Molecular Engineering of Redox Couples for Non-Aqueous Redox Flow Batteries
Abstract
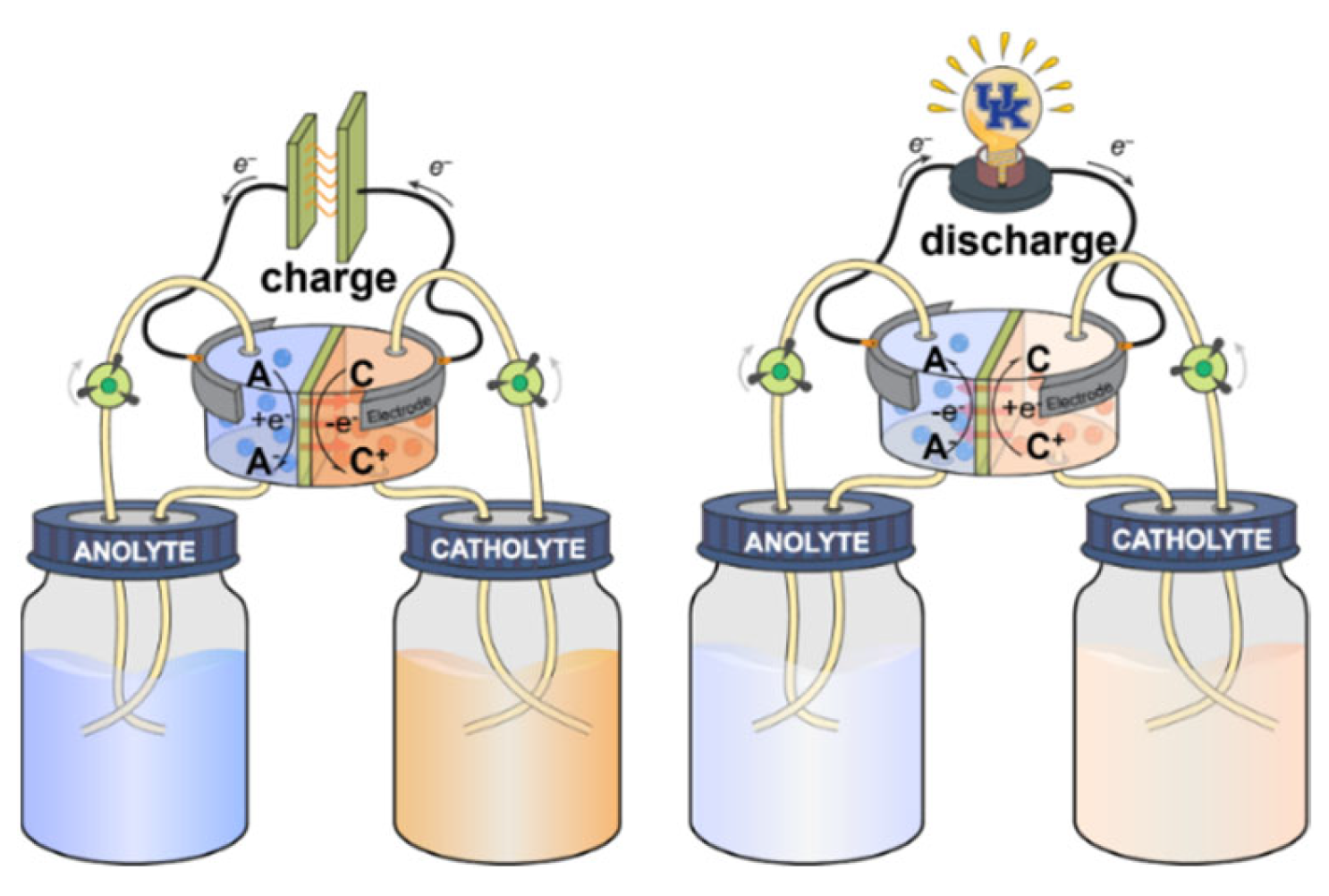
:1. Introduction
2. Cathodic Redox-Active Organic Molecules
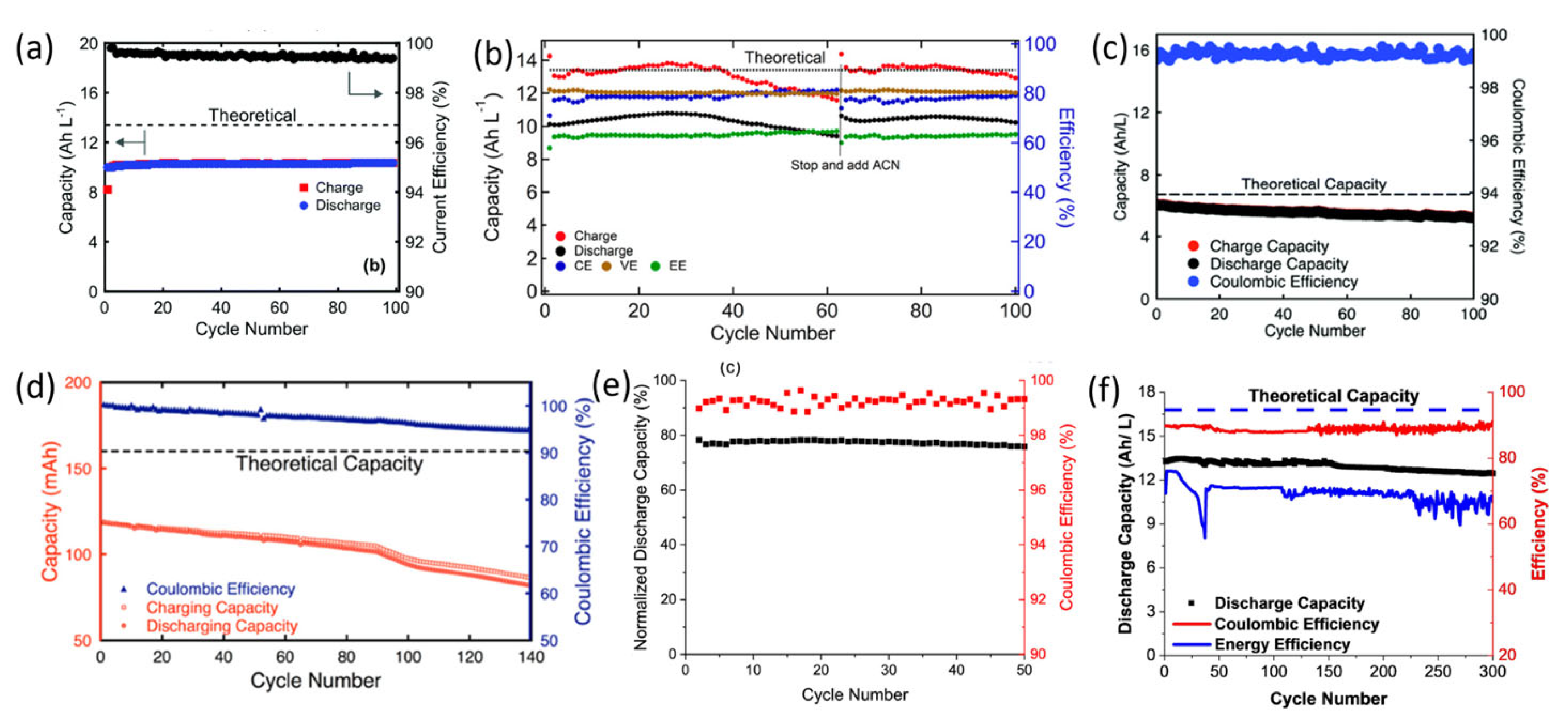
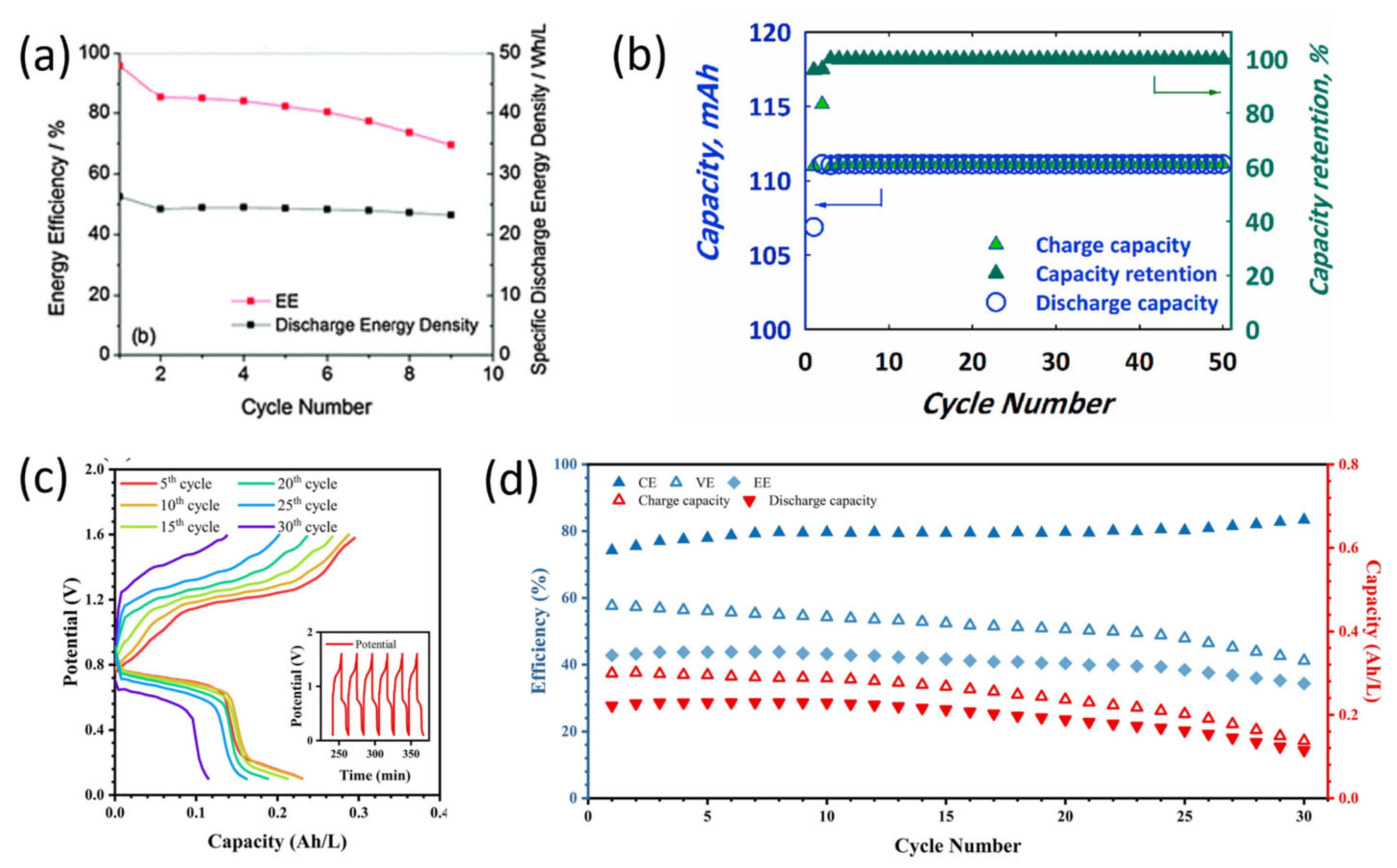
2.1. Quinones and Derivatives
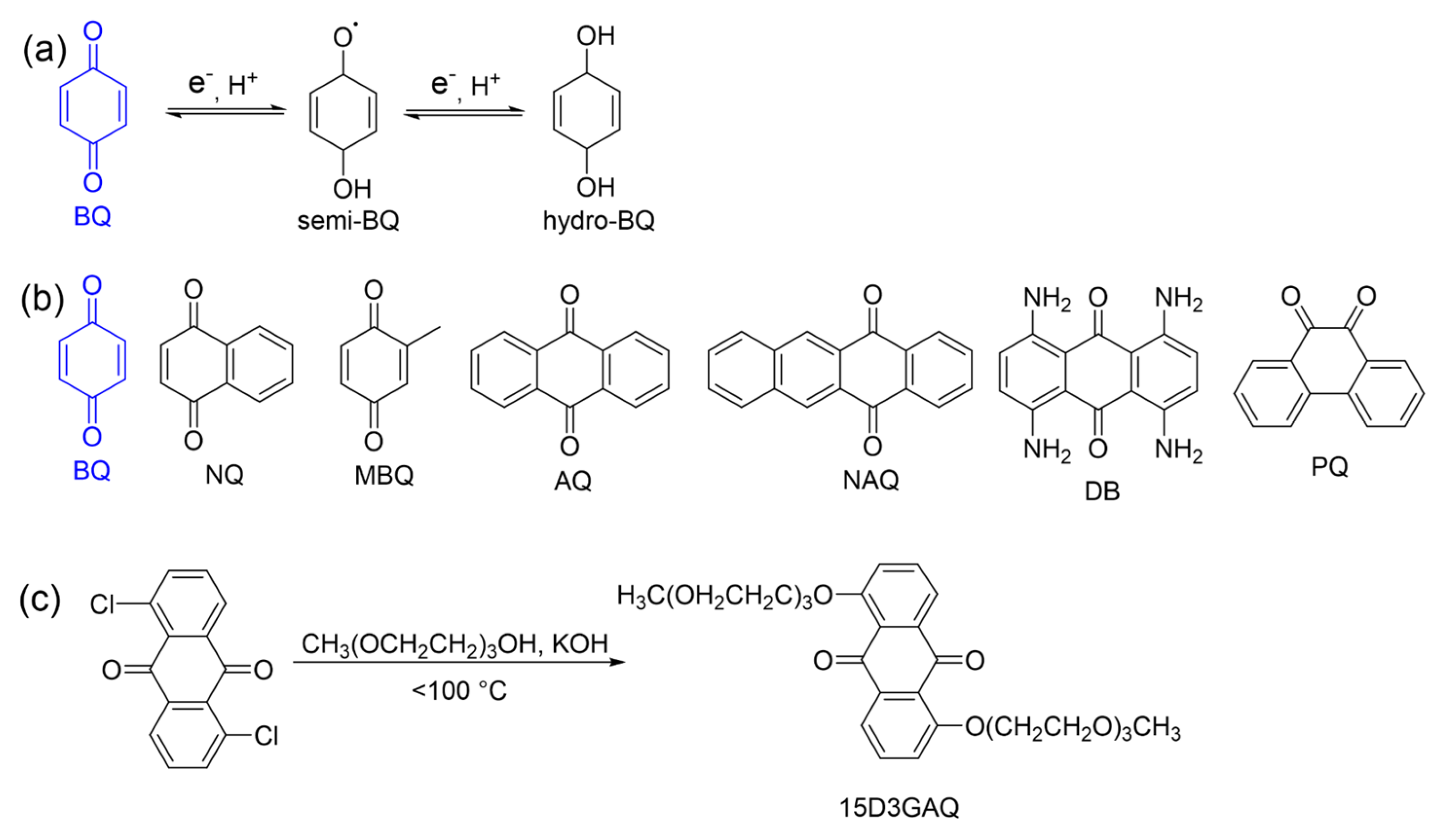
| Compound | Redox Potential (vs. Li+/Li) | Diffusion Coefficient (×10−6 cm2s−1) | Solubility (M) Neutral in DMA |
|---|---|---|---|
| AQ | 2.25 | 4.20 | <0.1 |
| NAQ | 2.2 | 4.29 | <0.1 |
| NQ | 2.6 | 4.63 | >0.1 |
| BQ | 2.8 | 5.00 | >0.1 |
| PQ | 2.7 | 4.96 | 0.3 |
| 15D3GAQ | 2.5 | - | >0.25 |
| DB-1 | 4 | - | 1 |
| MBQ | - | 0.034 | - |
2.2. Phenothiazines and Derivatives
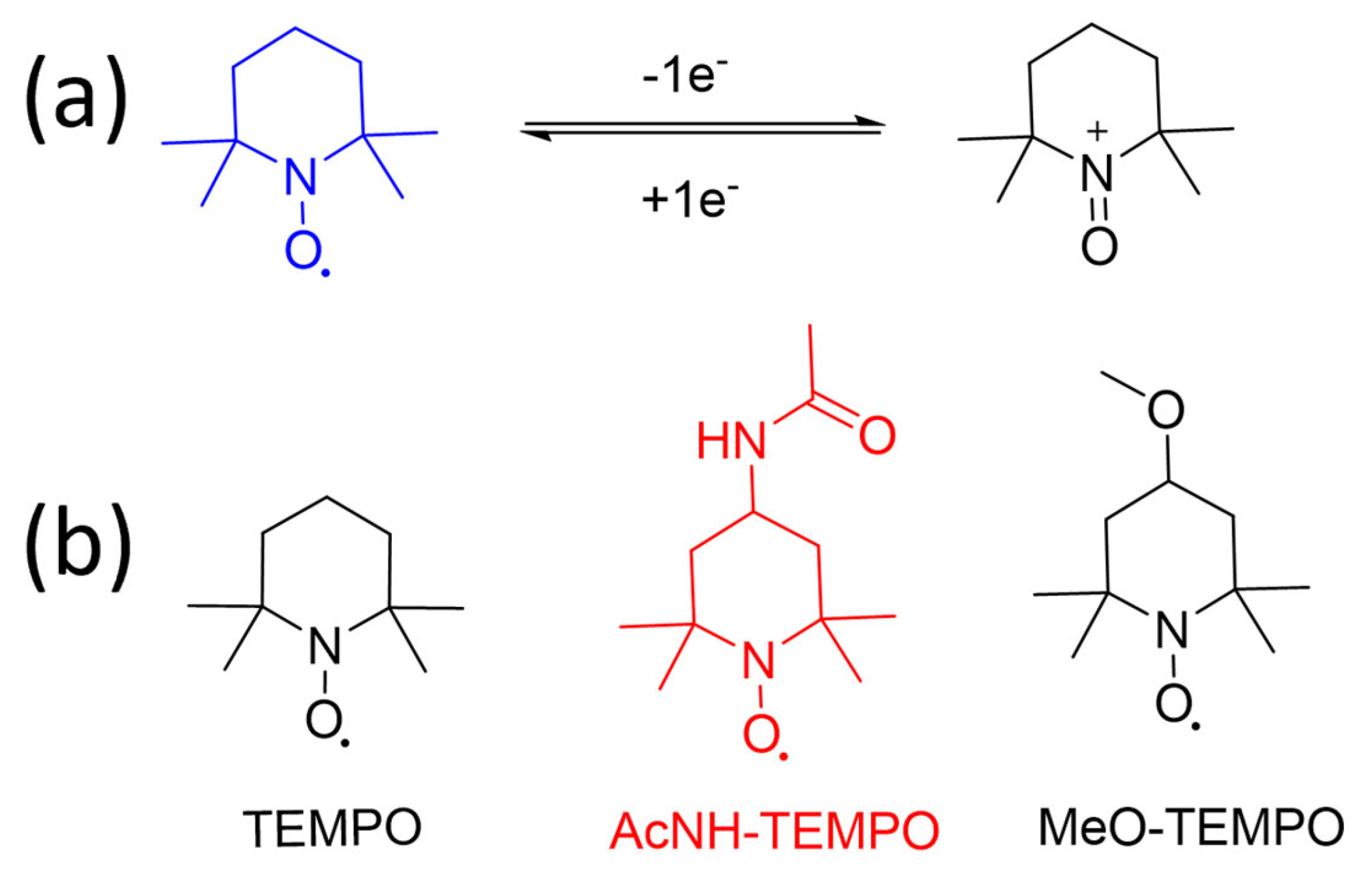
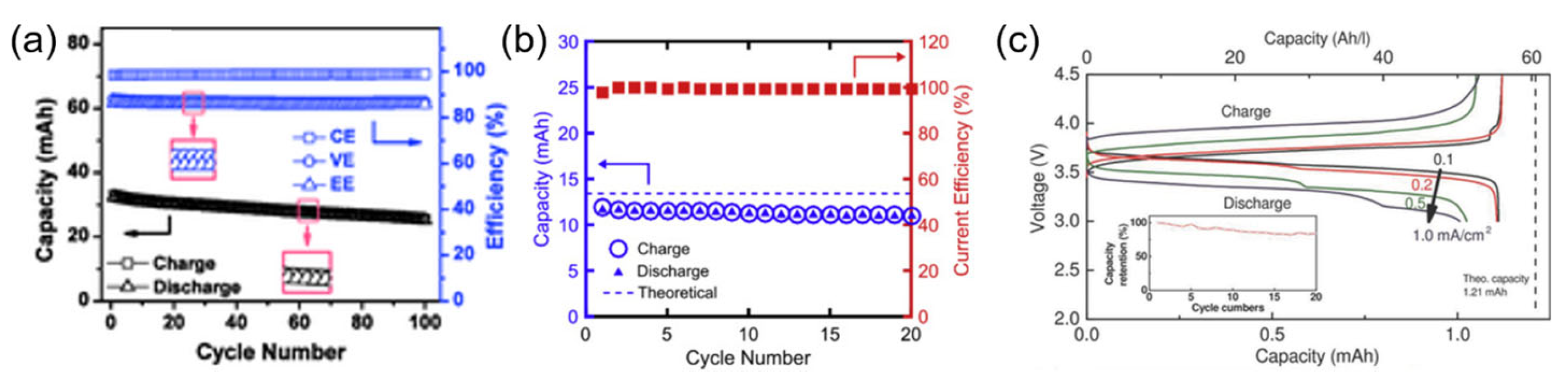
2.3. TEMPO and Derivatives
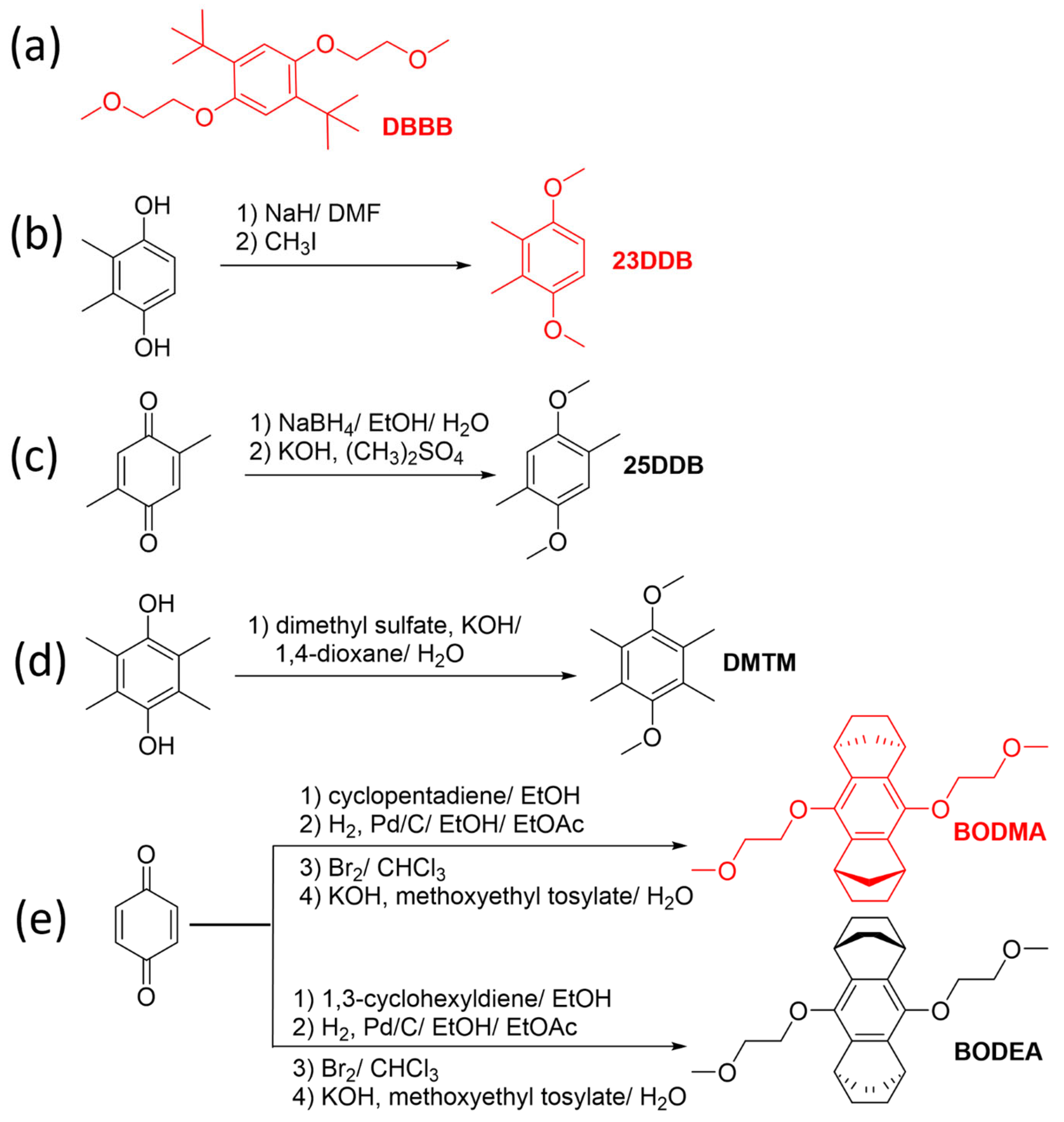
2.4. Dialkoxybenzenes and Derivatives
3. Anodic Redox-Active Organic Molecules
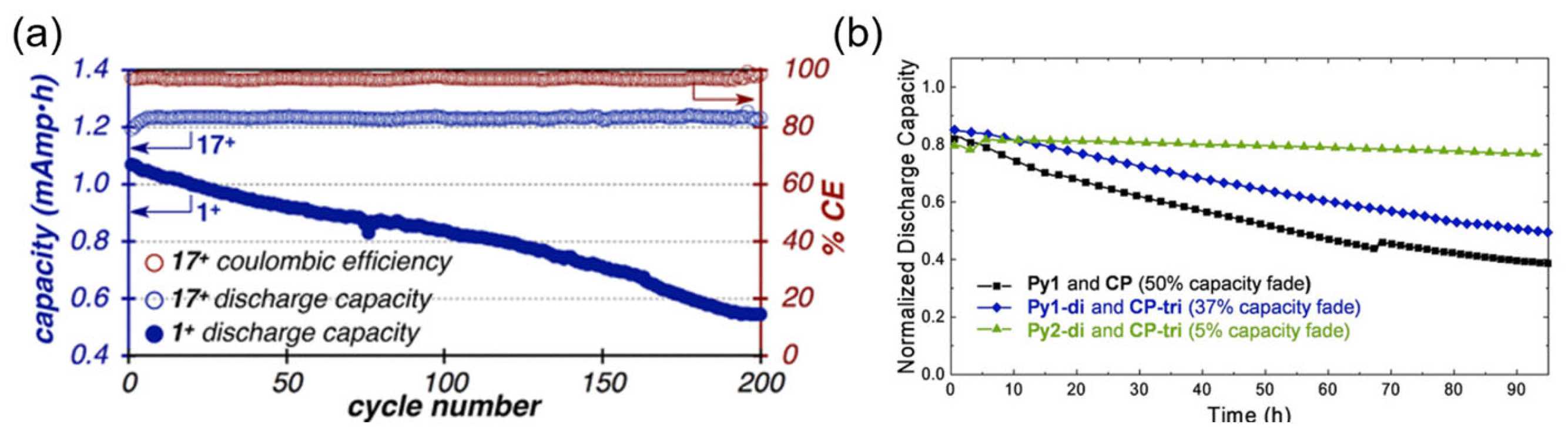
3.1. Pyridinium and Derivatives
3.2. Viologens and Derivatives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Z.; Nguyen, T.P.; Attanayake, N.H.; Easley, A.D.; Lutkenhaus, J.L.; Wooley, K.L.; Odom, S.A. Metal-free polypeptide redox flow batteries. Mater. Adv. 2022, 3, 6558–6565. [Google Scholar] [CrossRef]
- Li, M.; Rhodes, Z.; Cabrera-Pardo, J.R.; Minteer, S.D. Recent advancements in rational design of non-aqueous organic redox flow batteries. Sustain. Energy Fuels 2020, 4, 4370–4389. [Google Scholar] [CrossRef]
- Huang, Y.; Gu, S.; Yan, Y.; Li, S.F.Y. Nonaqueous redox-flow batteries: Features, challenges, and prospects. Curr. Opin. Chem. Eng. 2015, 8, 105–113. [Google Scholar] [CrossRef]
- Kwabi, D.G.; Ji, Y.; Aziz, M.J. Electrolyte Lifetime in Aqueous Organic Redox Flow Batteries: A Critical Review. Chem. Rev. 2020, 120, 6467–6489. [Google Scholar] [CrossRef]
- Kowalski, J.A.; Su, L.; Milshtein, J.D.; Brushett, F.R. Recent advances in molecular engineering of redox active organic molecules for nonaqueous flow batteries. Energy Environ. Eng. React. Eng. Catal. 2016, 13, 45–52. [Google Scholar] [CrossRef]
- Cameron, J.M.; Holc, C.; Kibler, A.J.; Peake, C.L.; Walsh, D.A.; Newton, G.N.; Johnson, L.R. Molecular redox species for next-generation batteries. Chem. Soc. Rev. 2021, 50, 5863–5883. [Google Scholar] [CrossRef]
- Hu, B.; De Bruler, C.; Rhodes, Z.; Liu, T.L. Long-Cycling Aqueous Organic Redox Flow Battery (AORFB) toward Sustainable and Safe Energy Storage. J. Am. Chem. Soc. 2017, 139, 1207–1214. [Google Scholar] [CrossRef]
- Luo, J.; Hu, B.; Hu, M.; Zhao, Y.; Liu, T.L. Status and Prospects of Organic Redox Flow Batteries toward Sustainable Energy Storage. ACS Energy Lett. 2019, 4, 2220–2240. [Google Scholar] [CrossRef]
- Liang, Z.; Jha, R.K.; Suduwella, T.M.; Attanayake, N.H.; Wang, Y.; Zhang, W.; Cao, C.; Kaur, A.P.; Landon, J.; Odom, S.A. A prototype of high-performance two-electron non-aqueous organic redox flow battery operated at −40 °C. J. Mater. Chem. A 2022, 10, 24685–24693. [Google Scholar] [CrossRef]
- Huang, Z.; Mu, A.; Wu, L.; Yang, B.; Qian, Y.; Wang, J. Comprehensive Analysis of Critical Issues in All-Vanadium Redox Flow Battery. ACS Sustain. Chem. Eng. 2022, 10, 7786–7810. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Xie, X.; Huang, Q.; Huang, C. Experimental study on efficiency improvement methods of vanadium redox flow battery for large-scale energy storage. Electrochim. Acta 2023, 466, 143025. [Google Scholar] [CrossRef]
- Chao, D.; Qiao, S.-Z. Toward High-Voltage Aqueous Batteries: Super- or Low-Concentrated Electrolyte? Joule 2020, 4, 1846–1851. [Google Scholar] [CrossRef]
- ECMWF. C3S. Available online: https://cds.climate.copernicus.eu/cdsapp#!/home (accessed on 31 August 2023).
- Gong, K.; Fang, Q.; Gu, S.; Li, S.F.Y.; Yan, Y. Nonaqueous redox-flow batteries: Organic solvents, supporting electrolytes, and redox pairs. Energy Environ. Sci. 2015, 8, 3515–3530. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Ji, X.; Fu, J.; Feng, G. Progress on predicting the electrochemical stability window of electrolytes. Curr. Opin. Electrochem. 2022, 34, 101030. [Google Scholar] [CrossRef]
- Izutsu, K. Electrochemistry in Nonaqueous Solutions; John Wiley & Sons: Weinheim, Germany, 2009. [Google Scholar]
- Ue, M.; Ida, K.; Mori, S. Electrochemical Properties of Organic Liquid Electrolytes Based on Quaternary Onium Salts for Electrical Double-Layer Capacitors. J. Electrochem. Soc. 1994, 141, 2989. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Ding, Y.; Peng, S.; Guo, X.; Zhao, Y.; He, G.; Yu, G. Progress and prospects of next-generation redox flow batteries. Energy Storage Mater. 2018, 15, 324–350. [Google Scholar] [CrossRef]
- Perry, M.L.; Weber, A.Z. Advanced Redox-Flow Batteries: A Perspective. J. Electrochem. Soc. 2015, 163, A5064. [Google Scholar] [CrossRef]
- Gregory, T.D.; Perry, M.L.; Albertus, P. Cost and price projections of synthetic active materials for redox flow batteries. J. Power Sources 2021, 499, 229965. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Kowalski, J.A.; Greco, K.V.; Casselman, M.D.; Milshtein, J.D.; Chapman, S.J.; Parkin, S.R.; Brushett, F.R.; Odom, S.A. Tailoring Two-Electron-Donating Phenothiazines To Enable High-Concentration Redox Electrolytes for Use in Nonaqueous Redox Flow Batteries. Chem. Mater. 2019, 31, 4353–4363. [Google Scholar] [CrossRef]
- Perera, A.S.; Suduwella, T.M.; Attanayake, N.H.; Jha, R.K.; Eubanks, W.L.; Shkrob, I.A.; Risko, C.; Kaur, A.P.; Odom, S.A. Large variability and complexity of isothermal solubility for a series of redox-active phenothiazines. Mater. Adv. 2022, 3, 8705–8715. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Liang, Z.; Wang, Y.; Kaur, A.P.; Parkin, S.R.; Mobley, J.K.; Ewoldt, R.H.; Landon, J.; Odom, S.A. Dual function organic active materials for nonaqueous redox flow batteries. Mater. Adv. 2021, 2, 1390–1401. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, C.; Zhang, L.; Zhou, Y.; Yu, G. Molecular engineering of organic electroactive materials for redox flow batteries. Chem. Soc. Rev. 2018, 47, 69–103. [Google Scholar] [CrossRef] [PubMed]
- Huskinson, B.; Marshak, M.P.; Suh, C.; Er, S.; Gerhardt, M.R.; Galvin, C.J.; Chen, X.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. A metal-free organic–inorganic aqueous flow battery. Nature 2014, 505, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, W.; Cosimbescu, L.; Choi, D.; Li, L.; Yang, Z. Anthraquinone with tailored structure for a nonaqueous metal–organic redox flow battery. Chem. Commun. 2012, 48, 6669–6671. [Google Scholar] [CrossRef]
- Cao, J.; Tao, M.; Chen, H.; Xu, J.; Chen, Z. A highly reversible anthraquinone-based anolyte for alkaline aqueous redox flow batteries. J. Power Sources 2018, 386, 40–46. [Google Scholar] [CrossRef]
- Wu, M.; Jing, Y.; Wong, A.A.; Fell, E.M.; Jin, S.; Tang, Z.; Gordon, R.G.; Aziz, M.J. Extremely Stable Anthraquinone Negolytes Synthesized from Common Precursors. Chem 2020, 6, 1432–1442. [Google Scholar] [CrossRef]
- Milshtein, J.D.; Barton, J.L.; Darling, R.M.; Brushett, F.R. 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxyl as a model organic redox active compound for nonaqueous flow batteries. J. Power Sources 2016, 327, 151–159. [Google Scholar] [CrossRef]
- Takechi, K.; Kato, Y.; Hase, Y. A Highly Concentrated Catholyte Based on a Solvate Ionic Liquid for Rechargeable Flow Batteries. Adv. Mater. 2015, 27, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, S.; Liu, S.; Huang, K.; Fang, D.; Wang, F.; Peng, S. Electrochemical Properties of an All-Organic Redox Flow Battery Using 2,2,6,6-Tetramethyl-1-Piperidinyloxy and N-Methylphthalimide. Electrochem. Solid-State Lett. 2011, 14, A171. [Google Scholar] [CrossRef]
- Wei, X.; Xu, W.; Vijayakumar, M.; Cosimbescu, L.; Liu, T.; Sprenkle, V.; Wang, W. TEMPO-Based Catholyte for High-Energy Density Nonaqueous Redox Flow Batteries. Adv. Mater. 2014, 26, 7649–7653. [Google Scholar] [CrossRef]
- Brushett, F.R.; Vaughey, J.T.; Jansen, A.N. An All-Organic Non-aqueous Lithium-Ion Redox Flow Battery. Adv. Energy Mater. 2012, 2, 1390–1396. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.; Shkrob, I.A.; Assary, R.S.; Tung, S.O.; Silcox, B.; Duan, W.; Zhang, J.; Su, C.C.; Hu, B.; et al. Annulated Dialkoxybenzenes as Catholyte Materials for Non-aqueous Redox Flow Batteries: Achieving High Chemical Stability through Bicyclic Substitution. Adv. Energy Mater. 2017, 7, 1701272. [Google Scholar] [CrossRef]
- Huang, J.; Pan, B.; Duan, W.; Wei, X.; Assary, R.S.; Su, L.; Brushett, F.R.; Cheng, L.; Liao, C.; Ferrandon, M.S.; et al. The lightest organic radical cation for charge storage in redox flow batteries. Sci. Rep. 2016, 6, 32102. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Attanayake, N.H.; Greco, K.V.; Neyhouse, B.J.; Barton, J.L.; Kaur, A.P.; Eubanks, W.L.; Brushett, F.R.; Landon, J.; Odom, S.A. Comparison of Separators vs Membranes in Nonaqueous Redox Flow Battery Electrolytes Containing Small Molecule Active Materials. ACS Appl. Energy Mater. 2021, 4, 5443–5451. [Google Scholar] [CrossRef]
- Kowalski, J.A.; Casselman, M.D.; Kaur, A.P.; Milshtein, J.D.; Elliott, C.F.; Modekrutti, S.; Attanayake, N.H.; Zhang, N.; Parkin, S.R.; Risko, C.; et al. A stable two-electron-donating phenothiazine for application in nonaqueous redox flow batteries. J. Mater. Chem. A 2017, 5, 24371–24379. [Google Scholar] [CrossRef]
- Milshtein, J.D.; Kaur, A.P.; Casselman, M.D.; Kowalski, J.A.; Modekrutti, S.; Zhang, P.L.; Harsha Attanayake, N.; Elliott, C.F.; Parkin, S.R.; Risko, C.; et al. High current density, long duration cycling of soluble organic active species for non-aqueous redox flow batteries. Energy Environ. Sci. 2016, 9, 3531–3543. [Google Scholar] [CrossRef]
- Chai, J.; Lashgari, A.; Wang, X.; Williams, C.K.; Jiang, J. “Jimmy” All-PEGylated redox-active metal-free organic molecules in non-aqueous redox flow battery. J. Mater. Chem. A 2020, 8, 15715–15724. [Google Scholar] [CrossRef]
- Janoschka, T.; Martin, N.; Hager, M.D.; Schubert, U.S. An Aqueous Redox-Flow Battery with High Capacity and Power: The TEMPTMA/MV System. Angew. Chem. Int. Ed. 2016, 55, 14427–14430. [Google Scholar] [CrossRef]
- Chai, J.; Lashgari, A.; Cao, Z.; Williams, C.K.; Wang, X.; Dong, J.; Jiang, J. “Jimmy” PEGylation-Enabled Extended Cyclability of a Non-aqueous Redox Flow Battery. ACS Appl. Mater. Interfaces 2020, 12, 15262–15270. [Google Scholar] [CrossRef]
- Hu, B.; Liu, T.L. Two electron utilization of methyl viologen anolyte in nonaqueous organic redox flow battery. J. Energy Chem. 2018, 27, 1326–1332. [Google Scholar] [CrossRef]
- Ahn, S.; Jang, J.H.; Kang, J.; Na, M.; Seo, J.; Singh, V.; Joo, J.M.; Byon, H.R. Systematic Designs of Dicationic Heteroarylpyridiniums as Negolytes for Nonaqueous Redox Flow Batteries. ACS Energy Lett. 2021, 6, 3390–3397. [Google Scholar] [CrossRef]
- Li, M.; Odom, S.A.; Pancoast, A.R.; Robertson, L.A.; Vaid, T.P.; Agarwal, G.; Doan, H.A.; Wang, Y.; Suduwella, T.M.; Bheemireddy, S.R.; et al. Experimental Protocols for Studying Organic Non-aqueous Redox Flow Batteries. ACS Energy Lett. 2021, 6, 3932–3943. [Google Scholar] [CrossRef]
- Yao, Y.; Lei, J.; Shi, Y.; Ai, F.; Lu, Y.-C. Assessment methods and performance metrics for redox flow batteries. Nat. Energy 2021, 6, 582–588. [Google Scholar] [CrossRef]
- Yuan, J.; Pan, Z.-Z.; Jin, Y.; Qiu, Q.; Zhang, C.; Zhao, Y.; Li, Y. Membranes in non-aqueous redox flow battery: A review. J. Power Sources 2021, 500, 229983. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Q. Next-Generation, High-Energy-Density Redox Flow Batteries. Chem. Plus Chem. 2015, 80, 312–322. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, G.; Xu, P.; Kashkooli, A.G.; Mousavi, M.; Yu, A.; Chen, Z. An all-aqueous redox flow battery with unprecedented energy density. Energy Environ. Sci. 2018, 11, 2010–2015. [Google Scholar] [CrossRef]
- Chen, H.; Cong, G.; Lu, Y.-C. Recent progress in organic redox flow batteries: Active materials, electrolytes and membranes. J. Energy Chem. 2018, 27, 1304–1325. [Google Scholar] [CrossRef]
- Pan, F.; Wang, Q. Redox Species of Redox Flow Batteries: A Review. Molecules 2015, 20, 20499–20517. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.; Zhang, L.; Qian, Y.; Zhang, C.; Yu, G. Molecular Engineering of Azobenzene-Based Anolytes Towards High-Capacity Aqueous Redox Flow Batteries. Angew. Chem. 2020, 132, 22347–22354. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, H.; Salla, M.; Zhuang, J.; Zhi, Y.; Wang, X.; Wang, Q. Molecular engineering of dihydroxyanthraquinone-based electrolytes for high-capacity aqueous organic redox flow batteries. Nat. Commun. 2022, 13, 4746. [Google Scholar] [CrossRef]
- Gerken, J.B.; Anson, C.W.; Preger, Y.; Symons, P.G.; Genders, J.D.; Qiu, Y.; Li, W.; Root, T.W.; Stahl, S.S. Comparison of Quinone-Based Catholytes for Aqueous Redox Flow Batteries and Demonstration of Long-Term Stability with Tetrasubstituted Quinones. Adv. Energy Mater. 2020, 10, 2000340. [Google Scholar] [CrossRef]
- Tabor, D.P.; Gómez-Bombarelli, R.; Tong, L.; Gordon, R.G.; Aziz, M.J.; Aspuru-Guzik, A. Mapping the frontiers of quinone stability in aqueous media: Implications for organic aqueous redox flow batteries. J. Mater. Chem. A 2019, 7, 12833–12841. [Google Scholar] [CrossRef]
- Tong, L.; Jing, Y.; Gordon, R.G.; Aziz, M.J. Symmetric All-Quinone Aqueous Battery. ACS Appl. Energy Mater. 2019, 2, 4016–4021. [Google Scholar] [CrossRef]
- Zhu, X.; Jing, Y. Natural quinone molecules as effective cathode materials for nonaqueous lithium-ion batteries. J. Power Sources 2022, 531, 231291. [Google Scholar] [CrossRef]
- Ouyang, Z.; Tranca, D.; Zhao, Y.; Chen, Z.; Fu, X.; Zhu, J.; Zhai, G.; Ke, C.; Kymakis, E.; Zhuang, X. Quinone-Enriched Conjugated Microporous Polymer as an Organic Cathode for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 9064–9073. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhao, Q.; Wang, J.; Pan, Z.; Chen, J. A Sulfur Heterocyclic Quinone Cathode and a Multifunctional Binder for a High-Performance Rechargeable Lithium-Ion Battery. Angew. Chem. Int. Ed. 2016, 55, 6428–6432. [Google Scholar] [CrossRef] [PubMed]
- Symons, P. Quinones for redox flow batteries. Curr. Opin. Electrochem. 2021, 29, 100759. [Google Scholar] [CrossRef]
- Wu, M.; Bahari, M.; Fell, E.M.; Gordon, R.G.; Aziz, M.J. High-performance anthraquinone with potentially low cost for aqueous redox flow batteries. J. Mater. Chem. A 2021, 9, 26709–26716. [Google Scholar] [CrossRef]
- Zhong, F.; Yang, M.; Ding, M.; Jia, C. Organic Electroactive Molecule-Based Electrolytes for Redox Flow Batteries: Status and Challenges of Molecular Design. Front. Chem. 2020, 8, 451. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Yu, G. Exploring Bio-inspired Quinone-Based Organic Redox Flow Batteries: A Combined Experimental and Computational Study. Chem 2016, 1, 790–801. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, W.; Wang, A.; Yu, Z.; Chen, S.; Yang, Y. A MC/AQ Parasitic Composite as Cathode Material for Lithium Battery. J. Electrochem. Soc. 2011, 158, A991. [Google Scholar] [CrossRef]
- Pahlevaninezhad, M.; Leung, P.; Velasco, P.Q.; Pahlevani, M.; Walsh, F.C.; Roberts, E.P.L.; Ponce de León, C. A nonaqueous organic redox flow battery using multi-electron quinone molecules. J. Power Sources 2021, 500, 229942. [Google Scholar] [CrossRef]
- Zheng, Y.; Ramos, Á.P.; Wang, H.; Álvarez, G.; Ridruejo, A.; Peng, J. Non-aqueous organic redox active materials for a bicontinuous microemulsion-based redox flow battery. Mater. Today Energy 2023, 34, 101286. [Google Scholar] [CrossRef]
- Kaur, A.P.; Ergun, S.; Elliott, C.F.; Odom, S.A. 3,7-Bis(trifluoromethyl)-N-ethylphenothiazine: A redox shuttle with extensive overcharge protection in lithium-ion batteries. J. Mater Chem. A 2014, 2, 18190–18193. [Google Scholar] [CrossRef]
- Elliott, C.F.; Fraser, K.E.; Odom, S.A.; Risko, C. Steric Manipulation as a Mechanism for Tuning the Reduction and Oxidation Potentials of Phenothiazines. J. Phys. Chem. A 2021, 125, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Preet Kaur, A.; Neyhouse, B.J.; Shkrob, I.A.; Wang, Y.; Harsha Attanayake, N.; Kant Jha, R.; Wu, Q.; Zhang, L.; Ewoldt, R.H.; Brushett, F.R.; et al. Concentration-dependent Cycling of Phenothiazine-based Electrolytes in Nonaqueous Redox Flow Cells. Chem.-Asian J. 2023, 18, e202201171. [Google Scholar] [CrossRef]
- Yan, Y.; Robinson, S.G.; Vaid, T.P.; Sigman, M.S.; Sanford, M.S. Simultaneously Enhancing the Redox Potential and Stability of Multi-Redox Organic Catholytes by Incorporating Cyclopropenium Substituents. J. Am. Chem. Soc. 2021, 143, 13450–13459. [Google Scholar] [CrossRef]
- Walser-Kuntz, R.; Yan, Y.; Sigman, M.; Sanford, M.S. A Physical Organic Chemistry Approach to Developing Cyclopropenium-Based Energy Storage Materials for Redox Flow Batteries. Acc. Chem. Res. 2023, 56, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Vaid, T.P.; Sanford, M.S. Bis(diisopropylamino)cyclopropenium-arene Cations as High Oxidation Potential and High Stability Catholytes for Non-aqueous Redox Flow Batteries. J. Am. Chem. Soc. 2020, 142, 17564–17571. [Google Scholar] [CrossRef]
- Yan, Y.; Sitaula, P.; Odom, S.A.; Vaid, T.P. High Energy Density, Asymmetric, Nonaqueous Redox Flow Batteries without a Supporting Electrolyte. ACS Appl. Mater. Interfaces 2022, 14, 49633–49640. [Google Scholar] [CrossRef]
- Robinson, S.G.; Yan, Y.; Hendriks, K.H.; Sanford, M.S.; Sigman, M.S. Developing a Predictive Solubility Model for Monomeric and Oligomeric Cyclopropenium-Based Flow Battery Catholytes. J. Am. Chem. Soc. 2019, 141, 10171–10176. [Google Scholar] [CrossRef]
- Yan, Y.; Vogt, D.B.; Vaid, T.P.; Sigman, M.S.; Sanford, M.S. Development of High Energy Density Diaminocyclopropenium-Phenothiazine Hybrid Catholytes for Non-Aqueous Redox Flow Batteries. Angew. Chem. 2021, 133, 27245–27251. [Google Scholar] [CrossRef]
- Armstrong, C.G.; Toghill, K.E. Stability of molecular radicals in organic non-aqueous redox flow batteries: A mini review. Electrochem. Commun. 2018, 91, 19–24. [Google Scholar] [CrossRef]
- Hu, B.; Hu, M.; Luo, J.; Liu, T.L. A Stable, Low Permeable TEMPO Catholyte for Aqueous Total Organic Redox Flow Batteries. Adv. Energy Mater. 2022, 12, 2102577. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Agarwal, G.; Yu, Z.; Corman, R.E.; Wang, Y.; Robertson, L.A.; Shi, Z.; Doan, H.A.; Ewoldt, R.H.; et al. TEMPO allegro: Liquid catholyte redoxmers for nonaqueous redox flow batteries. J. Mater. Chem. A 2021, 9, 16769–16775. [Google Scholar] [CrossRef]
- Liu, Y.; Goulet, M.-A.; Tong, L.; Liu, Y.; Ji, Y.; Wu, L.; Gordon, R.G.; Aziz, M.J.; Yang, Z.; Xu, T. A Long-Lifetime All-Organic Aqueous Flow Battery Utilizing TMAP-TEMPO Radical. Chem 2019, 5, 1861–1870. [Google Scholar] [CrossRef]
- Wilcox, D.A.; Agarkar, V.; Mukherjee, S.; Boudouris, B.W. Stable Radical Materials for Energy Applications. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 83–103. [Google Scholar] [CrossRef]
- Prakash, N.; Rajeev, R.; John, A.; Vijayan, A.; George, L.; Varghese, A. 2,2,6,6-Tetramethylpiperidinyloxyl (TEMPO) Radical Mediated Electro-Oxidation Reactions: A Review. Chem. Sel. 2021, 6, 7691–7710. [Google Scholar] [CrossRef]
- Wei, X.; Pan, W.; Duan, W.; Hollas, A.; Yang, Z.; Li, B.; Nie, Z.; Liu, J.; Reed, D.; Wang, W.; et al. Materials and Systems for Organic Redox Flow Batteries: Status and Challenges. ACS Energy Lett. 2017, 2, 2187–2204. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Redfern, P.C.; Curtiss, L.A.; Amine, K. Molecular engineering towards safer lithium-ion batteries: A highly stable and compatible redox shuttle for overcharge protection. Energy Environ. Sci. 2012, 5, 8204–8207. [Google Scholar] [CrossRef]
- Leonet, O.; Colmenares, L.C.; Kvasha, A.; Oyarbide, M.; Mainar, A.R.; Glossmann, T.; Blázquez, J.A.; Zhang, Z. Improving the Safety of Lithium-Ion Battery via a Redox Shuttle Additive 2,5-Di-tert-butyl-1,4-bis(2-methoxyethoxy)benzene (DBBB). ACS Appl. Mater. Interfaces 2018, 10, 9216–9219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shkrob, I.A.; Assary, R.S.; Clark, R.J.; Wilson, R.E.; Jiang, S.; Meisner, Q.J.; Zhu, L.; Hu, B.; Zhang, L. An extremely durable redox shuttle additive for overcharge protection of lithium-ion batteries. Mater. Today Energy 2019, 13, 308–311. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, L.; Assary, R.S.; Wang, P.; Xue, Z.; Burrell, A.K.; Curtiss, L.A.; Zhang, L. Liquid Catholyte Molecules for Nonaqueous Redox Flow Batteries. Adv. Energy Mater. 2015, 5, 1401782. [Google Scholar] [CrossRef]
- Lv, X.-L.; Sullivan, P.; Fu, H.-C.; Hu, X.; Liu, H.; Jin, S.; Li, W.; Feng, D. Dextrosil-Viologen: A Robust and Sustainable Anolyte for Aqueous Organic Redox Flow Batteries. ACS Energy Lett. 2022, 7, 2428–2434. [Google Scholar] [CrossRef]
- Jin, S.; Fell, E.M.; Vina-Lopez, L.; Jing, Y.; Michalak, P.W.; Gordon, R.G.; Aziz, M.J. Near Neutral pH Redox Flow Battery with Low Permeability and Long-Lifetime Phosphonated Viologen Active Species. Adv. Energy Mater. 2020, 10, 2000100. [Google Scholar] [CrossRef]
- Hu, M.; Wu, W.; Luo, J.; Liu, T.L. Desymmetrization of Viologen Anolytes Empowering Energy Dense, Ultra Stable Flow Batteries toward Long-Duration Energy Storage. Adv. Energy Mater. 2022, 12, 2202085. [Google Scholar] [CrossRef]
- Sevov, C.S.; Hickey, D.P.; Cook, M.E.; Robinson, S.G.; Barnett, S.; Minteer, S.D.; Sigman, M.S.; Sanford, M.S. Physical Organic Approach to Persistent, Cyclable, Low-Potential Electrolytes for Flow Battery Applications. J. Am. Chem. Soc. 2017, 139, 2924–2927. [Google Scholar] [CrossRef]
- Shrestha, A.; Hendriks, K.H.; Sigman, M.S.; Minteer, S.D.; Sanford, M.S. Realization of an Asymmetric Non-Aqueous Redox Flow Battery through Molecular Design to Minimize Active Species Crossover and Decomposition. Chem.-Eur. J. 2020, 26, 5369–5373. [Google Scholar] [CrossRef]
- Hu, B.; Tang, Y.; Luo, J.; Grove, G.; Guo, Y.; Liu, L.T. Improved radical stability of viologen anolytes in aqueous organic redox flow batteries. Chem. Commun. 2018, 54, 6871–6874. [Google Scholar] [CrossRef]
- Liu, T.; Wei, X.; Nie, Z.; Sprenkle, V.; Wang, W. A Total Organic Aqueous Redox Flow Battery Employing a Low Cost and Sustainable Methyl Viologen Anolyte and 4-HO-TEMPO Catholyte. Adv. Energy Mater. 2016, 6, 1501449. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zuo, P.; Chen, Q.; Tang, G.; Sun, P.; Yang, Z.; Xu, T. Screening Viologen Derivatives for Neutral Aqueous Organic Redox Flow Batteries. Chem. Sus. Chem. 2020, 13, 2245–2249. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-S.; Park, S.-K.; Yeon, S.-H.; Shin, K.-H.; Song, H.; Kim, H.-S.; Jung, Y.-S.; Jin, C.-S. Methyl Viologen Anolyte Introducing Nitrate as Counter-Anion for an Aqueous Redox Flow Battery. J. Electrochem. Soc. 2021, 168, 100532. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; Ramanujam, K.; Sankararaman, S. Benzylviologen/N-hexyl phenothiazine based non-aqueous organic redox flow battery in inert condition. J. Energy Storage 2023, 72, 108739. [Google Scholar] [CrossRef]
- Garcia, S.N.; Yang, X.; Bereczki, L.; Kónya, D. Aqueous Solubility of Organic Compounds for Flow Battery Applications: Symmetry and Counter Ion Design to Avoid Low-Solubility Polymorphs. Molecules 2021, 26, 1203. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Mao, J.; Chen, Q. Redox flow batteries toward more soluble anthraquinone derivatives. Curr. Opin. Electrochem. 2021, 29, 100748. [Google Scholar] [CrossRef]
- Roznyatovskaya, N.; Noack, J.; Mild, H.; Fühl, M.; Fischer, P.; Pinkwart, K.; Tübke, J.; Skyllas-Kazacos, M. Vanadium Electrolyte for All-Vanadium Redox-Flow Batteries: The Effect of the Counter Ion. Batteries 2019, 5, 13. [Google Scholar] [CrossRef]

| Freezing Point (°C) | Boiling Point (°C) | Viscosity (mPa s−1) | Size of Electrochemical Windows (V) | |
|---|---|---|---|---|
| Acetonitrile (ACN) | −44 | 82 | 0.34 | 6.1 |
| Propylene carbonate (PC) | −49 | 242 | 2.53 | 6.6 |
| N-methyl-2-pyrrolidone (NMP) | −24 | 204 | 1.7 | 5.4 |
| Dichloromethane (DCM) | −95 | 40 | 0.39 | 4.9 |
| Tetrahydronfuran (THF) | −108 | 66 | 0.46 | 5.5 |
| Compound | 1st Redox Potential (vs. Cp2 Fe0/+) | 2nd Redox Potential (vs. Cp2 Fe0/+) | Diffusion Coefficient (×10−6 cm2s−1) | Solubility (M) Neutral | Solubility (M) Radical Salt |
|---|---|---|---|---|---|
| EPT | 0.278 | 0.956 | 2.3 | 0.11 | 0.28 |
| MEEPT | 0.311 | 0.937 | 1.6 | miscible | 0.55 |
| DMeOEPT | 0.056 | 0.655 | 1.8 | 0.06 | 0.06 |
| DMeOMEEPT | 0.093 | 0.666 | 1.6 | miscible | 0.18 |
| B(MEEO)EPT | 0.064 | 0.654 | 0.8 | miscible | 0.63 |
| EPRT-TFSI | 0.55 | 1.12 | 15.3 | 1.27 | 1.27 |
| 2-DAC | 0.6 | 1.2 | 5.4 | - | 0.09 |
| 4-DMPP | 0.64 | 1 | 5.77 | miscible | 0.45 |
| Compound | Redox Potential (vs. Li+/Li) | Diffusion Coefficient (×10−6 cm2s−1) | Solubility (M) Neutral | Solubility (M) Radical Salt |
|---|---|---|---|---|
| TEMPO | 3.5 | 11 | 5.2 | - |
| AcNH-TEMPO | 3.63 | 0.43 | >0.5 | >0.5 |
| MeO-TEMPO | 3.6 | - | 2.5 | - |
| Compound | Redox Potential (vs. Li/Li+) | Diffusion Coefficient (×10−6 cm2s−1) | Solubility (M) Neutral |
|---|---|---|---|
| BODMA | 4.02 | 45 | 0.15 |
| BODEA | 4.18 | 11 | 0.10 |
| 6 (25DDB) | 3.92 | - | 0.6 |
| 7 (23DDB) | 3.98 | - | 2 |
| DBBB | 3.93 | 1.46 | 0.4 |
| Compound | 1st Redox Potential (vs. Fc/Fc+) | 2nd Redox Potential (vs. Fc/Fc+) | Solubility (M) |
|---|---|---|---|
| 1 | −1.08 | −1.78 | 1.5 |
| 17 | −1.23 | - | - |
| Py1-di | −1.01 | −1.55 | - |
| Py2-di | −0.626 | −0.976 | 1.70 |
| Compound | 1st Redox Potential (vs. Ag/Ag+) | 2nd Redox Potential (vs. Ag/Ag+) | Diffusion Coefficient (×10−6 cm2s−1) | Solubility (M) (MeCN) |
|---|---|---|---|---|
| PEG12-V | −0.74 | −1.15 | - | 0.79 |
| Me-V | −0.74 | −1.15 | 6 | - |
| MVTFSI | −0.79 | −1.20 | 7.54 | 0.98 |
| MEEVTFSI | −0.79 (vs. Fc+/Fc) | −1.26 (vs. Fc+/Fc) | 7 | 1.1 |
| BV | −0.65 | −1.1 | 6.2 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, C.M.; Boronski, C.E.; Yang, T.; Liu, T.; Liang, Z. Molecular Engineering of Redox Couples for Non-Aqueous Redox Flow Batteries. Batteries 2023, 9, 504. https://doi.org/10.3390/batteries9100504
Davis CM, Boronski CE, Yang T, Liu T, Liang Z. Molecular Engineering of Redox Couples for Non-Aqueous Redox Flow Batteries. Batteries. 2023; 9(10):504. https://doi.org/10.3390/batteries9100504
Chicago/Turabian StyleDavis, Casey M., Claire E. Boronski, Tianyi Yang, Tuo Liu, and Zhiming Liang. 2023. "Molecular Engineering of Redox Couples for Non-Aqueous Redox Flow Batteries" Batteries 9, no. 10: 504. https://doi.org/10.3390/batteries9100504










