Elucidating Spatial Distribution of Electrochemical Reaction in a Porous Electrode by Electrochemical Impedance Spectra for Flow Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
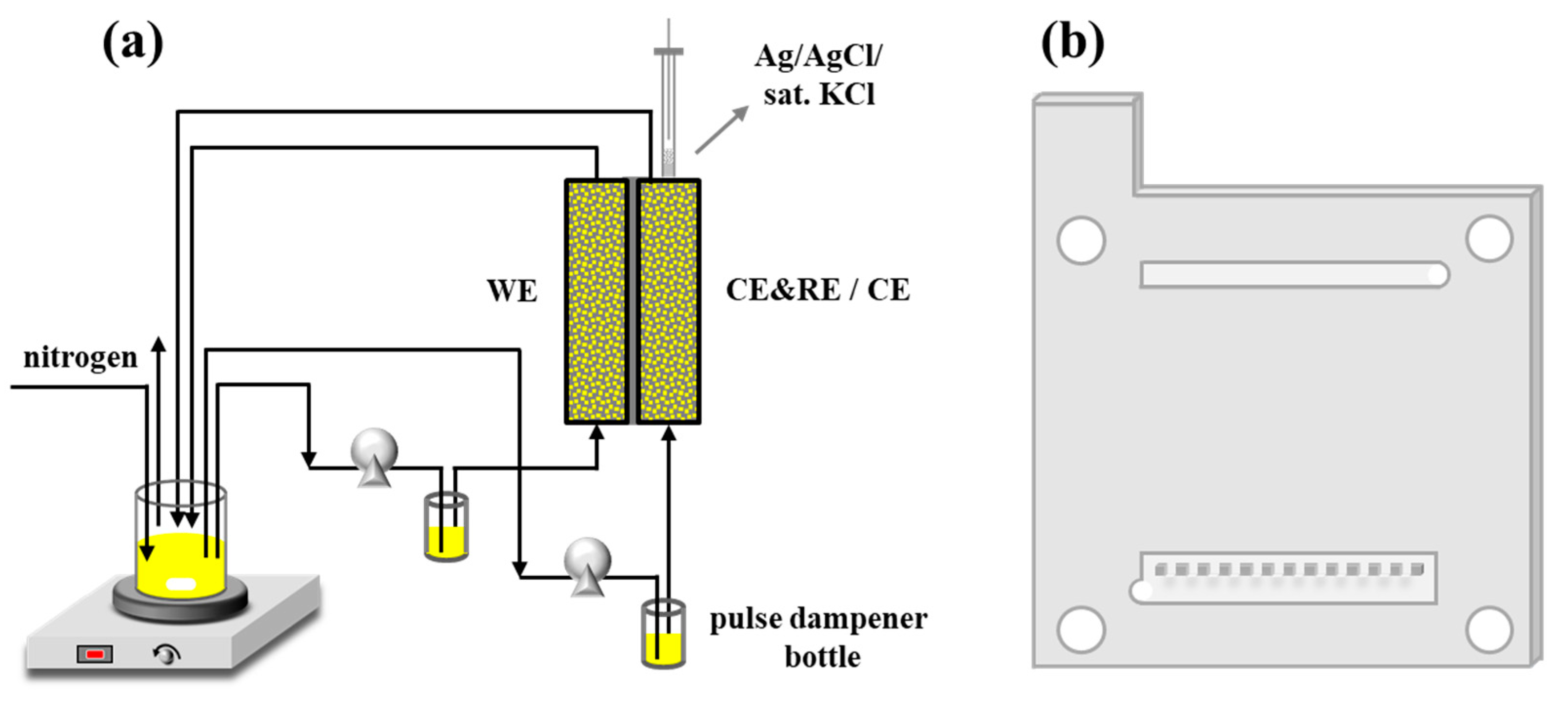
2.2. Experimental Setup
2.3. Electrochemical Measurements
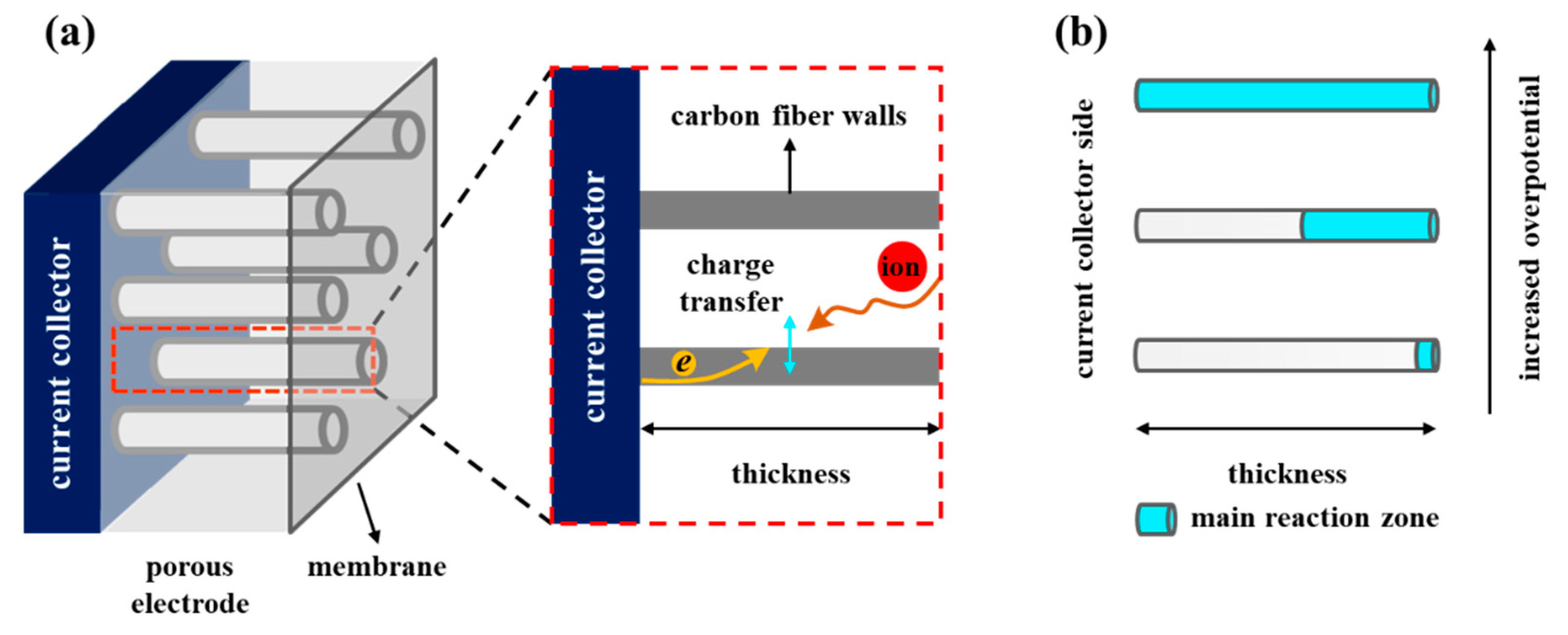
3. Results and Discussion
3.1. Full Cell Impedance at Open Circuit Voltage
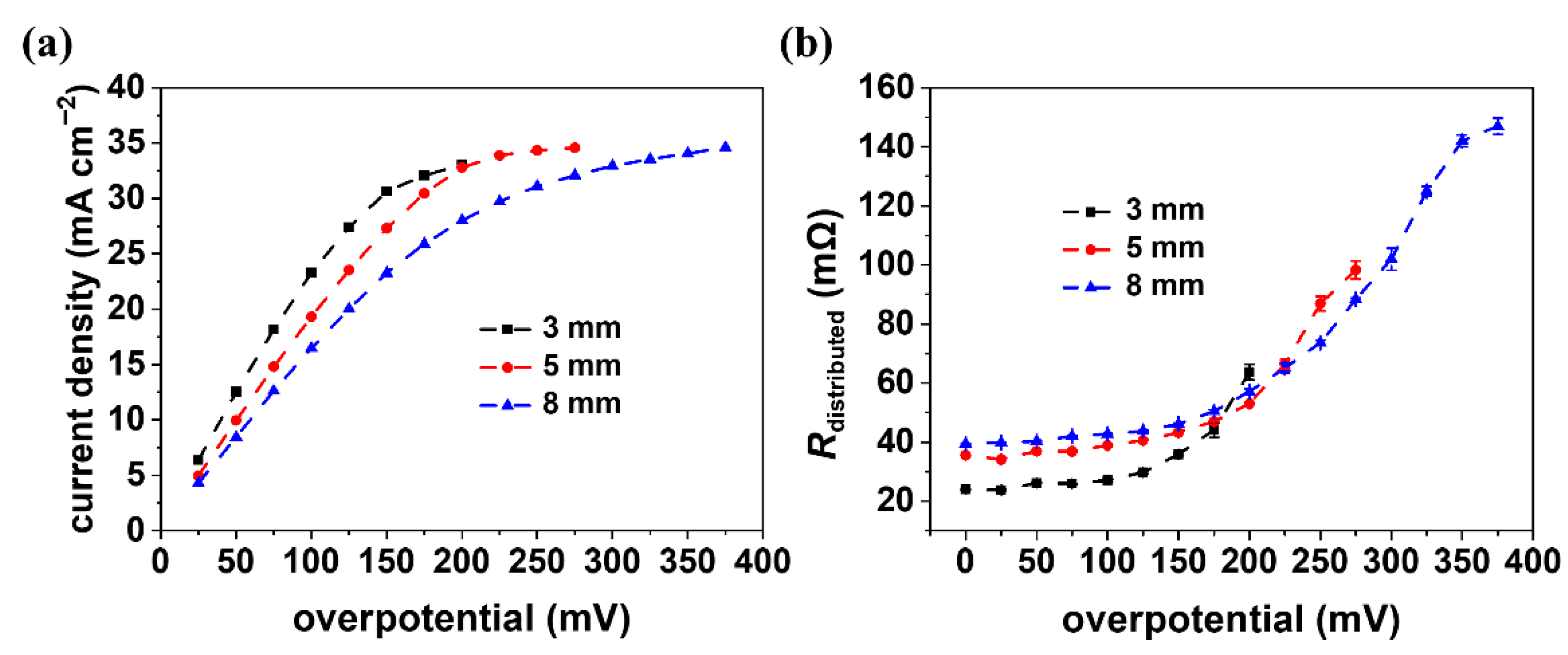
3.2. Impedance under Different Polarization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Xie, C.X.; Li, T.Y.; Li, X.F. A High Energy Density Bromine-Based Flow Battery with Two-Electron Transfer. ACS Energy Lett. 2022, 7, 1034–1039. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.Y.; Ge, M.M. Review of the Research Status of Cost-Effective Zinc–Iron Redox Flow Batteries. Batteries 2022, 8, 202. [Google Scholar] [CrossRef]
- Whitehead, A.H.; Robertson, A.; Martin, B.; Martin, E.; Wilson, E. Experimental Benchmarking of Redox Flow Cells. Batteries 2022, 8, 207. [Google Scholar] [CrossRef]
- Wu, W.D.; Luo, J.; Wang, F.; Yuan, B.; Liu, T.L. A Self-Trapping, Bipolar Viologen Bromide Electrolyte for Redox Flow Batteries. ACS Energy Lett. 2021, 6, 2891–2897. [Google Scholar] [CrossRef]
- Ai, F.; Wang, Z.Y.; Lai, N.C.; Zou, Q.L.; Liang, Z.; Lu, Y.C. Heteropoly Acid Negolytes for High-Power-Density Aqueous Redox Flow Batteries at Low Temperatures. Nat. Energy 2022, 7, 417–426. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Review of the Development of First-Generation Redox Flow Batteries: Iron-Chromium System. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef] [PubMed]
- Amini, K.; Gostick, J.; Pritzker, M.D. Metal and Metal Oxide Electrocatalysts for Redox Flow Batteries. Adv. Funct. Mater. 2020, 30, 1910564. [Google Scholar] [CrossRef]
- Esan, O.C.; Shi, X.Y.; Pan, Z.F.; Huo, X.Y.; An, L.; Zhao, T.S. Modeling and Simulation of Flow Batteries. Adv. Energy Mater. 2020, 10, 2000758. [Google Scholar] [CrossRef]
- Gao, J.X.; Amini, K.; George, T.Y.; Jing, Y.; Tsukamoto, T.; Xi, D.W.; Gordon, R.G.; Aziz, M.J. A High Potential, Low Capacity Fade Rate Iron Complex Posolyte for Aqueous Organic Flow Batteries. Adv. Energy Mater. 2022, 12, 2202444. [Google Scholar] [CrossRef]
- Huang, S.Q.; Zhang, H.; Salla, M.; Zhuang, J.H.; Zhi, Y.F.; Wang, X.; Wang, Q. Molecular Engineering of Dihydroxyanthraquinone-Based Electrolytes for High-Capacity Aqueous Organic Redox Flow Batteries. Nat. Commun. 2022, 13, 4746. [Google Scholar] [CrossRef]
- Chen, Q.R.; Lv, Y.G.; Yuan, Z.Z.; Li, X.F.; Yu, G.H.; Yang, Z.J.; Xu, T.W. Organic Electrolytes for pH-Neutral Aqueous Organic Redox Flow Batteries. Adv. Funct. Mater. 2021, 32, 2108777. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.S. Carbon Electrodes Improving Electrochemical Activity and Enhancing Mass and Charge Transports in Aqueous Flow Battery: Status and Perspective. Energy Storage Mater. 2020, 31, 230–251. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhao, T.S.; An, L.; Zeng, Y.K.; Yan, X.H. A Vanadium Redox Flow Battery Model Incorporating the Effect of Ion Concentrations on Ion Mobility. Appl. Energy 2015, 158, 157–166. [Google Scholar] [CrossRef]
- Forner-Cuenca, A.; Brushett, F.R. Engineering Porous Electrodes for Next-Generation Redox Flow Batteries: Recent Progress and Opportunities. Curr. Opin. Electrochem. 2019, 18, 113–122. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.S.; Liu, H.Y.; He, Y.L.; Hao, M.S. Sandwich-Like Multi-Scale Hierarchical Porous Carbon with a Highly Hydroxylated Surface for Flow Batteries. J. Mater. Chem. A 2021, 9, 2345–2356. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ye, X.L.; Huang, S.P.; Zhou, X.L. Promoting Pore-Level Mass Transport/Reaction in Flow Batteries: Bi Nanodot/Vertically Standing Carbon Nanosheet Composites on Carbon Fibers. ACS Appl. Mater. Interfaces 2021, 13, 37111–37122. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.S.; Wang, Y.N.; Fang, Z. Phosphorus-Doped Graphite Felt Allowing Stabilized Electrochemical Interface and Hierarchical Pore Structure for Redox Flow Battery. Appl. Energy 2020, 261, 114369. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Zhu, M.D.; Zhang, K.Y.; Zhang, X.H.; Xu, L.X.; Liu, J.G.; Liu, T.; Yan, C.A.W. Inspired by “Quenching-Cracking” Strategy: Structure-Based Design of Sulfur-Doped Graphite Felts for Ultrahigh-Rate Vanadium Redox Flow Batteries. Energy Stor. Mater. 2021, 39, 166–175. [Google Scholar] [CrossRef]
- Jin, C.X.; Lei, H.Y.; Liu, M.Y.; Tan, A.D.; Piao, J.H.; Fu, Z.Y.; Liang, Z.X.; Wang, H.H. Low-Dimensional Nitrogen-Doped Carbon for Br2/Br− Redox Reaction in Zinc-Bromine Flow Battery. Chem. Eng. J. 2020, 380, 122606. [Google Scholar] [CrossRef]
- Wan, S.B.; Liang, X.W.; Jiang, H.R.; Sun, J.; Djilali, N.; Zhao, T.S. A Coupled Machine Learning and Genetic Algorithm Approach to the Design of Porous Electrodes for Redox Flow Batteries. Appl. Energy 2021, 298, 117177. [Google Scholar] [CrossRef]
- Sun, J.; Wu, M.C.; Fan, X.Z.; Wan, Y.H.; Chao, C.Y.H.; Zhao, T.S. Aligned Microfibers Interweaved with Highly Porous Carbon Nanofibers: A Novel Electrode for High-Power Vanadium Redox Flow Batteries. Energy Storage Mater. 2021, 43, 30–41. [Google Scholar] [CrossRef]
- Wan, C.T.; Jacquemond, R.R.; Chiang, Y.M.; Nijmeijer, K.; Brushett, F.R.; Forner-Cuenca, A. Non-Solvent Induced Phase Separation Enables Designer Redox Flow Battery Electrodes. Adv. Mater. 2021, 33, e2006716. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Y.S.; He, Y.L. Achieving Gradient-Pore-Oriented Graphite Felt for Vanadium Redox Flow Batteries: Meeting Improved Electrochemical Activity and Enhanced Mass Transport from Nano-to Micro-Scale. J. Mater. Chem. A 2019, 7, 10962–10970. [Google Scholar] [CrossRef]
- Jin, S.J.; Fell, E.M.; Vina-Lopez, L.; Jing, Y.; Michalak, P.W.; Gordon, R.G.; Aziz, M.J. Near Neutral pH Redox Flow Battery with Low Permeability and Long-Lifetime Phosphonated Viologen Active Species. Adv. Energy Mater. 2020, 10, 2000100. [Google Scholar] [CrossRef]
- Liu, L.; Yao, Y.X.; Wang, Z.Y.; Lu, Y.C. Viologen Radical Stabilization by Molecular Spectators for Aqueous Organic Redox Flow Batteries. Nano Energy 2021, 84, 105897. [Google Scholar] [CrossRef]
- Luo, J.; Hu, B.; Debruler, C.; Bi, Y.J.; Zhao, Y.; Yuan, B.; Hu, M.W.; Wu, W.D.; Liu, T.L. Unprecedented Capacity and Stability of Ammonium Ferrocyanide Catholyte in pH Neutral Aqueous Redox Flow Batteries. Joule 2019, 3, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Leuaa, P.; Priyadarshani, D.; Tripathi, A.K.; Neergat, M. Internal and External Transport of Redox Species across the Porous Thin-Film Electrode/Electrolyte Interface. J. Phys. Chem. C 2019, 123, 21440–21447. [Google Scholar] [CrossRef]
- Sun, C.N.; Delnick, F.M.; Aaron, D.S.; Papandrew, A.B.; Mench, M.M.; Zawodzinski, T.A. Resolving Losses at the Negative Electrode in All-Vanadium Redox Flow Batteries Using Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2014, 161, A981–A988. [Google Scholar] [CrossRef]
- Pezeshki, A.M.; Sacci, R.L.; Delnick, F.M.; Aaron, D.S.; Mench, M.M. Elucidating Effects of Cell Architecture, Electrode Material, and Solution Composition on Overpotentials in Redox Flow Batteries. Electrochim. Acta 2017, 229, 261–270. [Google Scholar] [CrossRef] [Green Version]
- You, X.; Ye, Q.; Cheng, P. The Dependence of Mass Transfer Coefficient on the Electrolyte Velocity in Carbon Felt Electrodes: Determination and Validation. J. Electrochem. Soc. 2017, 164, E3386–E3394. [Google Scholar] [CrossRef]
- Schönleber, M.; Klotz, D.; Ivers-Tiffée, E. A Method for Improving the Robustness of Linear Kramers-Kronig Validity Tests. Electrochim. Acta 2014, 131, 20–27. [Google Scholar] [CrossRef]
- Joy, M.E.; Tripathi, A.K.; Priyadarshani, D.; Choudhury, D.; Neergat, M. What Contributes to the Internal Mass-Transport Resistance of Redox Species through Porous Thin-Film Electrodes? Phys. Chem. Chem. Phys. 2022, 24, 3886–3895. [Google Scholar] [CrossRef] [PubMed]
- Hitz, C.; Lasia, A. Experimental Study and Modeling of Impedance of the HER on Porous Ni Electrodes. J. Electroanal. Chem. 2001, 500, 213–222. [Google Scholar] [CrossRef]
- Kotz, R.; Carlen, M. Principles and Applications of Electrochemical Capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014. [Google Scholar]
- Schneider, J.; Tichter, T.; Khadke, P.; Zeis, R.; Roth, C. Deconvolution of Electrochemical Impedance Data for the Monitoring of Electrode Degradation in VRFB. Electrochim. Acta 2020, 336, 115510. [Google Scholar] [CrossRef]
- Simon, B.A.; Gayon-Lombardo, A.; Pino-Muñoz, C.A.; Wood, C.E.; Tenny, K.M.; Greco, K.V.; Cooper, S.J.; Forner-Cuenca, A.; Brushett, F.R.; Kucernak, A.R.; et al. Combining Electrochemical and Imaging Analyses to Understand the Effect of Electrode Microstructure and Electrolyte Properties on Redox Flow Batteries. Appl. Energy 2022, 306, 117678. [Google Scholar] [CrossRef]
- Song, H.K.; Jung, Y.H.; Lee, K.H.; Dao, L.H. Electrochemical Impedance Spectroscopy of Porous Electrodes the Effect of Pore Size Distribution. Electrochim. Acta 1999, 44, 3513–3519. [Google Scholar] [CrossRef]
- Huang, S.P.; Lu, Y.J. Numerical Parametric Investigation of Nonaqueous Vanadium Redox Flow Batteries. Batteries 2022, 8, 75. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Gan, Q.; Yuan, X.; Xiang, Z.; Fu, Z.; Liang, Z. Elucidating Spatial Distribution of Electrochemical Reaction in a Porous Electrode by Electrochemical Impedance Spectra for Flow Batteries. Batteries 2023, 9, 17. https://doi.org/10.3390/batteries9010017
Zhang J, Gan Q, Yuan X, Xiang Z, Fu Z, Liang Z. Elucidating Spatial Distribution of Electrochemical Reaction in a Porous Electrode by Electrochemical Impedance Spectra for Flow Batteries. Batteries. 2023; 9(1):17. https://doi.org/10.3390/batteries9010017
Chicago/Turabian StyleZhang, Jie, Qilong Gan, Xianzhi Yuan, Zhipeng Xiang, Zhiyong Fu, and Zhenxing Liang. 2023. "Elucidating Spatial Distribution of Electrochemical Reaction in a Porous Electrode by Electrochemical Impedance Spectra for Flow Batteries" Batteries 9, no. 1: 17. https://doi.org/10.3390/batteries9010017




