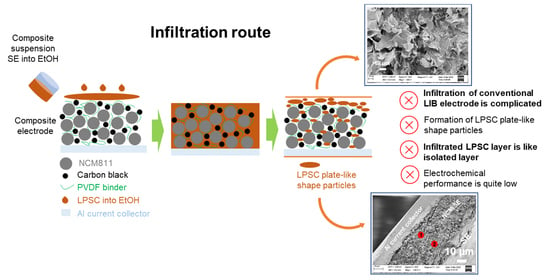
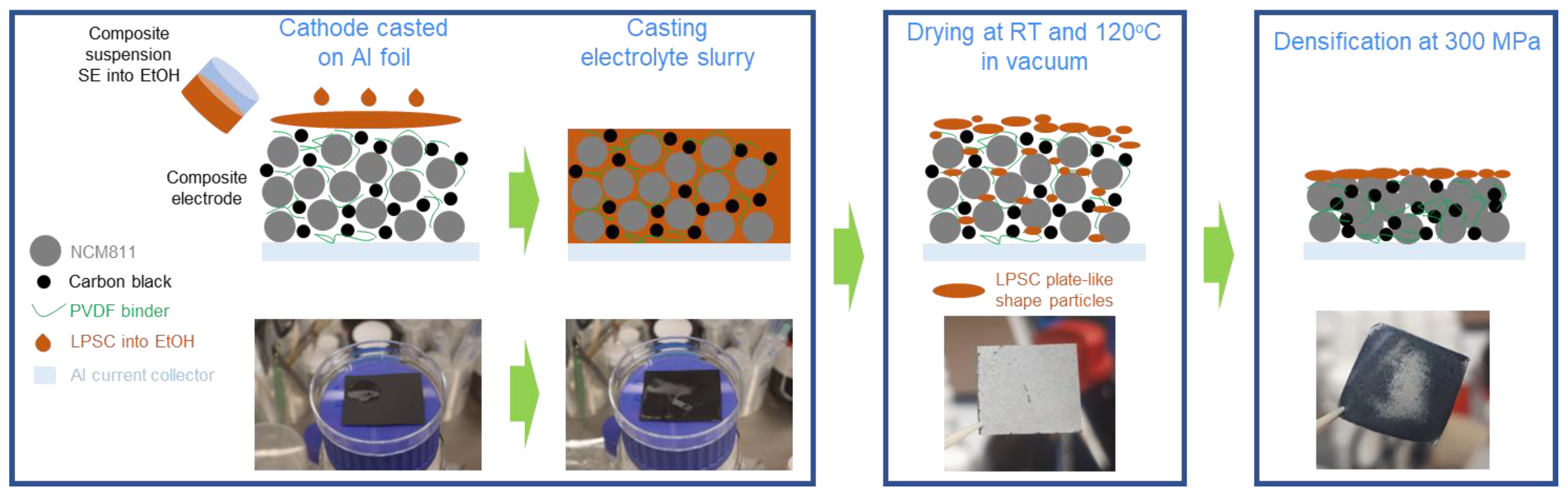
New Insights of Infiltration Process of Argyrodite Li6PS5Cl Solid Electrolyte into Conventional Lithium-Ion Electrodes for Solid-State Batteries
Abstract
:1. Introduction
2. Materials and Methods
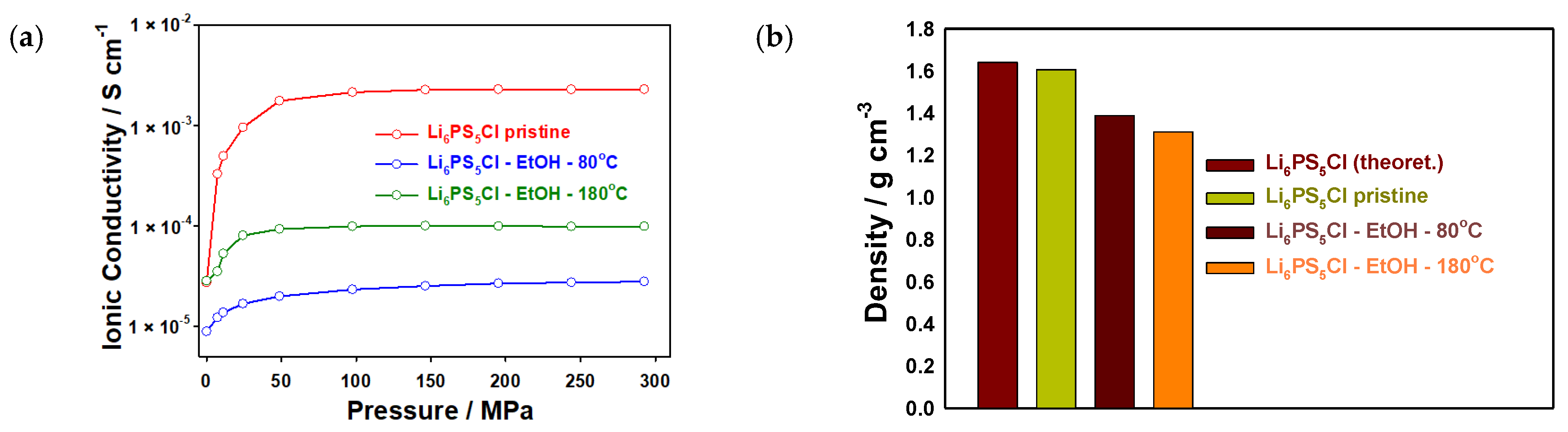
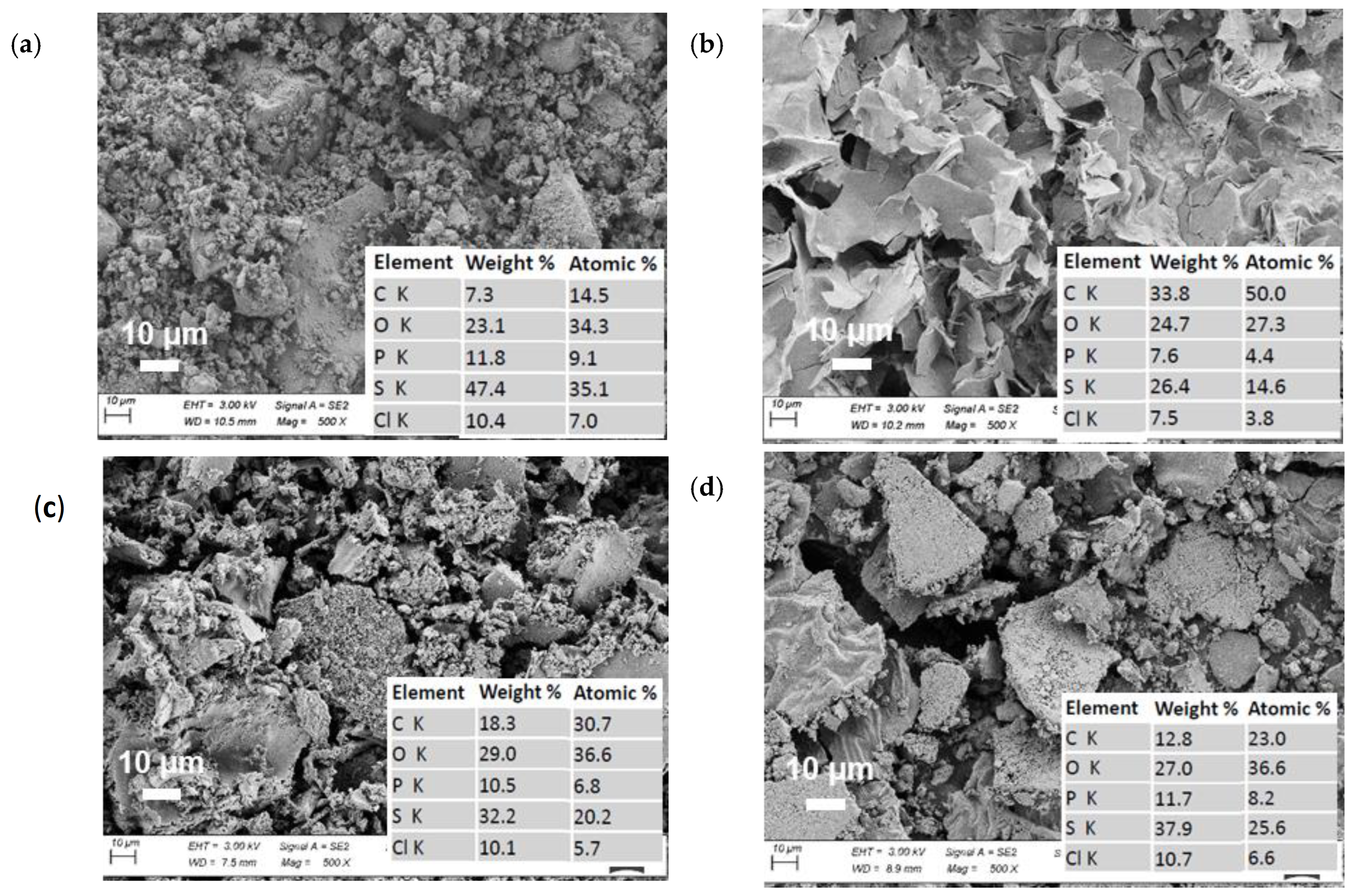
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horowitz, Y.; Schmidt, C.; Yoon, D.-H.; Riegger, L.M.; Katzenmeier, L.; Bosch, G.M.; Noked, M.; Ein-Eli, Y.; Janek, J.; Zeier, W.G.; et al. Between liquid and all solid: A prospect on electrolyte future in lithium-ion batteries for electric vehicles. Energy Technol. 2020, 8, 2000580. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Xia, G.B.; Cao, L.; Bi, G.L. A review on battery thermal management in electric vehicle application. J. Power Sources 2017, 367, 90–105. [Google Scholar] [CrossRef]
- Banerjee, A.; Wang, X.; Fang, C.; Wu, E.A.; Meng, Y.S. Interfaces and interphases in all-solid-state batteries with inorganic solid electrolytes. Chem. Rev. 2020, 120, 6878–6933. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.Z.; Yuan, H.; Cheng, X.B.; Huang, J.Q.; Zhang, Q. Critical current density in solid-state lithium metal batteries: Mechanism, influences, and strategies. Adv. Funct. Mater. 2021, 31, 2009925. [Google Scholar] [CrossRef]
- Tron, A.; Nosenko, A.; Park, Y.D.; Mun, J. Synthesis of the solid electrolyte Li2O–LiF–P2O5 and its application for lithium-ion batteries. Solid State Ion. 2017, 308, 40–45. [Google Scholar] [CrossRef]
- Tron, A.; Nosenko, A.; Park, Y.D.; Mun, J. The solid electrolytes Li2O–LiF–Li2WO4–B2O3 with enhanced ionic conductivity for lithium-ion battery. J. Ind. Eng. Chem. 2019, 73, 62–66. [Google Scholar] [CrossRef]
- López-Aranguren, P.; Reynaud, M.; Głuchowski, P.; Bustinza, A.; Galceran, M.; López del Amo, J.M.; Armand, M.; Casas-Cabanas, M. Crystalline LiPON as a bulk-type solid electrolyte. ACS Energy Lett. 2021, 6, 445–450. [Google Scholar] [CrossRef]
- Deng, Y.; Eames, C.; Fleutot, B.; David, R.; Chotard, J.-N.; Suard, E.; Masquelier, C.; Saiful Islam, M. Enhancing the lithium ion conductivity in lithium superionic Conductor (LISICON) solid electrolytes through a mixed polyanion effect. ACS Appl. Mater. Interfaces 2017, 8, 7050–7058. [Google Scholar] [CrossRef]
- Tao, B.; Ren, C.; Li, H.; Liu, B.; Jia, X.; Dong, X.; Zhang, S.; Chang, H. Thio-/LISICON and LGPS-type solid electrolytes for all-solid-state lithium-ion batteries. Adv. Funct. Mater. 2022, 32, 2203551. [Google Scholar] [CrossRef]
- Oh, J.A.S.; He, L.; Plewa, A.; Morita, M.; Zhao, Y.; Sakamoto, T.; Song, X.; Zhai, W.; Zeng, K.; Lu, L. Composite NASICON (Na3Zr2Si2PO12) solid-state electrolyte with enhanced Na+ ionic conductivity: Effect of liquid phase sintering. ACS Appl. Mater. Interfaces 2019, 43, 40125–40133. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-type solid-state electrolytes: Materials, interfaces, and batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Y. Perovskite-type Li-ion solid electrolytes: A review. J. Mater. Sci. Mater. Electron. 2021, 32, 9736–9754. [Google Scholar] [CrossRef]
- Kwak, H.; Wang, S.; Park, J.; Liu, Y.; Kim, K.T.; Choi, Y.; Mo, Y.; Jung, Y.S. Emerging halide superionic conductors for all-solid-state batteries: Design, synthesis, and practical applications. ACS Energy Lett. 2022, 7, 1776–1805. [Google Scholar] [CrossRef]
- Kim, K.J.; Balaish, M.; Wadaguchi, M.; Kong, L.; Rupp, J.L.M. Solid-state Li–metal batteries: Challenges and horizons of oxide and sulfide solid electrolytes and their interfaces. Adv. Energy Mater. 2021, 11, 2002689. [Google Scholar] [CrossRef]
- Adeli, P.; Bazak, J.D.; Park, K.H.; Kochetkov, I.; Huq, A.; Goward, G.R.; Nazar, L.F. Boosting solid-state diffusivity and conductivity in lithium superionic argyrodites by halide substitution. Angew. Chem. Int. Ed. 2019, 58, 8681–8686. [Google Scholar] [CrossRef]
- Xu, R.C.; Wang, X.L.; Zhang, S.Z.; Xia, Y.; Xia, X.H.; Wu, J.B.; Tu, J.P. Rational coating of Li7P3S11 solid electrolyte on MoS2 electrode for all-solid-state lithium ion batteries. J. Power Source 2018, 374, 107–112. [Google Scholar] [CrossRef]
- Tao, B.; Zhong, D.; Li, H.; Wanga, G.; Chang, H. Halide solid-state electrolytes for all-solid-state batteries: Structural design, synthesis, environmental stability, interface optimization and challenges. Chem. Sci. 2023, 14, 8693–8722. [Google Scholar] [CrossRef]
- Lee, K.; Kim, S.; Park, J.; Park, S.H.; Coskun, A.; Jung, D.S.; Cho, W.; Choi, J.W. Selection of binder and solvent for solution-processed all-solid-state battery. J. Electrochem. Soc. 2017, 164, A2075. [Google Scholar] [CrossRef]
- Koç, T.; Marchini, F.; Rousse, G.; Dugas, R.; Tarascon, J.-M. In the search for the best solid electrolyte-layered oxide pairing for assembling practical all-solid-state batteries. ACS Appl. Energy Mater. 2021, 12, 13575–13585. [Google Scholar] [CrossRef]
- Nam, Y.J.; Oh, D.Y.; Jung, S.H.; Jung, Y.S. Toward practical all-solid-state lithium-ion batteries with high energy density and safety: Comparative study for electrodes fabricated by dry- and slurry-mixing processes. J. Power Sources 2018, 375, 93–101. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.-Z.; Yuan, H.; Hu, J.-K.; Huang, J.-Q.; Zhang, Q. Dry electrode technology, the rising star in solid-state battery industrialization. Matter 2022, 5, 876–898. [Google Scholar] [CrossRef]
- Kim, K.T.; Kwon, T.Y.; Jung, Y.S. Scalable fabrication of sheet-type electrodes for practical all-solid-state batteries employing sulfide solid electrolytes. Curr. Opin. Electrochem. 2022, 34, 101026. [Google Scholar] [CrossRef]
- Cai, Y.; Li, C.; Zhao, Z.; Mu, D.; Wu, B. Air stability and interfacial compatibility of sulfide solid electrolytes for solid-state lithium batteries: Advances and perspectives. ChemElectroChem 2022, 9, e202101479. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Marple, M.A.T.; Tan, D.H.S.; Ham, S.-Y.; Sayahpour, B.; Li, W.-K.; Yang, H.; Lee, J.B.; Hah, H.J.; Wu, E.A.; et al. Investigating dry room compatibility of sulfide solid-state electrolytes for scalable manufacturing. J. Mater. Chem. A 2022, 10, 7155–7164. [Google Scholar] [CrossRef]
- Tron, A.; Hamid, R.; Zhang, N.; Beutl, A. Rational optimization of cathode composites for sulfide-based all-solid-state batteries. Nanomaterials 2023, 13, 327. [Google Scholar] [CrossRef]
- Han, Y.; Jung, S.H.; Kwak, H.; Jun, S.; Kwak, H.H.; Lee, J.H.; Hong, S.-T.; Jung, Y.S. Single- or poly-crystalline Ni-rich layered cathode, sulfide or halide solid electrolyte: Which will be the winners for all-solid-state batteries? Adv. Energy Mater. 2021, 11, 2100126. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Banerjee, A.; Deng, Z.; Wu, E.A.; Nguyen, H.; Doux, J.-M.; Wang, X.; Cheng, J.-H.; Ong, S.P.; Meng, Y.S.; et al. Enabling thin and flexible solid-state composite electrolytes by the scalable solution process. ACS Appl. Energy Mater. 2019, 2, 6542–6550. [Google Scholar] [CrossRef]
- Oh, D.Y.; Nam, Y.J.; Park, K.H.; Jung, S.H.; Kim, K.T.; Ha, A.R.; Jung, Y.S. Slurry-fabricable Li+-conductive polymeric binders for practical all-solid-state lithium-ion batteries enabled by solvate ionic liquids. Adv. Energy Mater. 2019, 9, 1802927. [Google Scholar] [CrossRef]
- Tron, A.; Hamid, R.; Zhang, N.; Paolella, A.; Wulfert-Holzmann, P.; Kolotygin, V.; López-Aranguren, P.; Beutl, A. Film processing of Li6PS5Cl electrolyte using different binders and their combinations. J. Energy Storage 2023, 66, 107480. [Google Scholar] [CrossRef]
- Nikodimos, Y.; Huang, C.-J.; Taklu, B.W.; Su, W.-N.; Hwang, B.J. Chemical stability of sulfide solid-state electrolytes: Stability toward humid air and compatibility with solvents and binders. Energy Environ. Sci. 2022, 15, 991–1033. [Google Scholar] [CrossRef]
- Inada, T.; Takada, K.; Kajiyama, A.; Kouguchi, M.; Sasaki, H.; Kondo, S.; Watanabe, M.; Murayama, M.; Kanno, R. Fabrications and properties of composite solid-state electrolytes. Solid State Ion. 2003, 158, 275–280. [Google Scholar] [CrossRef]
- Kim, D.H.; Oh, D.Y.; Park, K.H.; Choi, Y.E.; Nam, Y.J.; Lee, H.A.; Lee, S.-M.; Jung, Y.S. Infiltration of solution-processable solid electrolytes into conventional Li-ion-battery electrodes for all-solid-state Li-ion batteries. Nano Lett. 2017, 17, 3013–3020. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, H.A.; Song, Y.B.; Park, J.W.; Lee, S.-M.; Jung, Y.S. Sheet-type Li6PS5Cl-infiltrated Si anodes fabricated by solution process for all-solid-state lithium-ion batteries. J. Power Sources 2019, 426, 143–150. [Google Scholar] [CrossRef]
- Kim, M.-J.; Park, J.-W.; Kim, B.G.; Lee, Y.-J.; Ha, Y.-C.; Lee, S.-M.; Baeg, K.-J. Facile fabrication of solution-processed solid-electrolytes for high-energy-density all-solid-state-batteries by enhanced interfacial contact. Sci. Rep. 2020, 10, 11923. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Song, D.; Zhang, H.; Shi, X.; Wang, Z.; Zhang, L.; Zhu, L. LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 cathode with high discharge capacity and rate performance for all-solid-state lithium battery. J. Energy Chem. 2020, 40, 39–45. [Google Scholar] [CrossRef]
- Ohta, N.; Takada, K.; Sakaguchi, I.; Zhang, L.; Ma, R.; Fukuda, K.; Osada, M.; Sasaki, T. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem. Commun. 2007, 9, 1486–1490. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Hu, J.; Liu, W.; Sari, H.M.K.; Li, D.; Sun, Q.; Kou, L.; Tian, Z.; Shao, L.; et al. ZnO interface modified LiNi0.6Co0.2Mn0.2O2 toward boosting lithium storage. Energy Environ. Mater. 2020, 3, 522–528. [Google Scholar] [CrossRef]
- Tron, A.; Hong, M.; Park, Y.D.; Kim, J.; Mun, J. Ni-rich layered cathode materials by a mechanochemical method for high-energy Lithium-ion batteries. ChemistrySelect 2020, 5, 14596–14601. [Google Scholar] [CrossRef]
- Tron, A.; Park, Y.D.; Mun, J. AlF3-coated LiMn2O4 as cathode material for aqueous rechargeable lithium battery with improved cycling stability. J. Power Sources 2016, 325, 360–364. [Google Scholar] [CrossRef]
- Jaiser, S.; Müller, M.; Baunach, M.; Bauer, W.; Scharfer, P.; Schabel, W. Investigation of film solidification and binder migration during drying of Li-Ion battery anodes. J. Power Sources 2016, 318, 210–219. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Wu, E.A.; Nguyen, H.; Chen, Z.; Marple, M.A.T.; Doux, J.M.; Wang, X.; Yang, H.; Banerjee, A.; Meng, Y.S. Elucidating reversible electrochemical redox of Li6PS5Cl solid electrolyte. ACS Energy Lett. 2019, 4, 2418–2427. [Google Scholar] [CrossRef]
- Ruhl, J.; Riegger, L.M.; Ghidiu, M.; Zeier, W.G. Impact of solvent treatment of the superionic argyrodite Li6PS5Cl on solid-state battery performance. Adv. Energy Sustain. Res. 2021, 2, 2000077. [Google Scholar] [CrossRef]
- Koerver, R.; Aygün, I.; Leichtweiß, T.; Dietrich, C.; Zhang, W.; Binder, J.O.; Hartmann, P.; Zeier, W.G.; Janek, J. Capacity fade in solid-state batteries: Interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and lithium thiophosphate solid electrolytes. Chem. Mater. 2017, 29, 5574–5582. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Liu, Y.; Yan, X.; Xu, B.; Wang, L.-M. One-step solution process toward formation of Li6PS5Cl argyrodite solid electrolyte for all-solid-state lithium-ion batteries. J. Alloys Comp. 2020, 812, 152103. [Google Scholar] [CrossRef]
- Giraldo, S.; Nakagawa, K.; Vásquez, F.A.; Fujii, Y.; Wang, Y.; Miura, A.; Calderón, J.A.; Rosero-Navarro, N.C.; Tadanaga, K. Preparation of composite electrodes for all-solid-state batteries based on sulfide electrolytes: An electrochemical point of view. Batteries 2021, 7, 77. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Miura, A.; Tadanaga, K. Preparation of lithium ion conductive Li6PS5Cl solid electrolyte from solution for the fabrication of composite cathode of all-solid-state lithium battery. J. Sol-Gel Sci. Technol. 2019, 89, 303–309. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Miura, A.; Tadanaga, K. Composite cathode prepared by argyrodite precursor solution assisted by dispersant agents for bulk-type all-solid-state batteries. J. Power Sources 2018, 396, 33–40. [Google Scholar] [CrossRef]
- Yubuchi, S.; Teragawa, S.; Aso, K.; Tadanaga, K.; Hayashi, A.; Tatsumisago, M. Preparation of high lithium-ion conducting Li6PS5Cl solid electrolyte from ethanol solution for all-solid-state lithium batteries. J. Power Sources 2015, 293, 941–945. [Google Scholar] [CrossRef]
- Zhou, L.; Park, K.-H.; Sun, X.; Lalère, F.; Adermann, T.; Hartmann, P.; Nazar, L. Solvent-engineered design of argyrodite Li6PS5X (X = Cl, Br, I) solid electrolytes with high ionic conductivity. ACS Energy Lett. 2019, 4, 265–270. [Google Scholar] [CrossRef]
- Carbone, L.; Verrelli, R.; Gobet, M.; Peng, J.; Devany, M.; Scrosati, B.; Greenbaum, S.; Hassoun, J. Insight on the Li2S electrochemical process in a composite configuration electrode. New J. Chem. 2016, 40, 2935–2943. [Google Scholar] [CrossRef] [PubMed]
- Tron, A.; Jo, Y.N.; Oh, S.H.; Park, Y.D.; Mun, J. Surface modification of the LiFePO4 cathode for the aqueous rechargeable lithium ion battery. ACS Appl. Mater. Interfaces 2017, 9, 12391–12399. [Google Scholar] [CrossRef] [PubMed]
- Belharouak, I.; Johnson, C.; Amine, K. Synthesis and electrochemical analysis of vapor-deposited carbon-coated LiFePO4. Electrochem. Commun. 2005, 7, 983–988. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhang, X.; Liu, T.; Lin, Y.-H.; Shen, Y.; Li, L.; Nan, C.-W. High-conductivity argyrodite Li6PS5Cl solid electrolytes prepared via optimized sintering processes for all-solid-state lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 42279–42285. [Google Scholar] [CrossRef]
- Asano, T.; Sakai, A.; Ouchi, S.; Sakaida, M.; Miyazaki, A.; Hasegawa, S. Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state batteries. Adv. Mater. 2018, 30, 1803075. [Google Scholar] [CrossRef]
- Li, Y.; Song, S.; Kim, H.; Nomoto, K.; Kim, H.; Sun, X.; Hori, S.; Suzuki, K.; Matsui, N.; Hirayama, M.; et al. A lithium superionic conductor for millimeter-thick battery electrode. Science 2023, 381, 50–53. [Google Scholar] [CrossRef]
- Dielectric Constant of Common solvents.xls. Available online: washington.edu (accessed on 20 September 2023).
- Dielectric Constant Table.pdf. Available online: honeywellprocess.com (accessed on 20 September 2023).
- Physicochemical Properties of Solvents. Available online: SigmaAldrich.com (accessed on 20 September 2023).

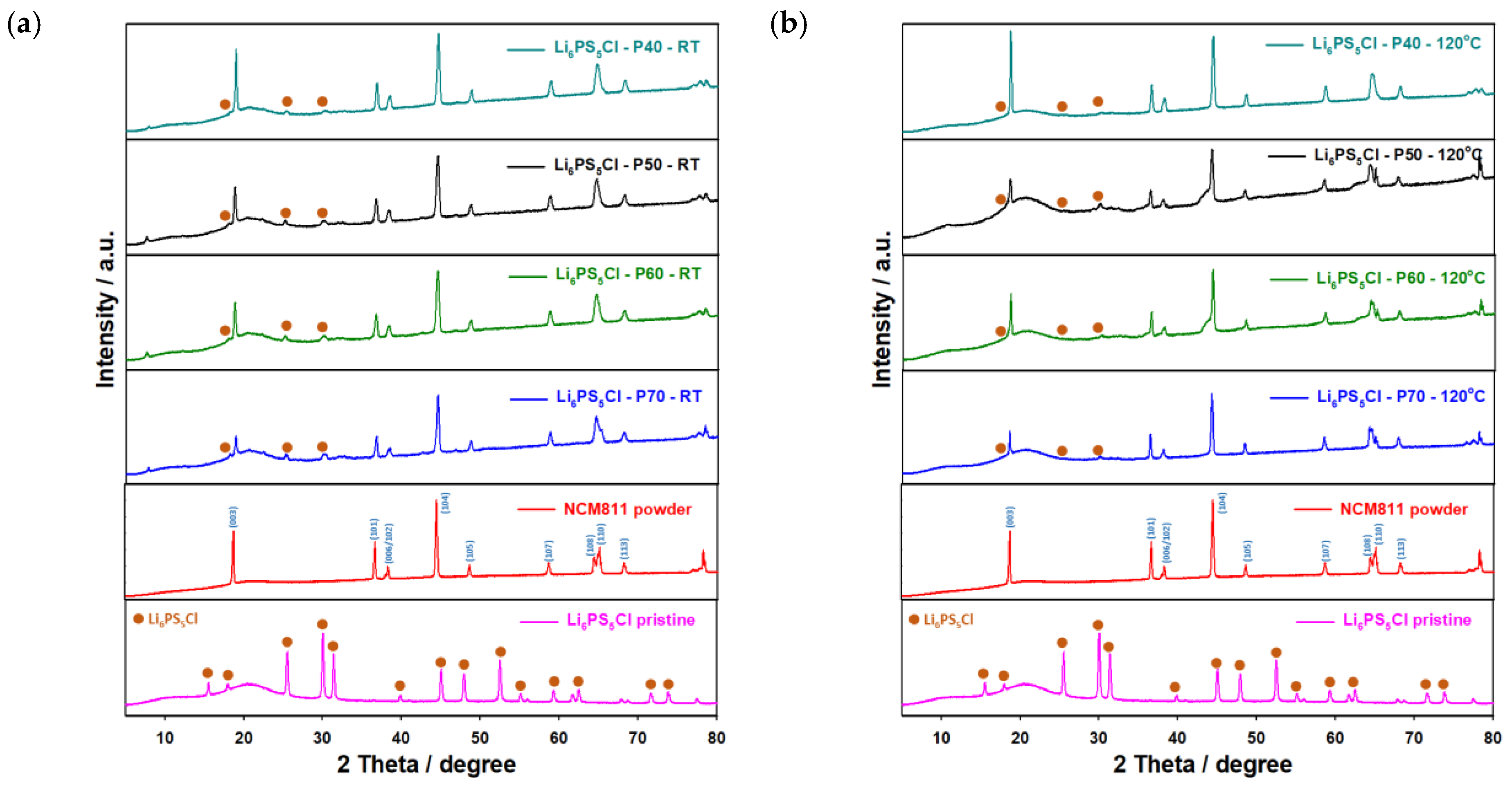



| Sulfide Electrolyte | Solvent | Post-Treatment (Heating Up to XX °C under Vacuum) | Ionic Conductivity after Dissolution, S cm−1 * | Density of Powder Pellets (Densified at 300 MPa), g cm−3 | Reference |
|---|---|---|---|---|---|
| Li6PS5Cl, 0.4LI-0.6Li4SnS4 | Ethanol, Methanol | 180 | 1.9 × 10−4 | - | [33] |
| Li6PS5Cl | Ethanol | 180 | 10−4 | - | [34] |
| Li6PS5Cl | Ethanol | 180 | 10−4 | - | [35] |
| Li6PS5Cl | Ethanol | 150 | 4 × 10−5 | - | [46] |
| Li6PS5Cl | Ethanol | 150 | 6 × 10−5 | - | [47] |
| Li6PS5Cl | Ethanol | 150 | 3 × 10−4 | - | [48] |
| Li6PS5Cl | Ethanol | 80 | 1.4 × 10−5 | - | [49] |
| Li6PS5Cl pristine | 2.14 × 10−3 | 1.61 | This work | ||
| Li6PS5Cl | Ethanol | 80 | 2.32 × 10−5 | 1.39 | This work |
| Li6PS5Cl | Ethanol | 180 | 9.87 × 10−5 | 1.31 | This work |
| Porosity of Pristine Cathodes | 70% | 60% | 50% | 40% |
| Loading level of electrode, mg cm−2 | 8–9 | 8–9 | 8–9 | 8–9 |
| Loading of SE, mg cm−2 | 3.34 | 4.27 | 3.59 | 2.08 |
| Thicknessofelectrode, µm | 57 | 51 | 38 | 28 |
| Wettabilitywithelectrolytesolution | Very good | Good | Medium | Poor |
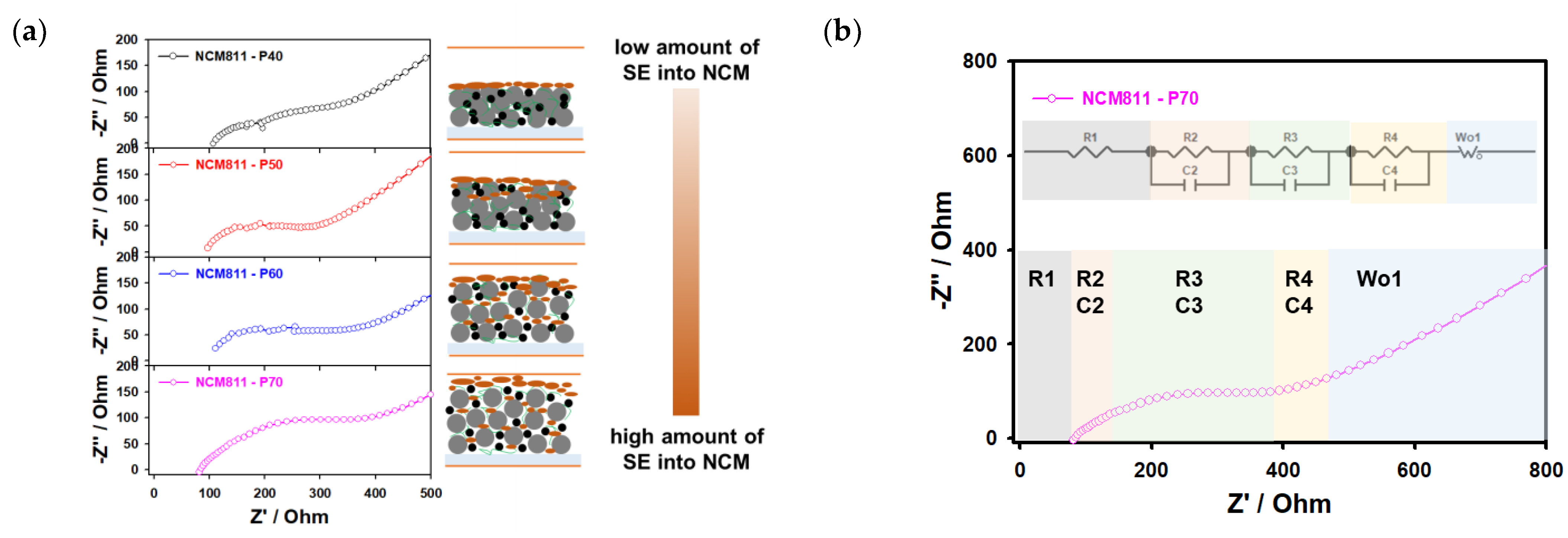
| Porosity of Electrode after Calendaring, % | EIS Resistance, Ohm | Specific Capacity, mAh g−1 | Adhesion between NCM811/SE and SE Film | |||
|---|---|---|---|---|---|---|
| R1 (SE Electrolyte) | Rc (Interface between CAM and SE) | (R4) Ra (Interface between AM and SE) | ||||
| R2 (Path Resistance) | R3 (Interfacial Resistance) | |||||
| P70 | 88.3 | 59.1 | 155.7 | 109.4 | 3.10 | poor |
| P60 | 112.4 | 61.8 | 78.9 | 93.9 | 2.64 | poor |
| P50 | 102.9 | 68.1 | 73.9 | 83.9 | 2.06 | medium |
| P40 | 117.6 | 73.4 | 62.7 | 65.1 | 0.06 | good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tron, A.; Paolella, A.; Beutl, A. New Insights of Infiltration Process of Argyrodite Li6PS5Cl Solid Electrolyte into Conventional Lithium-Ion Electrodes for Solid-State Batteries. Batteries 2023, 9, 503. https://doi.org/10.3390/batteries9100503
Tron A, Paolella A, Beutl A. New Insights of Infiltration Process of Argyrodite Li6PS5Cl Solid Electrolyte into Conventional Lithium-Ion Electrodes for Solid-State Batteries. Batteries. 2023; 9(10):503. https://doi.org/10.3390/batteries9100503
Chicago/Turabian StyleTron, Artur, Andrea Paolella, and Alexander Beutl. 2023. "New Insights of Infiltration Process of Argyrodite Li6PS5Cl Solid Electrolyte into Conventional Lithium-Ion Electrodes for Solid-State Batteries" Batteries 9, no. 10: 503. https://doi.org/10.3390/batteries9100503
APA StyleTron, A., Paolella, A., & Beutl, A. (2023). New Insights of Infiltration Process of Argyrodite Li6PS5Cl Solid Electrolyte into Conventional Lithium-Ion Electrodes for Solid-State Batteries. Batteries, 9(10), 503. https://doi.org/10.3390/batteries9100503