A Review of Sodium-Metal Chloride Batteries: Materials and Cell Design
Abstract
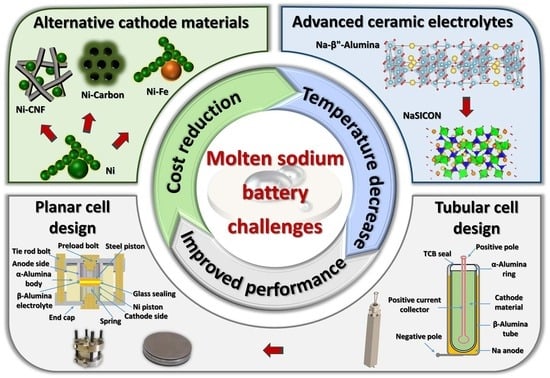
:1. Introduction
2. Cathode Materials
2.1. Standard Active Cathode Materials
2.1.1. Sulfur and Sulfide Additives
2.1.2. Sodium Iodide and Bromide Additives
2.2. Alternative Cathode Formulations
2.3. Effect of Temperature
3. Ceramic Electrolytes
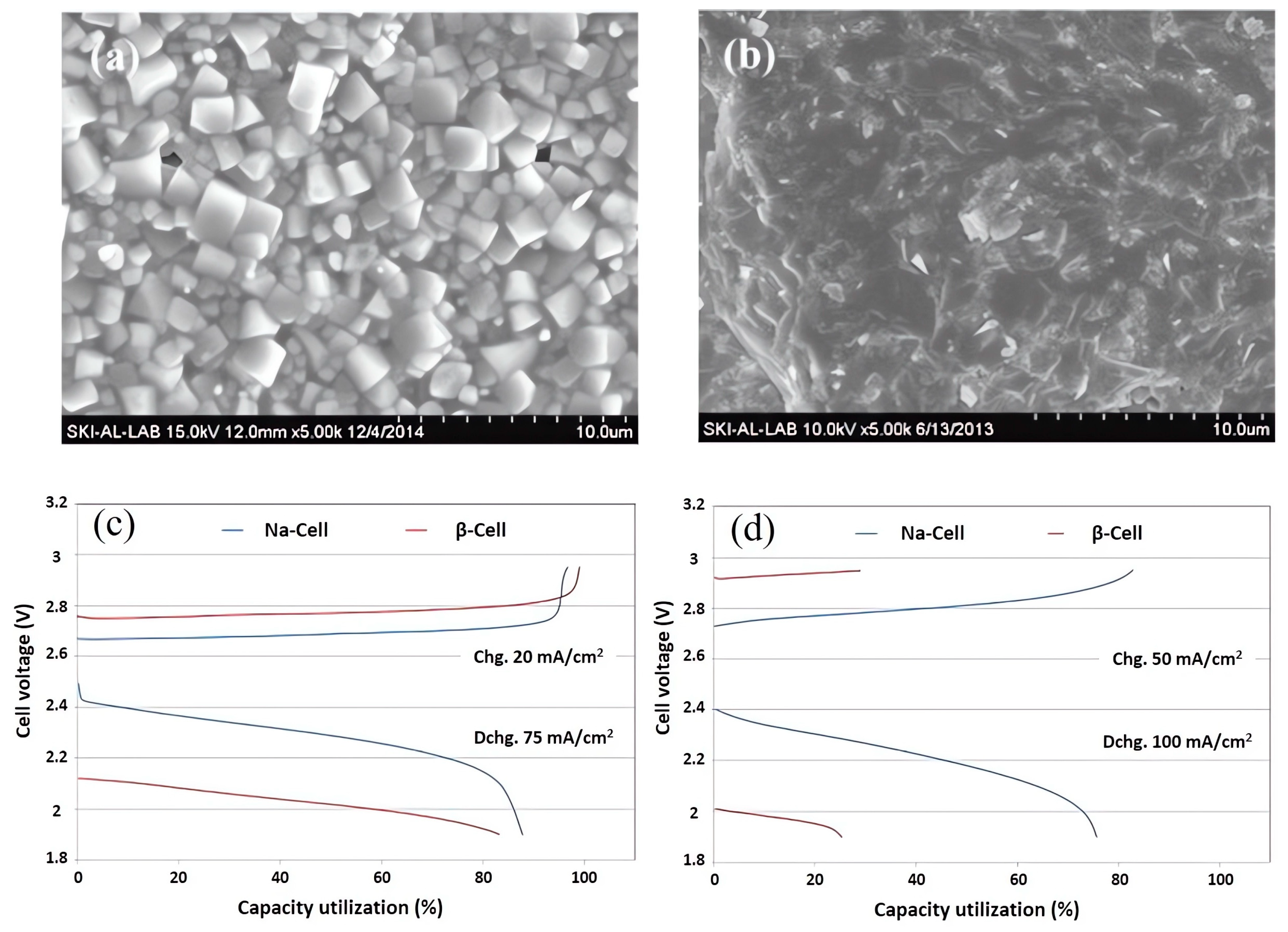
3.1. Beta-Alumina Solid Electrolyte
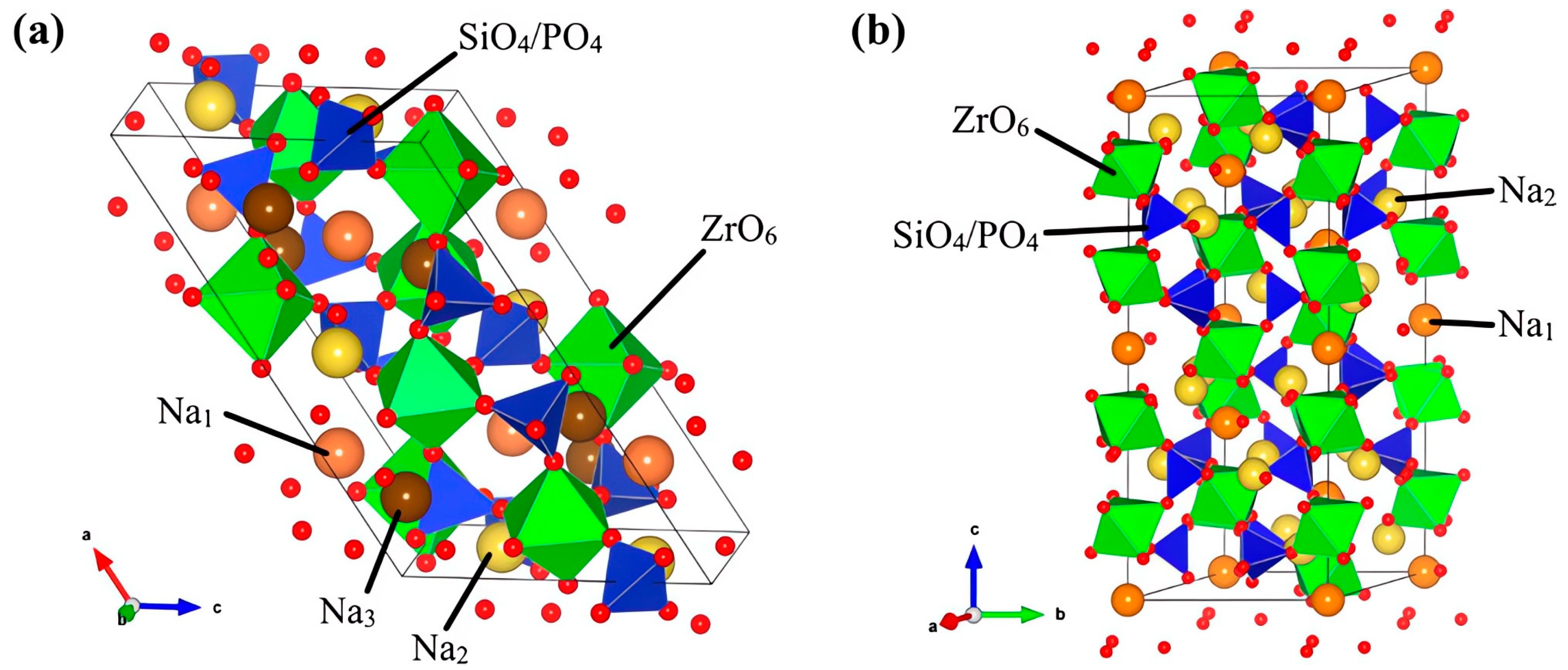
3.2. NaSICON Solid Electrolyte
4. Cell Design
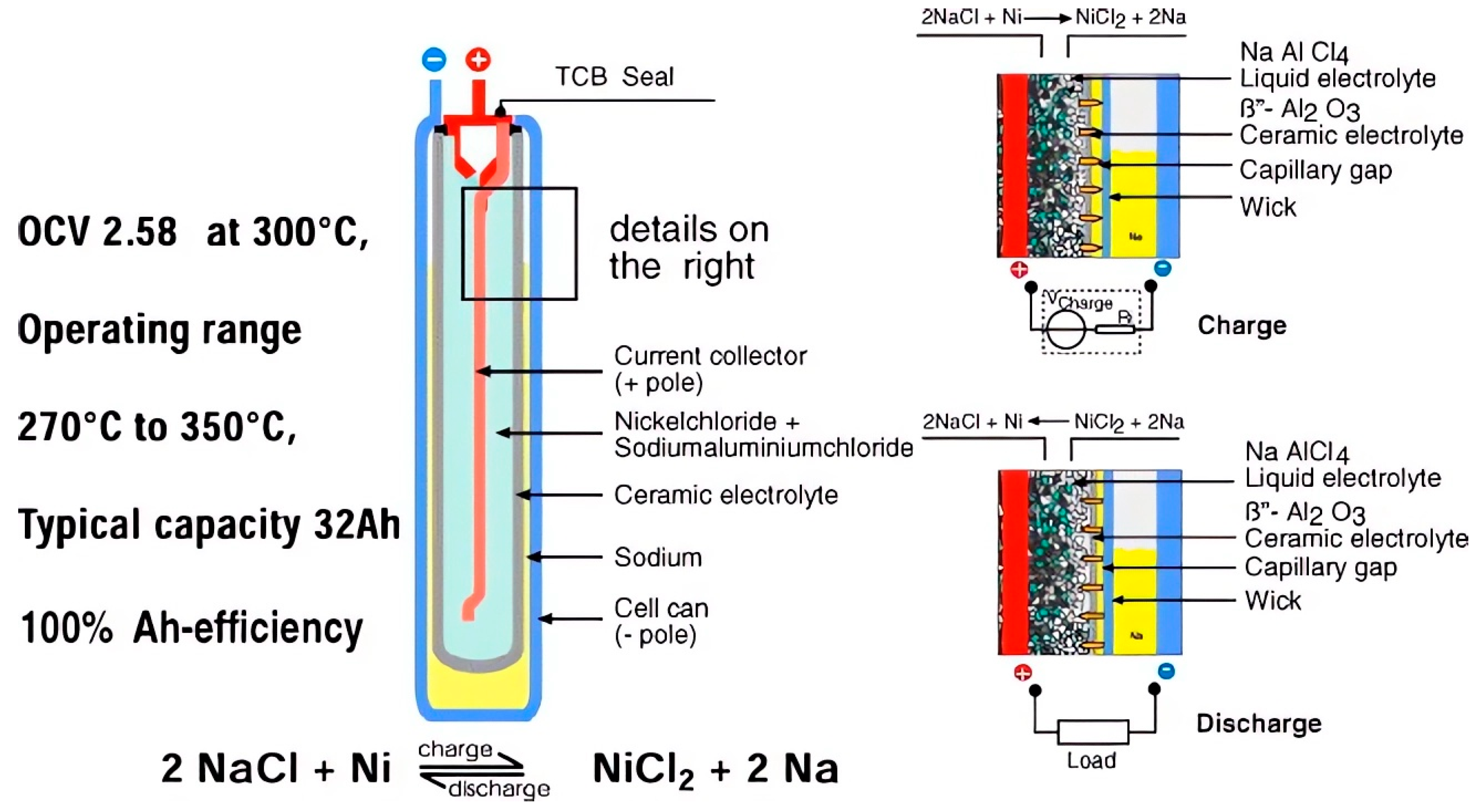
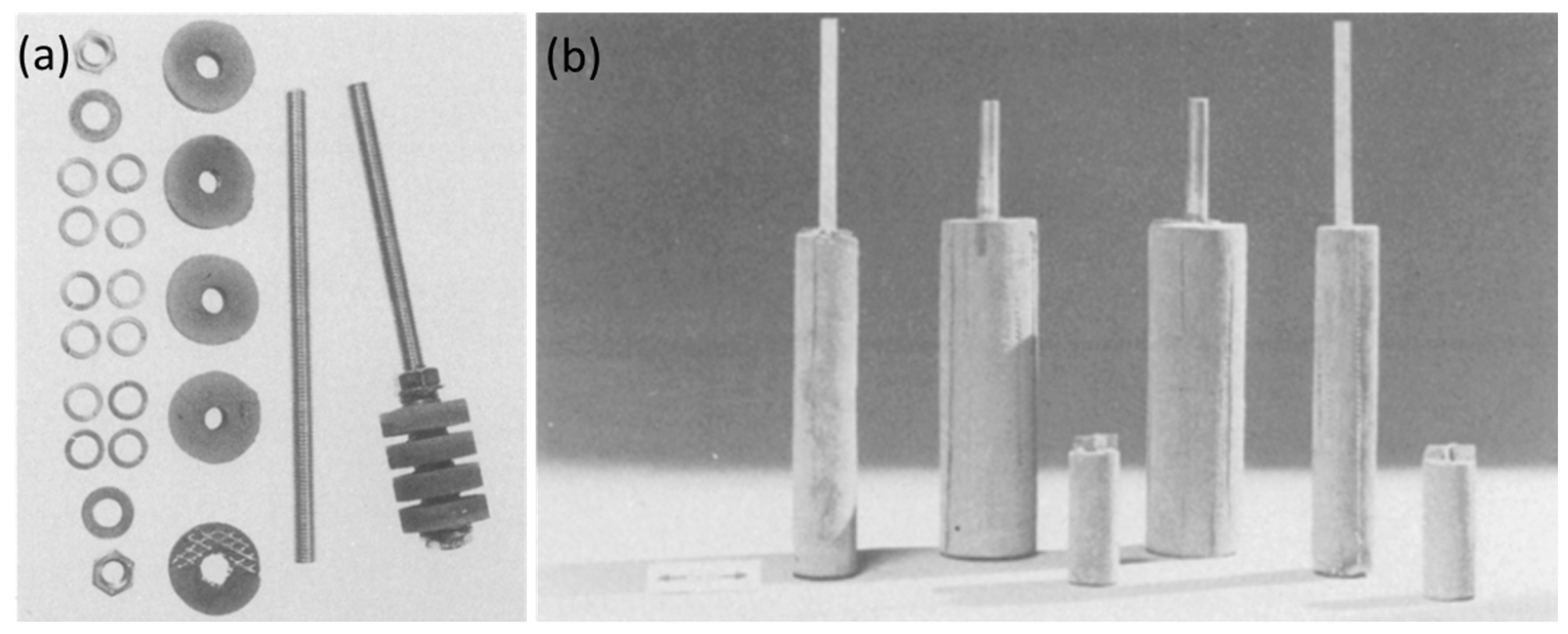
4.1. History and Evolution
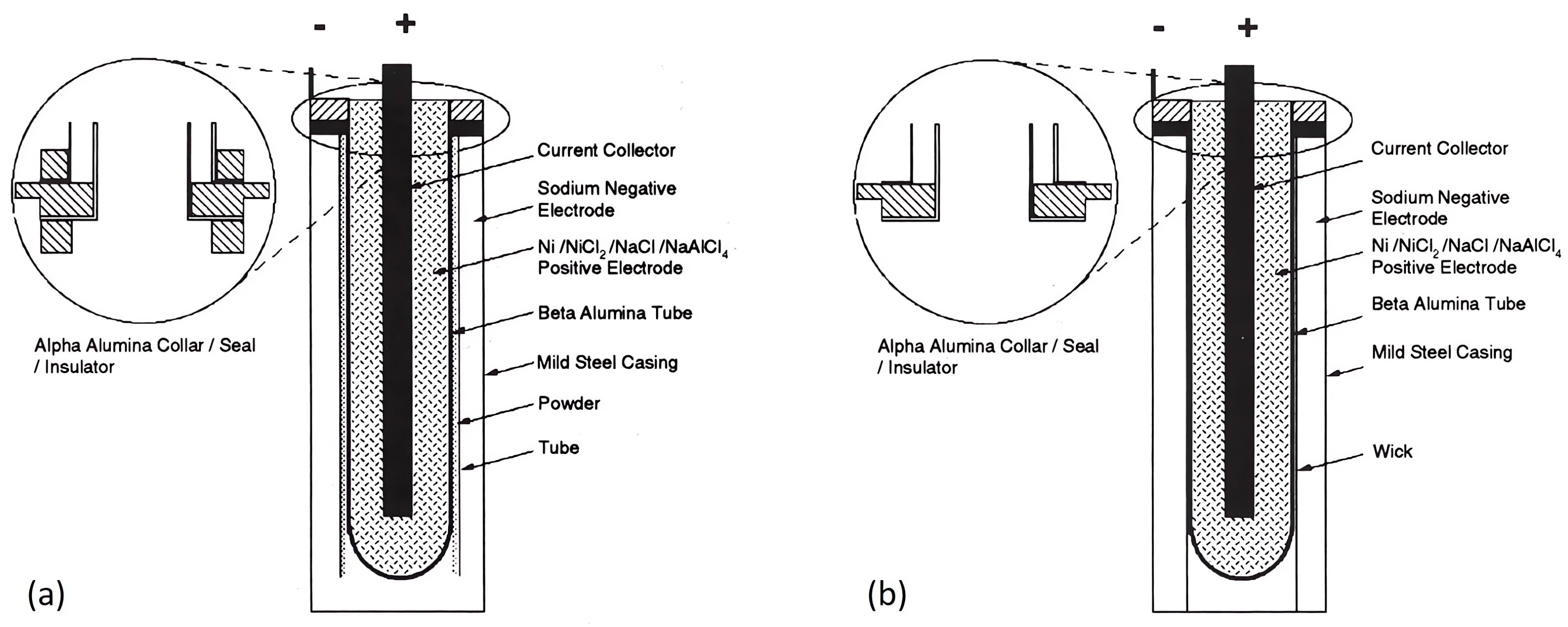
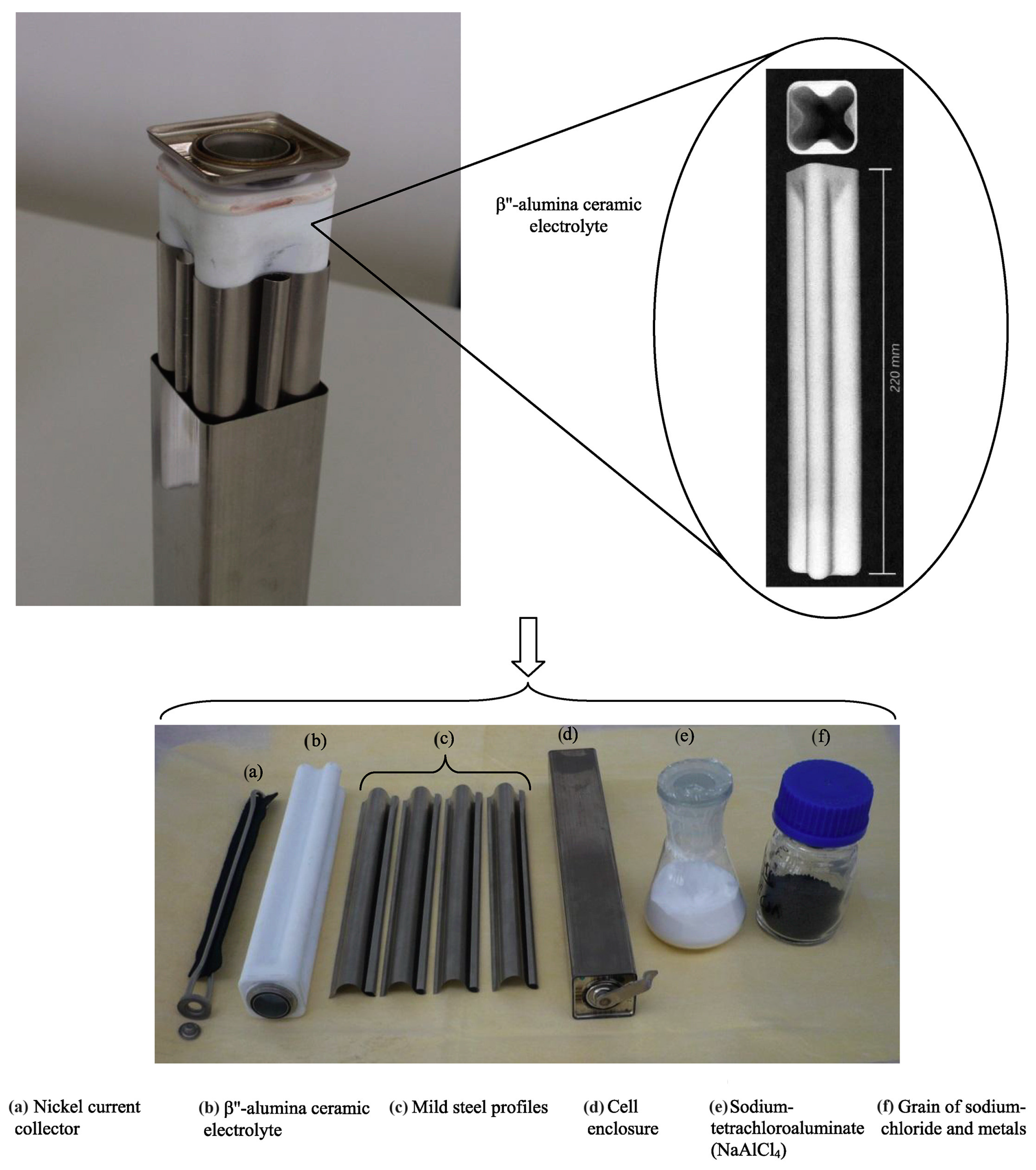
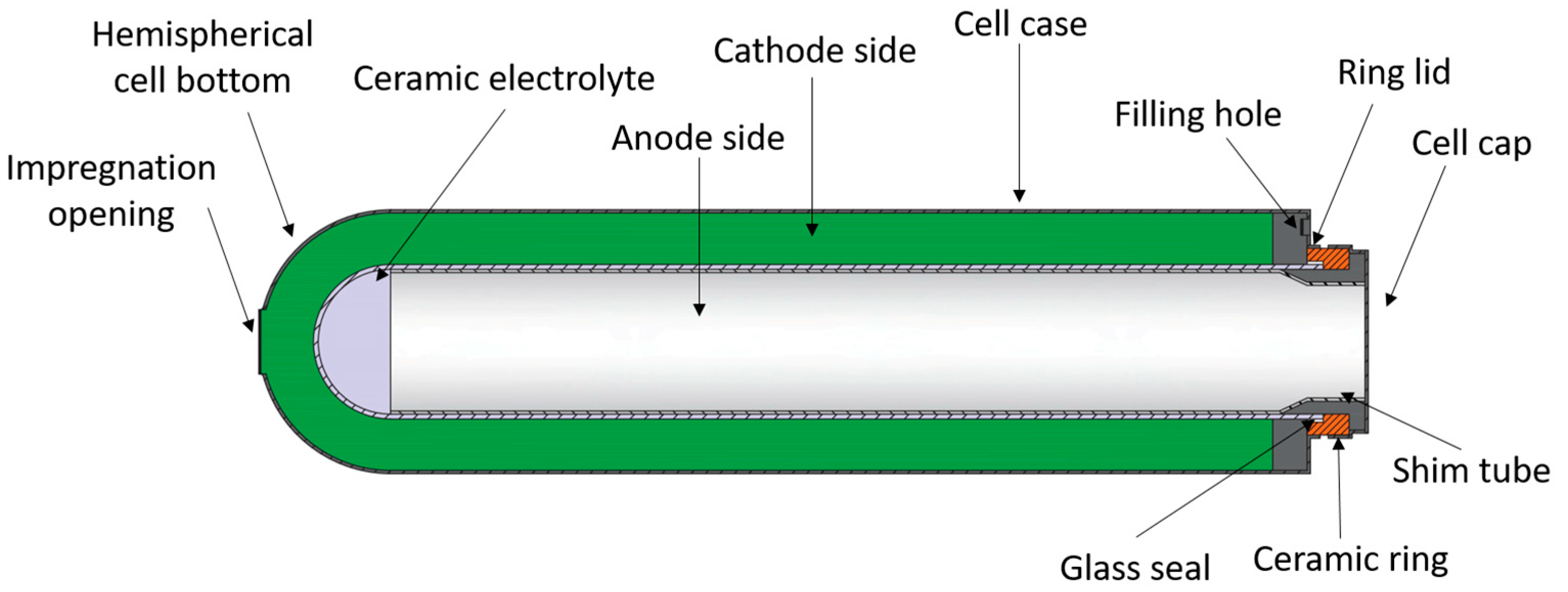
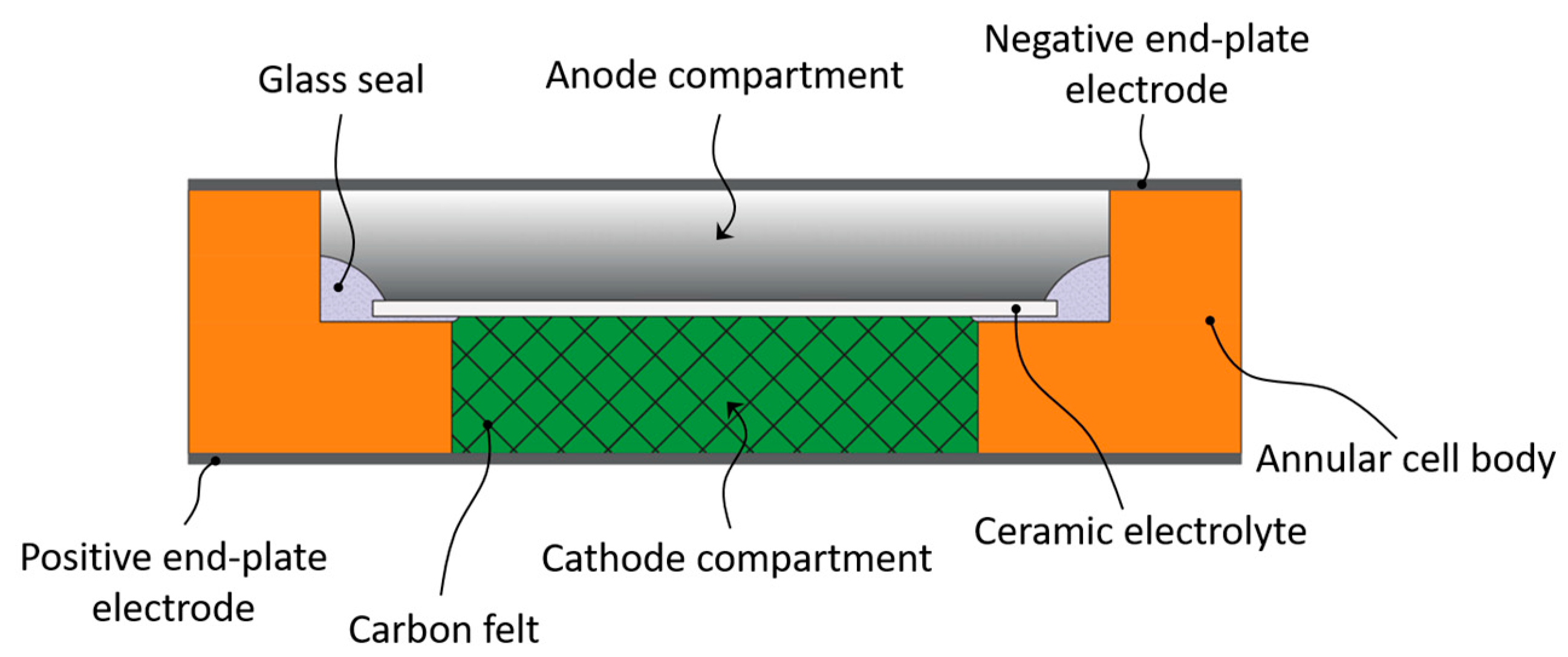
4.2. Tubular Cell
4.2.1. Cathode Side
4.2.2. Anode Side
4.2.3. Solid Electrolyte Ceramic Tube
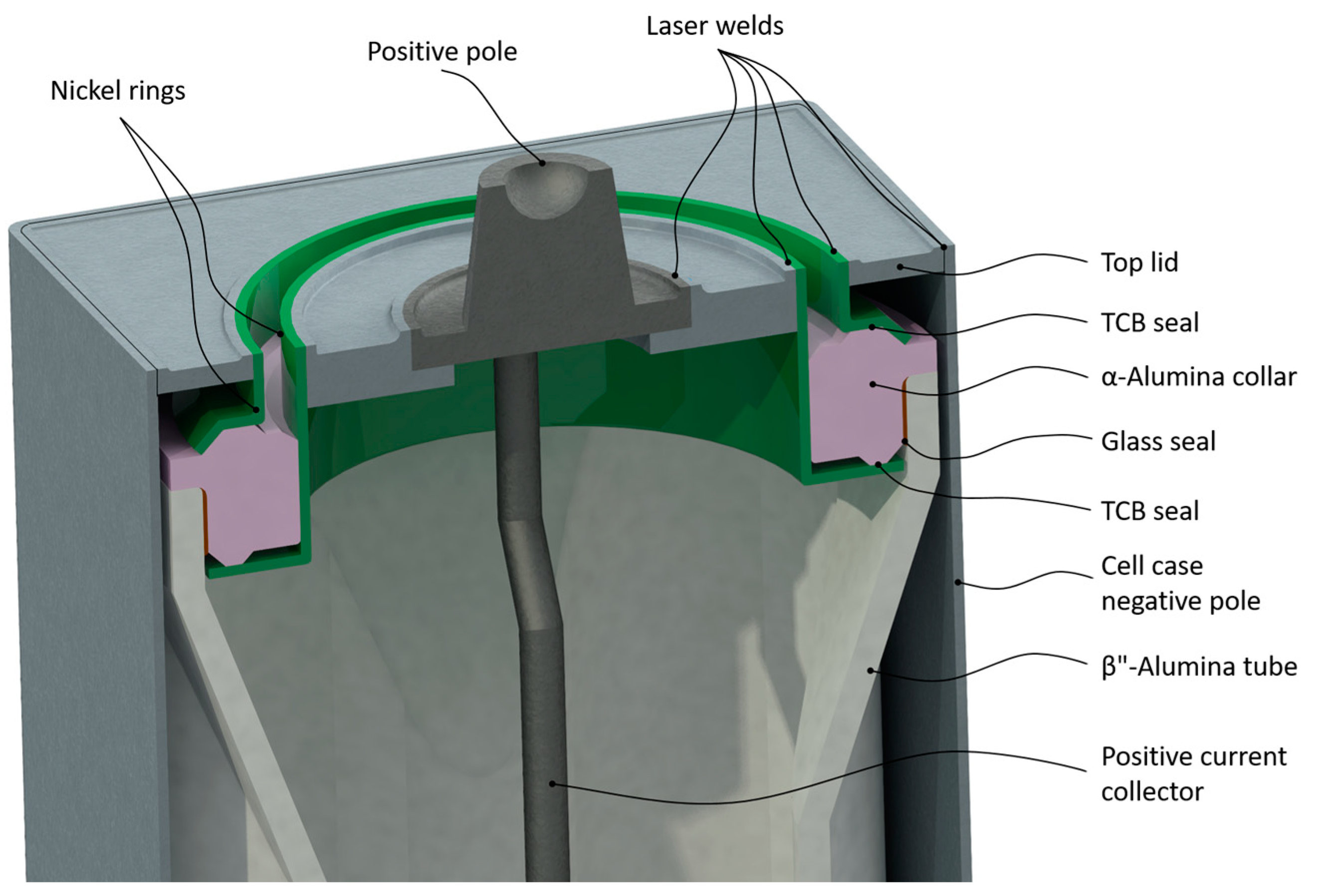
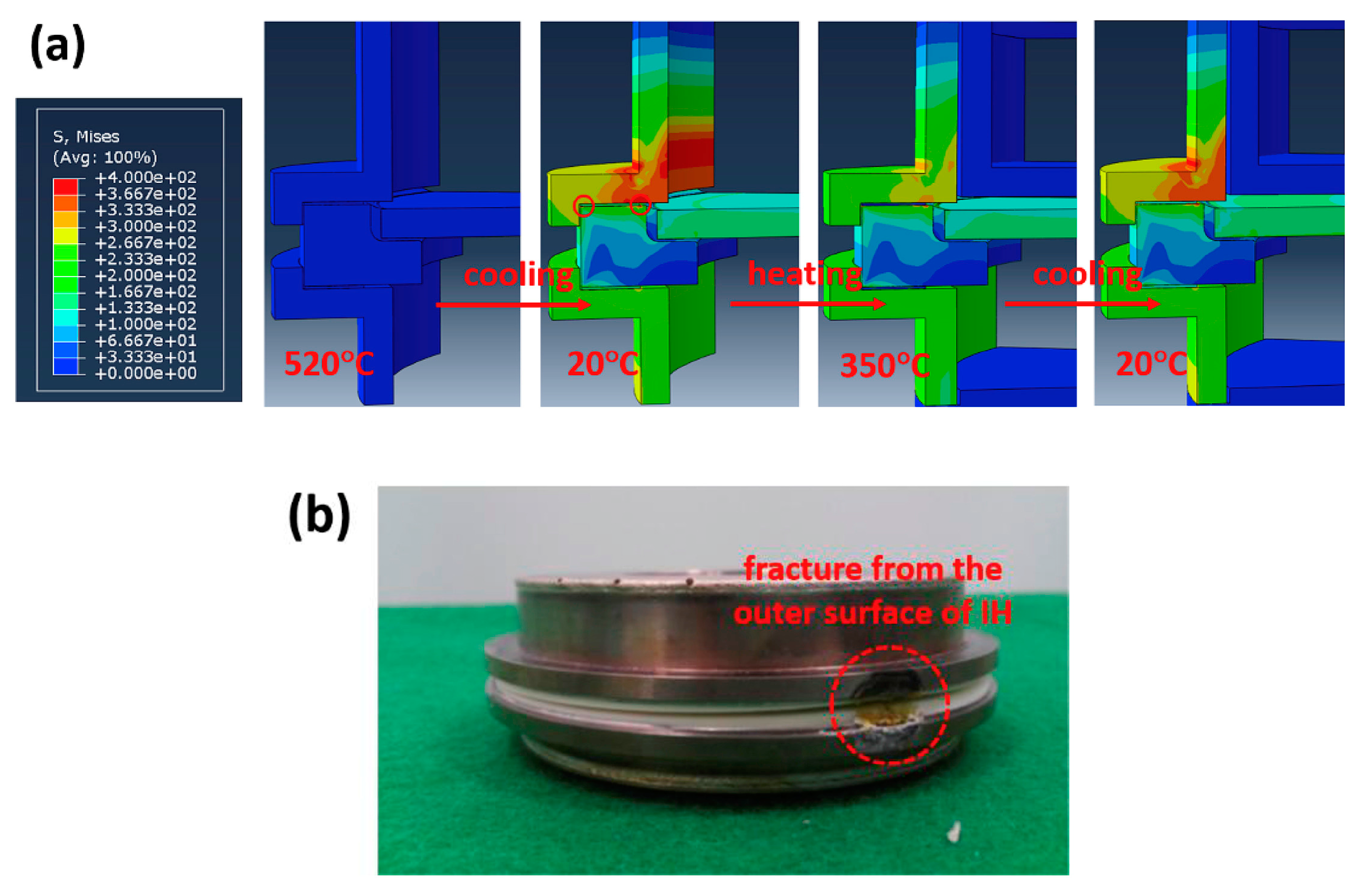
4.2.4. Cell Sealing
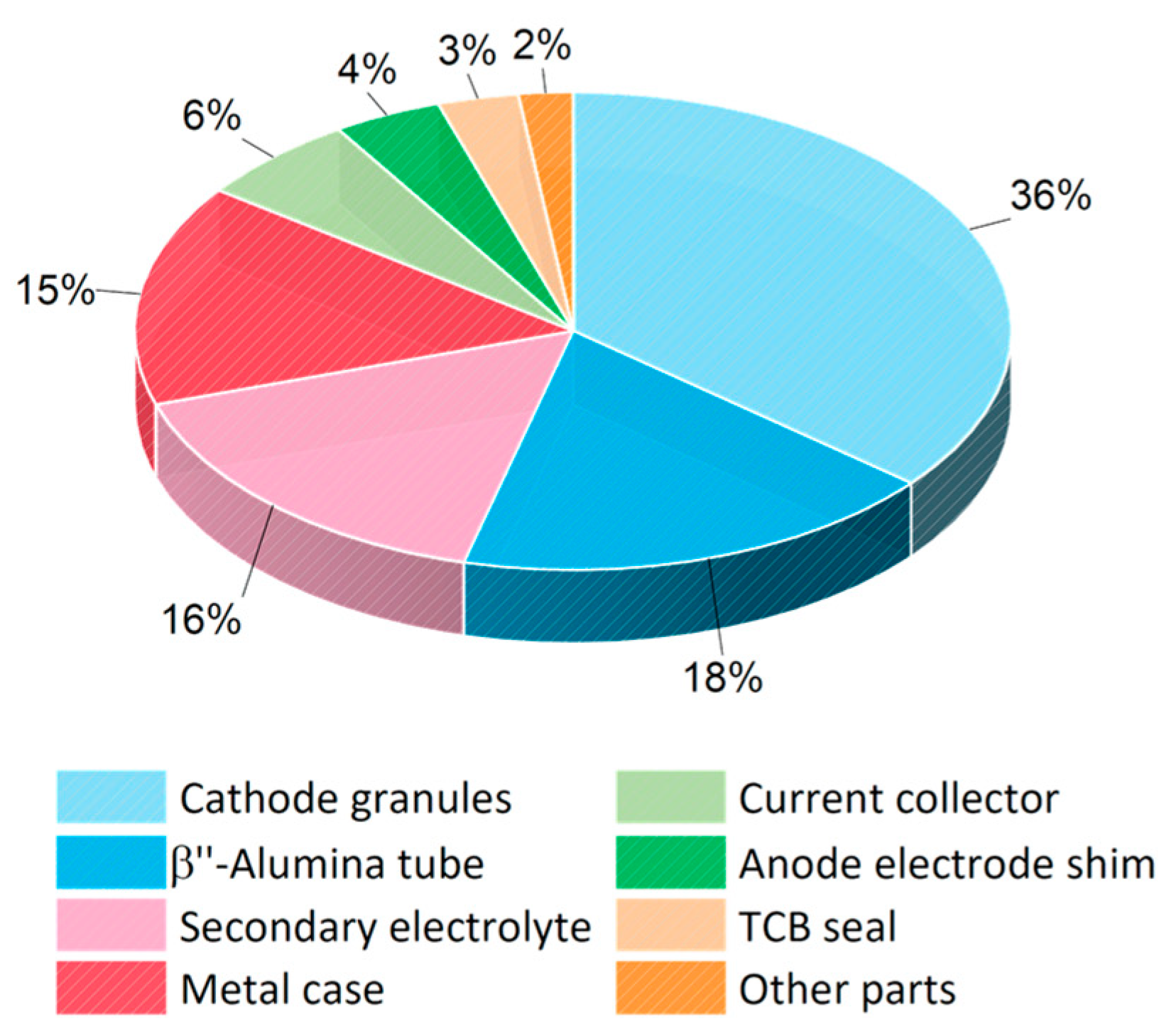
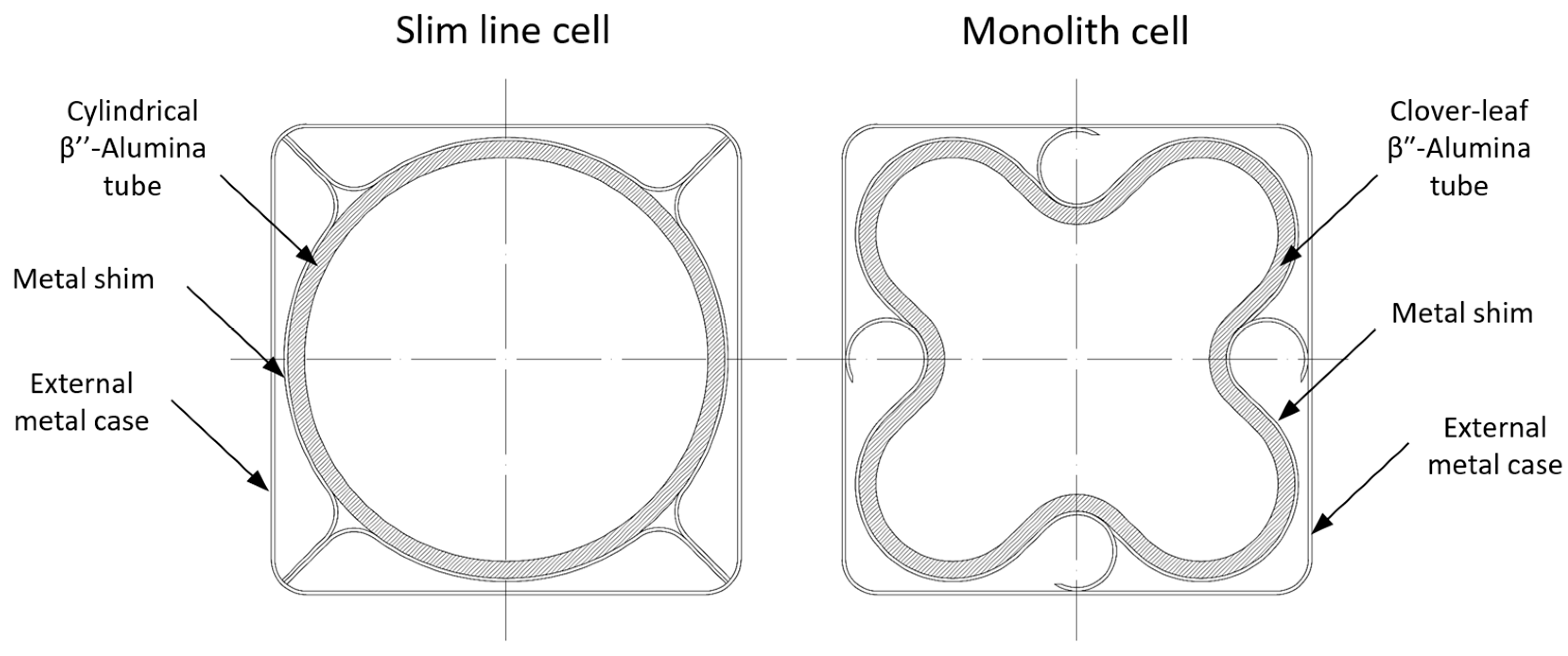
4.3. Advanced Tubular Cells and Prototypes
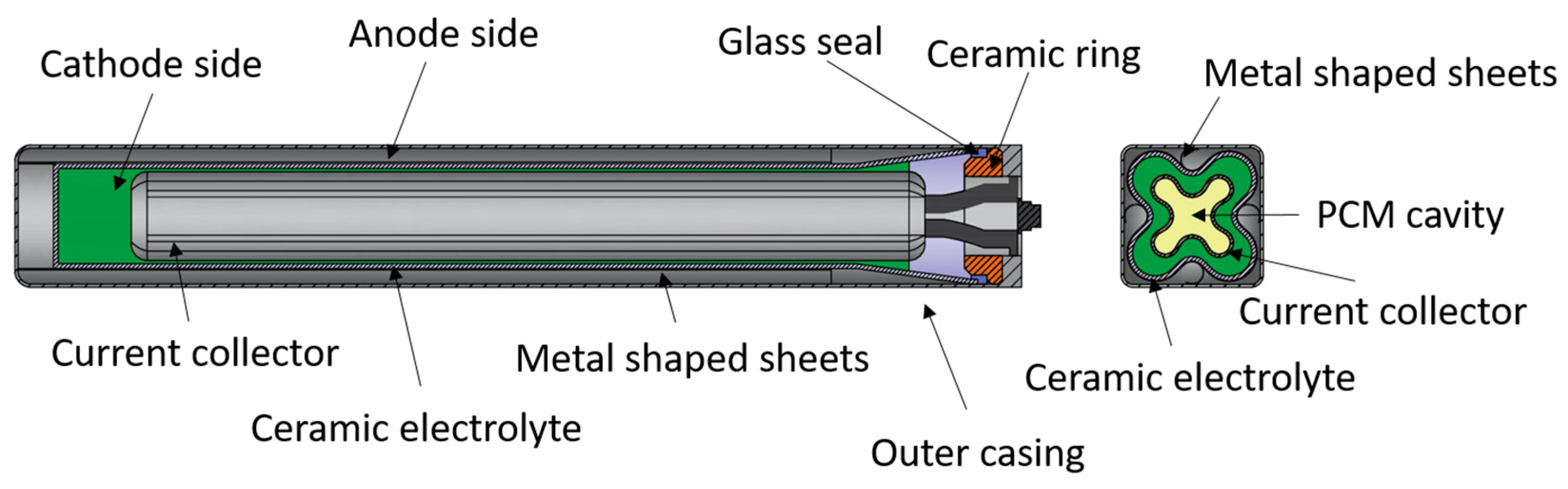
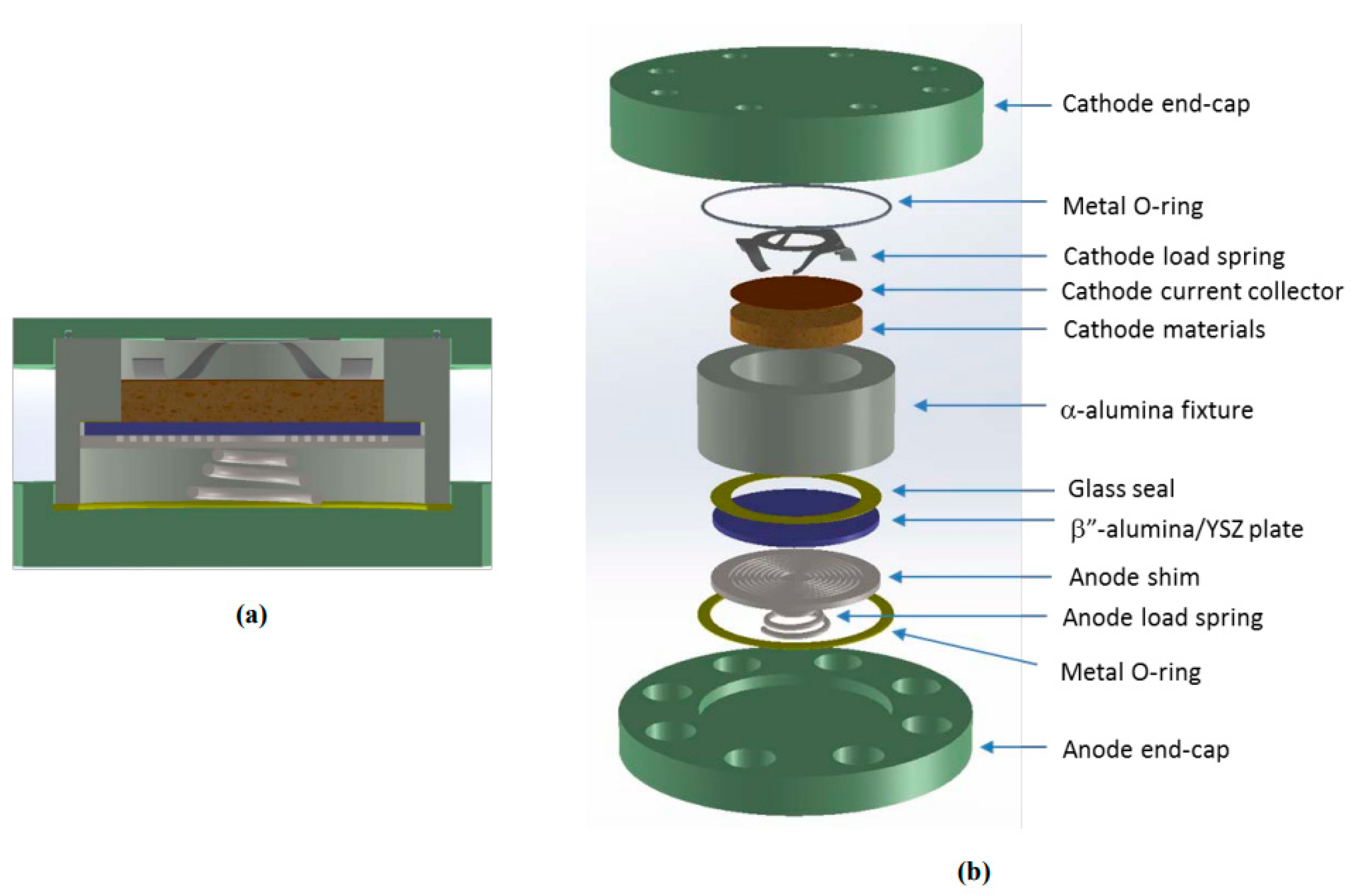
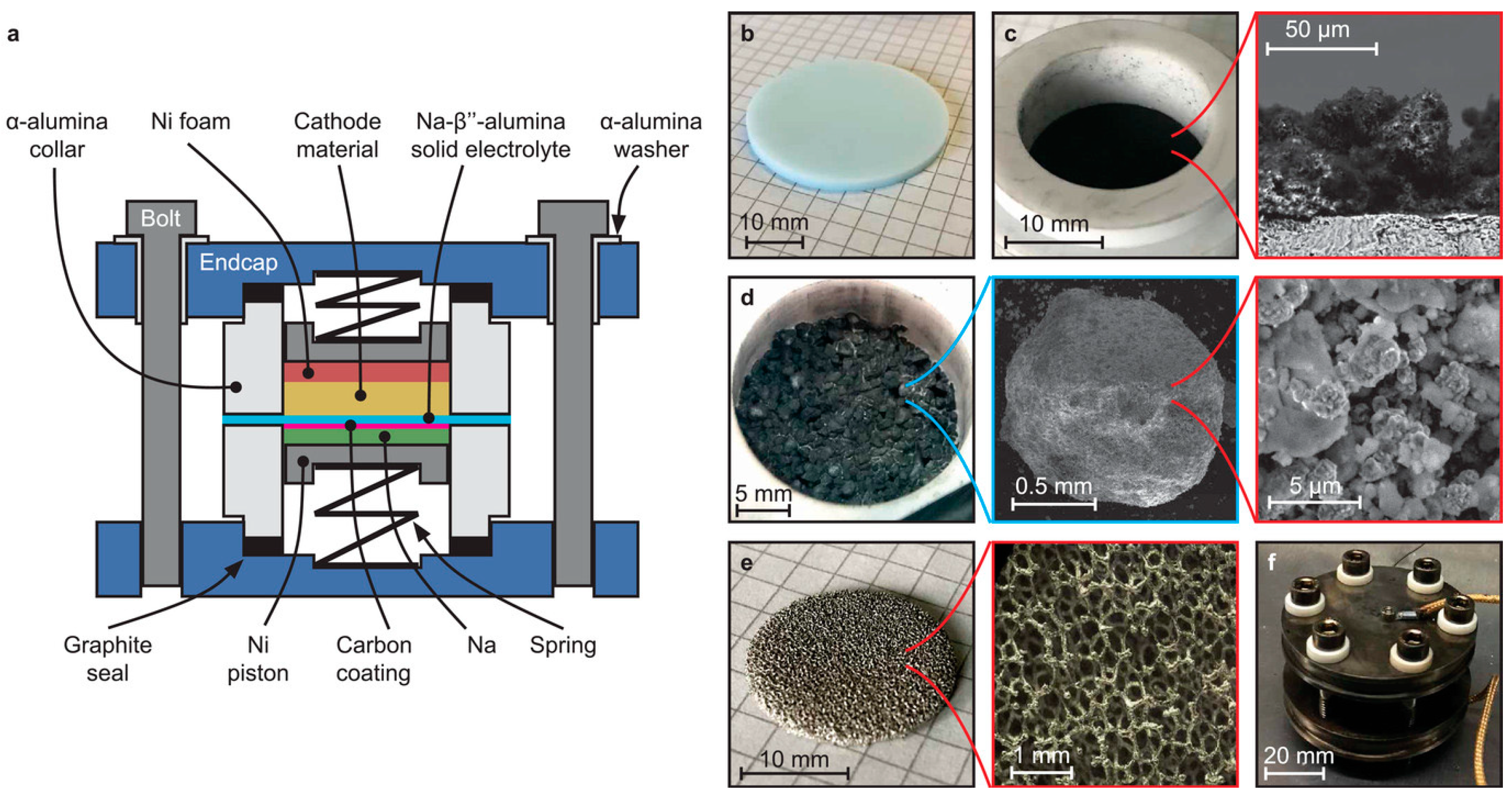
4.4. Planar Design
5. Summary and Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.-M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- James Abraham, J.; Arro, C.R.A.; Tariq, H.A.; Kahraman, R.; Al-Qaradawi, S.; Al tahtamouni, T.M.; Shakoor, R.A. Sodium and Lithium Incorporated Cathode Materials for Energy Storage Applications—A Focused Review. J. Power Sources 2021, 506, 230098. [Google Scholar] [CrossRef]
- Ellis, B.L.; Nazar, L.F. Sodium and Sodium-Ion Energy Storage Batteries. Curr. Opin. Solid State Mater. Sci. 2012, 16, 168–177. [Google Scholar] [CrossRef]
- Karabelli, D.; Singh, S.; Kiemel, S.; Koller, J.; Konarov, A.; Stubhan, F.; Miehe, R.; Weeber, M.; Bakenov, Z.; Birke, K.P. Sodium-Based Batteries: In Search of the Best Compromise Between Sustainability and Maximization of Electric Performance. Front. Energy Res. 2020, 8, 605129. [Google Scholar] [CrossRef]
- Usiskin, R.; Lu, Y.; Popovic, J.; Law, M.; Balaya, P.; Hu, Y.-S.; Maier, J. Fundamentals, Status and Promise of Sodium-Based Batteries. Nat. Rev. Mater. 2021, 6, 1020–1035. [Google Scholar] [CrossRef]
- Lu, X.; Lemmon, J.P.; Sprenkle, V.; Yang, Z. Sodium-Beta Alumina Batteries: Status and Challenges. JOM 2010, 62, 31–36. [Google Scholar] [CrossRef]
- Lu, X.; Li, G.; Kim, J.Y.; Mei, D.; Lemmon, J.P.; Sprenkle, V.L.; Liu, J. Liquid-Metal Electrode to Enable Ultra-Low Temperature Sodium-Beta Alumina Batteries for Renewable Energy Storage. Nat. Commun. 2014, 5, 4578. [Google Scholar] [CrossRef]
- Kluiters, E.C.; Schmal, D.; ter Veen, W.R.; Posthumus, K.J.C.M. Testing of a Sodium/Nickel Chloride (ZEBRA) Battery for Electric Propulsion of Ships and Vehicles. J. Power Sources 1999, 80, 261–264. [Google Scholar] [CrossRef]
- Capasso, C.; Veneri, O. Experimental Analysis of a Zebra Battery Based Propulsion System for Urban Bus under Dynamic Conditions. Energy Procedia 2014, 61, 1138–1141. [Google Scholar] [CrossRef]
- Dustmann, C.-H. Advances in ZEBRA Batteries. J. Power Sources 2004, 127, 85–92. [Google Scholar] [CrossRef]
- Sudworth, J.L. Zebra Batteries. J. Power Sources 1994, 51, 105–114. [Google Scholar] [CrossRef]
- Shamim, N.; Thomsen, E.C.; Viswanathan, V.V.; Reed, D.M.; Sprenkle, V.L.; Li, G. Evaluating ZEBRA Battery Module under the Peak-Shaving Duty Cycles. Materials 2021, 14, 2280. [Google Scholar] [CrossRef] [PubMed]
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A Comprehensive Review of Stationary Energy Storage Devices for Large Scale Renewable Energy Sources Grid Integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Benato, R.; Dambone Sessa, S.; Musio, M.; Palone, F.; Polito, R. Italian Experience on Electrical Storage Ageing for Primary Frequency Regulation. Energies 2018, 11, 2087. [Google Scholar] [CrossRef]
- Benato, R.; Cosciani, N.; Crugnola, G.; Dambone Sessa, S.; Lodi, G.; Parmeggiani, C.; Todeschini, M. Sodium Nickel Chloride Battery Technology for Large-Scale Stationary Storage in the High Voltage Network. J. Power Sources 2015, 293, 127–136. [Google Scholar] [CrossRef]
- Restello, S.; Lodi, G.; Miraldi, A.K. Sodium Nickel Chloride Batteries for Telecom Application: A Solution to Critical High Energy Density Deployment in Telecom Facilities. In Proceedings of the INTELEC, International Telecommunications Energy Conference (Proceedings), Scottsdale, AZ, USA, 30 September–4 October 2012. [Google Scholar]
- Restello, S.; Lodi, G.; Paolin, E. Sodium-Nickel Batteries for Telecom Applications: Design, Performance and Field Operational Overview. In Proceedings of the INTELEC, International Telecommunications Energy Conference (Proceedings), Amsterdam, The Netherlands, 9–13 October 2011. [Google Scholar]
- Rossi, F.; Parisi, M.L.; Greven, S.; Basosi, R.; Sinicropi, A. Life Cycle Assessment of Classic and Innovative Batteries for Solar Home Systems in Europe. Energies 2020, 13, 3454. [Google Scholar] [CrossRef]
- Accardo, A.; Dotelli, G.; Musa, M.L.; Spessa, E. Life Cycle Assessment of an NMC Battery for Application to Electric Light-Duty Commercial Vehicles and Comparison with a Sodium-Nickel-Chloride Battery. Appl. Sci. 2021, 11, 1160. [Google Scholar] [CrossRef]
- Gaillac, L.; Skaggs, D.; Pinsky, N. Sodium Nickel Chloride Battery Performance in a Stationary Application. In Proceedings of the INTELEC, International Telecommunications Energy Conference (Proceedings), Geneva, Switzerland, 10–14 September 2006. [Google Scholar]
- Ha, S.; Kim, J.K.; Choi, A.; Kim, Y.; Lee, K.T. Sodium-Metal Halide and Sodium-Air Batteries. ChemPhysChem 2014, 15, 1971–1982. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, X.; Wen, Z.; Hou, M.; Yi, B. Challenges and Thoughts on the Development of Sodium Battery Technology for Energy Storage. Chin. J. Eng. Sci. 2021, 23, 94. [Google Scholar] [CrossRef]
- Landmann, D.; Svaluto-Ferro, E.; Heinz, M.V.F.; Schmutz, P.; Battaglia, C. Elucidating the Rate-Limiting Processes in High-Temperature Sodium-Metal Chloride Batteries. Adv. Sci. 2022, 9, 2201019. [Google Scholar] [CrossRef]
- Zhan, X.; Sepulveda, J.P.; Lu, X.; Bonnett, J.F.; Canfield, N.L.; Lemmon, T.; Jung, K.; Reed, D.M.; Sprenkle, V.L.; Li, G. Elucidating the Role of Anionic Chemistry towards High-Rate Intermediate-Temperature Na-Metal Halide Batteries. Energy Storage Mater. 2020, 24, 177–187. [Google Scholar] [CrossRef]
- Li, G.; Lu, X.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L. Cell Degradation of a Na-NiCl2 (ZEBRA) Battery. J. Mater. Chem. A 2013, 1, 14935–14942. [Google Scholar] [CrossRef]
- Li, G.; Lu, X.; Kim, J.Y.; Engelhard, M.H.; Lemmon, J.P.; Sprenkle, V.L. The Role of FeS in Initial Activation and Performance Degradation of Na-NiCl2 batteries. J. Power Sources 2014, 272, 398–403. [Google Scholar] [CrossRef]
- Wu, T.; He, Q.; Wen, Z.; Ao, X.; Wu, X.; Hu, Y. Enhanced Cycle Performance of a Na/NiCl2 Battery Based on Ni Particles Encapsulated with Ni3S2 Layer. J. Power Sources 2016, 340, 411–418. [Google Scholar] [CrossRef]
- Kim, M.; Ahn, C.-W.; Hahn, B.-D.; Jung, K.; Park, Y.-C.; Cho, N.; Lee, H.; Choi, J.-H. Effects of Ni Particle Morphology on Cell Performance of Na/NiCl2 Battery. Met. Mater. Int. 2017, 23, 1234–1240. [Google Scholar] [CrossRef]
- Hosseinifar, M.; Petric, A. High Temperature versus Low Temperature Zebra (Na/NiCl2) Cell Performance. J. Power Sources 2012, 206, 402–408. [Google Scholar] [CrossRef]
- Prakash, J.; Redey, L.; Vissers, D.R. Electrochemical Behavior of Nonporous Ni/NiCl2 Electrodes in Chloroaluminate Melts. J. Electrochem. Soc. 2002, 147, 502. [Google Scholar] [CrossRef]
- Howie, R.C.; Macmillan, D.W. The Conductivity of the Binary Molten Salt System Aluminium Chloride/Sodium Chloride. J. Inorg. Nucl. Chem. 1971, 33, 3681–3686. [Google Scholar] [CrossRef]
- Li, G.; Lu, X.; Coyle, C.A.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L.; Yang, Z. Novel Ternary Molten Salt Electrolytes for Intermediate-Temperature Sodium/Nickel Chloride Batteries. J. Power Sources 2012, 220, 193–198. [Google Scholar] [CrossRef]
- Reed, D.; Coffey, G.; Mast, E.; Canfield, N.; Mansurov, J.; Lu, X.; Sprenkle, V. Wetting of Sodium on β″-Al2O3/YSZ Composites for Low Temperature Planar Sodium-Metal Halide Batteries. J. Power Sources 2013, 227, 94–100. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L.; Li, G.; Lu, X. Improved Cycling Behavior of ZEBRA Battery Operated at Intermediate Temperature of 175 °C. J. Power Sources 2013, 249, 414–417. [Google Scholar] [CrossRef]
- Fertig, M.P.; Skadell, K.; Schulz, M.; Dirksen, C.; Adelhelm, P.; Stelter, M. From High- to Low-Temperature: The Revival of Sodium-Beta Alumina for Sodium Solid-State Batteries. Batter. Supercaps 2022, 5, e202100131. [Google Scholar] [CrossRef]
- Koh, W.; Lee, J.; Kim, J.; Lee, S.; Park, D.; Lee, H.; Robins, M.; Eccleston, A.; Bhavaraju, S.; Kim, J. Development of Molten Sodium Battery Using NaSICON Solid Electrolyte Membrane for Stationary and Large-Scale Electrical Energy Storage System. In Electrochemical Society Meeting Abstracts 226; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2014; MA2014-02; p. 624. [Google Scholar] [CrossRef]
- Kim, J.; Jo, S.H.; Bhavaraju, S.; Eccleston, A.; Kang, S.O. Low Temperature Performance of Sodium-Nickel Chloride Batteries with NaSICON Solid Electrolyte. J. Electroanal. Chem. 2015, 759, 201–206. [Google Scholar] [CrossRef]
- Jo, S.H.; Kim, J.; Kang, S.O.; Kim, J.-S.; Bhavaraju, S. Investigation of Manufacturing Parameters for NaCl–Ni Granule Type Cathodes Used in Low Temperature NaSICON Sodium-Metal Chloride Batteries. J. Alloys Compd. 2016, 665, 288–293. [Google Scholar] [CrossRef]
- Jung, K.; Lee, S.; Park, Y.-C.; Kim, C.-S. Finite Element Analysis Study on the Thermomechanical Stability of Thermal Compression Bonding (TCB) Joints in Tubular Sodium Sulfur Cells. J. Power Sources 2014, 250, 1–14. [Google Scholar] [CrossRef]
- Jung, K.; Colker, J.P.; Cao, Y.; Kim, G.; Park, Y.-C.; Kim, C.-S. A Thermo-Mechanical Stress Prediction Model for Contemporary Planar Sodium Sulfur (NaS) Cells. J. Power Sources 2016, 324, 665–673. [Google Scholar] [CrossRef]
- Hueso, K.B.; Palomares, V.; Armand, M.; Rojo, T. Challenges and Perspectives on High and Intermediate-Temperature Sodium Batteries. Nano Res. 2017, 10, 4082–4114. [Google Scholar] [CrossRef]
- Hueso, K.B.; Armand, M.; Rojo, T. High Temperature Sodium Batteries: Status, Challenges and Future Trends. Energy Environ. Sci. 2013, 6, 734–749. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, D.; Palomares, V.; Shanmukaraj, D.; Sun, B.; Tang, X.; Wang, C.; Armand, M.; Rojo, T.; Wang, G. Revitalising Sodium–Sulfur Batteries for Non-High-Temperature Operation: A Crucial Review. Energy Environ. Sci. 2020, 13, 3848–3879. [Google Scholar] [CrossRef]
- Zhan, X.; Li, M.M.; Weller, J.M.; Sprenkle, V.L.; Li, G. Recent Progress in Cathode Materials for Sodium-Metal Halide Batteries. Materials 2021, 14, 3260. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sprenkle, V.L.; Li, G.; Canfield, N.L.; Kim, J.Y.; Chang, H.J.; Meinhardt, K.D. Advanced Intermediate Temperature Sodium–Nickel Chloride Batteries with Ultra-High Energy Density. Nat. Commun. 2016, 7, 10683. [Google Scholar] [CrossRef]
- Ratke, L.; Voorhees, P.W. Growth and Coarsening: Ostwald Ripening in Materials Processing. Choice Rev. Online 2002, 39, 39-6445. [Google Scholar] [CrossRef]
- Prakash, J.; Redey, L.; Vissers, D.R. Morphological Considerations of the Nickel Chloride Electrodes for Zebra Batteries. J. Power Sources 1999, 84, 63–69. [Google Scholar] [CrossRef]
- Bones, R.J.; Teagle, D.A.; Brooker, S.D.; Cullen, F.L. Development of a Ni, NiCl2 Positive Electrode for a Liquid Sodium (ZEBRA) Battery Cell. J. Electrochem. Soc. 1989, 136, 1274–1277. [Google Scholar] [CrossRef]
- Prakash, J.; Redey, L.; Vissers, D.R. Effect of Chemical Additives on the Performance of Na/NiCl2 Cells. Ionics 2000, 6, 210–217. [Google Scholar] [CrossRef]
- Ratnakumar, B.V.; Surampudi, S.; Halpert, G. Effects of Sulfur Additive on the Performance of Na/NiCl2 Cells. J. Power Sources 1994, 48, 349–360. [Google Scholar] [CrossRef]
- Lu, X.; Lemmon, J.P.; Kim, J.Y.; Sprenkle, V.L.; Yang, Z. High Energy Density Na-S/NiCl2 Hybrid Battery. J. Power Sources 2013, 224, 312–316. [Google Scholar] [CrossRef]
- Prakash, J.; Redey, L.; Vissers, D.R.; DeGruson, J. Effect of Sodium Iodide Additive on the Electrochemical Performance of Sodium/Nickel Chloride Cells. J. Appl. Electrochem. 2000, 30, 1229–1233. [Google Scholar] [CrossRef]
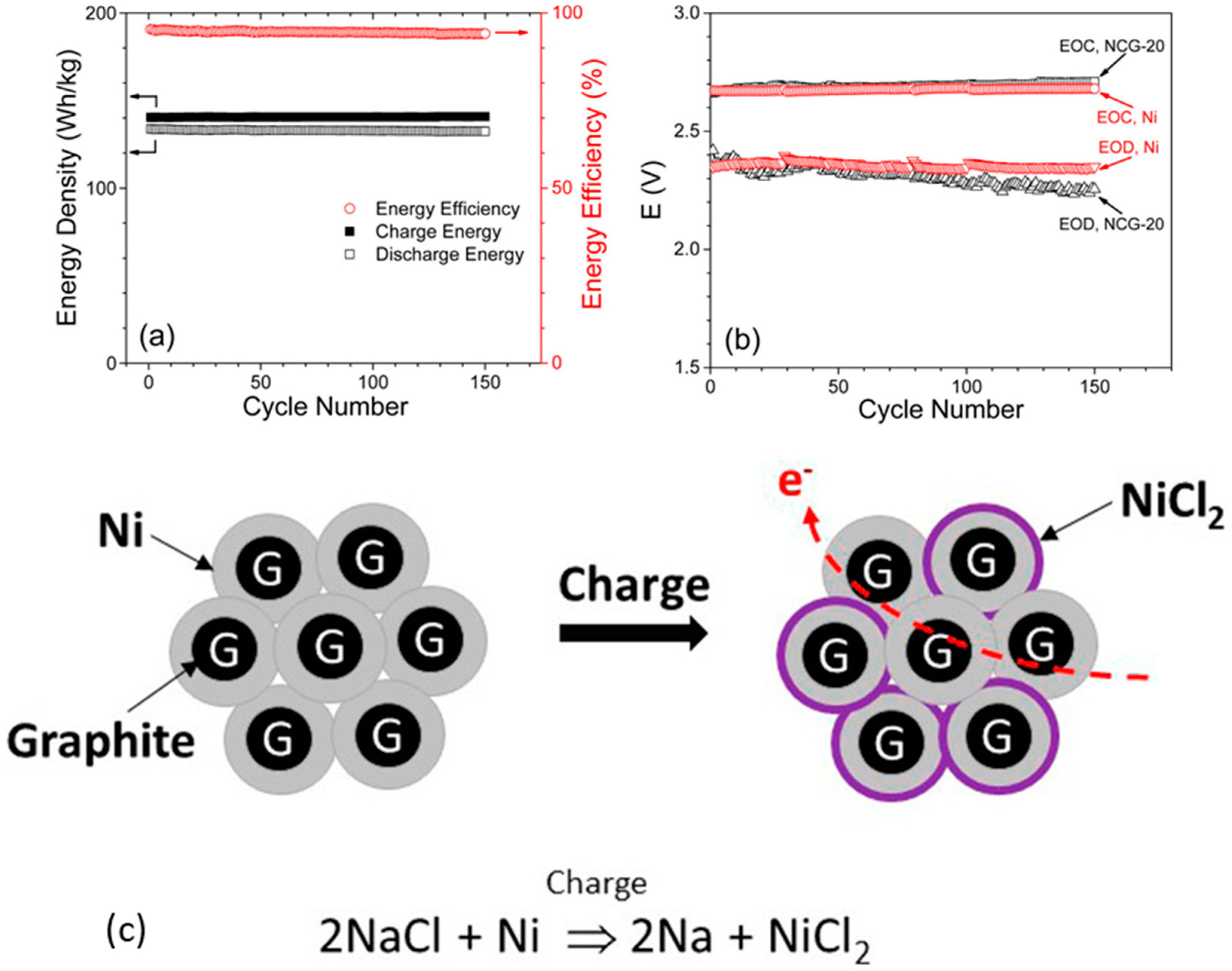
- Chang, H.J.; Canfield, N.L.; Jung, K.; Sprenkle, V.L.; Li, G. Advanced Na-NiCl2 Battery Using Nickel-Coated Graphite with Core-Shell Microarchitecture. ACS Appl. Mater. Interfaces 2017, 9, 11609–11614. [Google Scholar] [CrossRef]
- Chang, H.J.; Lu, X.; Bonnett, J.F.; Canfield, N.L.; Son, S.; Park, Y.C.; Jung, K.; Sprenkle, V.L.; Li, G. “Ni-Less” Cathodes for High Energy Density, Intermediate Temperature Na-NiCl2 Batteries. Adv. Mater. Interfaces 2018, 5, 1701592. [Google Scholar] [CrossRef]
- Bones, R.J.; Coetzer, J.; Galloway, R.C.; Teagle, D.A. A Sodium/Iron(II) Chloride Cell with a Beta Alumina Electrolyte. J. Electrochem. Soc. 1987, 134, 2379–2382. [Google Scholar] [CrossRef]
- Li, G.; Lu, X.; Kim, J.Y.; Viswanathan, V.V.; Meinhardt, K.D.; Engelhard, M.H.; Sprenkle, V.L. An Advanced Na-FeCl2 ZEBRA Battery for Stationary Energy Storage Application. Adv. Energy Mater. 2015, 5, 1500357. [Google Scholar] [CrossRef]
- Zhan, X.; Bowden, M.E.; Lu, X.; Bonnett, J.F.; Lemmon, T.; Reed, D.M.; Sprenkle, V.L.; Li, G. A Low-Cost Durable Na-FeCl2 Battery with Ultrahigh Rate Capability. Adv. Energy Mater. 2020, 10, 1903472. [Google Scholar] [CrossRef]
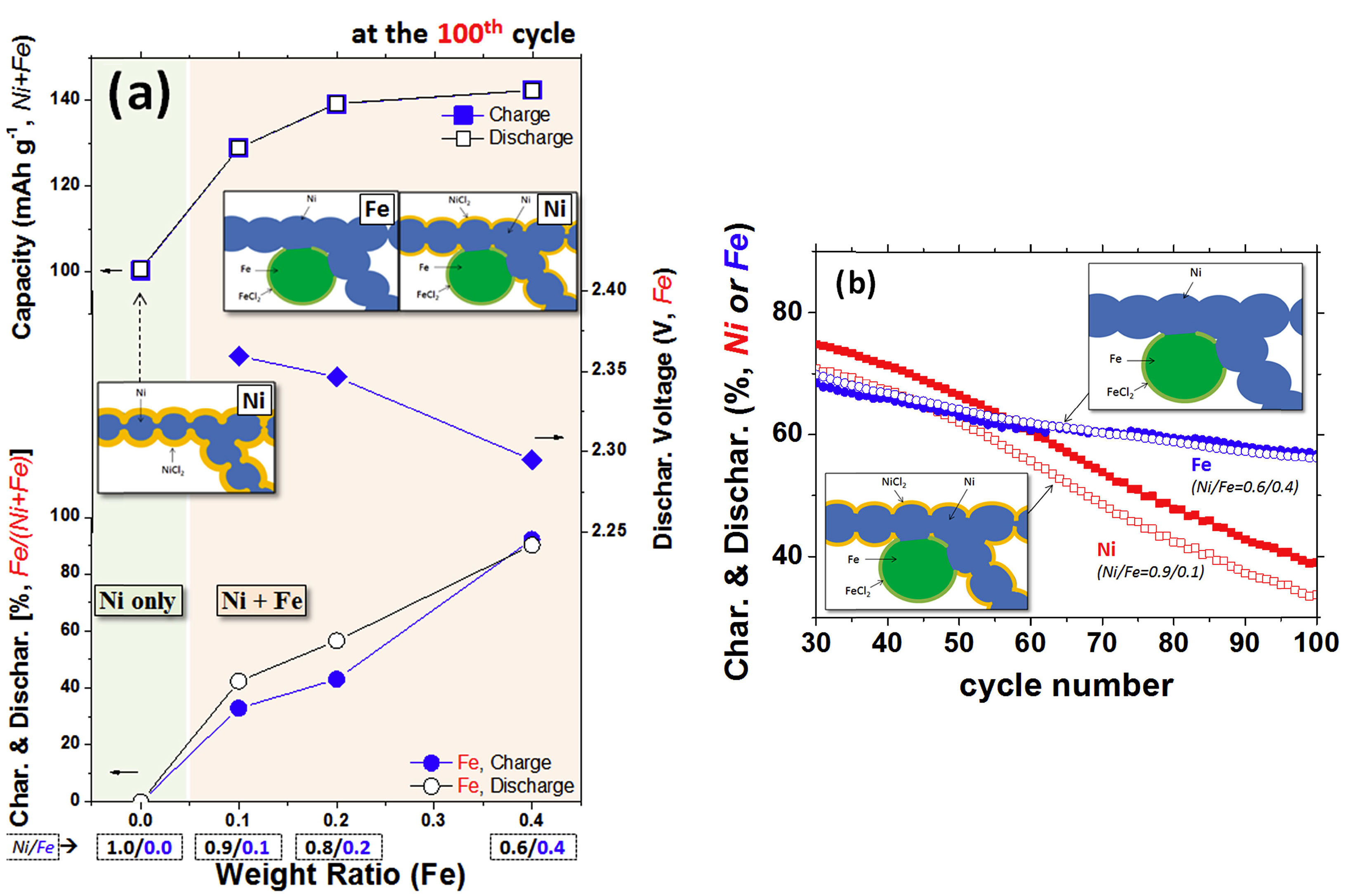
- Ahn, C.-W.; Kim, M.; Hahn, B.-D.; Hong, I.; Kim, W.; Moon, G.; Lee, H.; Jung, K.; Park, Y.-C.; Choi, J.-H. Microstructure Design of Metal Composite for Active Material in Sodium Nickel-Iron Chloride Battery. J. Power Sources 2016, 329, 50–56. [Google Scholar] [CrossRef]
- Ahn, B.-M.; Ahn, C.-W.; Hahn, B.-D.; Choi, J.-J.; Kim, Y.-D.; Lim, S.-K.; Jung, K.; Park, Y.-C.; Choi, J.-H. Easy Approach to Realize Low Cost and High Cell Capacity in Sodium Nickel-Iron Chloride Battery. Compos. Part B Eng. 2019, 168, 442–447. [Google Scholar] [CrossRef]
- Ahn, B.-M.; Ahn, C.-W.; Hahn, B.-D.; Choi, J.-J.; Kim, Y.-D.; Lim, S.-K.; Choi, J.-H. Effect of Cathode Microstructure on Electrochemical Properties of Sodium Nickel-Iron Chloride Batteries. Materials 2021, 14, 5605. [Google Scholar] [CrossRef]
- Frusteri, L.; Leonardi, S.G.; Samperi, M.; Antonucci, V.; D’Urso, C. Characterization and Testing of Cathode Materials for High Temperature Sodium Nickel-ironchloride Battery. J. Energy Storage 2022, 55, 105503. [Google Scholar] [CrossRef]
- Xue, L.; Xin, S.; Goodenough, J.B.; Angell, C.A. An Inverse Aluminum Battery: Putting the Aluminum as the Cathode. ACS Energy Lett. 2017, 2, 1534–1538. [Google Scholar] [CrossRef]
- Zhan, X.; Bonnett, J.F.; Engelhard, M.H.; Reed, D.M.; Sprenkle, V.L.; Li, G. A High-Performance Na-Al Battery Based on Reversible NaAlCl4 Catholyte. Adv. Energy Mater. 2020, 10, 2001378. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, H.-J.; Byun, D.-J.; Cho, K.-K.; Ahn, J.-H.; Kim, C.-S. Electrochemically Activated Na-ZnCl2 Battery Using a Carbon Matrix in the Cathode Compartment. J. Power Sources 2019, 440, 227110. [Google Scholar] [CrossRef]
- Lu, X.; Li, G.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L.; Yang, Z. A Novel Low-Cost Sodium–Zinc Chloride Battery. Energy Environ. Sci. 2013, 6, 1837. [Google Scholar] [CrossRef]
- Sprenkle, V.L.; Lu, X.; Jung, K.; Chang, H.J.; Canfield, N.L.; Li, G.; Bonnett, J.F. An Intermediate-Temperature High-Performance Na-ZnCl2 Battery. ACS Omega 2018, 3, 15702–15708. [Google Scholar] [CrossRef]
- Ratnakumar, B.V.; Di Stefano, S.; Halpert, G. Electrochemistry of Metal Chloride Cathodes in Sodium Batteries. J. Electrochem. Soc. 1990, 137, 2991–2997. [Google Scholar] [CrossRef]
- Pye, S.; Winnick, J.; Kohl, P.A. Iron, Copper, and Nickel Behavior in Buffered, Neutral Aluminum Chloride: 1-Methyl-3-ethylimidazolium Chloride Molten Salt. J. Electrochem. Soc. 1997, 144, 1933–1938. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Sun, I.-W. Electrochemical Study of Copper in a Basic 1-Ethyl-3-Methylimidazolium Tetrafluoroborate Room Temperature Molten Salt. Electrochim. Acta 1999, 45, 441–450. [Google Scholar] [CrossRef]
- Kim, B.R.; Jeong, G.; Kim, A.; Kim, Y.; Kim, M.G.; Kim, H.; Kim, Y.J. High Performance Na-CuCl2 Rechargeable Battery toward Room Temperature ZEBRA-Type Battery. Adv. Energy Mater. 2016, 6, 1600862. [Google Scholar] [CrossRef]
- Niu, C.; Zhang, Y.; Ma, S.; Wan, Y.; Yang, H.; Liu, X. An Intermediate Temperature Sodium Copper Chloride Battery Using Ionic Liquid Electrolyte and Its Degradation Mechanism. Ionics 2019, 25, 4189–4196. [Google Scholar] [CrossRef]
- Niu, C.; Ji, L.; Chen, Y.; Ma, S.; Zhang, Y.; Liu, X.; Yang, H. Low-Melting-Point Ionic Liquid Electrolyte for an Intermediate-Temperature Sodium–Copper Chloride Battery. Energy Fuels 2021, 35, 12538–12545. [Google Scholar] [CrossRef]
- Gao, X.; Hu, Y.; Li, Y.; Wang, J.; Wu, X.; Yang, J.; Wen, Z. High-Rate and Long-Life Intermediate-Temperature Na-NiCl2 Battery with Dual-Functional Ni–Carbon Composite Nanofiber Network. ACS Appl. Mater. Interfaces 2020, 12, 24767–24776. [Google Scholar] [CrossRef]
- Li, Y.; Shi, L.; Gao, X.; Wang, J.; Hu, Y.; Wu, X.; Wen, Z. Constructing a Charged-State Na-NiCl2 Battery with NiCl2/Graphene Aerogel Composite as Cathode. Chem. Eng. J. 2021, 421, 127853. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Wang, J.; Gao, X.; Hu, Y.; Wen, Z. Ni-Less Cathode with 3D Free-Standing Conductive Network for Planar Na-NiCl2 Batteries. Chem. Eng. J. 2020, 387, 124059. [Google Scholar] [CrossRef]
- Gerovasili, E.; May, J.F.; Sauer, D.U. Experimental Evaluation of the Performance of the Sodium Metal Chloride Battery below Usual Operating Temperatures. J. Power Sources 2014, 251, 137–144. [Google Scholar] [CrossRef]
- Lu, X.; Li, G.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L.; Yang, Z. The Effects of Temperature on the Electrochemical Performance of Sodium-Nickel Chloride Batteries. J. Power Sources 2012, 215, 288–295. [Google Scholar] [CrossRef]
- Yao, Y.-F.Y.; Kummer, J.T. Ion Exchange Properties of and Rates of Ionic Diffusion in Beta-Alumina. J. Inorg. Nucl. Chem. 1967, 29, 2453–2475. [Google Scholar] [CrossRef]
- Chi, C.; Katsui, H.; Goto, T. Effect of Li Addition on the Formation of Na-β/β″-Alumina Film by Laser Chemical Vapor Deposition. Ceram. Int. 2017, 43, 1278–1283. [Google Scholar] [CrossRef]
- Beckers, J. Ionic Conduction in Na+-β-Alumina Studied by Molecular Dynamics Simulation. Solid State Ionics 2000, 133, 217–231. [Google Scholar] [CrossRef]
- Stevens, R.; Binner, J.G.P. Structure, Properties and Production of β-Alumina. J. Mater. Sci. 1984, 19, 695–715. [Google Scholar] [CrossRef]
- Birnie, D.P. On the Structural Integrity of the Spinel Block in the β″-Alumina Structure. Acta Crystallogr. Sect. B Struct. Sci. 2012, 68, 118–122. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Koganei, K.; Oyama, T.; Inada, M.; Enomoto, N.; Hayashi, K. C-Axis Oriented β″-Alumina Ceramics with Anisotropic Ionic Conductivity Prepared by Spark Plasma Sintering. Solid State Ionics 2014, 267, 22–26. [Google Scholar] [CrossRef]
- Li, K.; Yang, Y.; Zhang, X.; Liang, S. Highly Oriented Β″-Alumina Ceramics with Excellent Ionic Conductivity and Mechanical Performance Obtained by Spark Plasma Sintering Technique. J. Mater. Sci. 2020, 55, 8435–8443. [Google Scholar] [CrossRef]
- Liang, S.; Yang, Y.; Li, K.; Zhang, X. A Study on the Preparation of Oriented Beta″-Alumina Ceramics Using Rod/Flake-like Boehmite as Precursors and Their Properties. J. Eur. Ceram. Soc. 2020, 40, 4047–4055. [Google Scholar] [CrossRef]
- Kim, J.Y.; Canfield, N.L.; Bonnett, J.F.; Sprenkle, V.L.; Jung, K.; Hong, I. A Duplex β″-Al2O3 Solid Electrolyte Consisting of a Thin Dense Layer and a Porous Substrate. Solid State Ionics 2015, 278, 192–197. [Google Scholar] [CrossRef]
- Canfield, N.L.; Kim, J.Y.; Bonnett, J.F.; Pearson, R.L.; Sprenkle, V.L.; Jung, K. Effects of Fabrication Conditions on Mechanical Properties and Microstructure of Duplex β″-Al2O3 Solid Electrolyte. Mater. Sci. Eng. B 2015, 197, 43–50. [Google Scholar] [CrossRef]
- Jung, K.; Chang, H.J.; Bonnett, J.F.; Canfield, N.L.; Sprenkle, V.L.; Li, G. An Advanced Na-NiCl2 Battery Using Bi-Layer (Dense/Micro-Porous) β″-Alumina Solid-State Electrolytes. J. Power Sources 2018, 396, 297–303. [Google Scholar] [CrossRef]
- Wen, Z.; Gu, Z.; Xu, X.; Cao, J.; Zhang, F.; Lin, Z. Research Activities in Shanghai Institute of Ceramics, Chinese Academy of Sciences on the Solid Electrolytes for Sodium Sulfur Batteries. J. Power Sources 2008, 184, 641–645. [Google Scholar] [CrossRef]
- Dirksen, C.L.; Skadell, K.; Schulz, M.; Fertig, M.P.; Stelter, M. Influence of 3d Transition Metal Doping on Lithium Stabilized Na-β″-Alumina Solid Electrolytes. Materials 2021, 14, 5389. [Google Scholar] [CrossRef]
- Fertig, M.P.; Dirksen, C.; Schulz, M.; Stelter, M. Humidity-Induced Degradation of Lithium-Stabilized Sodium-Beta Alumina Solid Electrolytes. Batteries 2022, 8, 103. [Google Scholar] [CrossRef]
- Zhu, L.; Virkar, A. V Conversion Kinetics and Ionic Conductivity in Na-β″-Alumina + YSZ (Naβ″AY) Sodium Solid Electrolyte via Vapor Phase Conversion Process. Membranes 2022, 12, 567. [Google Scholar] [CrossRef]
- Zhang, G.; Wen, Z.; Wu, X.; Zhang, J.; Ma, G.; Jin, J. Sol-Gel Synthesis of Mg2+ Stabilized Na-β″/β-Al2O3 Solid Electrolyte for Sodium Anode Battery. J. Alloys Compd. 2014, 613, 80–86. [Google Scholar] [CrossRef]
- Agustina, A.I.; Skadell, K.; Dirksen, C.L.; Schulz, M.; Kusumocahyo, S.P. Sol-Gel Method for Synthesis of Li+-Stabilized Na-β″-Alumina for Solid Electrolytes in Sodium-Based Batteries. In Proceedings of the AIP Conference Proceedings, Grenoble, France, 8–12 July 2019; p. 020070. [Google Scholar]
- Li, H.; Fan, H.; Wang, B.; Wang, C.; Zhang, M.; Chen, G.; Jiang, X.; Zhao, N.; Lu, J.; Zhang, J. Mechanical and Electrical Properties of Lithium Stabilized Sodium Beta Alumina Solid Electrolyte Shaping by Non-Aqueous Gelcasting. J. Eur. Ceram. Soc. 2020, 40, 3072–3079. [Google Scholar] [CrossRef]
- Mathews, T. Solution Combustion Synthesis of Magnesium Compensated Sodium-β-Aluminas. Mater. Sci. Eng. B 2000, 78, 39–43. [Google Scholar] [CrossRef]
- Mali, A.; Petric, A. Synthesis of Sodium β″-Alumina Powder by Sol–Gel Combustion. J. Eur. Ceram. Soc. 2012, 32, 1229–1234. [Google Scholar] [CrossRef]
- Sutorik, A.C.; Neo, S.S.; Treadwell, D.R.; Laine, R.M. Synthesis of Ultrafine β″-Alumina Powders via Flame Spray Pyrolysis of Polymeric Precursors. J. Am. Ceram. Soc. 2005, 81, 1477–1486. [Google Scholar] [CrossRef]
- Ghadbeigi, L.; Szendrei, A.; Moreno, P.; Sparks, T.D.; Virkar, A.V. Synthesis of Iron-Doped Na-β″-Alumina + Yttria-Stabilized Zirconia Composite Electrolytes by a Vapor Phase Process. Solid State Ionics 2016, 290, 77–82. [Google Scholar] [CrossRef]
- Park, H.C.; Lee, Y.B.; Lee, S.G.; Lee, C.H.; Kim, J.K.; Hong, S.S.; Park, S.S. Synthesis of Beta-Alumina Powders by Microwave Heating from Solution-Derived Precipitates. Ceram. Int. 2005, 31, 293–296. [Google Scholar] [CrossRef]
- Wei, X.; Xia, Y.; Liu, X.; Yang, H.; Shen, X. Preparation of Sodium Beta″-Alumina Electrolyte Thin Film by Electrophoretic Deposition Using Taguchi Experimental Design Approach. Electrochim. Acta 2014, 136, 250–256. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, Y.; Zeng, S.M.; Yang, J.H.; Liu, Y.W.; Zhan, Z.L.; Song, L. Preparation and Characterization of a ZrO2–TiO2-Co-Doped Na-Β″-Al2O3 Ceramic Thin Film. Ceram. Int. 2016, 42, 8990–8996. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, H.; Li, Y.; Li, L.; Li, M.; Hai, O. The Mechanical and Electrical Properties of Nb2O5 Doped Na-β″-Al2O3 Solid Electrolyte. Eur. Phys. J. Appl. Phys. 2016, 74, 10901. [Google Scholar] [CrossRef]
- Barison, S.; Fasolin, S.; Mortalò, C.; Boldrini, S.; Fabrizio, M. Effect of Precursors on β-Alumina Electrolyte Preparation. J. Eur. Ceram. Soc. 2015, 35, 2099–2107. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Wang, X.; Wang, Y.; Wang, D.; Mao, Z. Synthesis and Characterization of High Ionic-Conductive Sodium Beta-Alumina Solid Electrolyte Derived from Boehmite. J. Mater. Sci. Mater. Electron. 2020, 31, 17670–17678. [Google Scholar] [CrossRef]
- Zhu, C.; Xue, J.; Ji, G. Effect of Na2O Content on Properties of Beta Alumina Solid Electrolytes. Mater. Sci. Semicond. Process. 2015, 31, 487–492. [Google Scholar] [CrossRef]
- Bay, M.-C.; Heinz, M.V.F.; Figi, R.; Schreiner, C.; Basso, D.; Zanon, N.; Vogt, U.F.; Battaglia, C. Impact of Liquid Phase Formation on Microstructure and Conductivity of Li-Stabilized Na-β″-Alumina Ceramics. ACS Appl. Energy Mater. 2019, 2, 687–693. [Google Scholar] [CrossRef]
- Sudworth, J.L.; Barrow, P.; Dong, W.; Dunn, B.; Farrington, G.C.; Thomas, J.O. Toward Commercialization of the Beta-Alumina Family of Ionic Conductors. MRS Bull. 2000, 25, 22–26. [Google Scholar] [CrossRef]
- Zhu, C.; Xue, J. Structure and Properties Relationships of Beta-Al2O3 Electrolyte Materials. J. Alloys Compd. 2012, 517, 182–185. [Google Scholar] [CrossRef]
- Lee, S.T.; Lee, K.M.; Lee, D.H.; Haw, J.R.; Lim, S.K. Analysis of the Phase Formation of Na-β/β-Aluminas Using MgO and Li2O as Phase Stabilizers. J. Ceram. Process. Res. 2011, 12, 38–45. [Google Scholar]
- Chen, G.; Lu, J.; Zhou, X.; Chen, L.; Jiang, X. Solid-State Synthesis of High Performance Na-β″-Al2O3 Solid Electrolyte Doped with MgO. Ceram. Int. 2016, 42, 16055–16062. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, H.; Li, M.; Hai, O.; Zhang, Y. Synthesis and Characterization of Y2O3 Doped Na-β″-Al2O3 Solid Electrolyte by Double Zeta Process. Ceram. Int. 2015, 41, 5355–5361. [Google Scholar] [CrossRef]
- Li, H.; Fan, H.; Zhang, J.; Wen, Y.; Chen, G.; Zhu, Y.; Lu, J.; Jiang, X.; Hu, B.; Ning, L. Sintering Behavior and Properties of Lithium Stabilized Sodium β″—Alumina Ceramics with YSZ Addition. Ceram. Int. 2019, 45, 6744–6752. [Google Scholar] [CrossRef]
- Wu, J.; Shi, J.; Hong, Y.; Zhu, C. Effect of Chromium on Electrochemical and Mechanical Properties of Beta-Al2O3 Solid Electrolyte. Mater. Res. Express 2020, 7, 105502. [Google Scholar] [CrossRef]
- Zhu, C.; Hong, Y.; Huang, P. Synthesis and Characterization of NiO Doped Beta-Al2O3 Solid Electrolyte. J. Alloys Compd. 2016, 688, 746–751. [Google Scholar] [CrossRef]
- Lee, S.-T.; Lee, D.-H.; Kim, J.-S.; Lim, S.-K. Influence of Fe and Ti Addition on Properties of Na+-β/β″-Alumina Solid Electrolytes. Met. Mater. Int. 2017, 23, 246–253. [Google Scholar] [CrossRef]
- Lu, X.; Li, G.; Kim, J.Y.; Meinhardt, K.D.; Sprenkle, V.L. Enhanced Sintering of β″-Al2O3/YSZ with the Sintering Aids of TiO2 and MnO2. J. Power Sources 2015, 295, 167–174. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lee, D.-G.; Lim, S.-K. Influence of MnO2 and Ta2O5/YSZ Addition on Properties of Na+−β/β″-Alumina Solid Electrolytes Prepared by a Synthesizing-Cum-Sintering Process. Ceram. Int. 2021, 47, 24743–24751. [Google Scholar] [CrossRef]
- Wei, X.; Cao, Y.; Lu, L.; Yang, H.; Shen, X. Synthesis and Characterization of Titanium Doped Sodium Beta″-Alumina. J. Alloys Compd. 2011, 509, 6222–6226. [Google Scholar] [CrossRef]
- Wang, C.-J.; Huang, C.-Y. Effect of TiO2 Addition on the Sintering Behavior, Hardness and Fracture Toughness of an Ultrafine Alumina. Mater. Sci. Eng. A 2008, 492, 306–310. [Google Scholar] [CrossRef]
- Chen, G.; Lu, J.; Li, L.; Chen, L.; Jiang, X. Microstructure Control and Properties of β″-Al2O3 Solid Electrolyte. J. Alloys Compd. 2016, 673, 295–301. [Google Scholar] [CrossRef]
- Dirksen, C.L.; Skadell, K.; Schulz, M.; Stelter, M. Effects of TiO2 Doping on Li+-Stabilized Na-β″-Alumina for Energy Storage Applications. Sep. Purif. Technol. 2019, 213, 88–92. [Google Scholar] [CrossRef]
- Lee, D.-G.; Ahn, B.-M.; Ahn, C.-W.; Choi, J.-H.; Lee, D.-H.; Lim, S.-K. Fabrication of a Full-Scale Pilot Model of a Cost-Effective Sodium Nickel-Iron Chloride Battery Over 40 Ah. J. Electrochem. Sci. Technol. 2021, 12, 398–405. [Google Scholar] [CrossRef]
- Jin, D.; Choi, S.; Jang, W.; Soon, A.; Kim, J.; Moon, H.; Lee, W.; Lee, Y.; Son, S.; Park, Y.; et al. Bismuth Islands for Low-Temperature Sodium-Beta Alumina Batteries. ACS Appl. Mater. Interfaces 2018, 11, 2917–2924. [Google Scholar] [CrossRef]
- Chang, H.-J.; Lu, X.; Bonnett, J.F.; Canfield, N.L.; Han, K.; Engelhard, M.H.; Jung, K.; Sprenkle, V.L.; Li, G. Decorating Β″-Alumina Solid-State Electrolytes with Micron Pb Spherical Particles for Improving Na Wettability at Lower Temperatures. J. Mater. Chem. A 2018, 6, 19703–19711. [Google Scholar] [CrossRef]
- Li, M.M.; Lu, X.; Zhan, X.; Engelhard, M.H.; Bonnett, J.F.; Polikarpov, E.; Jung, K.; Reed, D.M.; Sprenkle, V.L.; Li, G. High Performance Sodium-Sulfur Batteries at Low Temperature Enabled by Superior Molten Na Wettability. Chem. Commun. 2021, 57, 45–48. [Google Scholar] [CrossRef]
- Sudworth, J.; Tiley, A.R. Sodium Sulphur Battery; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1985; ISBN 0412164906. [Google Scholar]
- Hu, Y.; Wen, Z.; Wu, X.; Lu, Y. Nickel Nanowire Network Coating to Alleviate Interfacial Polarization for Na-Beta Battery Applications. J. Power Sources 2013, 240, 786–795. [Google Scholar] [CrossRef]
- Ahlbrecht, K.; Bucharsky, C.; Holzapfel, M.; Tübke, J.; Hoffmann, M.J. Investigation of the Wetting Behavior of Na and Na Alloys on Uncoated and Coated Na-β″-Alumina at Temperatures below 150 °C. Ionics 2017, 23, 1319–1327. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.-P.; Kafalas, J.A. Fast Na+-Ion Transport in Skeleton Structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Tietz, F. Phase Relations of NASICON Materials and Compilation of the Quaternary Phase Diagram Na2O-P2O5-SiO2-ZrO2. AIMS Mater. Sci. 2017, 4, 1305–1318. [Google Scholar] [CrossRef]
- Hong, H.-P. Crystal Structures and Crystal Chemistry in the System Na1+xZr2SixP3−xO12. Mater. Res. Bull. 1976, 11, 173–182. [Google Scholar] [CrossRef]
- Oh, J.A.S.; He, L.; Plewa, A.; Morita, M.; Zhao, Y.; Sakamoto, T.; Song, X.; Zhai, W.; Zeng, K.; Lu, L. Composite NASICON (Na3Zr2Si2PO12) Solid-State Electrolyte with Enhanced Na+ Ionic Conductivity: Effect of Liquid Phase Sintering. ACS Appl. Mater. Interfaces 2019, 11, 40125–40133. [Google Scholar] [CrossRef]
- von Alpen, U.; Bell, M.F.; Wichelhaus, W. Phase Transition in Nasicon (Na3Zr2Si2PO12). Mater. Res. Bull. 1979, 14, 1317–1322. [Google Scholar] [CrossRef]
- Lalère, F.; Leriche, J.B.; Courty, M.; Boulineau, S.; Viallet, V.; Masquelier, C.; Seznec, V. An All-Solid State NASICON Sodium Battery Operating at 200 °C. J. Power Sources 2014, 247, 975–980. [Google Scholar] [CrossRef]
- Jolley, A.G.; Taylor, D.D.; Schreiber, N.J.; Wachsman, E.D. Structural Investigation of Monoclinic-Rhombohedral Phase Transition in Na3Zr2Si2PO12 and Doped NASICON. J. Am. Ceram. Soc. 2015, 98, 2902–2907. [Google Scholar] [CrossRef]
- Song, S.; Duong, H.M.; Korsunsky, A.M.; Hu, N.; Lu, L. A Na+ Superionic Conductor for Room-Temperature Sodium Batteries. Sci. Rep. 2016, 6, 32330. [Google Scholar] [CrossRef] [PubMed]
- Guin, M.; Tietz, F.; Guillon, O. New Promising NASICON Material as Solid Electrolyte for Sodium-Ion Batteries: Correlation between Composition, Crystal Structure and Ionic Conductivity of Na3+xSc3SixP3−xO12. Solid State Ionics 2016, 293, 18–26. [Google Scholar] [CrossRef]
- Ma, Q.; Guin, M.; Naqash, S.; Tsai, C.L.; Tietz, F.; Guillon, O. Scandium-Substituted Na3Zr2(SiO4)2(PO4) Prepared by a Solution-Assisted Solid-State Reaction Method as Sodium-Ion Conductors. Chem. Mater. 2016, 28, 4821–4828. [Google Scholar] [CrossRef]
- Chen, D.; Luo, F.; Zhou, W.; Zhu, D. Influence of Nb5+, Ti4+, Y3+ and Zn3+ Doped Na3Zr2Si2PO12 Solid Electrolyte on Its Conductivity. J. Alloys Compd. 2018, 757, 348–355. [Google Scholar] [CrossRef]
- Xie, B.; Jiang, D.; Wu, J.; Feng, T.; Xia, J.; Nian, H. Effect of Substituting Ce for Zr on the Electrical Properties of NASICON Materials. J. Phys. Chem. Solids 2016, 88, 104–108. [Google Scholar] [CrossRef]
- Naqash, S.; Tietz, F.; Guillon, O. Synthesis and Characterization of Equimolar Al/Y-Substituted NASICON Solid Solution Na1+2x+yAlxYxZr2−2xSiyP3−yO12. Solid State Ionics 2018, 319, 13–21. [Google Scholar] [CrossRef]
- Nonemacher, J.F.; Naqash, S.; Tietz, F.; Malzbender, J. Micromechanical Assessment of Al/Y-Substituted NASICON Solid Electrolytes. Ceram. Int. 2019, 45, 21308–21314. [Google Scholar] [CrossRef]
- Shao, Y.; Zhong, G.; Lu, Y.; Liu, L.; Zhao, C.; Zhang, Q.; Hu, Y.; Yang, Y.; Chen, L. A Novel NASICON-Based Glass-Ceramic Composite Electrolyte with Enhanced Na-Ion Conductivity. Energy Storage Mater. 2019, 23, 514–521. [Google Scholar] [CrossRef]
- Jalalian-Khakshour, A.; Phillips, C.O.; Jackson, L.; Dunlop, T.O.; Margadonna, S.; Deganello, D. Solid-State Synthesis of NASICON (Na3Zr2Si2PO12) Using Nanoparticle Precursors for Optimisation of Ionic Conductivity. J. Mater. Sci. 2020, 55, 2291–2302. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.T.; Lee, D.H.; Lee, S.H.; Han, S.S.; Lim, S.K. Effect of Particle Size on the Density and Ionic Conductivity of Na3Zr2Si2PO12 NASICON. J. Ceram. Process. Res. 2015, 16, 49–53. [Google Scholar]
- Fuentes, R.O.; Figueiredo, F.M.; Soares, M.R.; Marques, F.M.B. Submicrometric NASICON Ceramics with Improved Electrical Conductivity Obtained from Mechanically Activated Precursors. J. Eur. Ceram. Soc. 2005, 25, 455–462. [Google Scholar] [CrossRef]
- Park, H.; Jung, K.; Nezafati, M.; Kim, C.S.; Kang, B. Sodium Ion Diffusion in Nasicon (Na3Zr2Si2PO12) Solid Electrolytes: Effects of Excess Sodium. ACS Appl. Mater. Interfaces 2016, 8, 27814–27824. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Tsai, C.L.; Wei, X.K.; Heggen, M.; Tietz, F.; Irvine, J.T.S. Room Temperature Demonstration of a Sodium Superionic Conductor with Grain Conductivity in Excess of 0.01 S Cm−1 and Its Primary Applications in Symmetric Battery Cells. J. Mater. Chem. A 2019, 7, 7766–7776. [Google Scholar] [CrossRef]
- Naqash, S.; Tietz, F.; Yazhenskikh, E.; Müller, M.; Guillon, O. Impact of Sodium Excess on Electrical Conductivity of Na3Zr2Si2 PO12 + x Na2O Ceramics. Solid State Ionics 2019, 336, 57–66. [Google Scholar] [CrossRef]
- Naqash, S.; Sebold, D.; Tietz, F.; Guillon, O. Microstructure-Conductivity Relationship of Na3Zr2(SiO4)2(PO4) Ceramics. J. Am. Ceram. Soc. 2018, 102, 1057–1070. [Google Scholar] [CrossRef]
- Ruan, Y.; Song, S.; Liu, J.; Liu, P.; Cheng, B.; Song, X. Improved Structural Stability and Ionic Conductivity of Na3Zr2Si2PO12 Solid Electrolyte by Rare Earth Metal Substitutions. Ceram. Int. 2017, 43, 7810–7815. [Google Scholar] [CrossRef]
- Lee, G.; Olevsky, E.A.; Manière, C.; Maximenko, A.; Izhvanov, O.; Back, C.; McKittrick, J. Effect of Electric Current on Densification Behavior of Conductive Ceramic Powders Consolidated by Spark Plasma Sintering. Acta Mater. 2018, 144, 524–533. [Google Scholar] [CrossRef]
- Jiang, A.; Ke, D.; Xu, L.; Xu, Q.; Li, J.; Wei, J.; Hu, C.; Grasso, S. Cold Hydrostatic Sintering: From Shaping to 3D Printing. J. Mater. 2019, 5, 496–501. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Tang, Y.; Chen, J.; Wang, D.; Mao, Z. Low Temperature and Rapid Microwave Sintering of Na3Zr2Si2PO12 Solid Electrolytes for Na-Ion Batteries. J. Power Sources 2021, 481, 228924. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, Q.; Tsai, C.-L.; Tietz, F.; Guillon, O. A Novel Sol-Gel Method for Large-Scale Production of Nanopowders: Preparation of Li1.5Al0.5Ti1.5(PO4)3 as an Example. J. Am. Ceram. Soc. 2016, 99, 410–414. [Google Scholar] [CrossRef]
- Naqash, S.; Ma, Q.; Tietz, F.; Guillon, O. Na3Zr2(SiO4)2(PO4) Prepared by a Solution-Assisted Solid State Reaction. Solid State Ionics 2017, 302, 83–91. [Google Scholar] [CrossRef]
- Ignaszak, A.; Pasierb, P.; Gajerski, R.; Komornicki, S. Synthesis and Properties of Nasicon-Type Materials. Thermochim. Acta 2005, 426, 7–14. [Google Scholar] [CrossRef]
- Gordon, J.H.; Watkins, J.J. Sodium-Sulfur Battery with a Substantially Non-Porous Membrane and Enhanced Cathode Utilization. U.S. Patent US20100239893A1, 16 March 2010. [Google Scholar]
- Chae, J.; Kim, J.; Kim, J.; Bhavaraju, S. Sodium Secondary Battery. WO Patent WO2014092493A3, 23 October 2014. [Google Scholar]
- Bhavaraju, S.; Joshi, A.V.; Robins, M.; Eccleston, A. Intermediate Temperature Sodium-Metal Halide Battery. U.S. Patent US9537179B2, 25 September 2014. [Google Scholar]
- Kim, J.; Li, G.; Lu, X.; Sprenkle, V.L.; Lemmon, J.P.; Yang, Z.; Coyle, C.A. Intermediate Temperature Sodium Metal-Halide Energy Storage Devices. WO Patent WO2013116263A1, 30 January 2013. [Google Scholar]
- Bhavaraju, S.; Robins, M. Low Viscosity/High Conductivity Sodium Haloaluminate Electrolyte. U.S. Patent US9876253B2, 6 June 2014. [Google Scholar]
- Small, L.J.; Eccleston, A.; Lamb, J.; Read, A.C.; Robins, M.; Meaders, T.; Ingersoll, D.; Clem, P.G.; Bhavaraju, S.; Spoerke, E.D. Next Generation Molten NaI Batteries for Grid Scale Energy Storage. J. Power Sources 2017, 360, 569–574. [Google Scholar] [CrossRef]
- Gross, M.M.; Percival, S.J.; Small, L.J.; Lamb, J.; Peretti, A.S.; Spoerke, E.D. Low-Temperature Molten Sodium Batteries. ACS Appl. Energy Mater. 2020, 3, 11456–11462. [Google Scholar] [CrossRef]
- Gross, M.M.; Percival, S.J.; Lee, R.Y.; Peretti, A.S.; Spoerke, E.D.; Small, L.J. A High-Voltage, Low-Temperature Molten Sodium Battery Enabled by Metal Halide Catholyte Chemistry. Cell Reports Phys. Sci. 2021, 2, 100489. [Google Scholar] [CrossRef]
- Coetzer, J.; Sudworth, J. Out of Africa the Story of the Zebra Battery; Beta Resesarch & Development Ltd.: Burton-on-Trent, UK, 2000. [Google Scholar]
- Kurzweil, P. HISTORY|Secondary Batteries. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2009; pp. 565–578. ISBN 9780444527455. [Google Scholar]
- Galloway, R.C.; Haslam, S. The ZEBRA Electric Vehicle Battery: Power and Energy Improvements. J. Power Sources 1999, 80, 164–170. [Google Scholar] [CrossRef]
- Dustmann, C.-H. ZEBRA Battery Meets USABC Goals. J. Power Sources 1998, 72, 27–31. [Google Scholar] [CrossRef]
- Turconi, A. Developments and Improvements in Zebra Nickel Sodium Chloride Batteries. In Proceedings of the Electric Drive Transportation Association—23rd Int. Electric Vehicle Symposium and Exposition 2007, EVS 2007 (Battery, Hybrid, Fuel Cell) Conf. Proc.—Sustainability: The Future of Transportation, Anaheim, CA, USA, 2–5 December 2007; pp. 300–306. [Google Scholar]
- Frutschy, K.; Chatwin, T.; Mao, L.; Smith, C.R.; Bull, R. Sodium Nickel Chloride Battery Design and Testing. In Proceedings of the Volume 6: Energy, Parts A and B; American Society of Mechanical Engineers: New York, NY, USA, November 2012; pp. 429–438. [Google Scholar]
- Lu, X.; Coffey, G.; Meinhardt, K.; Sprenkle, V.; Yang, Z.; Lemmon, J.P. High Power Planar Sodium-Nickel Chloride Battery. ECS Trans. 2019, 28, 7. [Google Scholar] [CrossRef]
- Sudworth, J.L.; Galloway, R.C. Secondary Batteries—High Temperature Systems|Sodium–Nickel Chloride. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2009; pp. 312–323. ISBN 9780444527455. [Google Scholar]
- Sudworth, J.L.; Böhm, H. Performance Data from an Improved Sodium/Nickel Chloride Cell. J. Passeng. Cars 1991, 100, 1681–1686. [Google Scholar]
- Manzoni, R.; Metzger, M.; Crugnola, G. ZEBRA Electric Energy Storage System: From R&D to Market. Present. HTE Hi. Tech. Expo-Milan, 25th–28th Novemeber 2008, 25, 28. [Google Scholar]
- Hartenbach, A.; Bayer, M.; Dustmann, C.-H. The Sodium Metal Halide (ZEBRA) Battery. In Molten Salts Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 439–450. ISBN 9780123985385. [Google Scholar]
- Bull Roger, N.; Galloway, R.C.; Brady, C.D.A.; Barrow, P. Positive Electrode Composition for Overdischarge Protection. 2017. Available online: https://eureka.patsnap.com/patent-US20170104244A1 (accessed on 15 September 2023).
- Sudworth, J. The Sodium/Nickel Chloride (ZEBRA) Battery. J. Power Sources 2001, 100, 149–163. [Google Scholar] [CrossRef]
- Lu, X.; Xia, G.; Lemmon, J.P.; Yang, Z. Advanced Materials for Sodium-Beta Alumina Batteries: Status, Challenges and Perspectives. J. Power Sources 2010, 195, 2431–2442. [Google Scholar] [CrossRef]
- Vallance, M.A.; White, R.E. High-Temperature Sodium|Metal Chloride Storage Battery. In Proceedings of the Excerpt from the Proceedings of the COMSOL Conference Boston, Boston, MA, USA, 9–11 October 2008. [Google Scholar]
- Zappi, G.D.; Iacovangelo, C.D.; Rahmane, M.; Winkler, B.; Hale; Bull, R.N.; Sudworth, J.L. Electrochemical Cells, and Related Devices. 2011. [Google Scholar]
- Theodore, A.N.; Pett, R.A. Preparation of Beta Alumina Tubes by the Extrusion Process. U.S. Patent US4615851A, 21 February 1985. [Google Scholar]
- Avinash, K.; Suresh, M.; Khanra, A.; Johnson, R. Synthesis, Extrusion Processing and Ionic Conductivity Measurements of Sodium β-Alumina Tubes. Process. Appl. Ceram. 2015, 9, 131–138. [Google Scholar] [CrossRef]
- Hu, Y.; Heavens, S.N.; Blackburn, J.S.; Blackburn, S. Extrusion Process for the Manufacture of Beta’’-Alumina Solid Electrolyte Tubes. J. Ceram. Sci. Technol. 2017, 8, 25–30. [Google Scholar] [CrossRef]
- Lee, H.S.; Hong, I.K. Anode Bonding Part of Sodium Nickel Chloride Battery. KR Patent KR20160080192A, 29 December 2014. [Google Scholar]
- Baker, D.J.; Bugden, W.G.; Smith, P.R. Joining of Ceramic Components to Metal Components. U.S. Patent US5009357A, 23 April 1991. [Google Scholar]
- Park, D.-S.; Wu, J.; Nagesh, M.; Sundeep, K.; Stringer Digamber, C.; Hassan, V. Sealing Glass Composition, Method and Article. U.S. Patent US20100120602A1, 13 May 2010. [Google Scholar]
- Mali, A.; Petric, A. New Ceramic Seal for Na/NiCl2 Cells. J. Am. Ceram. Soc. 2011, 94, 3346–3349. [Google Scholar] [CrossRef]
- Smeacetto, F.; Radaelli, M.; Salvo, M.; Di Modugno, D.; Sabato, A.G.; Casalegno, V.; Broglia, M.; Ferraris, M. Glass-Ceramic Joining Material for Sodium-Based Battery. Ceram. Int. 2017, 43, 8329–8333. [Google Scholar] [CrossRef]
- Song, S.; Wen, Z.; Liu, Y.; Wu, X.; Lin, J. Bi-Doped Borosilicate Glass as Sealant for Sodium Sulfur Battery. J. Non. Cryst. Solids 2011, 357, 3074–3079. [Google Scholar] [CrossRef]
- Manthina, V.; Song, G.; Singh, P.; Mahapatra, M.K. Silica-free Sealing Glass for Sodium-beta Alumina Battery. Int. J. Appl. Ceram. Technol. 2019, 16, 887–895. [Google Scholar] [CrossRef]
- Song, S.; Wen, Z.; Liu, Y. The Effect of Substitution of Bi2O3 for Alkali Oxides on Thermal Properties, Structure and Wetting Behavior of the Borosilicate Glass. Mater. Lett. 2010, 64, 1025–1027. [Google Scholar] [CrossRef]
- Kim, S.; Joo, J.H.; Kim, S.-D.; Woo, S.-K. Evaluation of CaO–Al2O3 Adhesive Bonding Properties for β″-Al2O3 Solid Electrolyte Sealing for Alkali Metal Thermal Electric Converter. Ceram. Int. 2013, 39, 9223–9227. [Google Scholar] [CrossRef]
- Crugnola, G.; Lodi, G.; Residori, Z.; Restello, S.; Zanon, N. Glass Composition for Glass Welding ‘glass Welding’ of Ceramic Parts for Electrochemical Cells. ITMI20132155A1, 20 December 2013. [Google Scholar]
- Dustmann, C.-H.; Bayer, M. Electrochemical Energy Storage Device. CH10742019A, August 2019. [Google Scholar]
- Restello, S.; Zanon, N.; Residori, Z.; Crugnola, G.; Lodi, G. High-Efficiency, High-Temperature, Sodium-Based Electrochemical Cell. 2013. [Google Scholar]
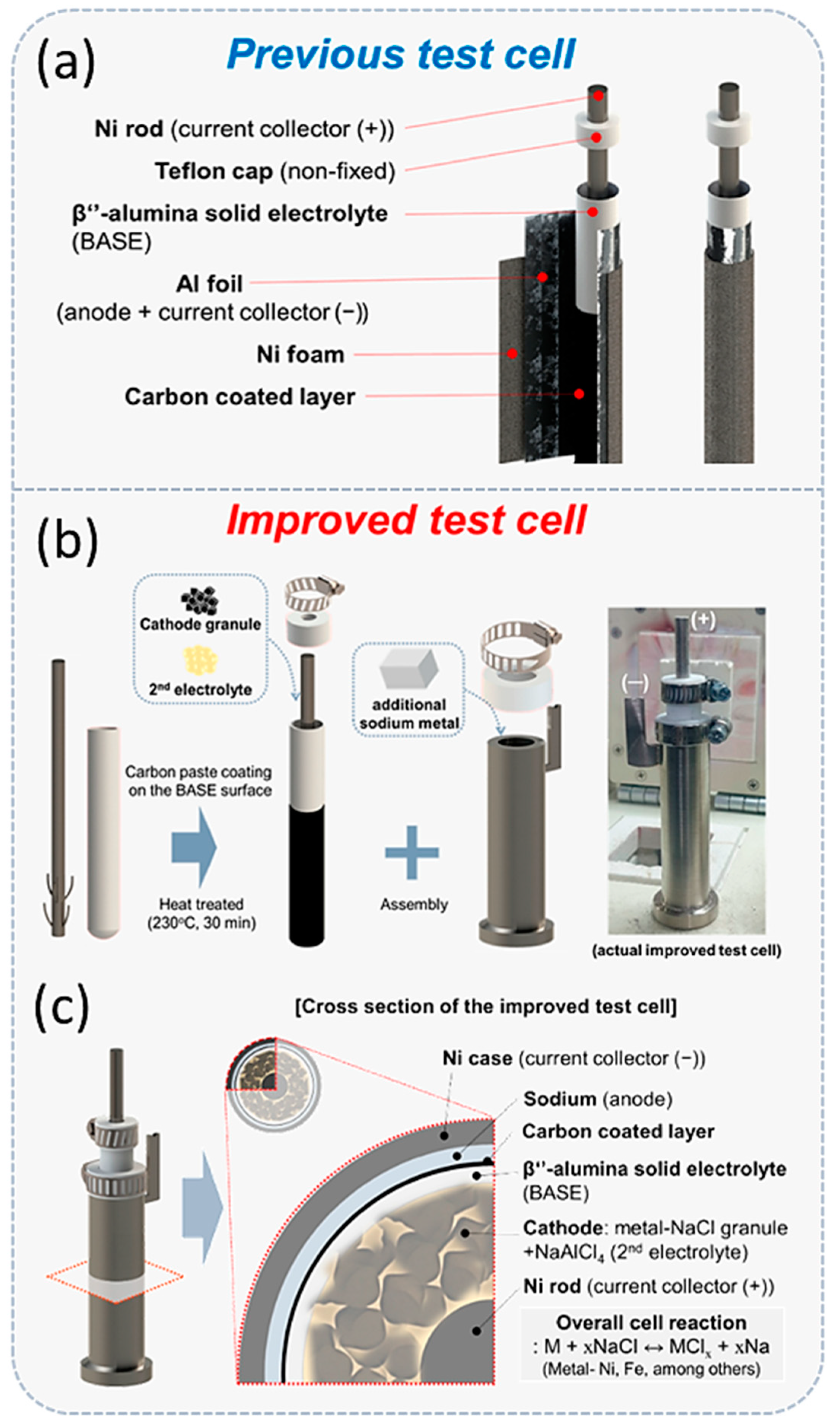
- Ahn, B.-M.; Ahn, C.-W.; Hahn, B.-D.; Choi, J.-J.; Kim, Y.-D.; Lim, S.-K.; Choi, J.-H. Improvement of Simple Test Cell Design for Cathode Microstructure Study in Tubular-Type Sodium–Metal Chloride Batteries. Batteries 2022, 8, 163. [Google Scholar] [CrossRef]
- Kim, G.; Park, Y.-C.; Lee, Y.; Cho, N.; Kim, C.-S.; Jung, K. The Effect of Cathode Felt Geometries on Electrochemical Characteristics of Sodium Sulfur (NaS) Cells: Planar vs. Tubular. J. Power Sources 2016, 325, 238–245. [Google Scholar] [CrossRef]
- Sudworth, J. Electric Cells and Batteries. US3765945A, 29 November 1970. [Google Scholar]
- Lemmon, J.P.; Meinhardt, K.D. Planar High Density Sodium Battery. U.S. Patent US9276294B2, 27 September 2011. [Google Scholar]
- Kim, S.-M.; Lee, S.-M.; Jung, K.; Park, Y.-C.; Cho, N.; Kim, H.-S. Feasibility Study of a Planar-Type Sodium-Nickel Chloride Battery. Bull. Korean Chem. Soc. 2016, 37, 695–699. [Google Scholar] [CrossRef]
- Ratnakumar, B.V.; Attia, A.I.; Halpert, G. Sodiummetal Chloride Battery Research at the Jet Propulsion Laboratory (JPL). J. Power Sources 1991, 36, 385–394. [Google Scholar] [CrossRef]
- Kim, J.Y.; Li, G.; Lu, X.; Sprenkle, V.L.; Lemmon, J.P. Metallization Pattern on Solid Electrolyte or Porous Support of Sodium Battery Process. U.S. Patent 9,356,314, 25 February 2013. [Google Scholar]
- Graeber, G.; Landmann, D.; Svaluto-Ferro, E.; Vagliani, F.; Basso, D.; Turconi, A.; Heinz, M.V.F.; Battaglia, C. Rational Cathode Design for High-Power Sodium-Metal Chloride Batteries. Adv. Funct. Mater. 2021, 31, 2106367. [Google Scholar] [CrossRef]
- Dustmann, C.-H.; Bayer, M. ZEBRA Battery Flat Plate Cell Design. In Proceedings of the South African Energy Storage Conference, Durban, South Africa, 25–27 June 2018; pp. 1–4. [Google Scholar]
- Dustmann, C.-H.; Bayer, M.; Hartenbach, A.; Kaninia, M.-E.; Leuthold, A. Electrochemical Energy Storage Device. WO Patent WO/2016/192051, 8 December 2016. [Google Scholar]
- Tao, G.; Weber, N.; Virkar, A.V. Planar Alkali Metal-Beta Battery. WO Patent WO2015006377A1, 15 January 2015. [Google Scholar]
- Ligon, S.C.; Bay, M.-C.; Heinz, M.V.F.; Battaglia, C.; Graule, T.; Blugan, G. Large Planar Na-β″-Al2O3 Solid Electrolytes for Next Generation Na-Batteries. Materials 2020, 13, 433. [Google Scholar] [CrossRef]
- D’Urso, C.; Briguglio, N.; Bonanno, A.; Ferraro, M.; Antonucci, V.; Vasta, S. Thermochemical Investigation on a Novel Sodium-Metal-Halide Battery Configuration: Experimental and FEM Model Results. J. Energy Storage 2019, 25, 100818. [Google Scholar] [CrossRef]
- Xu, Y.; Jung, K.; Park, Y.-C.; Kim, C.-S. Selection of Container Materials for Modern Planar Sodium Sulfur (NaS) Energy Storage Cells towards Higher Thermo-Mechanical Stability. J. Energy Storage 2017, 12, 215–225. [Google Scholar] [CrossRef]
- Heinz, M.V.F.; Graeber, G.; Landmann, D.; Battaglia, C. Pressure Management and Cell Design in Solid-Electrolyte Batteries, at the Example of a Sodium-Nickel Chloride Battery. J. Power Sources 2020, 465, 228268. [Google Scholar] [CrossRef]




| Sample | Temp (°C) | C/Rate | Energy Density (Wh/kg) | Energy Efficiency (%) | Reference |
|---|---|---|---|---|---|
| Ni/NaCl ratio 1.8 | 280 | C/3 | ~150 | 96 | [25] |
| Ni/NaCl ratio 1.0 | 190 | C/5 | 285 | 60 | [55] |
| Ni/NaCl ratio 1.25 | 190 | C/5 | 330 | 96 | [55] |
| Ni/NaCl ratio 1.5 | 190 | C/5 | 390 | 98 | [55] |
| Ni/NaCl ratio 1.8 | 190 | C/5 | 420 | 98 | [55] |
| Na (10µm)/NaCl (50 μm) | 280 | C/5 | ~250 | 92 | [46] |
| Na (1µm)/NaCl (5 μm) | 190 | C/5 | 340 | 88 | [46] |
| Na-S NiCl2 hybrid | 280 | C/5 | 248 | 90 | [52] |
| Na-FeCl2 | 190 | C/8 | 135 | 92 | [57] |
| Ni-Fe/NaCl (ratio Ni-Fe 1:1) | 300 | C/5 | 275 | 91 | [62] |
| NaAl2Cl7–NaAlCl4 | 200 | C/10 | 366 | 96 | [63] |
| Na-Al/NaAlCl4 | 190 | C/3 | 447 | 95 | [64] |
| Na-ZnCl2 | 280 | - | ~225 | - | [66] |
| Na-CuCl2 | 175 | - | ~388 | - | [73] |
| Na-NCG | 190 | C/4 | 133 | 94 | [54] |
| MWCNT/CF/Ni/NaCl | 190 | - | 263 | - | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardi, S.G.; Samperi, M.; Frusteri, L.; Antonucci, V.; D’Urso, C. A Review of Sodium-Metal Chloride Batteries: Materials and Cell Design. Batteries 2023, 9, 524. https://doi.org/10.3390/batteries9110524
Leonardi SG, Samperi M, Frusteri L, Antonucci V, D’Urso C. A Review of Sodium-Metal Chloride Batteries: Materials and Cell Design. Batteries. 2023; 9(11):524. https://doi.org/10.3390/batteries9110524
Chicago/Turabian StyleLeonardi, Salvatore Gianluca, Mario Samperi, Leone Frusteri, Vincenzo Antonucci, and Claudia D’Urso. 2023. "A Review of Sodium-Metal Chloride Batteries: Materials and Cell Design" Batteries 9, no. 11: 524. https://doi.org/10.3390/batteries9110524
APA StyleLeonardi, S. G., Samperi, M., Frusteri, L., Antonucci, V., & D’Urso, C. (2023). A Review of Sodium-Metal Chloride Batteries: Materials and Cell Design. Batteries, 9(11), 524. https://doi.org/10.3390/batteries9110524