Application of TiS2 as an Active Material for Aqueous Calcium-Ion Batteries: Electrochemical Calcium Intercalation into TiS2 from Aqueous Solutions
Abstract
:1. Introduction
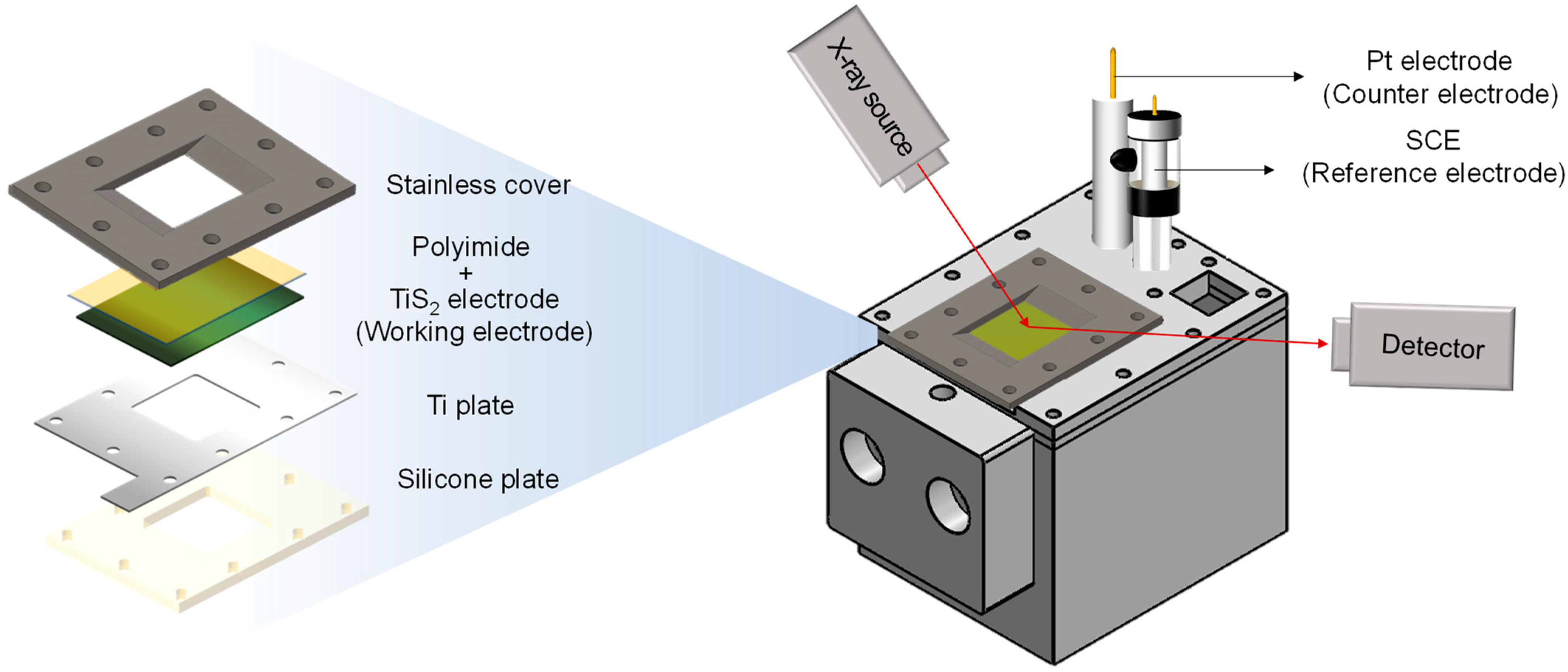
2. Materials and Methods
2.1. Preparation of Electrode and Electrolytes
2.2. Electrochemical Measurements
2.3. Structure and Surface Analysis
3. Results and Discussion
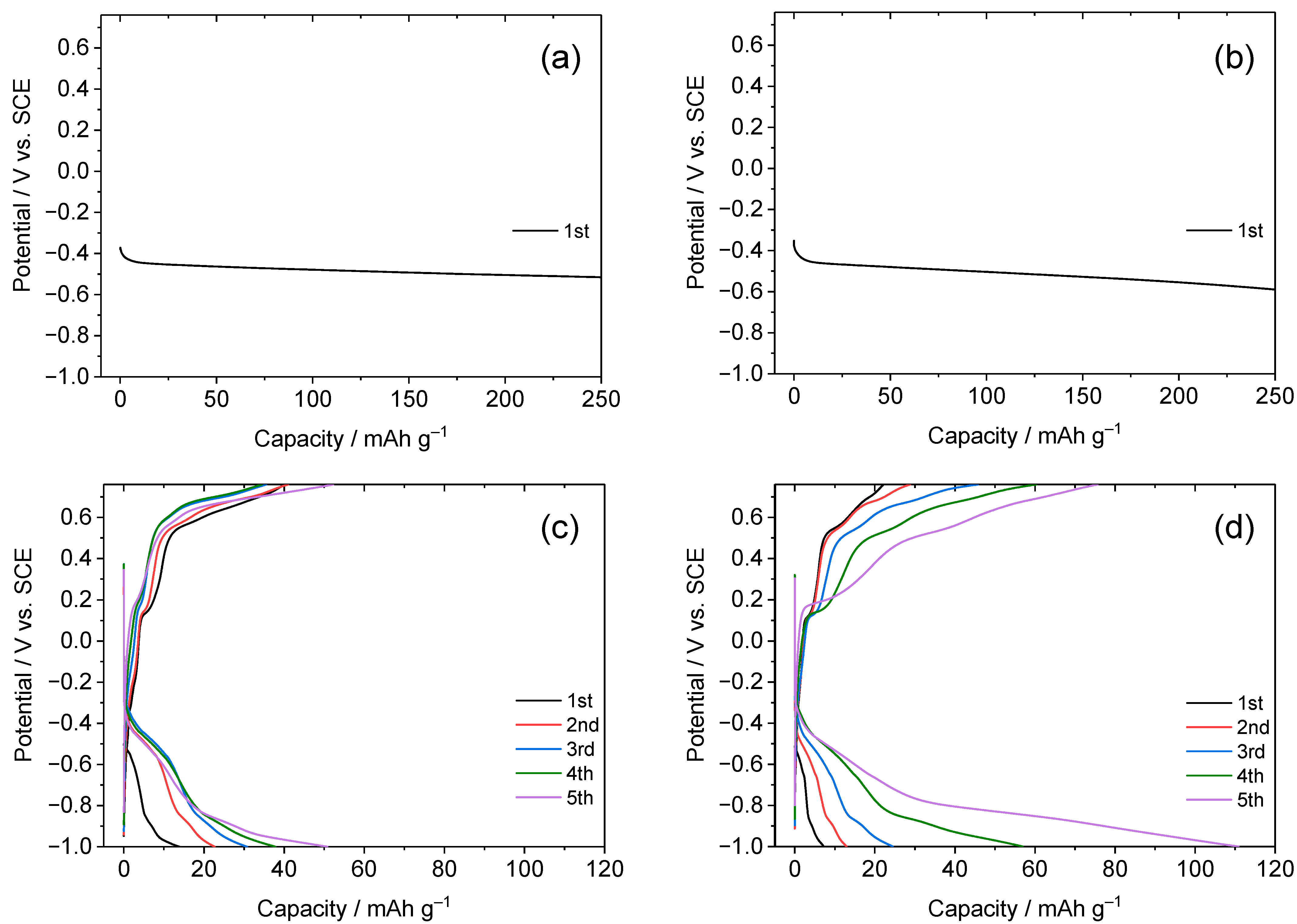
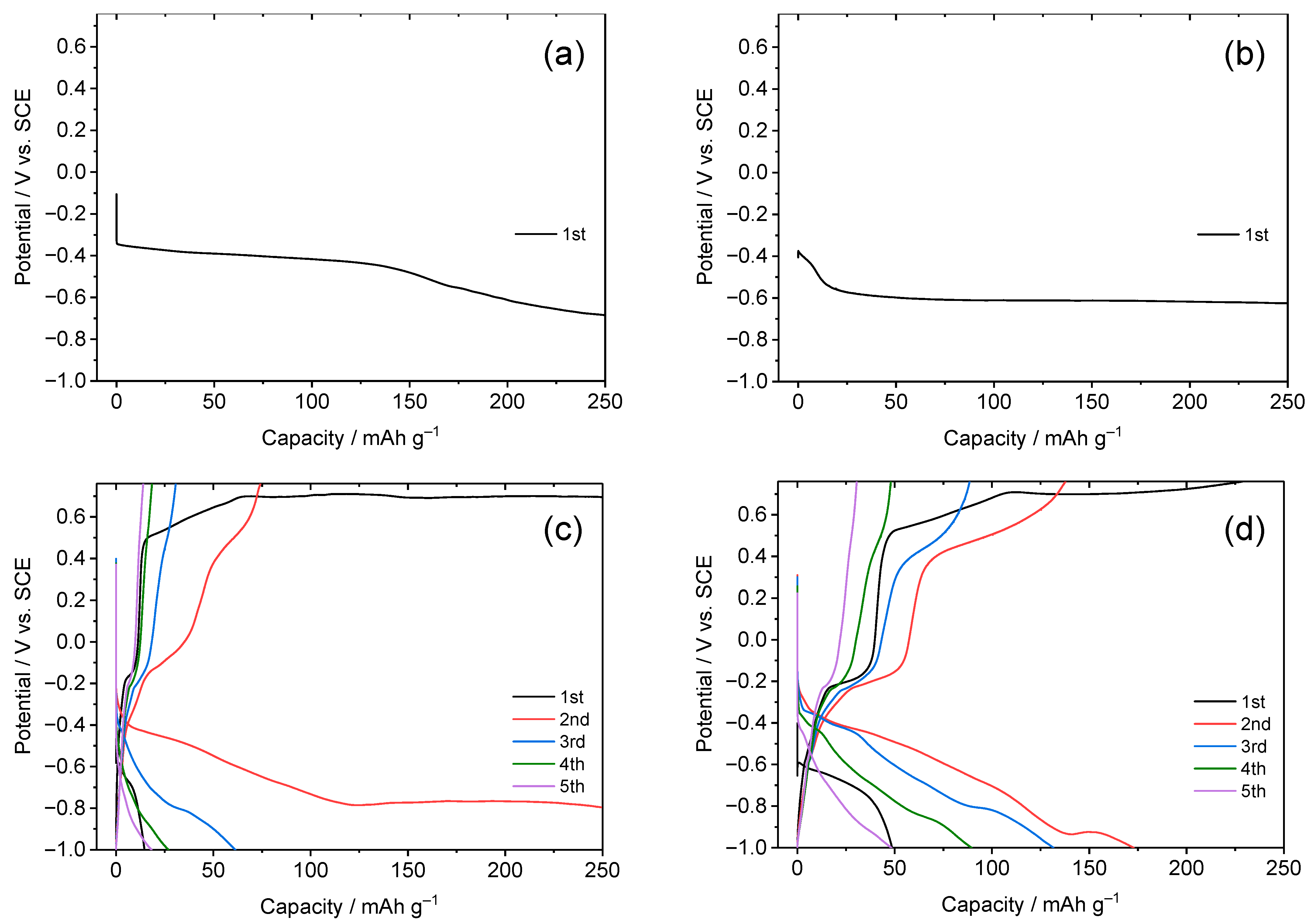
3.1. Dependence of Charge–Discharge Behavior on Electrolyte Concentration
3.2. In Situ Structural Analysis of TiS2 during Charging and Discharging
3.3. XPS Analysis of TiS2 Electrode before and after Discharging
3.4. Anion Dependence of Charge–Discharge Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zaman, W.; Hatzell, K.B. Processing and manufacturing of next generation lithium-based all solid-state batteries. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101003. [Google Scholar] [CrossRef]
- Chen, S.; Jeong, S.R.; Tao, S. Key materials and future perspective for aqueous rechargeable lithium-ion batteries. Mater. Rep. Energy 2022, 2, 100096. [Google Scholar] [CrossRef]
- Shin, J.; Choi, J.W. Opportunities and reality of aqueous rechargeable batteries. Adv. Energy Mater. 2020, 10, 2001386. [Google Scholar] [CrossRef]
- Tran, M.-K.; Mevawalla, A.; Aziz, A.; Panchal, S.; Xie, Y.; Fowler, M. A review of lithium-ion battery thermal runaway modeling and diagnosis approaches. Processes 2022, 10, 1192. [Google Scholar] [CrossRef]
- Ji, B.; He, H.; Yao, W.; Tang, Y. Recent advances and perspectives on calcium-ion storage: Key materials and devices. Adv. Mater. 2021, 33, 2005501. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Ma, L.; Huang, W. Solvation structures in aqueous metal-ion batteries. Adv. Energy Mater. 2022, 12, 2202068. [Google Scholar] [CrossRef]
- Gheytani, S.; Liang, Y.; Wu, F.; Jing, Y.; Dong, H.; Rao, K.K.; Chi, X.; Fang, F.; Yao, Y. An aqueous Ca-ion battery. Adv. Sci. 2017, 4, 1700465. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, S.-K. A novel strategy to improve the electrochemical performance of a prussian blue analogue electrode for calcium-ion batteries. Electrochemistry 2018, 86, 134–137. [Google Scholar] [CrossRef]
- Purbarani, M.E.; Hyoung, J.; Hong, S.-T. Crystal-water-free potassium vanadium bronze (K0.5V2O5) as a cathode material for Ca-ion batteries. ACS Appl. Energy Mater. 2021, 4, 7487–7491. [Google Scholar] [CrossRef]
- Alvarez Ferrero, G.; Åvall, G.; Mazzio, K.A.; Son, Y.; Janßen, K.; Risse, S.; Adelhelm, P. Co-intercalation batteries (CoIBs): Role of TiS2 as electrode for storing solvated Na ions. Adv. Energy Mater. 2022, 12, 2202377. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical energy storage and intercalation chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef]
- Whittingham, M.S. Chemistry of intercalation compounds: Metal guests in chalcogenide hosts. Prog. Solid State Chem. 1978, 12, 41–99. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, Z.; Xia, W.; Ming, C.; Han, Y.; Cao, L.; Lu, J.; Zhang, P.; Zhang, S.; Xu, H. Semimetal or semiconductor: The nature of high intrinsic electrical conductivity in TiS2. J. Phys. Chem. Lett. 2019, 10, 6996–7001. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bates, J.; Hart, F.; Sales, B.; Zuhr, R.; Robertson, J. Characterization of thin-film rechargeable lithium batteries with lithium cobalt oxide cathodes. J. Electrochem. Soc. 1996, 143, 3203. [Google Scholar] [CrossRef]
- Striebel, K.; Deng, C.; Wen, S.; Cairns, E. Electrochemical behavior of LiMn2O4 and LiCoO2 Thin films produced with pulsed laser deposition. J. Electrochem. Soc. 1996, 143, 1821. [Google Scholar] [CrossRef]
- Prosini, P.P.; Lisi, M.; Zane, D.; Pasquali, M. Determination of the chemical diffusion coefficient of lithium in LiFePO4. Solid State Ion. 2002, 148, 45–51. [Google Scholar] [CrossRef]
- Sun, X.; Bonnick, P.; Nazar, L.F. Layered TiS2 positive electrode for Mg batteries. ACS Energy Lett. 2016, 1, 297–301. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, X.; Edström, K.; Berg, E.J. Reactivity of TiS2 anode towards electrolytes in aqueous lithium-ion batteries. Batter. Supercaps 2022, 5, e202200336. [Google Scholar] [CrossRef]
- Sun, W.; Suo, L.; Wang, F.; Eidson, N.; Yang, C.; Han, F.; Ma, Z.; Gao, T.; Zhu, M.; Wang, C. “Water-in-salt” electrolyte enabled LiMn2O4/TiS2 lithium-ion batteries. Electrochem. Commun. 2017, 82, 71–74. [Google Scholar] [CrossRef]
- Chung, S.-H.; Luo, L.; Manthiram, A. TiS2–polysulfide hybrid cathode with high sulfur loading and low electrolyte consumption for lithium–sulfur batteries. ACS Energy Lett. 2018, 3, 568–573. [Google Scholar] [CrossRef]
- Tian, B.; Tang, W.; Leng, K.; Chen, Z.; Tan, S.J.R.; Peng, C.; Ning, G.-H.; Fu, W.; Su, C.; Zheng, G.W. Phase transformations in TiS2 during K intercalation. ACS Energy Lett. 2017, 2, 1835–1840. [Google Scholar] [CrossRef]
- Wang, L.; Zou, J.; Chen, S.; Zhou, G.; Bai, J.; Gao, P.; Wang, Y.; Yu, X.; Li, J.; Hu, Y.-S. TiS2 as a high performance potassium ion battery cathode in ether-based electrolyte. Energy Storage Mater. 2018, 12, 216–222. [Google Scholar] [CrossRef]
- Hu, Z.; Tai, Z.; Liu, Q.; Wang, S.W.; Jin, H.; Wang, S.; Lai, W.; Chen, M.; Li, L.; Chen, L. Ultrathin 2D TiS2 nanosheets for high capacity and long-life sodium ion batteries. Adv. Energy Mater. 2019, 9, 1803210. [Google Scholar] [CrossRef]
- Tchitchekova, D.S.; Ponrouch, A.; Verrelli, R.; Broux, T.; Frontera, C.; Sorrentino, A.; Bardé, F.; Biskup, N.; Arroyo-de Dompablo, M.E.; Palacin, M.R. Electrochemical intercalation of calcium and magnesium in TiS2: Fundamental studies related to multivalent battery applications. Chem. Mater. 2018, 30, 847–856. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, Y.-T.; Nogales, P.M.; Song, H.-Y.; Kim, Y.; Yin, R.-Z.; Jeong, S.-K. Electrochemical intercalation of Ca2+ ions into TiS2 in organic electrolytes at room temperature. Electrochem. Commun. 2019, 98, 115–118. [Google Scholar] [CrossRef]
- Huang, C.; Liu, Y.; Li, J.; Miao, Z.; Cai, X.; Wu, Z.; Yu, H.; Yan, L.; Zhang, L.; Shu, J. Organic interlayer engineering of TiS2 for enhanced aqueous Zn ions storage. J. Mater. Sci. Technol. 2023, 140, 135–141. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef]
- Yamada, Y.; Usui, K.; Sodeyama, K.; Ko, S.; Tateyama, Y.; Yamada, A. Hydrate-melt electrolytes for high-energy-density aqueous batteries. Nat. Energy 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Kühnel, R.-S.; Reber, D.; Battaglia, C. A high-voltage aqueous electrolyte for sodium-ion batteries. ACS Energy Lett. 2017, 2, 2005–2006. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, S.J.; Chang, D.; Kim, J.; Moon, S.; Oh, K.; Park, K.-Y.; Seong, W.M.; Park, H.; Kwon, G. Toward a low-cost high-voltage sodium aqueous rechargeable battery. Mater. Today 2019, 29, 26–36. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, D.; Zhang, B.; Wang, S.; Li, P.; Liu, H.; Guo, X.; Jaumaux, P.; Gao, X.; Fu, Y. A universal strategy towards high–energy aqueous multivalent–ion batteries. Nat. Commun. 2021, 12, 2857. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, D.; Hu, E.; Xu, J.; Deng, T.; Ma, L.; Wang, Y.; Yang, X.-Q.; Wang, C. Solvation structure design for aqueous Zn metal batteries. J. Am. Chem. Soc. 2020, 142, 21404–21409. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Pan, L.; Peng, Z.; Sun, Z.; Lin, H.; Mao, C.; Wang, L.; Dai, L.; Liu, H.; Pan, K. Electrolyte additive engineering for aqueous Zn ion batteries. Energy Storage Mater. 2022, 51, 733–755. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, S.-K. Modulating the hydration number of calcium ions by varying the electrolyte concentration: Electrochemical performance in a prussian blue electrode/aqueous electrolyte system for calcium-ion batteries. Electrochim. Acta 2018, 265, 430–436. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Ji, X.; Fu, J.; Feng, G. Progress on predicting the electrochemical stability window of electrolytes. Curr. Opin. Electrochem. 2022, 34, 101030. [Google Scholar] [CrossRef]
- Chen, M.; Feng, G.; Qiao, R. Water-in-salt electrolytes: An interfacial perspective. Curr. Opin. Colloid Interface Sci. 2020, 47, 99–110. [Google Scholar] [CrossRef]
- Bi, S.; Wang, R.; Liu, S.; Yan, J.; Mao, B.; Kornyshev, A.A.; Feng, G. Minimizing the electrosorption of water from humid ionic liquids on electrodes. Nat. Commun. 2018, 9, 5222. [Google Scholar] [CrossRef]
- Chen, M.; Wu, J.; Ye, T.; Ye, J.; Zhao, C.; Bi, S.; Yan, J.; Mao, B.; Feng, G. Adding salt to expand voltage window of humid ionic liquids. Nat. Commun. 2020, 11, 5809. [Google Scholar] [CrossRef]
- Vatamanu, J.; Borodin, O. Ramifications of water-in-salt interfacial structure at charged electrodes for electrolyte electrochemical stability. J. Phys. Chem. Lett. 2017, 8, 4362–4367. [Google Scholar] [CrossRef]
- Lv, T.; Suo, L. Water-in-salt widens the electrochemical stability window: Thermodynamic and kinetic factors. Curr. Opin. Electrochem. 2021, 29, 100818. [Google Scholar] [CrossRef]
- Adil, M.; Ghosh, A.; Mitra, S. Water-in-salt electrolyte-based extended voltage range, safe, and long-cycle-life aqueous calcium-ion cells. ACS Appl. Mater. Interfaces 2022, 14, 25501–25515. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Oh, D.; Lin, Y.; Zhuo, Z.; Borodin, O.; Gao, T.; Wang, F.; Kushima, A.; Wang, Z.; Kim, H.-C. How solid-electrolyte interphase forms in aqueous electrolytes. J. Am. Chem. Soc. 2017, 139, 18670–18680. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, J.; Luo, B.; Knibbe, R.; Lin, T.; Hu, H.; Rana, M.; Hu, Y.; Zhu, X.; Gu, Q. Sandwich-like ultrathin TiS2 nanosheets confined within N, S codoped porous carbon as an effective polysulfide promoter in lithium-sulfur batteries. Adv. Energy Mater. 2019, 9, 1901872. [Google Scholar] [CrossRef]
- Starnberg, H. Recent developments in alkali metal intercalation of layered transition metal dichalcogenides. Mod. Phys. Lett. B 2000, 14, 455–471. [Google Scholar] [CrossRef]
- Bezerra, C.d.S.; Valerio, M.E.G. Structural and optical study of CaF2 nanoparticles produced by a microwave-assisted hydrothermal method. Phys. B Condens. Matter 2016, 501, 106–112. [Google Scholar] [CrossRef]
- Demri, B.; Muster, D. XPS study of some calcium compounds. J. Mater. Process. Technol. 1995, 55, 311–314. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Lawson, S.; Rownaghi, A.A.; Rezaei, F. Improving adsorptive performance of CaO for high-temperature CO2 capture through Fe and Ga doping. Energy Fuels 2019, 33, 1404–1413. [Google Scholar] [CrossRef]
- Rudolph, W.W.; Irmer, G. Hydration of the calcium (II) ion in an aqueous solution of common anions (ClO4−, Cl−, Br−, and NO3−). Dalton Trans. 2013, 42, 3919–3935. [Google Scholar] [CrossRef]
- Li, M.; Duan, Z.; Zhang, Z.; Zhang, C.; Weare, J. The structure, dynamics and solvation mechanisms of ions in water from long time molecular dynamics simulations: A case study of CaCl2 (aq) aqueous solutions. Mol. Phys. 2008, 106, 2685–2697. [Google Scholar] [CrossRef]
- Han, C.; Li, H.; Li, Y.; Zhu, J.; Zhi, C. Proton-assisted calcium-ion storage in aromatic organic molecular crystal with coplanar stacked structure. Nat. Commun. 2021, 12, 2400. [Google Scholar] [CrossRef]
- Qin, Z.; Song, Y.; Liu, Y.; Liu, X.X. Aqueous calcium-ion storage in amorphous molybdenum oxide. Chem. Eng. J. 2023, 451, 138681. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Deng, X.; Hao, J.; Zhao, X.; Li, H.; Li, B. A covalent organic framework for high-rate aqueous calcium-ion batteries. J. Mater. Chem. A 2022, 10, 20827–20836. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, S.; Lee, H.; Lee, S.; Nogales, P.M.; Lee, C.; Kim, Y.; Jeong, S.-K. Application of TiS2 as an Active Material for Aqueous Calcium-Ion Batteries: Electrochemical Calcium Intercalation into TiS2 from Aqueous Solutions. Batteries 2023, 9, 500. https://doi.org/10.3390/batteries9100500
Seong S, Lee H, Lee S, Nogales PM, Lee C, Kim Y, Jeong S-K. Application of TiS2 as an Active Material for Aqueous Calcium-Ion Batteries: Electrochemical Calcium Intercalation into TiS2 from Aqueous Solutions. Batteries. 2023; 9(10):500. https://doi.org/10.3390/batteries9100500
Chicago/Turabian StyleSeong, Sujin, Hajin Lee, Sangyup Lee, Paul Maldonado Nogales, Changhee Lee, Yangsoo Kim, and Soon-Ki Jeong. 2023. "Application of TiS2 as an Active Material for Aqueous Calcium-Ion Batteries: Electrochemical Calcium Intercalation into TiS2 from Aqueous Solutions" Batteries 9, no. 10: 500. https://doi.org/10.3390/batteries9100500
APA StyleSeong, S., Lee, H., Lee, S., Nogales, P. M., Lee, C., Kim, Y., & Jeong, S.-K. (2023). Application of TiS2 as an Active Material for Aqueous Calcium-Ion Batteries: Electrochemical Calcium Intercalation into TiS2 from Aqueous Solutions. Batteries, 9(10), 500. https://doi.org/10.3390/batteries9100500








