Synchrotron-Based X-ray Photoelectron Microscopy of LMO/LAGP/Cu Thin-Film Solid-State Lithium Metal Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Microfabrication of the SSB
2.2. Scanning PhotoElectron Microscopy (SPEM)
3. Results and Discussion
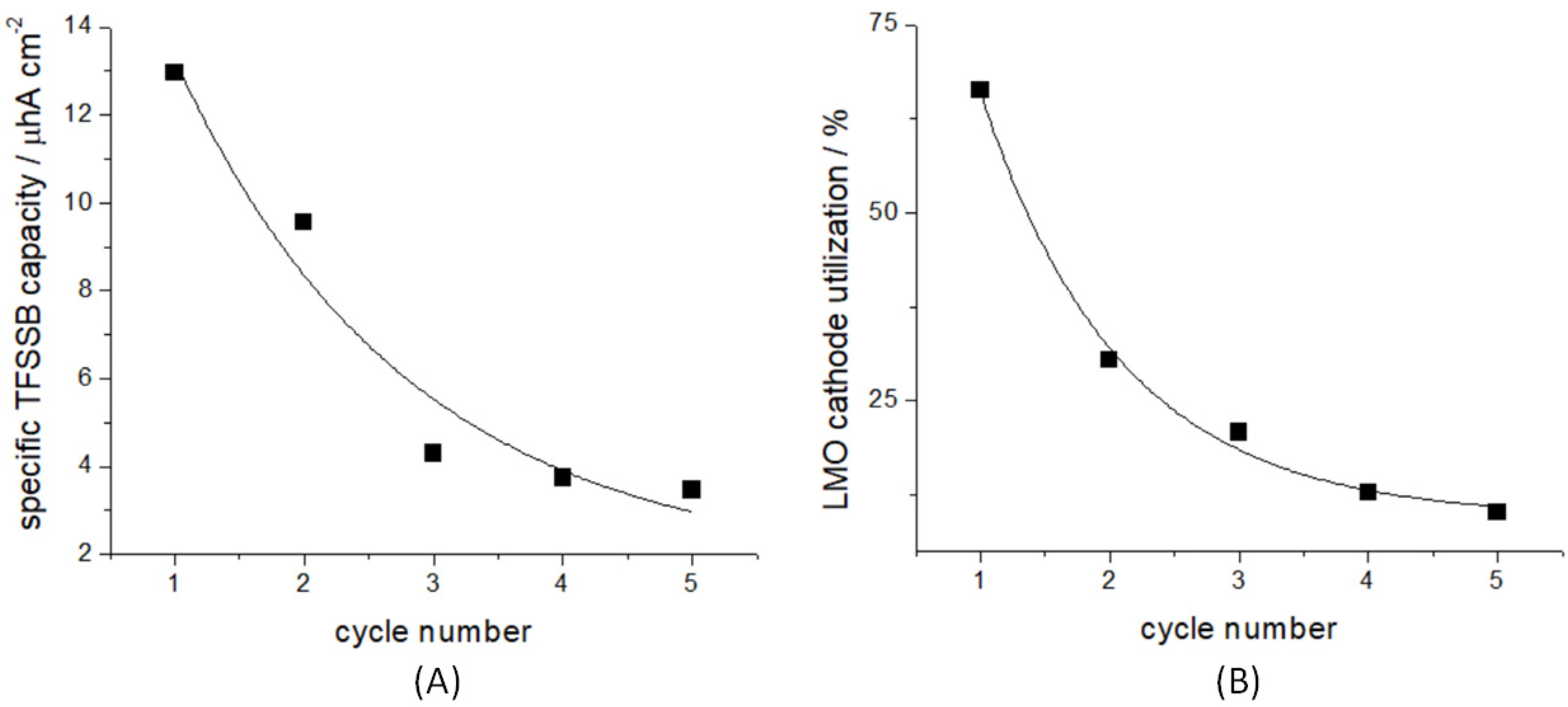
3.1. Electrochemical Ageing
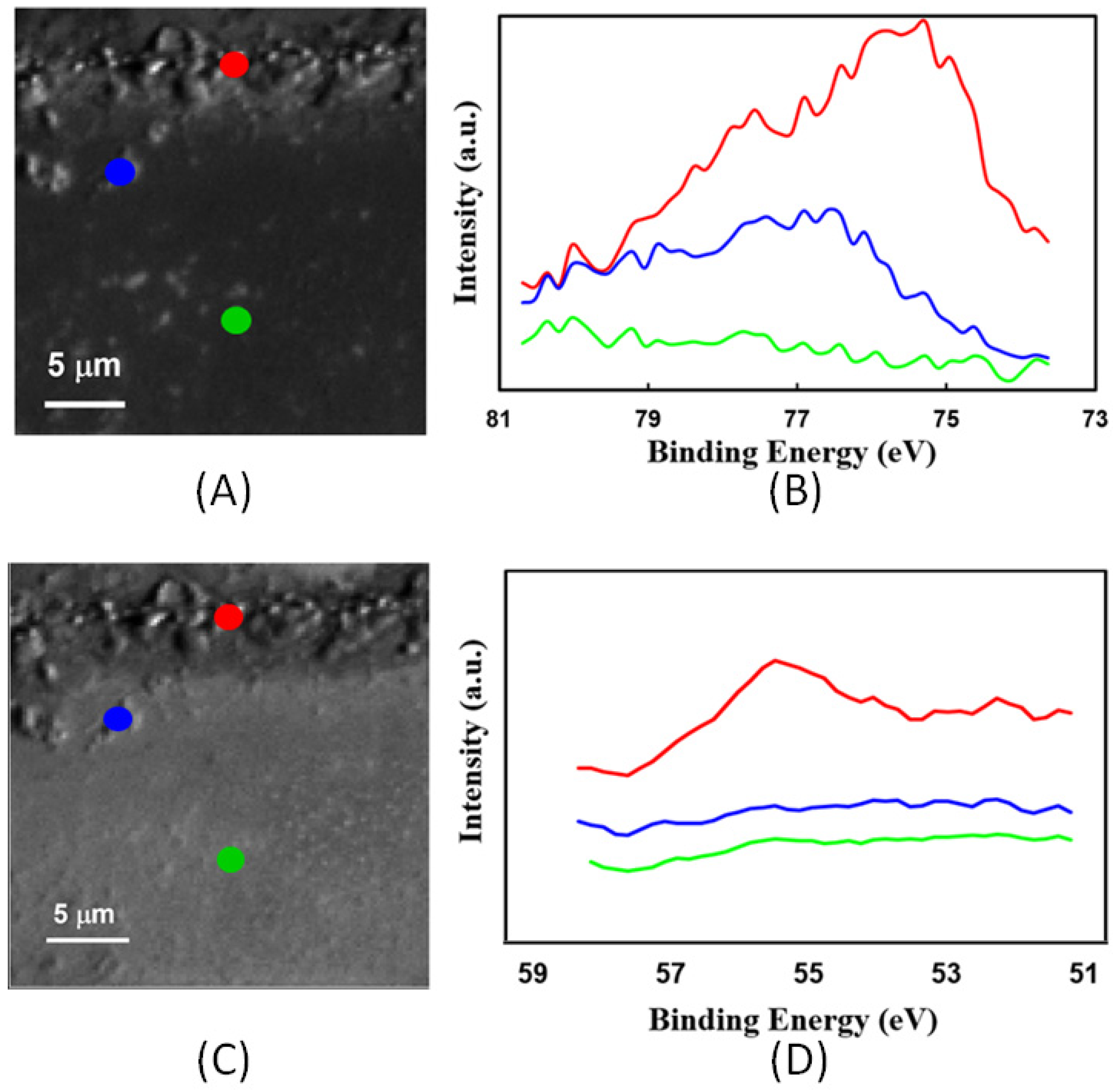
3.2. Chemical Mapping by Scanning Photoelectron Microscopy (SPEM)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monroe, C.; Newman, J. The Impact of Elastic Deformation on Deposition Kinetics at Lithium/Polymer Interfaces. J. Electrochem. Soc. 2005, 152, A396–A404. [Google Scholar] [CrossRef]
- Moitzheim, S.; Put, B.; Vereecken, P.M. Advances in 3D Thin-Film Li-Ion Batteries. Adv. Mater. Interfaces 2019, 6, 1900805. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.-Y.; He, Z.-J.; Li, Y.-J.; Mao, J.; Dai, K.-H.; Yan, C.; Zheng, J.-C. An advance review of solid-state battery: Challenges, progress and prospects Sust. Mater. Technol. 2021, 29, e00297. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Yu, X.; Chen, L.; Li, H. Approaching Practically Accessible Solid-State Batteries: Stability Issues Related to Solid Electrolytes and Interfaces. Chem. Rev. 2020, 120, 6820–6877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Stalin, S.; Zhao, C.-Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, L.; Yang, B.; Li, Q.; Wu, B.; Zhao, J.; Ma, L.; Liu, Y.; An, H. Preparation and ion conduction of Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte films using radio frequency sputtering. Solid State Ion. 2020, 346, 115224. [Google Scholar] [CrossRef]
- DeWees, R.; Wang, H. Synthesis and Properties of NaSICON-type LATP and LAGP Solid Electrolytes. ChemSusChem 2019, 12, 3713–3725. [Google Scholar] [CrossRef]
- Meesala, Y.; Chen, C.; Jena, A.; Liao, Y.; Hu, F. All-Solid-State Li-Ion Battery Using Li1.5Al0.5Ge1.5(PO4)3 As Electrolyte Without Polymer Interfacial Adhesion. J. Phys. Chem. C 2018, 122, 14383–14389. [Google Scholar] [CrossRef]
- Giarola, M.; Sanson, A.; Tietz, F.; Pristat, S.; Dashjav, E.; Rettenwander, D.; Redhammer, G.J.; Mariotto, G. Structure and Vibrational Dynamics of Nasicon-Type LiTi2(PO4)3. J. Phys. Chem. C 2017, 121, 3697–3706. [Google Scholar] [CrossRef]
- Xiong, L.; Ren, Z.; Xu, Y.; Mao, S.; Lei, P.; Sun, M. LiF Assisted Synthesis of LiTi2(PO4)3 Solid Electrolyte with Enhanced Ionic Conductivity. Solid State Ion. 2017, 309, 22–26. [Google Scholar] [CrossRef]
- Fu, J. Fast Li+ Ion Conducting Glass-Ceramics in the System Li2O–Al2O3–GeO2–P2O5. Solid State Ion. 1997, 104, 191–194. [Google Scholar] [CrossRef]
- Hartmann, P.; Leichtweiss, T.; Busche, M.R.; Schneider, M.; Reich, M.; Sann, J.; Adelhelm, P.; Janek, J. Degradation of NASICON-Type Materials in Contact with Lithium Metal: Formation of Mixed Conducting Interphases (MCI) on Solid Electrolytes. J. Phys. Chem. C 2013, 117, 21064–21074. [Google Scholar] [CrossRef]
- He, L.; Sun, Q.; Chen, C.; Oh, J.A.S.; Sun, J.; Li, M.; Tu, W.; Zhou, H.; Zeng, K.; Lu, L. Failure Mechanism and Interface Engineering for NASICON Structured All-Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2019, 11, 20895–20904. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.K.; Lu, L.; Lai, M.O. Lithium Storage Capability of Lithium Ion Conductor Li1.5Al0.5Ge1.5(PO4)3. J. Alloys Compd. 2010, 501, 255–258. [Google Scholar] [CrossRef]
- Wenzel, S.; Leichtweiss, T.; Kruger, D.; Sann, J.; Janek, J. Interphase Formation on Lithium Solid Electrolytes-an in Situ Approach to Study Interfacial Reactions by Photoelectron Spectroscopy. Solid State Ion. 2015, 278, 98–105. [Google Scholar] [CrossRef]
- Robinson, J.P.; Kichambare, P.D.; Deiner, J.L.; Miller, R.; Rottmayer, M.A.; Koenig, G.M. High Temperature Electrode-Electrolyte Interface Formation between LiMn1.5Ni0.5O4 and Li1.4Al0.4Ge1.6(PO4)3. J. Am. Ceram. Soc. 2018, 101, 1087–1094. [Google Scholar] [CrossRef]
- Gellert, M.; Dashjav, E.; Gruner, D.; Ma, Q.L.; Tietz, F. Compatibility Study of Oxide and Olivine Cathode Materials with Lithium Aluminum Titanium Phosphate. Ionics 2018, 24, 1001–1006. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and Stress in Electrode Materials for Li-Ion Batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- McGrogan, F.P.; Swamy, T.; Bishop, S.R.; Eggleton, E.; Porz, L.; Chen, X.; Chiang, Y.-M.; Van Vliet, K.J. Compliant yet Brittle Mechanical Behavior of Li2S–P2S5 Lithium-Ion-Conducting Solid Electrolyte. Adv. Energy Mater. 2017, 7, 1602011. [Google Scholar] [CrossRef]
- Porz, L.; Swamy, T.; Sheldon, B.W.; Rettenwander, D.; Froemling, T.; Thaman, H.L.; Berendts, S.; Uecker, R.; Carter, W.C.; Chiang, Y.-M. Mechanism of Lithium Metal Penetration through Inorganic Solid Electrolytes. Adv. Energy Mater. 2017, 7, 1701003. [Google Scholar] [CrossRef]
- Swamy, T.; Park, R.; Sheldon, B.W.; Rettenwander, D.; Porz, L.; Berendts, S.; Uecker, R.; Carter, W.C.; Chiang, Y.-M. Lithium Metal Penetration Induced by Electrodeposition through Solid Electrolytes: Example in Single-Crystal Li6La3ZrTaO12 Garnet. J. Electrochem. Soc. 2018, 165, A3648. [Google Scholar] [CrossRef]
- Han, F.; Westover, A.S.; Yue, J.; Fan, X.; Wang, F.; Chi, M.; Leonard, D.N.; Dudney, N.J.; Wang, H.; Wang, C. High Electronic Conductivity as the Origin of Lithium Dendrite Formation within Solid Electrolytes. Nat. Energy 2019, 4, 187–196. [Google Scholar] [CrossRef]
- Krauskopf, T.; Dippel, R.; Hartmann, H.; Peppler, K.; Mogwitz, B.; Richter, F.H.; Zeier, W.G.; Janek, J. Lithium-Metal Growth Kinetics on LLZO Garnet-Type Solid Electrolytes. Joule 2019, 3, 2030–2049. [Google Scholar] [CrossRef]
- Chung, H.; Kang, B. Mechanical and Thermal Failure Induced by Contact between Li1.5Al0.5Ge1.5(PO4)3 Solid Electrolyte and Li Metal in an All Solid-State Li Cell. Chem. Mater. 2017, 29, 8611–8619. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, C.; Li, B.J.; Song, H.C.; Cheng, Z.; Chen, M.R.; He, P.; Zhou, H.S. Germanium Thin Film Protected Lithium Aluminum Germanium Phosphate for Solid-State Li Batteries. Adv. Energy Mater. 2018, 8, 1702374. [Google Scholar] [CrossRef]
- Liu, Y.L.; Sun, Q.; Zhao, Y.; Wang, B.Q.; Kaghazchi, P.; Adair, K.R.; Li, R.Y.; Zhang, C.; Liu, J.R.; Kuo, L.Y.; et al. Stabilizing the Interface of NASICON Solid Electrolyte against Li Metal with Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2018, 10, 31240. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Chen, X.; Lu, X.; Cui, Z.; Xin, S.; Xue, L.; Jia, Q.; Goodenough, J.B. Mastering the Interface for Advanced All-Solid-State Lithium Rechargeable Batteries. Proc. Natl. Acad. Sci. USA 2016, 113, 13313–13317. [Google Scholar] [CrossRef]
- Hao, X.G.; Zhao, Q.; Su, S.M.; Zhang, S.Q.; Ma, J.B.; Shen, L.; Yu, Q.P.; Zhao, L.; Liu, Y.; Kang, F.Y.; et al. Constructing Multifunctional Interphase between Li1.4Al0.4Ti1.6(PO4)3 and Li Metal by Magnetron Sputtering for Highly Stable Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2019, 9, 1901604. [Google Scholar] [CrossRef]
- Hou, G.M.; Ma, X.X.; Sun, Q.D.; Ai, Q.; Xu, X.Y.; Li, D.P.; Chen, J.H.; Zhong, H.; Li, Y.; Feng, J.; et al. Lithium Dendrite Suppression and Enhanced Interfacial Compatibility Enabled by an Ex Situ SEI on Li Anode for LAGP-Based All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2018, 10, 18610. [Google Scholar] [CrossRef]
- Wenzel, S.; Randau, S.; Leichtweiss, T.; Weber, D.A.; Sann, J.; Zeier, W.G.; Janek, J. Direct Observation of the Interfacial Instability of the Fast Ionic Conductor Li10GeP2S12 at the Lithium Metal Anode. Chem. Mater. 2016, 28, 2400–2407. [Google Scholar] [CrossRef]
- Schwobel, A.; Hausbrand, R.; Jaegermann, W. Interface Reactions between LiPON and Lithium Studied by In-Situ X-ray Photoemission. Solid State Ion. 2015, 273, 51–54. [Google Scholar] [CrossRef]
- Wenzel, S.; Weber, D.A.; Leichtweiss, T.; Busche, M.R.; Sann, J.; Janek, J. Interphase Formation and Degradation of Charge Transfer Kinetics between a Lithium Metal Anode and Highly Crystalline Li7P3S11 Solid Electrolyte. Solid State Ion. 2016, 286, 24–33. [Google Scholar] [CrossRef]
- Wood, K.N.; Steirer, K.X.; Hafner, S.E.; Ban, C.M.; Santhanagopalan, S.; Lee, S.H.; Teeter, G. Operando X-ray Photoelectron Spectroscopy of Solid Electrolyte Interphase Formation and Evolution in Li2S-P2S5 Solid-State Electrolytes. Nat. Commun. 2018, 9, 2490. [Google Scholar] [CrossRef] [PubMed]
- Auvergniot, J.; Cassel, A.; Ledeuil, J.; Viallet, V.; Seznec, V.; Dedryvère, R. Interface Stability of Argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in Bulk All-Solid-State Batteries. Chem. Mater. 2017, 29, 3883–3890. [Google Scholar] [CrossRef]
- Wang, C.; Gong, Y.; Dai, J.; Zhang, L.; Xie, H.; Pastel, G.; Liu, B.; Wachsman, E.; Wang, H.; Hu, L. In Situ Neutron Depth Profiling of Lithium Metal-Garnet Interfaces for Solid State Batteries. J. Am. Chem. Soc. 2017, 139, 14257–14264. [Google Scholar] [CrossRef]
- Yamamoto, K.; Iriyama, Y.; Asaka, T.; Hirayama, T.; Fujita, H.; Nonaka, K.; Miyahara, K.; Sugita, Y.; OgumiZ, Z. Direct Observation of Lithium-Ion Movement around an In-situ-Formed-Negative-Electrode/Solid-State-Electrolyte Interface during Initial Charge–Discharge Reaction. Electrochem. Commun. 2012, 20, 113–116. [Google Scholar] [CrossRef]
- Yamamoto, K.; Iriyama, Y.; Asaka, T.; Hirayama, T.; Fujita, H.; Fisher, C.A.J.; Nonaka, K.; Sugita, Y.; Ogumi, Z. Dynamic Visualization of the Electric Potential in an All-Solid-State Rechargeable Lithium Battery. Angew. Chem. Int. Ed. 2010, 49, 4414–4417. [Google Scholar] [CrossRef]
- Kazemian, M.; Kiskinova, M.; Bozzini, B. X-ray absorption spectromicroscopy gives access to LAGP local degradation at the anode-electrolyte interface. J. Power Sources Adv. 2022, 17–18, 100106. [Google Scholar] [CrossRef]
- Zhang, T.; Imanishi, N.; Shimonishi, Y.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Sammes, N. A Novel High Energy Density Rechargeable Lithium/Air Battery. Chem. Commun. 2010, 46, 1661–1663. [Google Scholar] [CrossRef]
- Shimonishi, Y.; Zhang, T.; Imanishi, N.; Im, D.; Lee, D.J.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Sammes, N. A Study on Lithium/Air Secondary Batteries-Stability of the Nasicon-Type Lithium Ion Conducting Solid Electrolyte in Alkaline Aqueous Solutions. J. Power Sources 2011, 196, 5128–5132. [Google Scholar] [CrossRef]
- Dashjav, E.; Ma, Q.L.; Xu, Q.; Tsai, C.L.; Giarola, M.; Mariotto, G.; Tietz, F. The Influence of Water on the Electrical Conductivity of Aluminum-Substituted Lithium Titanium Phosphates. Solid State Ion. 2018, 321, 83–90. [Google Scholar] [CrossRef]
- Benabed, Y.; Rioux, M.; Rousselot, S.; Hautier, G.; Dollé, M. Assessing the Electrochemical Stability Window of NASICON-Type Solid Electrolytes. Front. Energy Res. 2021, 9, 682008. [Google Scholar] [CrossRef]
- Tan, G.; Wu, F.; Li, L.; Liu, Y.; Chen, R. Magnetron sputtering preparation of nitrogen-incorporated lithium–aluminum–titanium phosphate based thin film electrolytes for all-solid-state lithium ion batteries. J. Phys. Chem. C 2012, 116, 3817–3826. [Google Scholar] [CrossRef]
- Neudecker, B.J.; Dudney, N.J.; Bates, J.B. Lithium-Free” Thin-Film Battery with In Situ Plated Li Anode. J. Electrochem. Soc. 2000, 147, 517–523. [Google Scholar] [CrossRef]
- Qin, J.; Adams, B.D.; Zheng, J.; Xu, W.; Henderson, W.A.; Wang, J.; Bowden, M.E.; Xu, S.; Hu, J.; Zhang, J. Anode-Free Rechargeable Lithium Metal Batteries. Adv. Funct. Mater. 2016, 26, 7094–7102. [Google Scholar] [CrossRef]
- Bates, J.B.; Dudney, N.J.; Neudecker, B.J.; Hart, F.X.; Jun, H.P.; Hackney, S.A. Preferred Orientation of Polycrystalline LiCoO2 Films. J. Electrochem. Soc. 2000, 147, 59. [Google Scholar] [CrossRef]
- Lee, K.L.; Jung, J.Y.; Lee, S.W.; Moon, H.S.; Park, J.W. Electrochemical characteristics and cycle performance of LiMn2O4/a-Si microbattery. J. Power Sources 2004, 130, 241–246. [Google Scholar] [CrossRef]
- Otsuji, H.; Kawahara, K.; Ikegami, T.; Ebihara, K. LiMn2O4 thin films prepared by pulsed laser deposition for rechargeable batteries. Thin Solid Film. 2006, 506, 120–122. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, J.G.; Turner, J.A.; Tracy, C.E.; Benson, D.K. Lithium-Manganese-Oxide Thin-Film Cathodes Prepared by Plasma-Enhanced Chemical Vapor Deposition. J. Electrochem. Soc. 1999, 146, 2001–2005. [Google Scholar] [CrossRef]
- Delluva, A.A.; Dudoff, J.; Teeter, G.; Holewinski, A. Cathode Interface Compatibility of Amorphous LiMn2O4 (LMO) and Li7La3Zr2O12 (LLZO) Characterized with Thin-Film Solid-State Electrochemical Cells. ACS Appl. Mater. Interfaces 2020, 12, 24992–24999. [Google Scholar] [CrossRef]
- Bates, J.B.; Dudney, N.J.; Neudecker, B.; Ueda, A.; Evans, C.D. Thin-film lithium and lithium-ion batteries. Solid State Ion. 2000, 135, 33–45. [Google Scholar] [CrossRef]
- Dudney, N.J. Solid-state thin-film rechargeable batteries. Mater. Sci. Eng. B 2005, 116, 245–249. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, T.; Shen, Y.; Lin, Y.; Nan, C.W. Chemical Compatibility between Garnet-like Solid State Electrolyte Li6.75La3Zr1.75Ta0.25O12 and Major Commercial Lithium Battery Cathode Materials. J. Mater. 2016, 2, 256–264. [Google Scholar] [CrossRef]
- Mousavi, T.; Chen, X.; Doerrer, C.; Jagger, B.; Speller, S.C.; Grovenor, C.R.M. Fabrication of Li1+xAlxGe2-x(PO4)3 thin films by sputtering for solid. Solid State Ion. 2020, 354, 115397. [Google Scholar] [CrossRef]
- Tian, L.; Yuan, A. Electrochemical performance of nanostructured spinel LiMn2O4 in different aqueous electrolytes. J. Power Sources 2009, 192, 693–697. [Google Scholar] [CrossRef]
- Liu, X.; Tan, J.; Fu, J.; Yuan, R.; Wen, H.; Zhang, C. Facile Synthesis of Nanosized Lithium-Ion-Conducting Solid Electrolyte Li1.4Al0.4Ti1.6(PO4)3 and Its Mechanical Nanocomposites with LiMn2O4 for Enhanced Cyclic Performance in Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 11696–11703. [Google Scholar] [CrossRef]
- Das, A.; Krishna, P.S.R.; Goswami, M.; Krishnan, M. Structural analysis of Al and Si substituted lithium germanium phosphate glass-ceramics using neutron and X-ray diffraction. J. Solid State Chem. 2019, 271, 74–80. [Google Scholar] [CrossRef]
- Yu, Q.; Han, D.; Lu, Q.; He, Y.-B.; Li, S.; Liu, Q.; Han, C.; Kang, F.; Li, B. Constructing Effective Interfaces for Li1.5Al0.5Ge1.5(PO4)3 Pellets To Achieve Room-Temperature Hybrid Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2019, 11, 9911–9918. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Qian, K.; Li, T.; Wei, G.; Kang, F. Efficient Construction of a C60 Interlayer for Mechanically Robust, Dendrite-free, and Ultrastable Solid-State Batteries. iScience 2020, 23, 101636. [Google Scholar] [CrossRef]
- Lam, Y.C.; Zheng, H.Y.; Tjeung, R.T.; Chen, X. Seeing the invisible laser markings. J. Phys. D: Appl. Phys. 2009, 42, 042004. [Google Scholar] [CrossRef]
- Yan, W.; Li, H.; Liu, J.; Guo, J. EPMA and XRD study on nickel metal thin film for temperature sensor. Sens. Actuators A 2007, 136, 212–215. [Google Scholar] [CrossRef]
- Tarte, P.; Rulmont, A.; Merckaert-Ansay, C. Vibrational spectrum of nasicon-like, rhombohedral orthophosphates MIMIV2(PO4)3. Spectrochim. Acta A 1986, 42, 1009–1016. [Google Scholar] [CrossRef]
- Francisco, B.E.; Stoldt, C.R.; M’Peko, J.C. Lithium-Ion Trapping from Local Structural Distortions in Sodium Super Ionic Conductor (NASICON) Electrolytes. Chem. Mater. 2014, 26, 4741–4749. [Google Scholar] [CrossRef]
- Pershina, S.V.; Pankratov, A.A.; Vovkotrub, E.G.; Antonov, B.D. Promising high-conductivity Li1.5Al0.5Ge1.5(PO4)3 solid electrolytes: The effect of crystallization temperature on the microstructure and transport properties. Ionics 2019, 25, 4713–4725. [Google Scholar] [CrossRef]
- Abyaneh, M.K.; Gregoratti, L.; Amati, M.; Dalmiglio, M.; Kiskinova, M. Scanning photoelectron microscopy: A powerful technique for probing micro and nano-structures. J. Surf. Sci. Nanotechnol. 2011, 9, 158–162. [Google Scholar] [CrossRef]
- Dudney, N.J. Evolution of the lithium morphology from cycling of thin film solid state batteries. J. Electroceramics 2017, 38, 222–229. [Google Scholar] [CrossRef]
- Guo, X.; Hao, L.; Yang, Y.; Wang, Y.; Yu, Y.L.H. High cathode utilization efficiency through interface engineering in all-solid state lithium-metal batteries. J. Mater. Chem. A 2019, 7, 25915–25924. [Google Scholar] [CrossRef]
- Sorianello, V.; Colace, L.; Armani, N.; Rossi, F.; Ferrari, C.; Lazzarini, L.; Assanto, G. Low-temperature germanium thin films on silicon. Opt. Mater. Express 2011, 1, 856–865. [Google Scholar] [CrossRef]
- Parker, J.H., Jr.; Feldman, D.W.; Ashkin, M. Raman scattering by Silicon and Germanium. Phys. Rev. 1967, 155, 712. [Google Scholar] [CrossRef]
- D’Costa, V.R.; Tolle, J.; Poweleit, C.D.; Kouvetakis, J. Compositional dependence of the Raman frequencies in ternary Ge1-x-ySixSny alloys. Phys. Rev. B 2007, 76, 035211. [Google Scholar] [CrossRef]
- Zanatta, A.R. Temperature-dependent Raman scattering of the Ge+GeOx system and its potential as an optical thermometer. Results Phys. 2020, 19, 103500. [Google Scholar] [CrossRef]
- Gregoratti, L.; Mentes, T.O.; Locatelli, A.; Kiskinova, M. Beam-induced effects in soft X-ray photoelectron emission microscopy experiments. J. Electron Spectrosc. Relat. Phenom. 2009, 170, 13–18. [Google Scholar] [CrossRef]
- Cao, X.; Cao, Y.; Peng, H.; Cao, Y.; Zhu, H.; Wang, N.; Dong, X.; Wang, C.; Liu, Y.; Wu, J.; et al. A New Germanium-Based Anode Material with High Stability for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 11883–11890. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Allen, J.L.; Read, J.; Sakamoto, J. Chemical Stability of Cubic Li7La3Zr2O12 with Molten Lithium at Elevated Temperature. J. Mater. Sci. 2013, 48, 5846–5851. [Google Scholar] [CrossRef]
- Zhu, Y.; Connell, J.G.; Tepavcevic, S.; Zapol, P.; Garcia-Mendez, R.; Taylor, N.J.; Sakamoto, J.; Ingram, B.J.; Curtiss, L.A.; Freeland, J.W.; et al. Dopant-Dependent Stability of Garnet Solid Electrolyte Interfaces with Lithium Metal. Adv. Energy Mater. 2019, 9, 1803440. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Huang, T.; Geng, Z.; Yu, A. Reducing interfacial resistance of a Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte/electrode interface by polymer interlayer protection. RSC Adv. 2020, 10, 10038–10045. [Google Scholar] [CrossRef]
- Etxebarria, A.; Yun, D.-J.; Blum, M.; Ye, Y.; Sun, M.; Lee, K.-J.; Su, H.; Muñoz-Márquez, M.Á.; Ross, P.N.; Crumlin, E.J. Revealing In Situ Li Metal Anode Surface Evolution upon Exposure to CO2 Using Ambient Pressure X-ray Photoelectron Spectroscopy. ACS Appl. Mater. Interfaces 2020, 12, 26607–26613. [Google Scholar] [CrossRef]
- Bensalah, N.; Matalkeh, M.; Mustafa, N.K.; Merabet, H. Binary Si–Ge Alloys as High-Capacity Anodes for Li-Ion Batteries. Phys. Status Solidi A 2020, 217, 1900414. [Google Scholar] [CrossRef]
- Hoque, E.; DeRose, J.A.; Kulik, G.; Hoffmann, P.; Mathieu, H.J.; Bhishan, B. Alkylphosphonate Modified Aluminium Oxide Surfaces. J. Phys. Chem. B 2006, 110, 10855–10861. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazemian, M.; Amati, M.; Gregoratti, L.; Kiskinova, M.; Bozzini, B. Synchrotron-Based X-ray Photoelectron Microscopy of LMO/LAGP/Cu Thin-Film Solid-State Lithium Metal Batteries. Batteries 2023, 9, 506. https://doi.org/10.3390/batteries9100506
Kazemian M, Amati M, Gregoratti L, Kiskinova M, Bozzini B. Synchrotron-Based X-ray Photoelectron Microscopy of LMO/LAGP/Cu Thin-Film Solid-State Lithium Metal Batteries. Batteries. 2023; 9(10):506. https://doi.org/10.3390/batteries9100506
Chicago/Turabian StyleKazemian, Majid, Matteo Amati, Luca Gregoratti, Maya Kiskinova, and Benedetto Bozzini. 2023. "Synchrotron-Based X-ray Photoelectron Microscopy of LMO/LAGP/Cu Thin-Film Solid-State Lithium Metal Batteries" Batteries 9, no. 10: 506. https://doi.org/10.3390/batteries9100506
APA StyleKazemian, M., Amati, M., Gregoratti, L., Kiskinova, M., & Bozzini, B. (2023). Synchrotron-Based X-ray Photoelectron Microscopy of LMO/LAGP/Cu Thin-Film Solid-State Lithium Metal Batteries. Batteries, 9(10), 506. https://doi.org/10.3390/batteries9100506






