The Stabilizing of 1T-MoS2 for All-Solid-State Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of MoS2 Hollow Spheres
2.2. Electrochemical Measurements
2.3. Characterization of the Samples
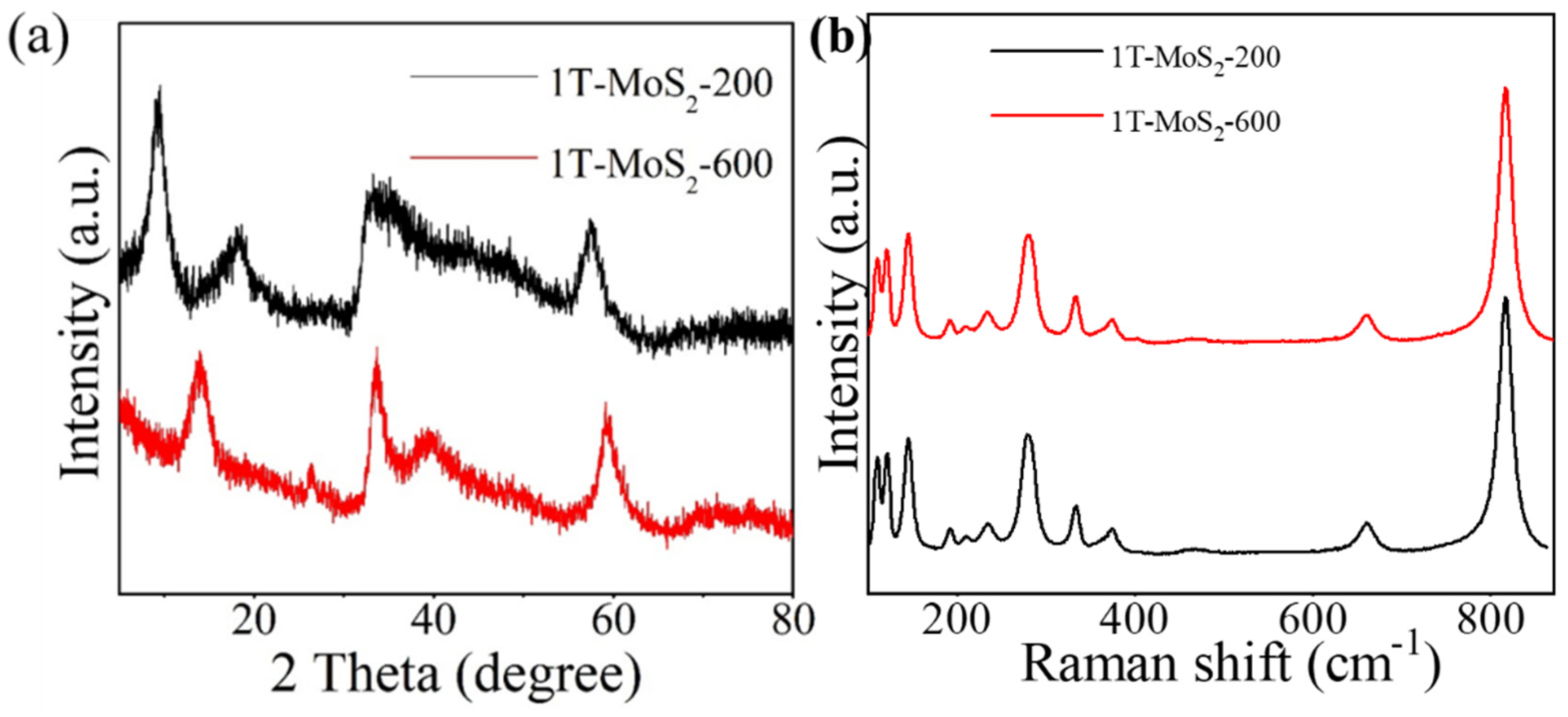
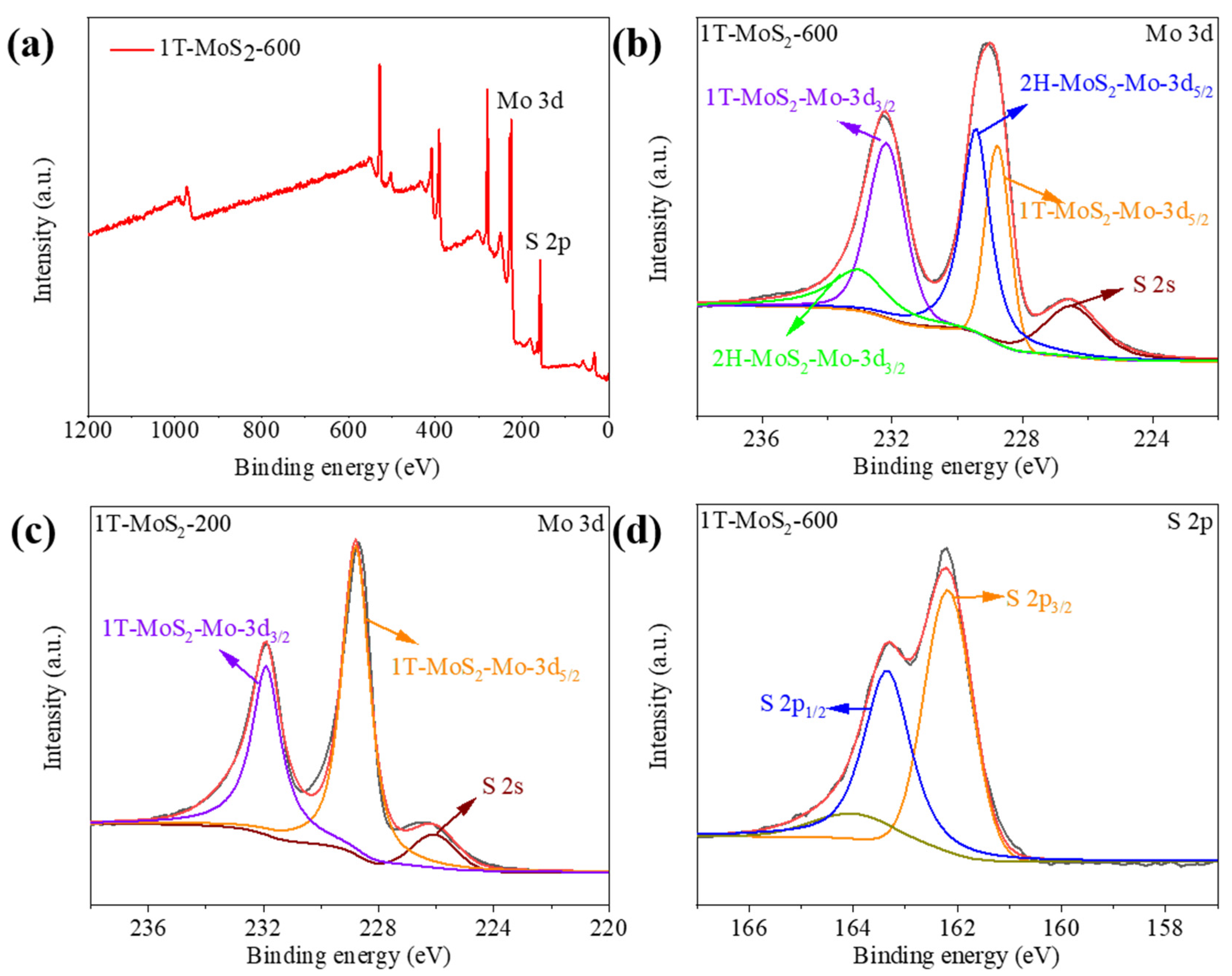
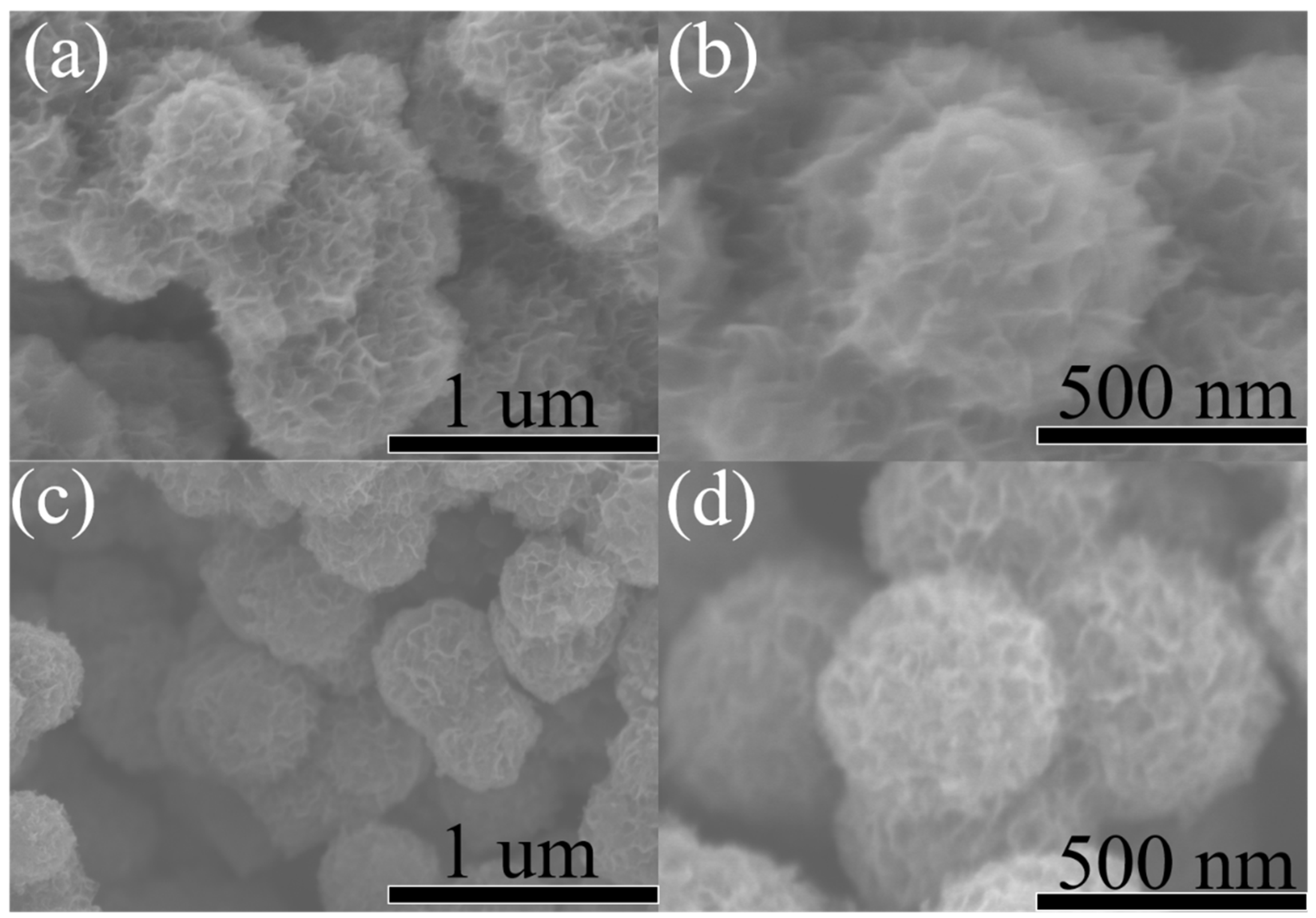
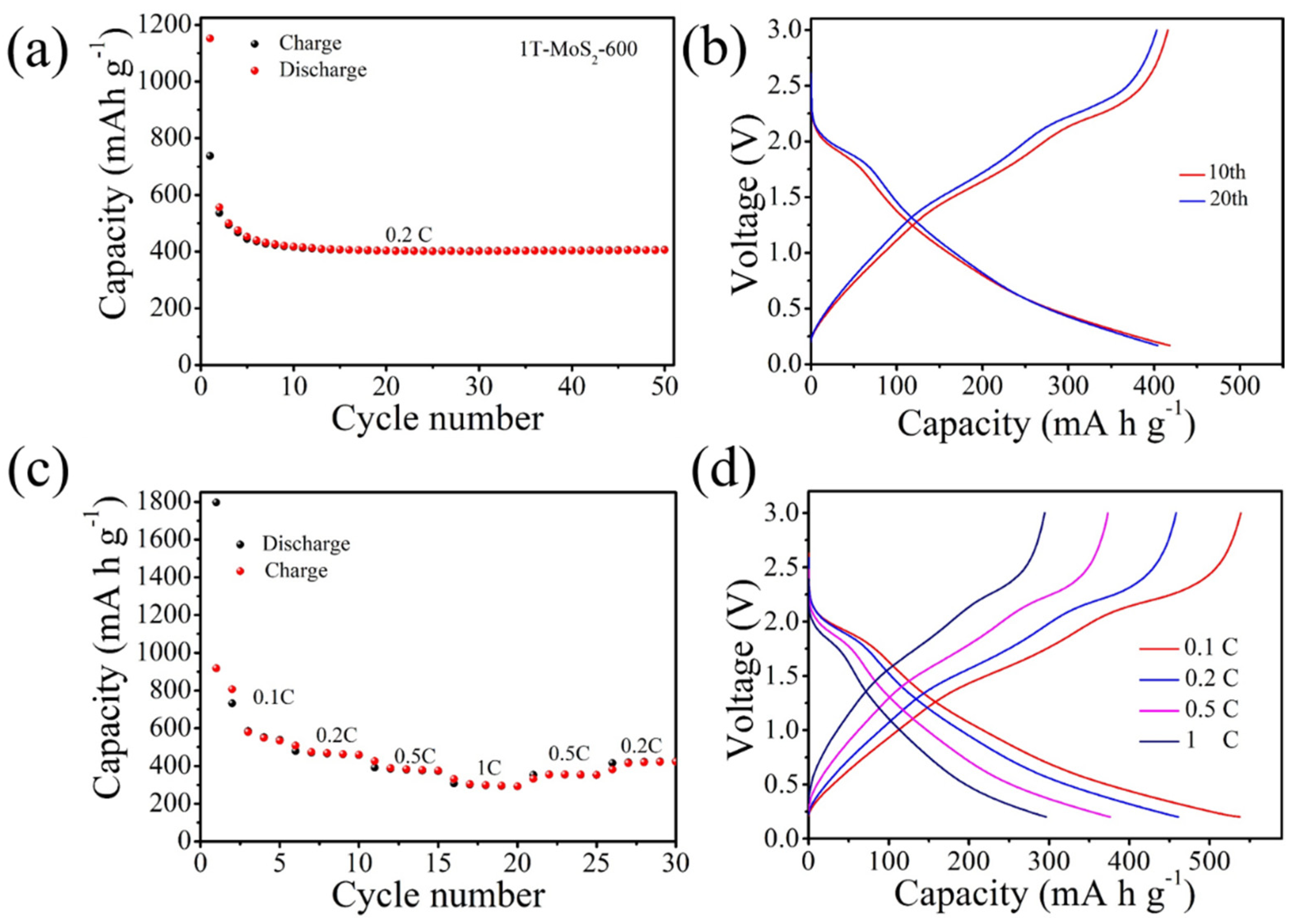
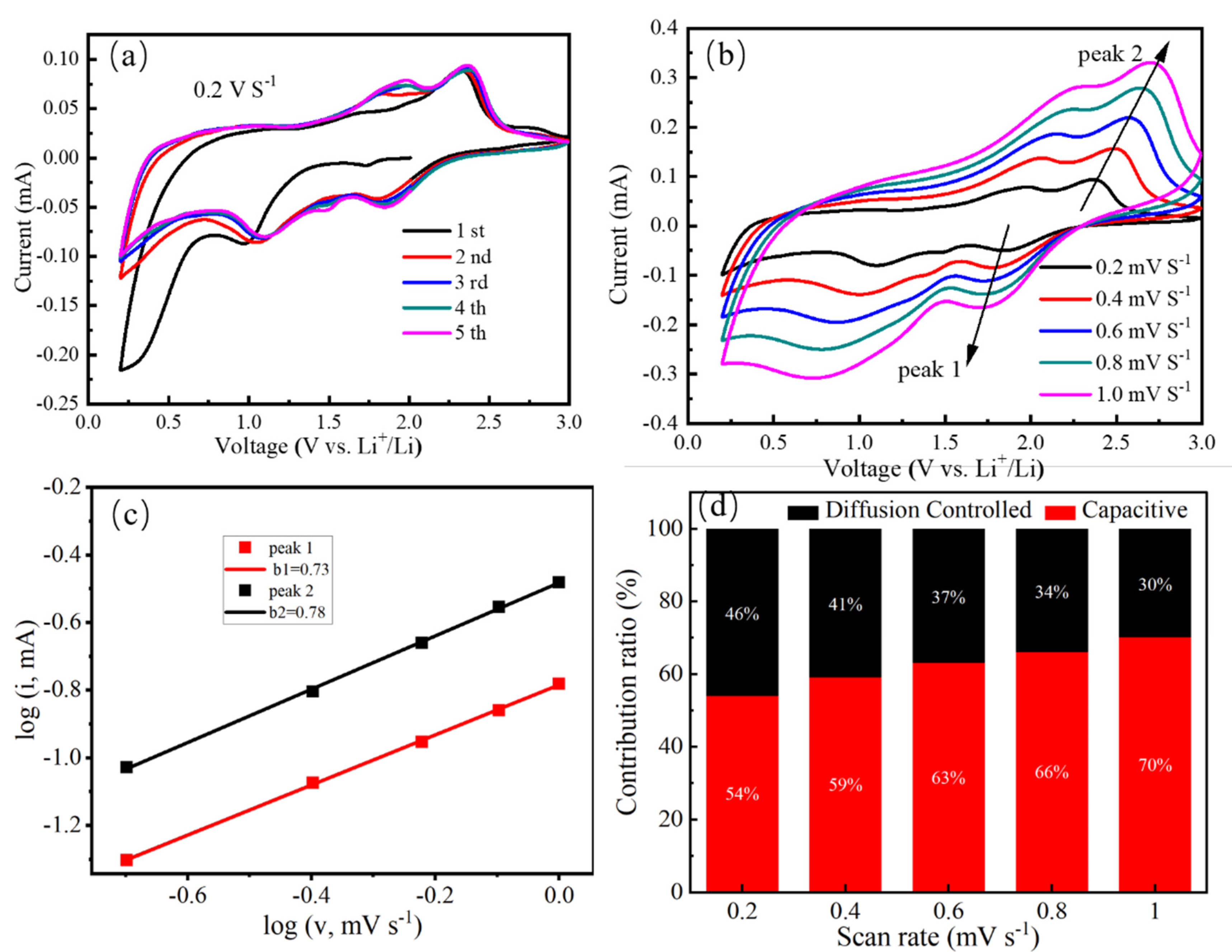
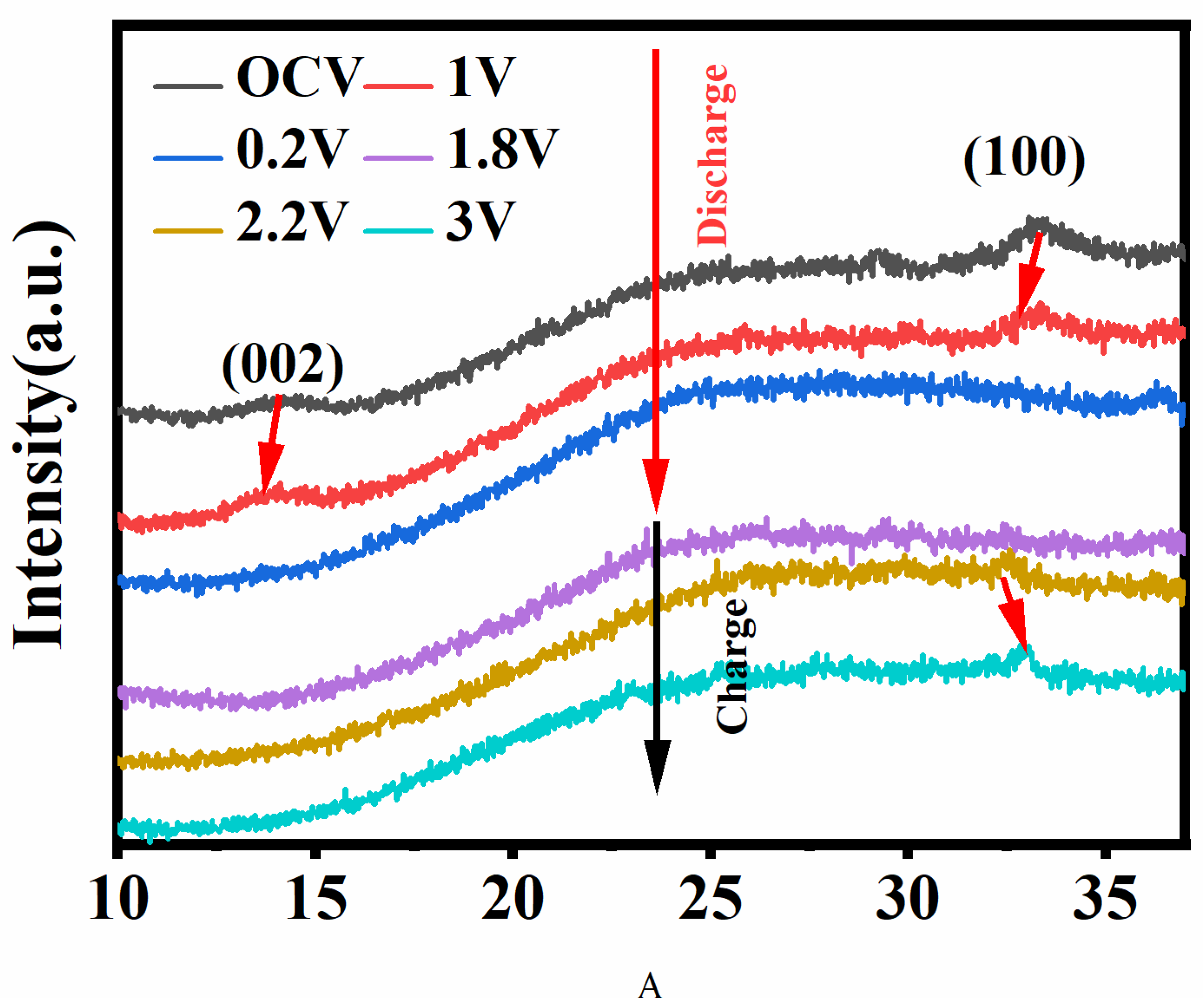
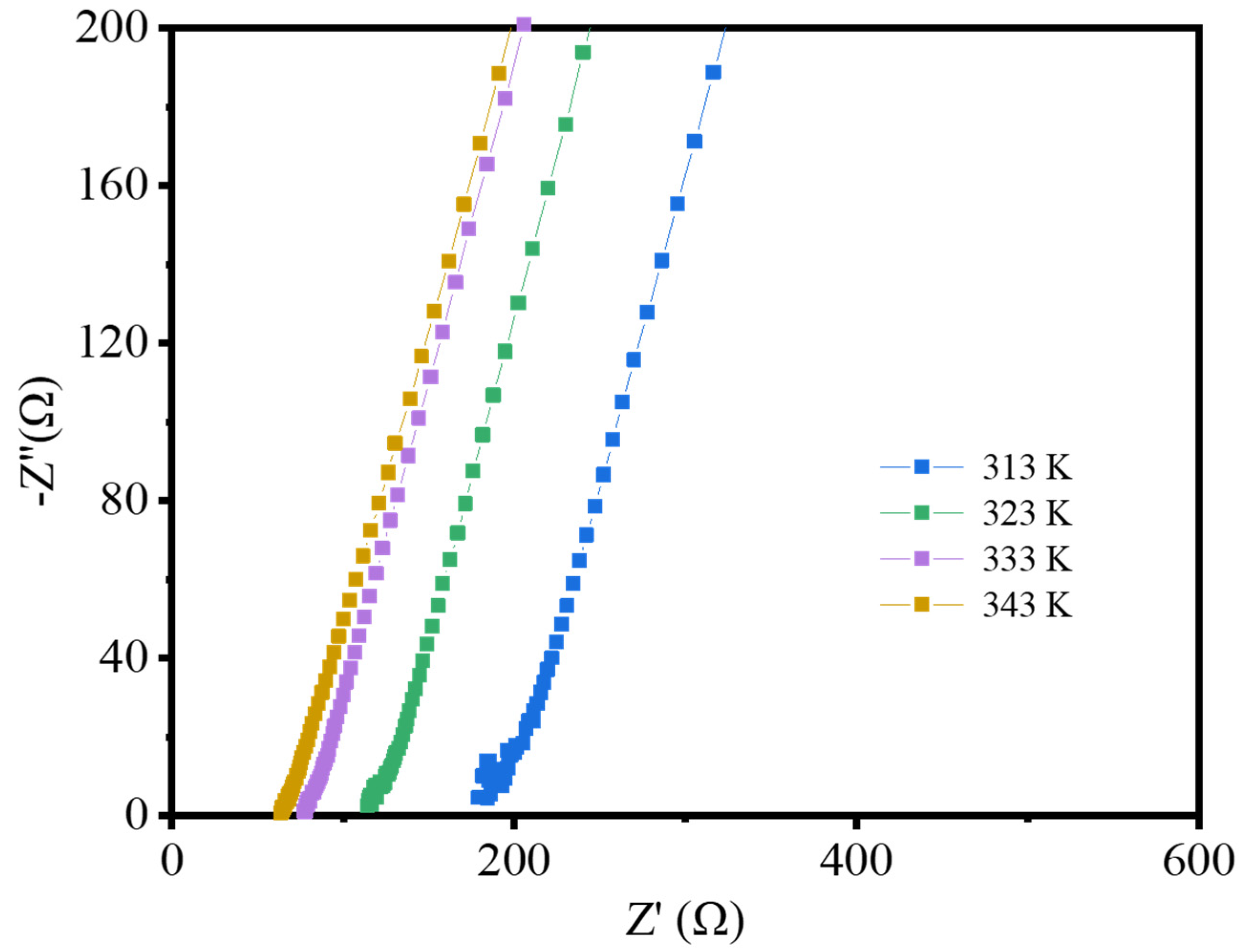
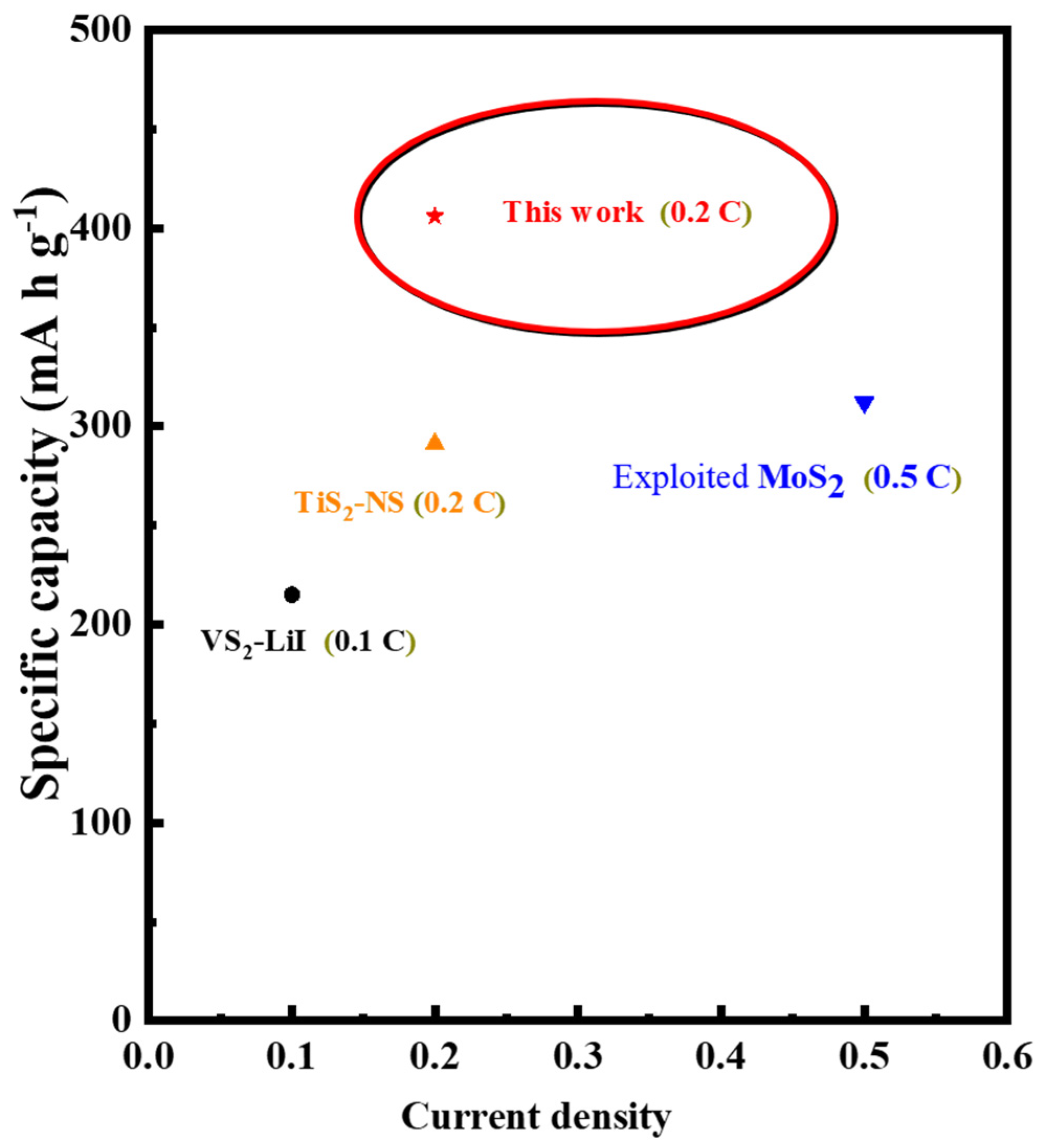
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiling, M.; Pfeiffer, F.; Baghernejad, M. Vibrational Spectroscopy Insight into the Electrode|electrolyte Interface/Interphase in Lithium Batteries. Adv. Energy Mater. 2022, 12, 2202504. [Google Scholar] [CrossRef]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-Energy Lithium Ion Batteries: Recent Progress and A Promising Future in Applications. Energy Environ. Mater. 2022, 1–60. [Google Scholar] [CrossRef]
- Oh, H.J.; Kang, H.K.; Ahn, H.; Park, J.; Choi, J.; Kim, H.Y.; Lee, E.; Yeo, S.Y.; Choi, Y.O.; Yeang, B.J.; et al. Layered oxide cathode-inspired secondary hard carbon microsphere anode material for high-power and long-life rechargeable batteries. Chem. Eng. J. 2023, 454, 140252. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Min, X.; Xu, G.; Xie, B.; Guan, P.; Sun, M.; Cui, G. Challenges of prelithiation strategies for next generation high energy lithium-ion batteries. Energy Stor. Mater. 2022, 47, 297–318. [Google Scholar] [CrossRef]
- Gao, H.; Neale, A.R.; Zhu, Q.; Bahri, M.; Wang, X.; Yang, H.; Xu, Y.; Clowes, R.; Browning, N.D.; Little, M.A.; et al. A Pyrene-4,5,9,10-Tetraone-Based Covalent Organic Framework Delivers High Specific Capacity as a Li-Ion Positive Electrode. J. Am. Chem. Soc. 2022, 144, 9434–9442. [Google Scholar] [CrossRef]
- Gao, H.; Tian, B.; Yang, H.; Neale, A.R.; Little, M.A.; Sprick, R.S.; Hardwick, L.J.; Cooper, A.I. Crosslinked Polyimide and Reduced Graphene Oxide Composites as Long Cycle Life Positive Electrode for Lithium-Ion Cells. ChemSusChem 2020, 13, 5571–5579. [Google Scholar] [CrossRef]
- Lewis, J.A.; Cavallaro, K.A.; Liu, Y.; McDowell, M.T. The promise of alloy anodes for solid-state batteries. Joule 2022, 6, 1418–1430. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Ma, J.; Chen, B.; Li, C.; Li, J.; Chang, L.; Yu, X.; Chan, T.-S.; Hu, Z.; et al. Bidirectionally Compatible Buffering Layer Enables Highly Stable and Conductive Interface for 4.5 V Sulfide-Based All-Solid-State Lithium Batteries. Adv. Energy Mater. 2021, 11, 2100881. [Google Scholar] [CrossRef]
- Cao, D.; Zhao, Y.; Sun, X.; Natan, A.; Wang, Y.; Xiang, P.; Wang, W.; Zhu, H. Processing Strategies to Improve Cell-Level Energy Density of Metal Sulfide Electrolyte-Based All-Solid-State Li Metal Batteries and Beyond. ACS Energy Lett. 2020, 5, 3468–3489. [Google Scholar] [CrossRef]
- Wang, J.; Okabe, J.; Urita, K.; Moriguchi, I.; Wei, M. Cu2S hollow spheres as an anode for high-rate sodium storage performance. J. Electroanal. Chem. 2020, 874, 114523. [Google Scholar] [CrossRef]
- Oh, P.; Yun, J.; Choi, J.H.; Saqib, K.S.; Embleton, T.J.; Park, S.; Lee, C.; Ali, J.; Ko, K.; Cho, J. Development of High-Energy Anodes for All-Solid-State Lithium Batteries Based on Sulfide Electrolytes. Angew. Chem. Int. Ed. 2022, 61, e202201249. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, R.; Chen, B.; Yu, X.; Ma, J.; Li, C.; Hu, Z.; Sun, X.; Xu, C.; Dong, S.; et al. In-situ visualization of the space-charge-layer effect on interfacial lithium-ion transport in all-solid-state batteries. Nat. Commun. 2020, 11, 5889. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, K.; Ren, F.; Ha, Y.; Liang, Z.; Zheng, X.; Wang, Z.; Yang, W.; Zhang, M.; Luo, M.; et al. Highly reversible Li2RuO3 cathodes in sulfide-based all solid-state lithium batteries. Energy Environ. Sci. 2022, 15, 3470–3482. [Google Scholar] [CrossRef]
- Fan, Z.; Xiang, J.; Yu, Q.; Wu, X.; Li, M.; Wang, X.; Xia, X.; Tu, J. High Performance Single-Crystal Ni-Rich Cathode Modification via Crystalline LLTO Nanocoating for All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yamamoto, K.; Mei, X.; Sakuda, A.; Uchiyama, T.; Watanabe, T.; Takami, T.; Hayashi, A.; Tatsumisago, M.; Uchimoto, Y. High Rate Capability from a Graphite Anode through Surface Modification with Lithium Iodide for All-Solid-State Batteries. ACS Appl. Energy Mater. 2022, 5, 667–673. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Meng, Y.S.; Jang, J. Scaling up high-energy-density sulfidic solid-state batteries: A lab-to-pilot perspective. Joule 2022, 6, 1755–1769. [Google Scholar] [CrossRef]
- Ohashi, A.; Kodama, M.; Xueying, S.; Hori, S.; Suzuki, K.; Kanno, R.; Hirai, S. Stress distribution in the composite electrodes of sulfide all-solid-state lithium-ion batteries. J. Power Sources 2020, 470, 228437. [Google Scholar] [CrossRef]
- Gregory, G.L.; Gao, H.; Liu, B.; Gao, X.; Rees, G.J.; Pasta, M.; Bruce, P.G.; Williams, C.K. Buffering Volume Change in Solid-State Battery Composite Cathodes with CO2-Derived Block Polycarbonate Ethers. J. Am. Chem. Soc. 2022, 144, 17477–17486. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Duan, H.; Zhang, L.; Deng, Y.; Chen, G. Optimized functional additive enabled stable cathode and anode interfaces for high-voltage all-solid-state lithium batteries with significantly improved cycling performance. J. Mater. Chem. A 2022, 10, 20331–20342. [Google Scholar] [CrossRef]
- Ye, L.; Li, X. A dynamic stability design strategy for lithium metal solid state batteries. Nature 2021, 593, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lai, Y.; Lv, N.; Hu, Y.; Li, B.; Jing, S.; Jiang, L.; Jia, M.; Li, J.; Chen, S.; et al. Boosting the Electrochemical Performance of All-Solid-State Batteries with Sulfide Li6PS5Cl Solid Electrolyte Using Li2WO4-Coated LiCoO2 Cathode. Adv. Mater. Interfaces 2021, 8, 2100624. [Google Scholar] [CrossRef]
- Wan, H.; Zhang, J.; Xia, J.; Ji, X.; He, X.; Liu, S.; Wang, C. F and N Rich Solid Electrolyte for Stable All-Solid-State Battery. Adv. Funct. Mater. 2022, 32, 2110876. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Y.; Yin, G.; Gao, Y. The stable cycling of a high-capacity Bi anode enabled by an in situ-generated Li3PO4 transition layer in a sulfide-based all-solid-state battery. ChemComm 2020, 56, 15458–15461. [Google Scholar] [CrossRef] [PubMed]
- Priyadharsini, N.; Rupa Kasturi, P.; Shanmugavani, A.; Surendran, S.; Shanmugapriya, S.; Kalai Selvan, R. Effect of chelating agent on the sol-gel thermolysis synthesis of LiNiPO4 and its electrochemical properties for hybrid capacitors. J. Phys. Chem. Solids 2018, 119, 183–192. [Google Scholar] [CrossRef]
- Priyadharsini, N.; Shanmugapriya, S.; Kasturi, P.R.; Surendran, S.; Selvan, R.K. Morphology-dependent electrochemical properties of sol-gel synthesized LiCoPO4 for aqueous hybrid capacitors. Electrochim. Acta 2018, 289, 516–526. [Google Scholar] [CrossRef]
- Gao, Y.; Hai, P.; Liu, L.; Yin, J.; Gan, Z.; Ai, W.; Wu, C.; Cheng, Y.; Xu, X. Balanced Crystallinity and Nanostructure for SnS2 Nanosheets through Optimized Calcination Temperature toward Enhanced Pseudocapacitive Na+ Storage. ACS Nano 2022, 16, 14745–14753. [Google Scholar] [CrossRef]
- Geng, P.; Zheng, S.; Tang, H.; Zhu, R.; Zhang, L.; Cao, S.; Xue, H.; Pang, H. Transition Metal Sulfides Based on Graphene for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1703259. [Google Scholar] [CrossRef]
- Priyadharsini, N.; Shanmugavani, A.; Surendran, S.; Senthilkumar, B.; Vasylechko, L.; Kalai Selvan, R. Improved electrochemical performances of LiMnPO4 synthesized by a hydrothermal method for Li-ion supercapatteries. J. Mater. Sci. Mater. Electron. 2018, 29, 18553–18565. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Lv, T.A.; Chu, P.K.; Huo, K. Two-Dimensional Transition Metal Chalcogenides for Alkali Metal Ions Storage. ChemSusChem 2020, 13, 1114–1154. [Google Scholar] [CrossRef]
- Yin, X.; Tang, C.S.; Zheng, Y.; Gao, J.; Wu, J.; Zhang, H.; Chhowalla, M.; Chen, W.; Wee, A.T.S. Recent developments in 2D transition metal dichalcogenides: Phase transition and applications of the (quasi-)metallic phases. Chem. Soc. Rev. 2021, 50, 10087–10115. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, D.; Dai, L.; Liang, Z.; Ci, L. Structural Engineering of SnS2 Encapsulated in Carbon Nanoboxes for High-Performance Sodium/Potassium-Ion Batteries Anodes. Small 2020, 16, 2005023. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, Q.; Wang, D.; Chen, G.; Cheng, X.; Kups, T.; Schaaf, P. Disordered surface formation of WS2via hydrogen plasma with enhanced anode performances for lithium and sodium ion batteries. Sustain. Energy Fuels 2019, 3, 865–874. [Google Scholar] [CrossRef]
- Santhosha, A.L.; Nayak, P.K.; Pollok, K.; Langenhorst, F.; Adelhelm, P. Exfoliated MoS2 as Electrode for All-Solid-State Rechargeable Lithium-Ion Batteries. J. Phys. Chem. C 2019, 123, 12126–12134. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhao, X.; Cui, K.; Shen, Q.; Li, P.; Qu, X.; Jiao, L. Molecular Engineering on MoS2 Enables Large Interlayers and Unlocked Basal Planes for High-Performance Aqueous Zn-Ion Storage. Angew. Chem. Int. Ed. 2021, 60, 20286–20293. [Google Scholar] [CrossRef]
- Mirabal, N.; Aguirre, P.; Santa Ana, M.A.; Benavente, E.; González, G. Thermal stability and electrical conductivity in polyethers-molybdenum disulfide nanocomposites. Electrochim. Acta 2003, 48, 2123–2127. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Zhang, D.; Wang, Y.; Luo, X.; Liu, X.; Kim, J.-K.; Luo, Y. Inter-overlapped MoS2/C composites with large-interlayer-spacing for high-performance sodium-ion batteries. Nanoscale Horiz. 2020, 5, 1127–1135. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, B.; Zhou, J.; Si, J.; Fang, Z.; Li, K.; Ma, H.; Dai, J.; Zhu, X.; Sun, Y. Glucose-Induced Synthesis of 1T-MoS2/C Hybrid for High-Rate Lithium-Ion Batteries. Small 2019, 15, 1805420. [Google Scholar] [CrossRef]
- Zhuo, Y.; Prestat, E.; Kinloch, I.A.; Bissett, M.A. Self-Assembled 1T-MoS2/Functionalized Graphene Composite Electrodes for Supercapacitor Devices. ACS Appl. Energy Mater 2022, 5, 61–70. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Zhang, F.; Zheng, W.; Zhao, L.; Feng, Q. Metal-semiconductor 1T/2H-MoS2 by a heteroatom-doping strategy for enhanced electrocatalytic hydrogen evolution. Catal. Commun. 2021, 156, 106325. [Google Scholar] [CrossRef]
- Fayed, M.G.; Attia, S.Y.; Barakat, Y.F.; El-Shereafy, E.E.; Rashad, M.M.; Mohamed, S.G. Carbon and nitrogen co-doped MoS2 nanoflakes as an electrode material for lithium-ion batteries and supercapacitors. SM&T 2021, 29, e00306. [Google Scholar]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, L.; Zhu, Y.; Peng, H.; Du, C.; Yang, X.; Zhao, Q.; Hou, J.; Cao, C. Ammonium-Modified Synthesis of Vanadium Sulfide Nanosheet Assemblies toward High Sodium Storage. ACS Nano 2022, 16, 12900–12909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Weng, W.; Gu, H.; Hong, Z.; Xiao, W.; Wang, F.; Li, W.; Gu, D. Versatile Preparation of Mesoporous Single-Layered Transition-Metal Sulfide/Carbon Composites for Enhanced Sodium Storage. Adv. Mater. 2022, 34, 2104427. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, G.; Huang, H.; Liu, C.; Lyu, P.; Zhang, N. Interlayer-expanded MoS2 nanoflowers anchored on the graphene: A high-performance Li+/Mg2+ co-intercalation cathode material. Chem. Eng. J. 2022, 428, 131214. [Google Scholar] [CrossRef]
- Lu, H.; Tian, K.; Bu, L.; Huang, X.; Li, X.; Zhao, Y.; Wang, F.; Bai, J.; Gao, L.; Zhao, J. Synergistic effect from coaxially integrated CNTs@MoS2/MoO2 composite enables fast and stable lithium storage. J. Energy Chem. 2021, 55, 449–458. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhao, X.; Shen, Q.; Zhao, W.; Tan, Q.; Zhang, N.; Li, P.; Jiao, L.; Qu, X. Sandwich-Like Heterostructures of MoS2/Graphene with Enlarged Interlayer Spacing and Enhanced Hydrophilicity as High-Performance Cathodes for Aqueous Zinc-Ion Batteries. Adv. Mater. 2021, 33, 2007480. [Google Scholar] [CrossRef]
- Gong, F.; Ye, S.; Liu, M.; Zhang, J.; Gong, L.; Zeng, G.; Meng, E.; Su, P.; Xie, K.; Zhang, Y.; et al. Boosting electrochemical oxygen evolution over yolk-shell structured O–MoS2 nanoreactors with sulfur vacancy and decorated Pt nanoparticles. Nano Energy 2020, 78, 105284. [Google Scholar] [CrossRef]
- Zhou, L.-F.; Gao, X.-W.; Du, T.; Gong, H.; Liu, L.-Y.; Luo, W.-B. New Phosphate Zn2Fe(PO4)2 Cathode Material for Nonaqueous Zinc Ion Batteries with Long Life Span. ACS Appl. Mater. Interfaces 2022, 14, 8888–8895. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Huang, S.; Komine, Y.; Notohara, H.; Urita, K.; Moriguchi, I.; Wei, M. Regulating the effects of SnS shrinkage in all-solid-state lithium-ion batteries with excellent electrochemical performance. Chem. Eng. J. 2022, 429, 132424. [Google Scholar] [CrossRef]
- Wu, D.; Sun, F.; Qu, Z.; Wang, H.; Lou, Z.; Wu, B.; Zhao, G. Multi-scale structure optimization of boron-doped hard carbon nanospheres boosting the plateau capacity for high performance sodium ion batteries. J. Mater. Chem. A 2022, 10, 17225–17236. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Zhang, Z.; Xu, M.; Lai, Y.; Chou, S.-L. Full Activation of Mn4+/Mn3+ Redox in Na4MnCr(PO4)3 as a High-Voltage and High-Rate Cathode Material for Sodium-Ion Batteries. Small 2020, 16, 2001524. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Yang, Y.; Wang, H.; Zheng, Y.; Hao, H.; Song, W.; Shi, J.; Huang, M.; Mitlin, D. Sulfur-nitrogen rich carbon as stable high capacity potassium ion battery anode: Performance and storage mechanisms. Energy Stor. Mater. 2020, 27, 212–225. [Google Scholar] [CrossRef]
- Oh, D.Y.; Choi, Y.E.; Kim, D.H.; Lee, Y.-G.; Kim, B.-S.; Park, J.; Sohn, H.; Jung, Y.S. All-solid-state lithium-ion batteries with TiS2 nanosheets and sulphide solid electrolytes. J. Mater. Chem. A 2016, 4, 10329–10335. [Google Scholar] [CrossRef]
- Wang, J.; Okabe, J.; Komine, Y.; Notohara, H.; Urita, K.; Moriguchi, I.; Wei, M. The optimized interface engineering of VS2 as cathodes for high performance all-solid-state lithium-ion battery. Sci. China Technol. Sci. 2022, 65, 1859–1866. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, P.; Zhou, Z.; Wang, K.; Zhai, W.; Li, Y.; Wang, J.; Wei, M. The Stabilizing of 1T-MoS2 for All-Solid-State Lithium-Ion Batteries. Batteries 2023, 9, 26. https://doi.org/10.3390/batteries9010026
Chong P, Zhou Z, Wang K, Zhai W, Li Y, Wang J, Wei M. The Stabilizing of 1T-MoS2 for All-Solid-State Lithium-Ion Batteries. Batteries. 2023; 9(1):26. https://doi.org/10.3390/batteries9010026
Chicago/Turabian StyleChong, Peidian, Ziwang Zhou, Kaihong Wang, Wenhao Zhai, Yafeng Li, Jianbiao Wang, and Mingdeng Wei. 2023. "The Stabilizing of 1T-MoS2 for All-Solid-State Lithium-Ion Batteries" Batteries 9, no. 1: 26. https://doi.org/10.3390/batteries9010026
APA StyleChong, P., Zhou, Z., Wang, K., Zhai, W., Li, Y., Wang, J., & Wei, M. (2023). The Stabilizing of 1T-MoS2 for All-Solid-State Lithium-Ion Batteries. Batteries, 9(1), 26. https://doi.org/10.3390/batteries9010026





