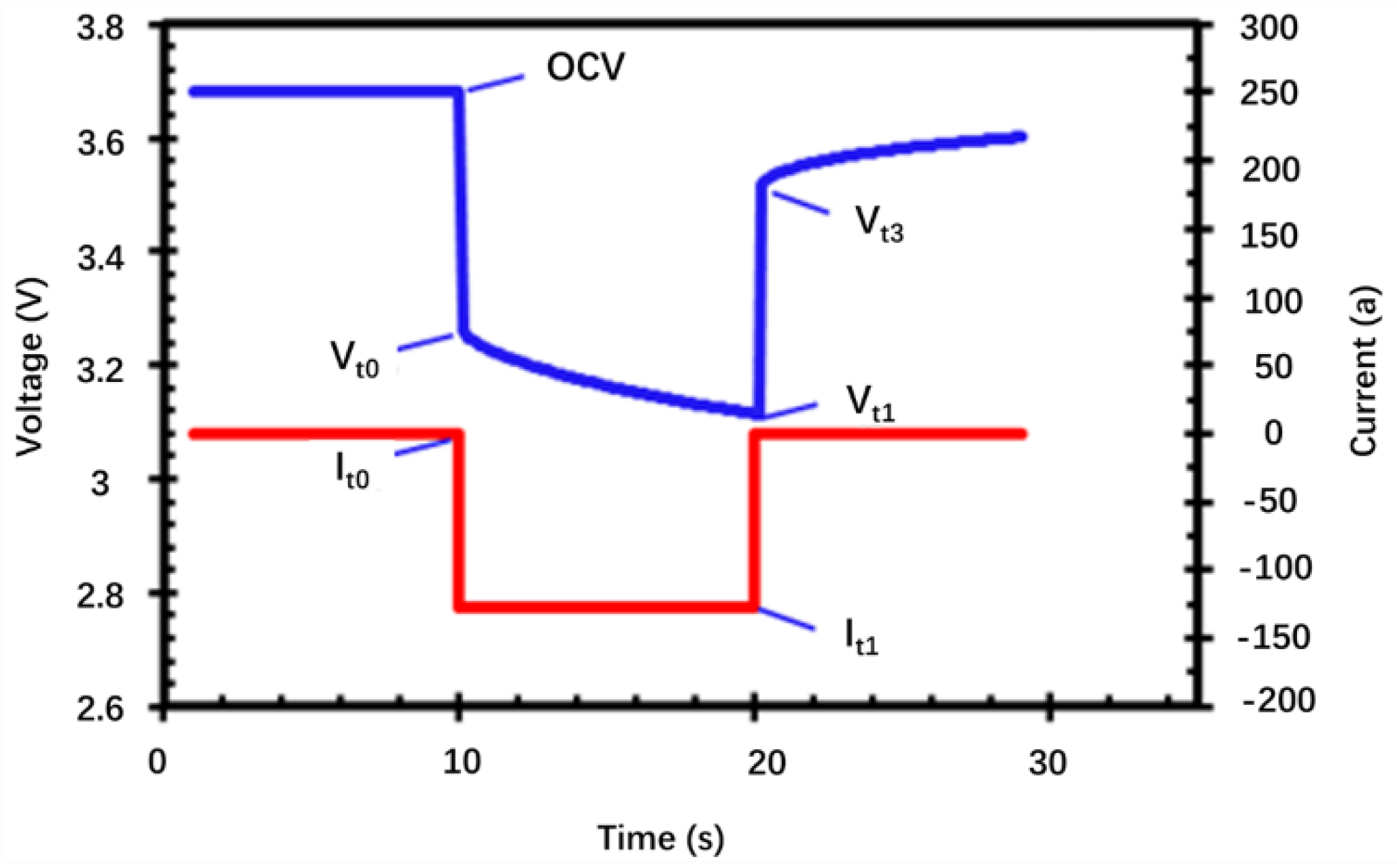
| Electrode Thickness (μm) | OCV | Vt0 | OCV-Vt0 | Vt1 | Vt0–Vt1 | OCV-Vt1 |
|---|---|---|---|---|---|---|
| 71.8 | 3.682 | 3.392 | 0.290 | 3.314 | 0.078 | 0.368 |
| 65.4 | 3.683 | 3.426 | 0.257 | 3.354 | 0.072 | 0.329 |
| 52.6 | 3.687 | 3.47 | 0.217 | 3.407 | 0.064 | 0.281 |
| 39.3 | 3.673 | 3.217 | 0.456 | 3.061 | 0.156 | 0.612 |
| 32.9 | 3.680 | 3.259 | 0.421 | 3.115 | 0.144 | 0.565 |
| 26.2 | 3.678 | 3.279 | 0.399 | 3.148 | 0.131 | 0.53 |
| Electrode Thickness (μm) | Power Density (W/Kg) | Rdis (mΩ) |
|---|---|---|
| 71.8 | 1813.2 | 7.50 |
| 65.4 | 2009.7 | 6.72 |
| 52.6 | 2455.8 | 5.26 |
| 39.3 | 2938.0 | 4.04 |
| 32.9 | 3200.6 | 3.58 |
| 26.2 | 3204.3 | 3.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Lv, Q.; Zhang, C.; Zhou, W.; Guo, H.; Jiang, S.; Li, Z. The Effect of Electrode Thickness on the High-Current Discharge and Long-Term Cycle Performance of a Lithium-Ion Battery. Batteries 2022, 8, 101. https://doi.org/10.3390/batteries8080101
Li D, Lv Q, Zhang C, Zhou W, Guo H, Jiang S, Li Z. The Effect of Electrode Thickness on the High-Current Discharge and Long-Term Cycle Performance of a Lithium-Ion Battery. Batteries. 2022; 8(8):101. https://doi.org/10.3390/batteries8080101
Chicago/Turabian StyleLi, Dongjian, Qiqi Lv, Chunmei Zhang, Wei Zhou, Hongtao Guo, Shaohua Jiang, and Zhuan Li. 2022. "The Effect of Electrode Thickness on the High-Current Discharge and Long-Term Cycle Performance of a Lithium-Ion Battery" Batteries 8, no. 8: 101. https://doi.org/10.3390/batteries8080101
APA StyleLi, D., Lv, Q., Zhang, C., Zhou, W., Guo, H., Jiang, S., & Li, Z. (2022). The Effect of Electrode Thickness on the High-Current Discharge and Long-Term Cycle Performance of a Lithium-Ion Battery. Batteries, 8(8), 101. https://doi.org/10.3390/batteries8080101







