Electrocatalytic and Conductive Vanadium Oxide on Carbonized Bacterial Cellulose Aerogel for the Sulfur Cathode in Li-S Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Characterization
2.3. Electrochemical Measurement
3. Results
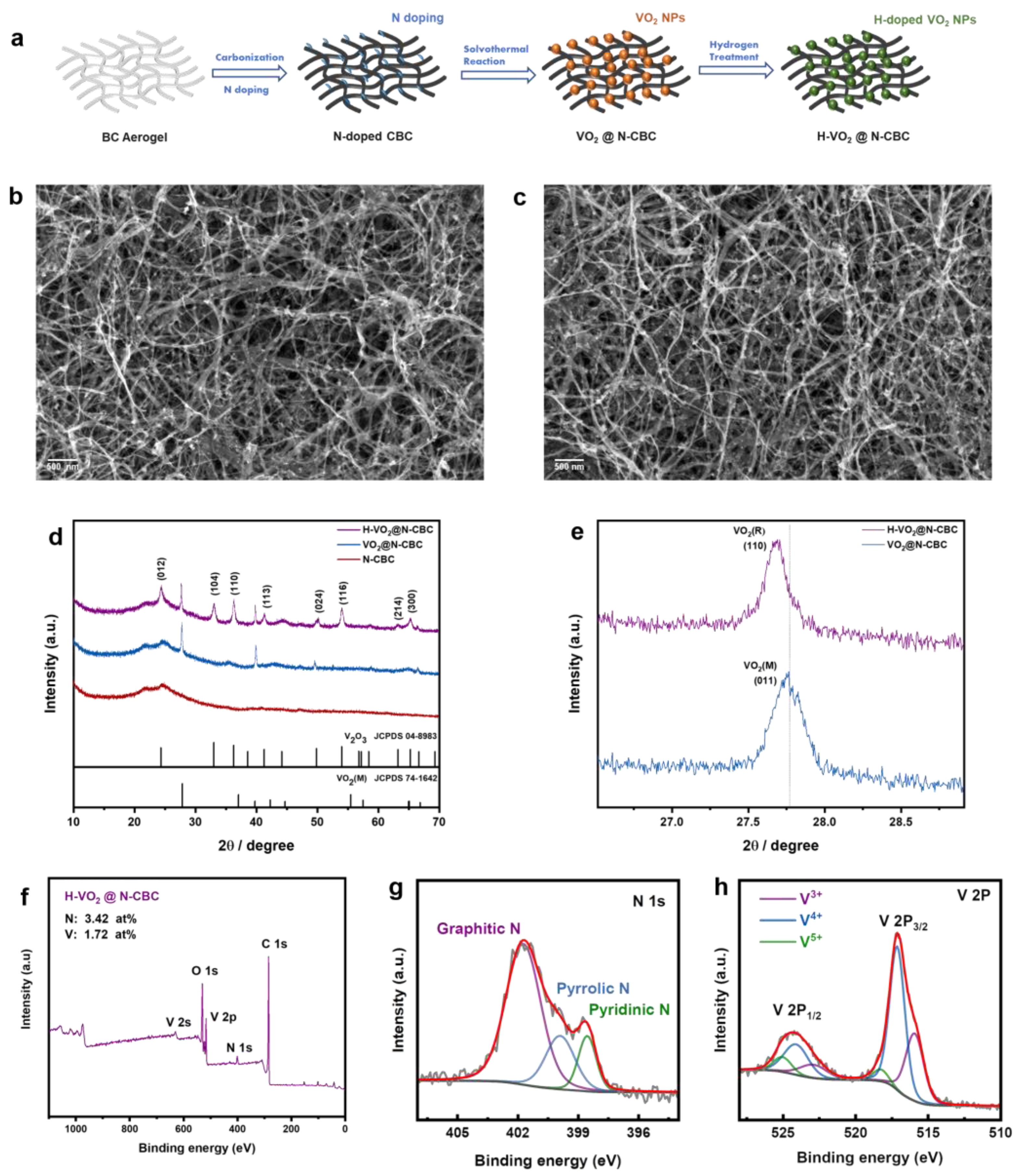
3.1. Material Characterization
3.2. Polysulfide Binding and Li2S Precipitation
3.3. Activation Energy Barriers
3.4. Charge Transportation Evaluation
3.5. Li-S Battery Performance
3.6. Catalyst Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Tang, T.; Ali, Z.; Huang, X.; Hou, Y.J.S. Integrated design of MnO2@ carbon hollow nanoboxes to synergistically encapsulate polysulfides for empowering lithium sulfur batteries. Small 2017, 13, 1700087. [Google Scholar] [CrossRef] [PubMed]
- Gueon, D.; Ju, M.-Y.; Moon, J.H. Complete encapsulation of sulfur through interfacial energy control of sulfur solutions for high-performance Li-S batteries. Proc. Natl. Acad. Sci. USA 2020, 117, 12686–12692. [Google Scholar] [CrossRef] [PubMed]
- Xi, K.; Kidambi, P.R.; Chen, R.; Gao, C.; Peng, X.; Ducati, C.; Hofmann, S.; Kumar, R.V. Binder free three-dimensional sulphur/few-layer graphene foam cathode with enhanced high-rate capability for rechargeable lithium sulphur batteries. Nanoscale 2014, 6, 5746–5753. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Chi, Y.; Hu, Q.; Tang, H.; Jiang, X.; Zhang, L.; Zhang, S.; Pang, H.; Xu, Q. Carbon nanotube-based materials for lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 17204–17241. [Google Scholar] [CrossRef]
- Doñoro, Á.; Muñoz-Mauricio, Á.; Etacheri, V. High-Performance Lithium Sulfur Batteries Based on Multidimensional Graphene-CNT-Nanosulfur Hybrid Cathodes. Batteries 2021, 7, 26. [Google Scholar] [CrossRef]
- Li, S.; Mou, T.; Ren, G.; Warzywoda, J.; Wang, B.; Fan, Z. Confining Sulfur Species in Cathodes of Lithium–Sulfur Batteries: Insight into Nonpolar and Polar Matrix Surfaces. ACS Energy Lett. 2016, 1, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Pei, F.; An, T.; Zang, J.; Zhao, X.; Fang, X.; Zheng, M.; Dong, Q.; Zheng, N. From Hollow Carbon Spheres to N-Doped Hollow Porous Carbon Bowls: Rational Design of Hollow Carbon Host for Li-S Batteries. Adv. Energy Mater. 2016, 6, 1502539. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, S.; Jiang, S.; Huang, C.; Wang, X.; Yang, Z.; Xiang, K.; Zhang, Y. Suppressing the Polysulfide Shuttle Effect by Heteroatom-Doping for High-Performance Lithium–Sulfur Batteries. ACS Sustain. Chem. Eng. 2018, 6, 7545–7557. [Google Scholar] [CrossRef]
- Li, N.; Yu, L.; Yang, J.; Zheng, B.; Qiu, X.; Xi, J. Identifying the active sites and multifunctional effects in nitrogen-doped carbon microtube interlayer for confining-trapping-catalyzing polysulfides. Nano Energy 2021, 79, 105466. [Google Scholar] [CrossRef]
- Laverde, J.; Rosero-Navarro, N.C.; Miura, A.; Buitrago-Sierra, R.; Tadanaga, K.; López, D. Impact of Sulfur Infiltration Time and Its Content in an N-doped Mesoporous Carbon for Application in Li-S Batteries. Batteries 2022, 8, 58. [Google Scholar] [CrossRef]
- Waluś, S.; Barchasz, C.; Bouchet, R.; Leprêtre, J.-C.; Colin, J.-F.; Martin, J.-F.; Elkaïm, E.; Baehtz, C.; Alloin, F. Lithium/Sulfur Batteries Upon Cycling: Structural Modifications and Species Quantification by In Situ and Operando X-Ray Diffraction Spectroscopy. Adv. Energy Mater. 2015, 5, 1500165. [Google Scholar] [CrossRef]
- Lang, X.; Zhao, Y.; Cai, K.; Li, L.; Chen, D.; Zhang, Q. A facile synthesis of stable TiO2/TiC composite material as sulfur immobilizers for cathodes of lithium–sulfur batteries with excellent electrochemical performances. Energy Technol. 2019, 7, 1900543. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Zhang, Y.; Xiang, M.; Wu, H.; Liu, H.; Dou, S. Interwoven V2O5 nanowire/graphene nanoscroll hybrid assembled as efficient polysulfide-trapping-conversion interlayer for long-life lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 19358–19370. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.; Fan, H.; Cheng, F.; Su, D.; Wang, G. Promoting lithium polysulfide/sulfide redox kinetics by the catalyzing of zinc sulfide for high performance lithium-sulfur battery. Nano Energy 2018, 51, 73–82. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Liao, J.; Sun, Q.; Zhao, F.; Luo, J.; Lin, X.; Niu, X.; Wu, M.; Li, R.; et al. Efficient trapping and catalytic conversion of polysulfides by VS4 nanosites for Li–S batteries. ACS Energy Lett. 2019, 4, 755–762. [Google Scholar] [CrossRef]
- Cho, J.; Ryu, S.; Gong, Y.J.; Pyo, S.; Yun, H.; Kim, H.; Lee, J.; Yoo, J.; Kim, Y.S. Nitrogen-doped MoS2 as a catalytic sulfur host for lithium-sulfur batteries. Chem. Eng. J. 2022, 439, 135568. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Bernussi, A.A.; Fan, Z. 3-D edge-oriented electrocatalytic NiCo2S4 nanoflakes on vertical graphene for Li-S batteries. Energy Mater. Adv. 2021, 2021, 2712391. [Google Scholar]
- Wang, S.; Feng, S.; Liang, J.; Su, Q.; Zhao, F.; Song, H.; Zheng, M.; Sun, Q.; Song, Z.; Jia, X.; et al. Insight into MoS2–MoN Heterostructure to Accelerate Polysulfide Conversion toward High-Energy-Density Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2003314. [Google Scholar]
- He, J.; Chen, Y.; Manthiram, A. Vertical Co9S8 hollow nanowall arrays grown on a Celgard separator as a multifunctional polysulfide barrier for high-performance Li–S batteries. Energy Environ. Sci. 2018, 11, 2560–2568. [Google Scholar]
- Lim, W.-G.; Kim, S.; Jo, C.; Lee, J. A Comprehensive Review of Materials with Catalytic Effects in Li–S Batteries: Enhanced Redox Kinetics. Angew. Chem. 2019, 58, 18746–18757. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L. Nanostructured metal oxides and sulfides for lithium–sulfur batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, C.; Deng, Y.; Huang, Z.; Zhou, G.; Lv, W.; He, Y.B.; Wan, Y.; Kang, F.; Yang, Q.-H. Optimized catalytic WS2–WO3 heterostructure design for accelerated polysulfide conversion in lithium–sulfur batteries. Adv. Energy Mater. 2020, 10, 2000091. [Google Scholar] [CrossRef]
- Liang, Z.; Zheng, G.; Li, W.; Seh, Z.W.; Yao, H.; Yan, K.; Kong, D.; Cui, Y. Sulfur Cathodes with Hydrogen Reduced Titanium Dioxide Inverse Opal Structure. ACS Nano 2014, 8, 5249–5256. [Google Scholar]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Ono, L.K.; Tong, G.; Liu, Y.; Qi, Y. Long-life lithium-sulfur batteries with high areal capacity based on coaxial CNTs@TiN-TiO2 sponge. Nat. Commun. 2021, 12, 4738. [Google Scholar] [CrossRef]
- Zhao, Y.; Lee, J.H.; Zhu, Y.; Nazari, M.; Chen, C.; Wang, H.; Bernussi, A.; Holtz, M.; Fan, Z. Structural, electrical, and terahertz transmission properties of VO2 thin films grown on c-, r-, and m-plane sapphire substrates. J. Appl. Phys. 2012, 111, 053533. [Google Scholar]
- Zhao, Y.; Karaoglan-Bebek, G.; Pan, X.; Holtz, M.; Bernussi, A.A.; Fan, Z. Hydrogen-doping stabilized metallic VO2 (R) thin films and their application to suppress Fabry-Perot resonances in the terahertz regime. Appl. Phys. Lett. 2014, 104, 241901. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Feng, F.; Feng, J.; Dai, J.; Peng, L.; Zhao, J.; Yang, J.; Si, C.; Wu, Z.; Xie, Y. Hydrogen-Incorporation Stabilization of Metallic VO2(R) Phase to Room Temperature, Displaying Promising Low-Temperature Thermoelectric Effect. J. Am. Chem. Soc. 2011, 133, 13798–13801. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, Y.; Zhao, S.; Wang, Z.; Liu, Z.; Zhu, L.; Wang, B.; Zhang, Q. Modulation of VO2 metal-insulator transition by co-doping of hydrogen and oxygen vacancy. Sol. Energy Mater. Sol. Cells 2020, 212, 110562. [Google Scholar] [CrossRef]
- Wei, J.; Ji, H.; Guo, W.; Nevidomskyy, A.H.; Natelson, D. Hydrogen stabilization of metallic vanadium dioxide in single-crystal nanobeams. Nat. Nanotechnol. 2012, 7, 357–362. [Google Scholar] [CrossRef]
- Pan, X.; Ren, G.; Hoque, M.N.F.; Bayne, S.; Zhu, K.; Fan, Z. Fast Supercapacitors Based on Graphene-Bridged V2O3/VOx Core–Shell Nanostructure Electrodes with a Power Density of 1 MW kg−1. Adv. Mater. Interfaces 2014, 1, 1400398. [Google Scholar] [CrossRef]
- Li, S.; Mou, T.; Ren, G.; Warzywoda, J.; Wei, Z.; Wang, B.; Fan, Z. Gel based sulfur cathodes with a high sulfur content and large mass loading for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 1650–1657. [Google Scholar] [CrossRef]
- Robinson, J.B.; Xi, K.; Kumar, R.V.; Ferrari, A.C.; Au, H.; Titirici, M.-M.; Parra-Puerto, A.; Kucernak, A.; Fitch, S.D.; Garcia-Araez, N.; et al. 2021 roadmap on lithium sulfur batteries. J. Phys. Energy 2021, 3, 031501. [Google Scholar] [CrossRef]
- Wang, J.; Han, W.-Q. A Review of Heteroatom Doped Materials for Advanced Lithium–Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2107166. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z.; Zheng, M.; Wang, T.; Yang, J.; Yuan, F.; Lu, X.; Liu, L.; Sun, D. Amorphous Fe2O3 nanoshells coated on carbonized bacterial cellulose nanofibers as a flexible anode for high-performance lithium ion batteries. J. Power Sources 2016, 307, 649–656. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Y.; Meng, C.; Wang, X.; Liu, C.; Bo, M.; Pei, X.; Wei, Y.; Lv, T.; Cao, G. V2O3/C nanocomposites with interface defects for enhanced intercalation pseudocapacitance. Electrochim. Acta 2019, 318, 635–643. [Google Scholar] [CrossRef]
- Arteaga-Cardona, F.; Franco-Bacca, A.P.; Cervantes-Alvarez, F.; Alvarado-Gil, J.J.; Silva-González, N.R.; Salazar-Kuri, U. Simple thermal decomposition synthesis of monoclinic VO2. Appl. Phys. A 2021, 127, 159. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Li, J.; Jin, H.; Rehman, F.; Chen, P.; Jiang, Y.; Chen, C.; Cao, M.; Zhao, Y. Evolution of Structural and Electrical Properties of Oxygen-Deficient VO2 under Low Temperature Heating Process. ACS Appl. Mater. Interfaces 2017, 9, 27135–27141. [Google Scholar] [CrossRef]
- Ning, Y.; Wang, B.; Jin, F.; Yang, J.; Zhang, J.; Luo, H.; Wu, F.; Zhang, Z.; Zhang, H.; Zhou, Y.; et al. A rational VO2 nanotube/graphene binary sulfur host for superior lithium-sulfur batteries. J. Alloy. Compd. 2020, 838, 155504. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Yue, W.-C.; Li, X.; Gao, N.; Zhang, Y.-J.; Hu, C.-Q. V2O3/VN Catalysts Decorated Free-Standing Multifunctional Interlayer for High-Performance Li-S Battery. Chem. Eng. J. 2022, 441, 136082. [Google Scholar] [CrossRef]
- Lai, Q.; Zheng, L.; Liang, Y.; He, J.; Zhao, J.; Chen, J. Metal-Organic-Framework-Derived Fe-N/C Electrocatalyst with Five-Coordinated Fe-Nx Sites for Advanced Oxygen Reduction in Acid Media. ACS Catal. 2017, 7, 1655–1663. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Shlyakhova, E.V.; Stolyarova, S.G.; Vorfolomeeva, A.A.; Grebenkina, M.A.; Makarova, A.A.; Shubin, Y.V.; Okotrub, A.V.; Bulusheva, L.G. Brominated Porous Nitrogen-Doped Carbon Materials for Sodium-Ion Storage. Batteries 2022, 8, 114. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, Y.; Zong, M.; Huo, S.; Khan, I.U.; Li, K.; Lv, C. Hierarchically porous N-doped carbon encapsulating CoO/MgO as superior cathode catalyst for microbial fuel cell. Chem. Eng. J. 2020, 385, 123861. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, G.; Zhong, J.; Shi, Z.; Zhu, Y.; Su, D.S.; Wang, J.; Bao, X.; Ma, D. Nitrogen-Doped sp2-Hybridized Carbon as a Superior Catalyst for Selective Oxidation. Angew. Chem. Int. Ed. 2013, 52, 2109–2113. [Google Scholar] [CrossRef]
- Liang, X.; Kwok, C.Y.; Lodi-Marzano, F.; Pang, Q.; Cuisinier, M.; Huang, H.; Hart, C.J.; Houtarde, D.; Kaup, K.; Sommer, H.; et al. Tuning Transition Metal Oxide–Sulfur Interactions for Long Life Lithium Sulfur Batteries: The “Goldilocks” Principle. Adv. Energy Mater. 2016, 6, 1501636. [Google Scholar] [CrossRef]
- Zhang, J.; You, C.; Lin, H.; Wang, J. Electrochemical Kinetic Modulators in Lithium–Sulfur Batteries: From Defect-Rich Catalysts to Single Atomic Catalysts. Energy Environ. Mater. 2022, 5, 731–750. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Zhu, L.; Zhou, J.; Guan, Y.; Chen, L.; Niu, S.; Cai, J.; Sun, D.; Zhu, Y.; et al. Deciphering the Modulation Essence of p Bands in Co-Based Compounds on Li-S Chemistry. Joule 2018, 2, 2681–2693. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhou, Y.; Wang, Y.; Lu, Y.-C. Solvent-Mediated Li2S Electrodeposition: A Critical Manipulator in Lithium–Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1802207. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.Y.; Carter, W.C.; Chiang, Y.M. Mechanism and kinetics of Li2S precipitation in lithium–sulfur batteries. Adv. Mater. 2015, 27, 5203–5209. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Z.; Knibbe, R.; Luo, B.; Ahad, S.A.; Sun, D.; Wang, L. Cyclic voltammetry in lithium–sulfur batteries—Challenges and opportunities. Energy Technol. 2019, 7, 1801001. [Google Scholar] [CrossRef] [Green Version]
- Hua, W.; Li, H.; Pei, C.; Xia, J.; Sun, Y.; Zhang, C.; Lv, W.; Tao, Y.; Jiao, Y.; Zhang, B.; et al. Selective Catalysis Remedies Polysulfide Shuttling in Lithium-Sulfur Batteries. Adv. Mater. 2021, 33, 2101006. [Google Scholar] [CrossRef] [PubMed]
- Jing, E.; Chen, L.; Xu, S.; Tian, W.; Zhang, D.; Wang, N.; Bai, Z.; Zhou, X.; Liu, S.; Duan, D.; et al. Dual redox catalysis of VN/nitrogen-doped graphene nanocomposites for high-performance lithium-sulfur batteries. J. Energy Chem. 2022, 64, 574–582. [Google Scholar] [CrossRef]
- Sun, Z.; Vijay, S.; Heenen, H.H.; Eng, A.Y.S.; Tu, W.; Zhao, Y.; Koh, S.W.; Gao, P.; Seh, Z.W.; Chan, K.; et al. Catalytic Polysulfide Conversion and Physiochemical Confinement for Lithium–Sulfur Batteries. Adv. Energy Mater. 2020, 10, 1904010. [Google Scholar] [CrossRef]
- Luo, C.; Liang, X.; Sun, Y.; Lv, W.; Sun, Y.; Lu, Z.; Hua, W.; Yang, H.; Wang, R.; Yan, C.; et al. An organic nickel salt-based electrolyte additive boosts homogeneous catalysis for lithium-sulfur batteries. Energy Storage Mater. 2020, 33, 290–297. [Google Scholar] [CrossRef]
- Zhou, G.; Tian, H.; Jin, Y.; Tao, X.; Liu, B.; Zhang, R.; Seh, Z.W.; Zhuo, D.; Liu, Y.; Sun, J.; et al. Catalytic oxidation of Li2S on the surface of metal sulfides for Li-S batteries. Proc. Natl. Acad. Sci. USA 2017, 114, 840–845. [Google Scholar] [CrossRef] [Green Version]
- Lei, T.; Chen, W.; Lv, W.; Huang, J.; Zhu, J.; Chu, J.; Yan, C.; Wu, C.; Yan, Y.; He, W.; et al. Inhibiting Polysulfide Shuttling with a Graphene Composite Separator for Highly Robust Lithium-Sulfur Batteries. Joule 2018, 2, 2091–2104. [Google Scholar] [CrossRef] [Green Version]
- Waluś, S.; Barchasz, C.; Bouchet, R.; Alloin, F. Electrochemical impedance spectroscopy study of lithium–sulfur batteries: Useful technique to reveal the Li/S electrochemical mechanism. Electrochim. Acta 2020, 359, 136944. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Wu, X.; Zhang, Y.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. Double-Shelled Co3O4/C Nanocages Enabling Polysulfides Adsorption for High-Performance Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2019, 2, 8153–8162. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Zhang, M.; Chen, W.; Cai, X.; Rao, H.; Chang, J.; Fang, Y.; Zhong, X.; Yang, Y.; Yang, Z.; et al. Vanadium Nitride Quantum Dots/Holey Graphene Matrix Boosting Adsorption and Conversion Reaction Kinetics for High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 30746–30755. [Google Scholar] [CrossRef]
- Wang, S.; Liao, J.; Yang, X.; Liang, J.; Sun, Q.; Liang, J.; Zhao, F.; Koo, A.; Kong, F.; Yao, Y.; et al. Designing a highly efficient polysulfide conversion catalyst with paramontroseite for high-performance and long-life lithium-sulfur batteries. Nano Energy 2019, 57, 230–240. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, W.; Zhu, X.; Zhang, L.; Li, Q.; Ding, F.; Liu, Z.; Sun, J. Vanadium Dioxide-Graphene Composite with Ultrafast Anchoring Behavior of Polysulfides for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 15733–15741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Lv, W.; Li, J.; Zhou, G.; Zhao, Y.; Fan, S.; Liu, B.; Li, B.; Kang, F.; Yang, Q.-H. Twinborn TiO2–TiN heterostructures enabling smooth trapping–diffusion–conversion of polysulfides towards ultralong life lithium–sulfur batteries. Energy Environ. Sci. 2017, 10, 1694–1703. [Google Scholar] [CrossRef]
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Li, W.; Pan, X.; Wang, S.; Fan, Z. Electrocatalytic and Conductive Vanadium Oxide on Carbonized Bacterial Cellulose Aerogel for the Sulfur Cathode in Li-S Batteries. Batteries 2023, 9, 14. https://doi.org/10.3390/batteries9010014
Lin X, Li W, Pan X, Wang S, Fan Z. Electrocatalytic and Conductive Vanadium Oxide on Carbonized Bacterial Cellulose Aerogel for the Sulfur Cathode in Li-S Batteries. Batteries. 2023; 9(1):14. https://doi.org/10.3390/batteries9010014
Chicago/Turabian StyleLin, Xueyan, Wenyue Li, Xuan Pan, Shu Wang, and Zhaoyang Fan. 2023. "Electrocatalytic and Conductive Vanadium Oxide on Carbonized Bacterial Cellulose Aerogel for the Sulfur Cathode in Li-S Batteries" Batteries 9, no. 1: 14. https://doi.org/10.3390/batteries9010014
APA StyleLin, X., Li, W., Pan, X., Wang, S., & Fan, Z. (2023). Electrocatalytic and Conductive Vanadium Oxide on Carbonized Bacterial Cellulose Aerogel for the Sulfur Cathode in Li-S Batteries. Batteries, 9(1), 14. https://doi.org/10.3390/batteries9010014









