Li-ion Electrode Microstructure Evolution during Drying and Calendering
Abstract
:1. Introduction
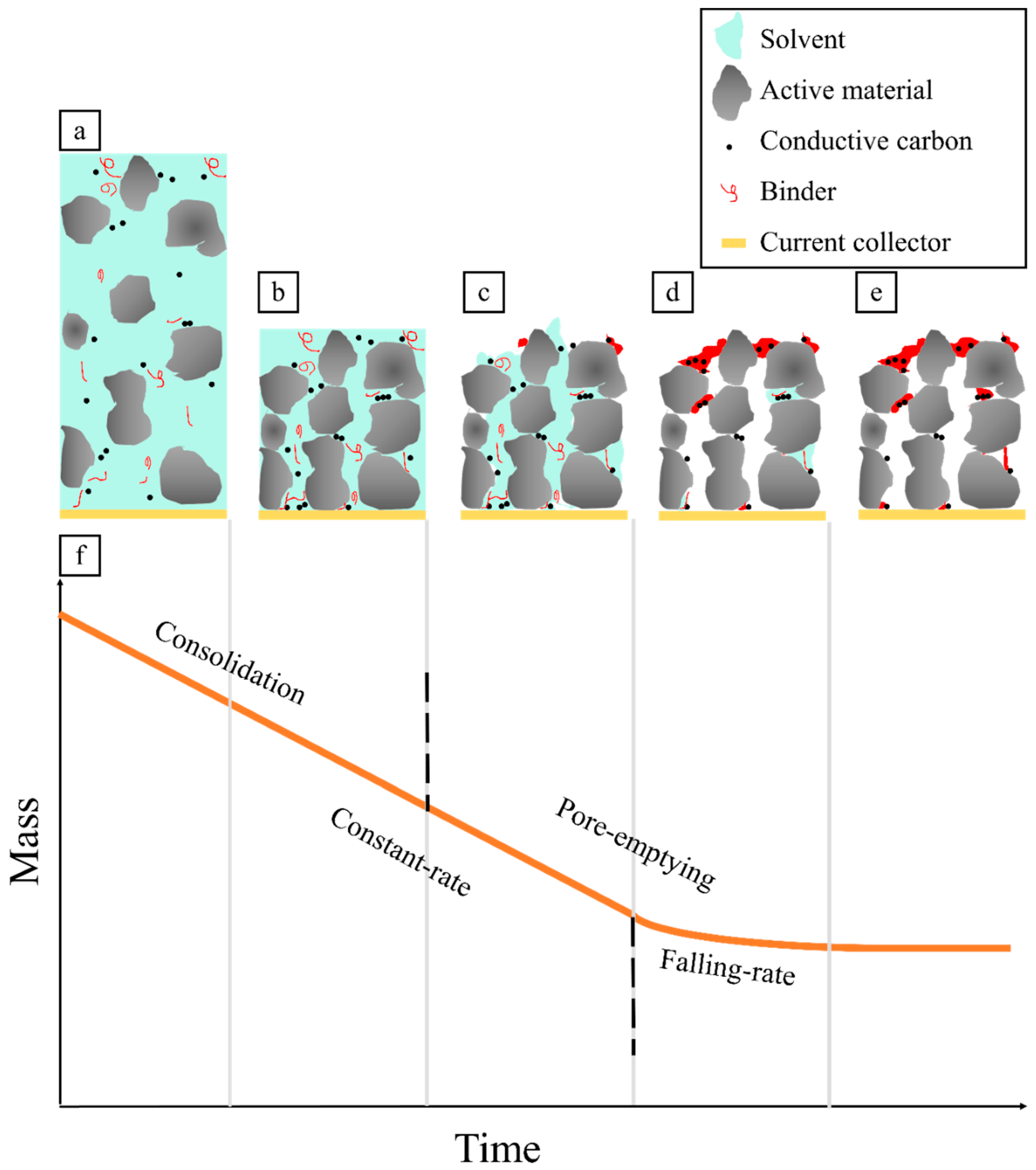
1.1. Electrode Drying Process
1.2. Electrode Defects from the Drying Process
1.3. Defect Mitigation Methods
1.4. Calendering Process
2. Experimental Design
2.1. Electrode Fabrication
2.2. Electrode Cross-Section Imaging and Analysis
2.3. Electrode Testing
3. Results and Discussion
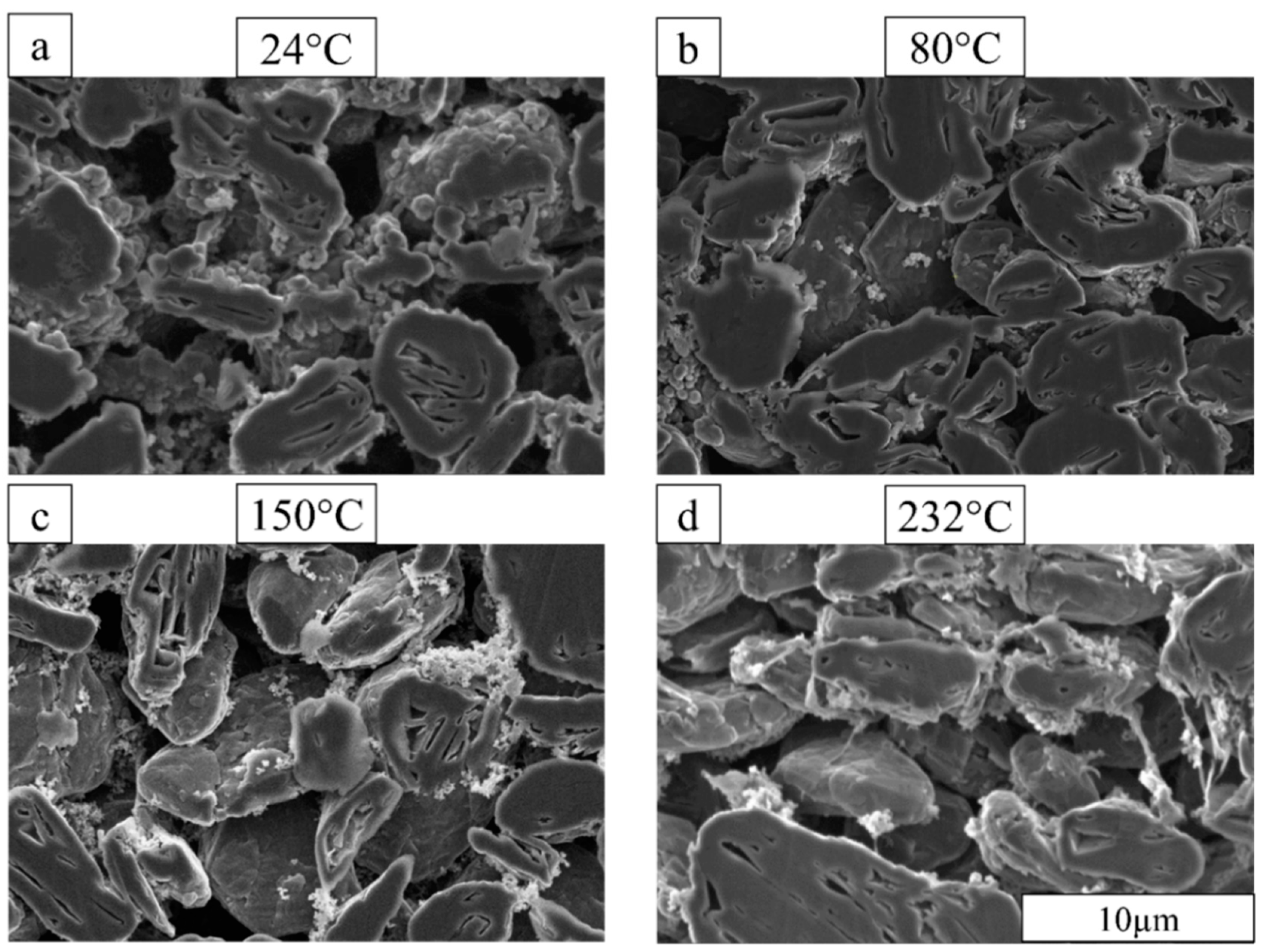
3.1. Anode Microstructure Changes with Drying Temperature
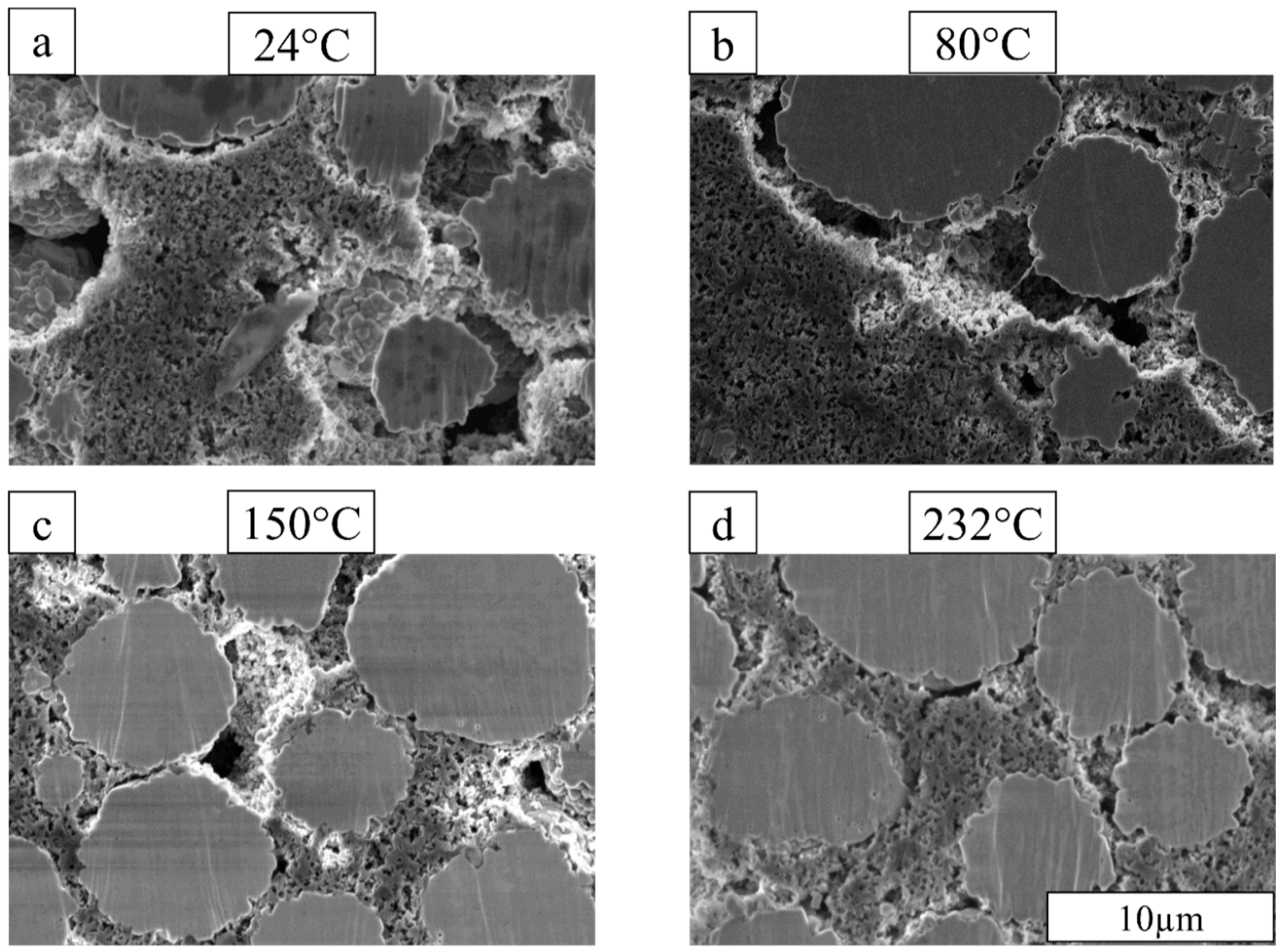
3.2. Cathode Microstructure Changes with Drying Temperature
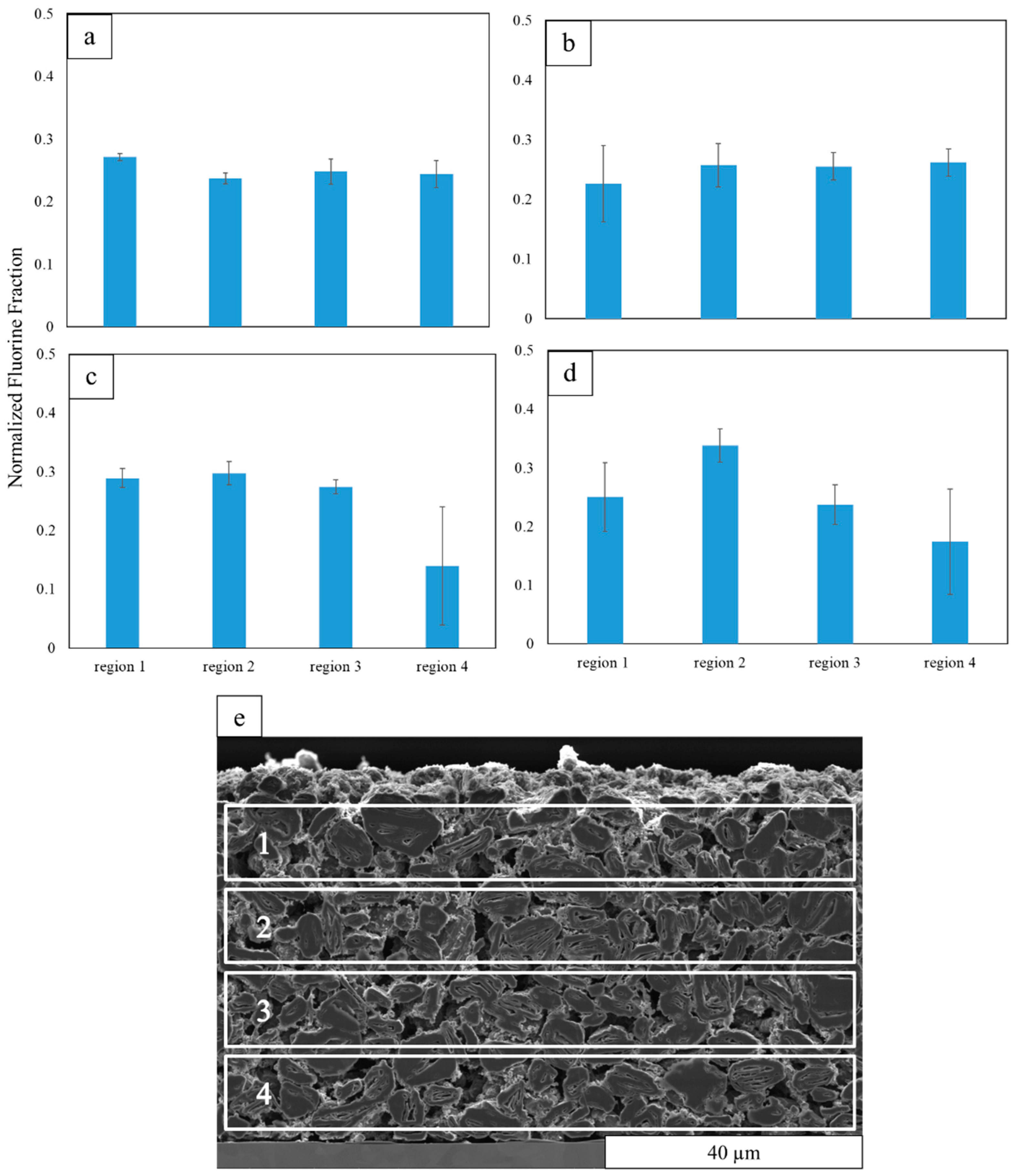
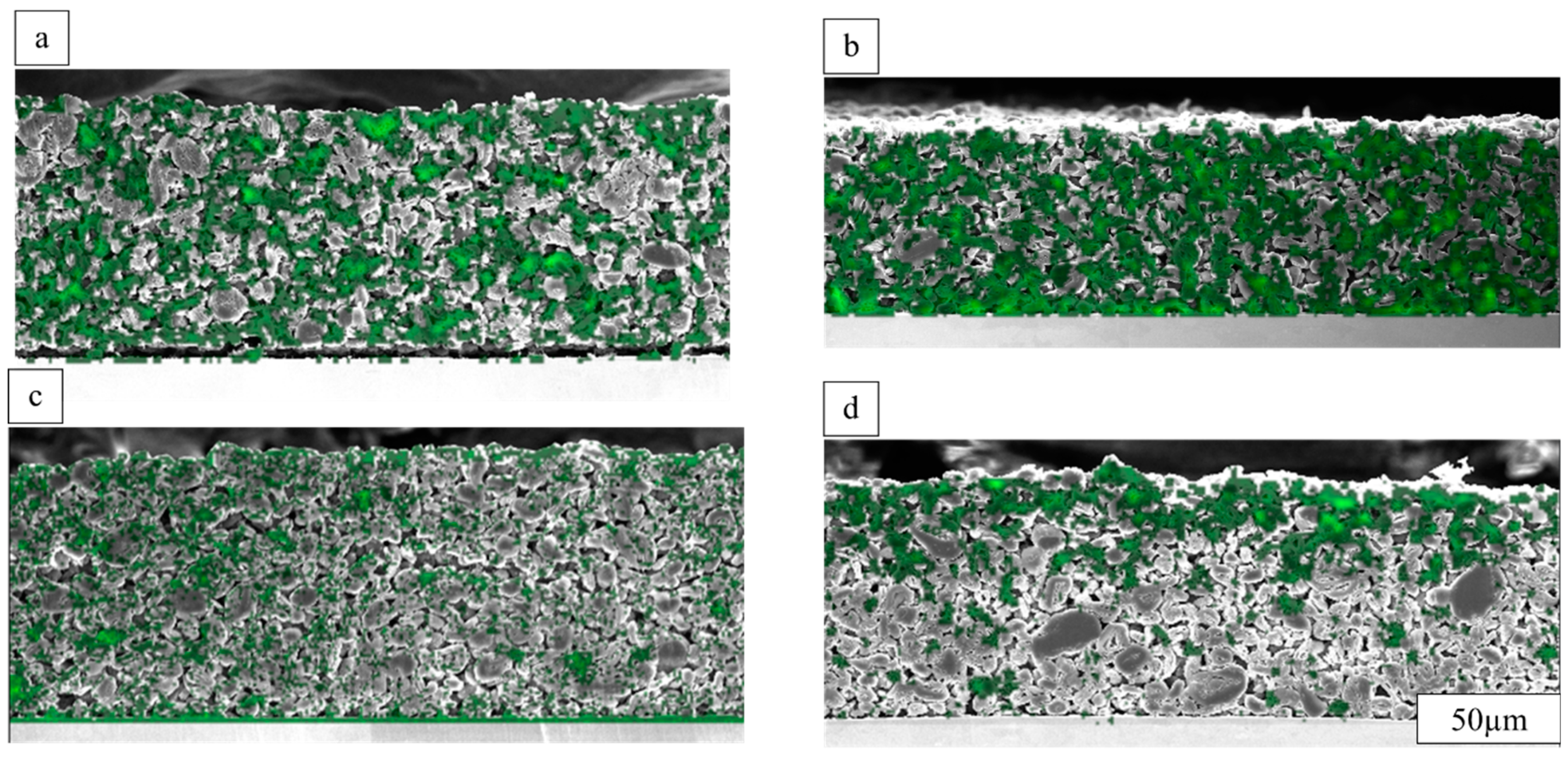
3.3. Binder Distribution in through- and in-Plane Directions
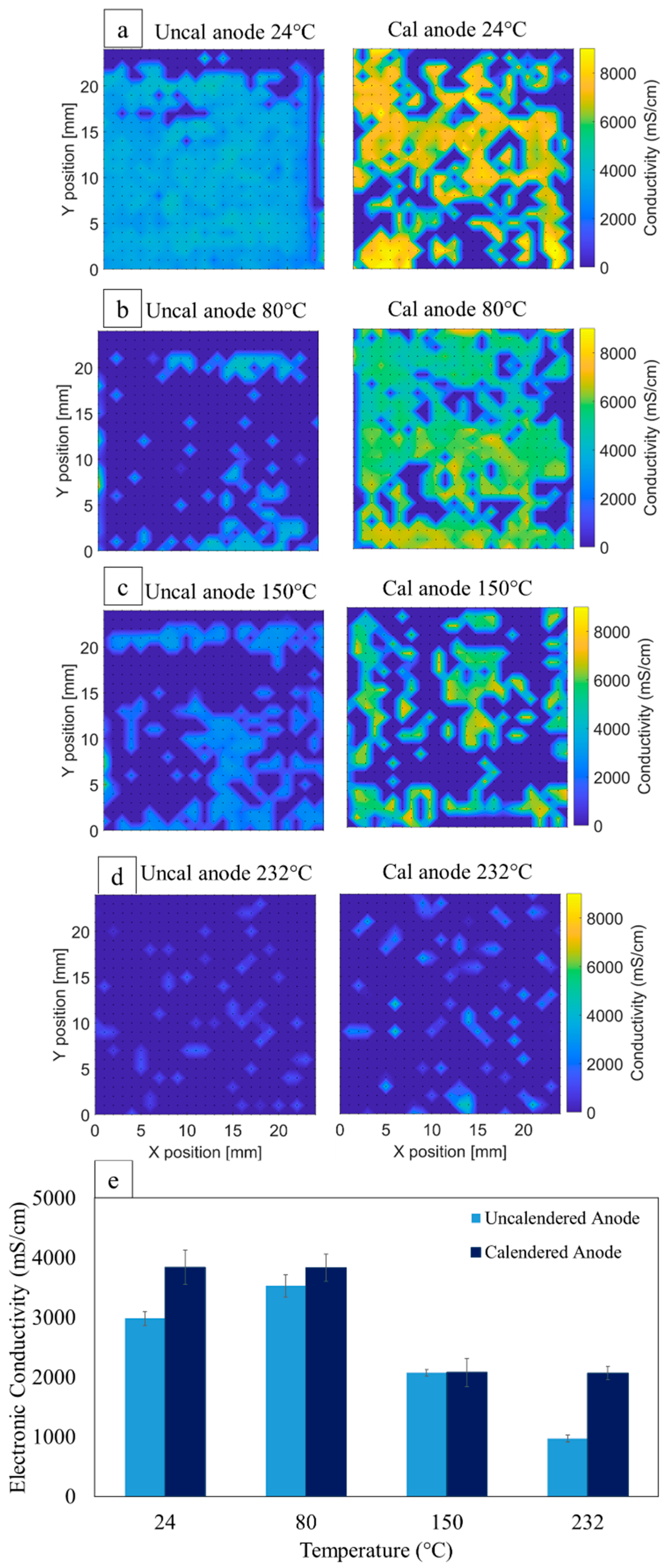
3.4. Anode Electronic Conductivity
3.5. Cathode Electronic Conductivity
3.6. Anode Contact Resistance
3.7. Cathode Contact Resistance
3.8. Anode Ionic Resistance
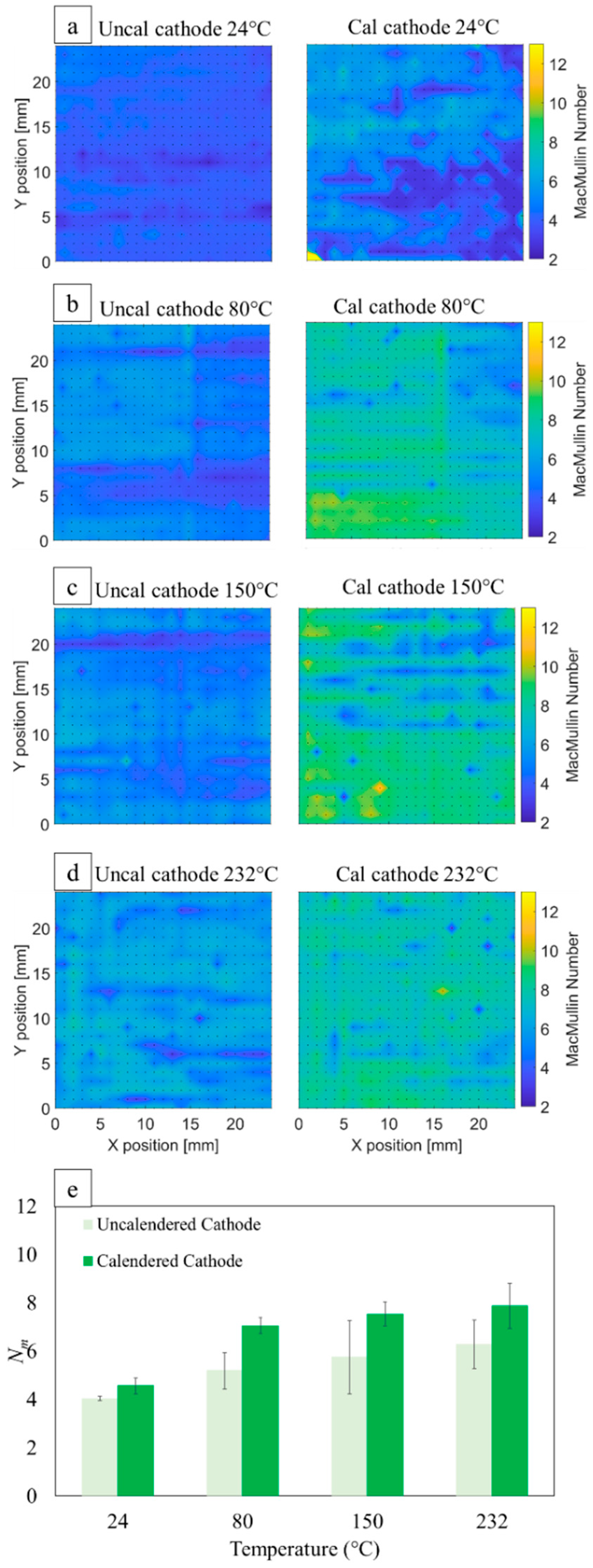
3.9. Cathode Ionic Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tsai, F.-Y.; Jhang, J.H.; Hsieh, H.W.; Li, C.C. Dispersion, agglomeration, and gelation of LiFePO4 in water-based slurry. J. Power Sources 2016, 310, 47–53. [Google Scholar] [CrossRef]
- Mohanty, D.; Hockaday, E.; Li, J.; Hensley, D.K.; Daniel, C.; Wood III, D.L. Effect of electrode manufacturing defects on electrochemical performance of lithium-ion batteries: Cognizance of the battery failure sources. J. Power Sources 2016, 312, 70–79. [Google Scholar] [CrossRef]
- Jaiser, S.; Kumberg, J.; Klaver, J.; Urai, J.L.; Schabel, W.; Schmatz, J.; Scharfer, P. Microstructure formation of lithium-ion battery electrodes during drying—An ex-situ study using cryogenic broad ion beam slope-cutting and scanning electron microscopy (Cryo-BIB-SEM). J. Power Sources 2017, 345, 97–107. [Google Scholar] [CrossRef]
- Zheng, H.; Tan, L.; Liu, G.; Song, X.; Battaglia, V.S. Calendering effects on the physical and electrochemical properties of Li[Ni1/3Mn1/3Co1/3]O2 cathode. J. Power Sources 2012, 208, 52–57. [Google Scholar] [CrossRef]
- Font, F.; Protas, B.; Richardson, G.; Foster, J.M. Binder migration during drying of lithium-ion battery electrodes: Modelling and comparison to experiment. J. Power Sources 2018, 393, 177–185. [Google Scholar] [CrossRef]
- Kumberg, J.; Müller, M.; Diehm, R.; Spiegel, S.; Wachsmann, C.; Bauer, W.; Scharfer, P.; Schabel, W. Drying of Lithium-Ion Battery Anodes for Use in High-Energy Cells: Influence of Electrode Thickness on Drying Time, Adhesion, and Crack Formation. Energy Technol. 2019, 7, 1900722. [Google Scholar] [CrossRef]
- Morasch, R.; Landesfeind, J.; Suthar, B.; Gasteiger, H.A. Detection of binder gradients using impedance spectroscopy and their influence on the tortuosity of Li-ion battery graphite electrodes. J. Electrochem. Soc. 2018, 165, A3459. [Google Scholar] [CrossRef]
- Saraka, R.; Morelly, S.L.; Tang, M.H.; Alvarez, N.J. Effect of Shear Rate and Drying Speed in Lithium-Ion Battery Slurry Processing. In Proceedings of the 2018 AIChE Annual Meeting, Pittsburgh, PA, USA, 28 October–2 November 2018. [Google Scholar]
- Jaiser, S.; Müller, M.; Baunach, M.; Bauer, W.; Scharfer, P.; Schabel, W. Investigation of film solidification and binder migration during drying of Li-Ion battery anodes. J. Power Sources 2016, 318, 210–219. [Google Scholar] [CrossRef]
- Jaiser, S.; Funk, L.; Baunach, M.; Scharfer, P.; Schabel, W. Experimental investigation into battery electrode surfaces: The distribution of liquid at the surface and the emptying of pores during drying. J. Colloid Interface Sci. 2017, 494, 22–31. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Z.; Zhang, S.; Chen, G.; Dong, S.; Cui, G. Constructing in-situ polymerized electrolyte on lithiophilic anode for high-performance lithium–air batteries operating in ambient conditions. Energy Storage Mater. 2021, 43, 221–228. [Google Scholar] [CrossRef]
- Saraka, R.M. Correlating Processing Conditions to Microstructural Order and Performance of Lithium-Ion Battery Electrodes; Drexel University: Philadelphia, PA, USA, 2021. [Google Scholar]
- Nikpour, M.; Barrett, N.; Hillman, Z.; Thompson, A.; Mazzeo, B.A.; Wheeler, D. A Model for Investigating Sources of Li-Ion Battery Electrode Heterogeneity: Part I. Electrode Drying and Calendering Processes. J. Electrochem. Soc. 2021, 168, 060547. [Google Scholar] [CrossRef]
- Vogel, J.E.; Hunter, E.E.; Wheeler, D.R.; Mazzeo, B.A. Micro-Flexible-Surface Probe for Determining Spatially Heterogeneous Electronic Conductivity of Lithium-Ion Battery Electrode Films. J. Electrochem. Soc. 2021, 168, 100504. [Google Scholar] [CrossRef]
- Liu, B.; Prugue, K.; Nikpour, M.; Ward, K.; Mazzeo, B.A.; Wheeler, D.R. Heterogeneity in MacMullin Number of Li-ion Battery Electrodes Studied by Means of an Aperture Probe. J. Electrochem. Soc. 2021, 1, 010517. [Google Scholar] [CrossRef]
- Hawley, W.B.; Li, J. Beneficial rheological properties of lithium-ion battery cathode slurries from elevated mixing and coating temperatures. J. Energy Storage 2019, 26, 100994. [Google Scholar] [CrossRef]
- Baesch, S.; Price, K.; Scharfer, P.; Francis, L.; Schabel, W. Influence of the drying conditions on the particle distribution in particle filled polymer films: Experimental validation of predictive drying regime maps. Chem. Eng. Process.-Process. Intensif. 2018, 123, 138–147. [Google Scholar] [CrossRef]
- Westphal, B.G.; Kwade, A. Critical electrode properties and drying conditions causing component segregation in graphitic anodes for lithium-ion batteries. J. Energy Storage 2018, 18, 509–517. [Google Scholar] [CrossRef]
- Park, I.-H.; Xu, Z.Y.; Ling, Y.; Kim, B.-S.; Lee, J.-O. Existence of critical aggregation concentration at the very dilute regime of poly (vinylidene fluoride)/propylene carbonate system. Bull. Korean Chem. Soc. 2007, 28, 1425–1428. [Google Scholar]
- Jaiser, S.; Sanchez Salach, N.; Baunach, M.; Scharfer, P.; Schabel, W. Impact of drying conditions and wet film properties on adhesion and film solidification of lithium-ion battery anodes. Dry. Technol. 2017, 35, 1807–1817. [Google Scholar] [CrossRef]
- Xu, P.; Mujumdar, A.; Yu, B. Drying-induced cracks in thin film fabricated from colloidal dispersions. Dry. Technol. 2009, 27, 636–652. [Google Scholar] [CrossRef]
- Susarla, N.; Ahmed, S.; Dees, D.W. Modeling and analysis of solvent removal during Li-ion battery electrode drying. J. Power Sources 2018, 378, 660–670. [Google Scholar] [CrossRef]
- Pfaffmann, L.; Jaiser, S.; Müller, M.; Scharfer, P.; Schabel, W.; Bauer, W.; Scheiba, F.; Ehrenberg, H. New method for binder and carbon black detection at nanometer scale in carbon electrodes for lithium ion batteries. J. Power Sources 2017, 363, 460–469. [Google Scholar] [CrossRef]
- Schlünder, E.-U. Drying of porous material during the constant and the falling rate period: A critical review of existing hypotheses. Dry. Technol. 2004, 22, 1517–1532. [Google Scholar] [CrossRef]
- Delgado, J.; de Lima, A.G.B. Drying and Energy Technologies; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Routh, A.F. Drying of thin colloidal films. Rep. Prog. Phys. 2013, 76, 046603. [Google Scholar] [CrossRef]
- Sung, S.H.; Kim, S.; Park, J.H.; Park, J.D.; Ahn, K.H. Role of PVDF in Rheology and Microstructure of NCM Cathode Slurries for Lithium-Ion Battery. Materials 2020, 13, 4544. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-L.; Pan, Y.; Wang, J.-Z.; Liu, H.-K.; Dou, S.-X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-C.; Wang, Y.-W. Binder distributions in water-based and organic-based LiCoO2 electrode sheets and their effects on cell performance. J. Electrochem. Soc. 2011, 158, A1361. [Google Scholar] [CrossRef]
- Baunach, M.; Jaiser, S.; Schmelzle, S.; Nirschl, H.; Scharfer, P.; Schabel, W. Delamination behavior of lithium-ion battery anodes: Influence of drying temperature during electrode processing. Dry. Technol. 2016, 34, 462–473. [Google Scholar] [CrossRef]
- Pouraghajan, F.; Thompson, A.I.; Hunter, E.E.; Mazzeo, B.; Christensen, J.; Subbaraman, R.; Wray, M.; Wheeler, D. The effects of cycling on ionic and electronic conductivities of Li–ion battery electrodes. J. Power Sources 2021, 492, 229636. [Google Scholar] [CrossRef]
- Du, Z.; Rollag, K.; Li, J.; An, S.J.; Wood, M.; Sheng, Y.; Mukherjee, P.; Daniel, C.; Wood Iii, D. Enabling aqueous processing for crack-free thick electrodes. J. Power Sources 2017, 354, 200–206. [Google Scholar] [CrossRef]
- Mampallil, D. Some physics inside drying droplets. Resonance 2014, 19, 123–134. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.E.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B. Optimizing areal capacities through understanding the limitations of lithium-ion electrodes. J. Electrochem. Soc. 2015, 163, A138. [Google Scholar] [CrossRef]
- Kermad, C.; Chehdi, K. Multi-bands image segmentation: A scalar approach. In Proceedings of the 2000 International Conference on Image Processing (Cat. No. 00CH37101), Vancouver, BC, Canada, 10–13 September 2000; IEEE: Manhattan, NY, USA, 2000; pp. 468–471. [Google Scholar]
- Lombardo, T.; Ngandjong, A.C.; Belhcen, A.; Franco, A.A. Carbon-Binder Migration: A Three-Dimensional Drying Model for Lithium-ion Battery Electrodes. Energy Storage Mater. 2021, 43, 337–347. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Horibe, H.; Sasaki, Y.; Oshiro, H.; Hosokawa, Y.; Kono, A.; Takahashi, S.; Nishiyama, T. Quantification of the solvent evaporation rate during the production of three PVDF crystalline structure types by solvent casting. Polym. J. 2014, 46, 104–110. [Google Scholar] [CrossRef]
- Wang, M.; Hu, J.; Wang, Y.; Cheng, Y.-T. The Influence of Polyvinylidene Fluoride (PVDF) Binder Properties on LiNi0.33Co0.33Mn0.33O2 (NMC) Electrodes Made by a Dry-Powder-Coating Process. J. Electrochem. Soc. 2019, 166, A2151. [Google Scholar] [CrossRef]






| Active Name | Active (wt%) | Carbon Black (wt%) | Binder (wt%) |
|---|---|---|---|
| NMC | 90 | 5 | 5 |
| Graphite | 92 | 2 | 6 |
| Temperature | 24 °C | 80 °C | 150 °C | 232 °C |
|---|---|---|---|---|
| Drying rate | 0.005 | 2.1 | 16 | 35 |
| T (°C) | Anode | Cathode |
|---|---|---|
| 24 | 48.4 | 53.9 |
| 80 | 49.1 | 41.5 |
| 150 | 44.8 | 39.5 |
| 232 | 48.4 | 39.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikpour, M.; Liu, B.; Minson, P.; Hillman, Z.; Mazzeo, B.A.; Wheeler, D.R. Li-ion Electrode Microstructure Evolution during Drying and Calendering. Batteries 2022, 8, 107. https://doi.org/10.3390/batteries8090107
Nikpour M, Liu B, Minson P, Hillman Z, Mazzeo BA, Wheeler DR. Li-ion Electrode Microstructure Evolution during Drying and Calendering. Batteries. 2022; 8(9):107. https://doi.org/10.3390/batteries8090107
Chicago/Turabian StyleNikpour, Mojdeh, Baichuan Liu, Paul Minson, Zachary Hillman, Brian A. Mazzeo, and Dean R. Wheeler. 2022. "Li-ion Electrode Microstructure Evolution during Drying and Calendering" Batteries 8, no. 9: 107. https://doi.org/10.3390/batteries8090107
APA StyleNikpour, M., Liu, B., Minson, P., Hillman, Z., Mazzeo, B. A., & Wheeler, D. R. (2022). Li-ion Electrode Microstructure Evolution during Drying and Calendering. Batteries, 8(9), 107. https://doi.org/10.3390/batteries8090107






