Silicon Negative Electrodes—What Can Be Achieved for Commercial Cell Energy Densities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Design
2.2. Electrode Expansion at 100% State of Charge
2.3. Full Cell Design
2.4. Assumptions Related to Cell Characteristics
- Silicon is known to have high irreversible capacity loss, not only on the first cycle, but on subsequent cycles. The results assume silicon delivers a reversible capacity of 3000 mAh g−1. This can either be interpreted as the first discharge energy density or as a future prediction of energy density on cells containing silicon, where the continued ICL has been mitigated through research and development.
- The consumption of available reversible lithium as silicon SEI is mitigated. LCO and NMC811 deliver 150 mAh g−1 and 200 mAh g−1, respectively.
- The expansion of silicon and graphite occurs as cells are charged. The porosity of the electrode does not change during expansion.
- No increase in electrode thickness occurs after electrode calendering. Depending on the target calender porosity, electrodes may “spring back” or relax following calendering. As this relaxation is typically minimal, it has been omitted.
- No separator compression occurs during electrode expansion at 100% SOC.
3. Results
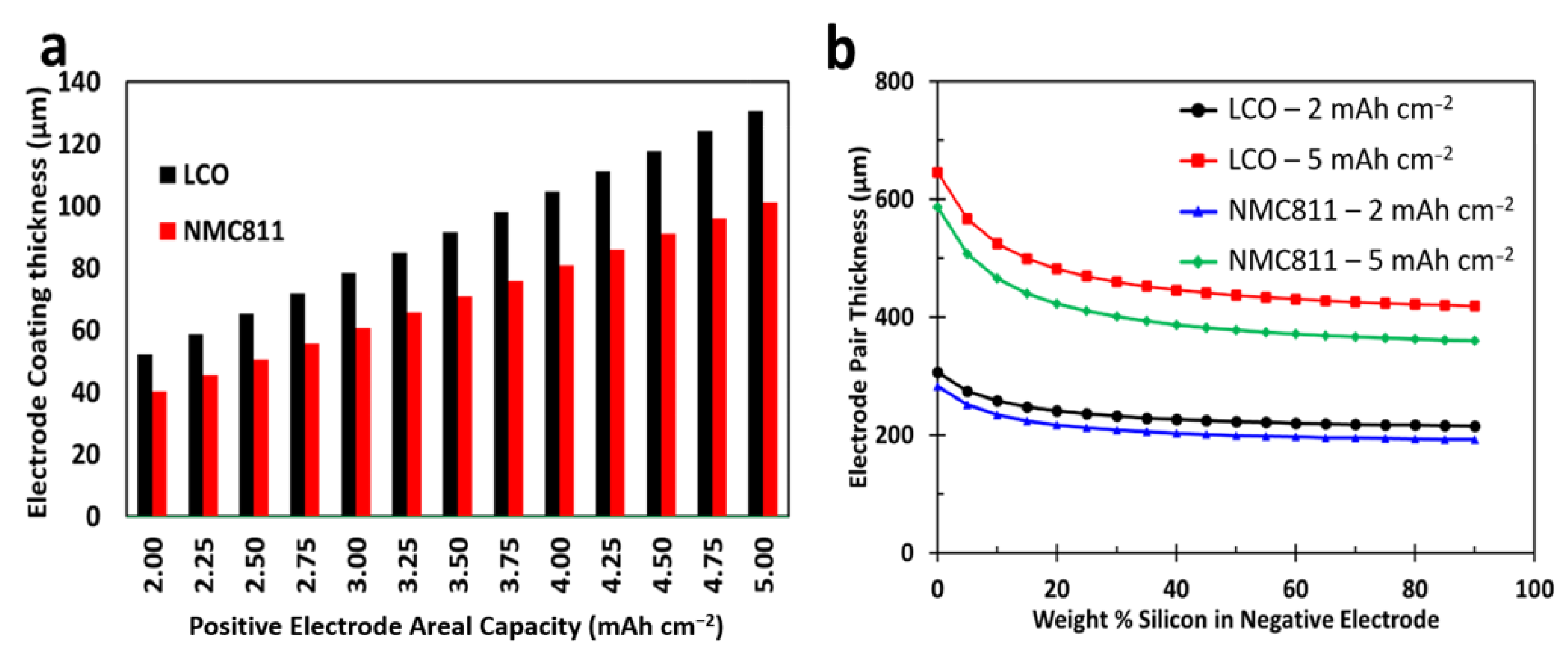
3.1. Influence of Percentage Silicon on Electrode Thickness
3.2. Positive Electrode Thickness LCO versus NMC 811
3.3. Stack Characteristics
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Lipu, M.S.H.; Al Mamun, A.; Ansari, S.; Miah, M.S.; Hasan, K.; Meraj, S.T.; Abdolrasol, M.G.M.; Rahman, T.; Maruf, M.H.; Sarker, M.R.; et al. Battery Management, Key Technologies, Methods, Issues, and Future Trends of Electric Vehicles: A Pathway toward Achieving Sustainable Development Goals. Batteries 2022, 8, 119. [Google Scholar] [CrossRef]
- Kennedy, B.; Patterson, D.; Camilleri, S. Use of Lithium-Ion Batteries in Electric Vehicles. J. Power Sources 2000, 90, 156–162. [Google Scholar] [CrossRef]
- Salgado, R.M.; Danzi, F.; Oliveira, J.E.; El-Azab, A.; Camanho, P.P.; Braga, M.H. The Latest Trends in Electric Vehicles Batteries. Molecules 2021, 26, 3188. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Placke, T.; Chau, K.T. Overview of Batteries and Battery Management for Electric Vehicles. Energy Rep. 2022, 8, 4058–4084. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium Batteries: Status, Prospects and Future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Märker, K.; Reeves, P.J.; Xu, C.; Griffith, K.J.; Grey, C.P. Evolution of Structure and Lithium Dynamics in LiNi0.8Mn0.1Co0.1O2 (NMC811) Cathodes during Electrochemical Cycling. Chem. Mater. 2019, 31, 2545–2554. [Google Scholar] [CrossRef]
- Beltrop, K.; Klein, S.; Nölle, R.; Wilken, A.; Lee, J.J.; Köster, T.K.J.; Reiter, J.; Tao, L.; Liang, C.; Winter, M.; et al. Triphenylphosphine Oxide as Highly Effective Electrolyte Additive for Graphite/NMC811 Lithium Ion Cells. Chem. Mater. 2018, 30, 2726–2741. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, J.; Wang, C.; Gu, M. Designing Principle for Ni-Rich Cathode Materials with High Energy Density for Practical Applications. Nano Energy 2018, 49, 434–452. [Google Scholar] [CrossRef]
- Noh, H.J.; Youn, S.; Yoon, C.S.; Sun, Y.K. Comparison of the Structural and Electrochemical Properties of Layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) Cathode Material for Lithium-Ion Batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity Fading of Ni-Rich Li[NixCoyMn1−x−y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Lee, S.; Manthiram, A. Can Cobalt Be Eliminated from Lithium-Ion Batteries? ACS Energy Lett. 2022, 7, 3058–3063. [Google Scholar] [CrossRef]
- Banza Lubaba Nkulu, C.; Casas, L.; Haufroid, V.; De Putter, T.; Saenen, N.D.; Kayembe-Kitenge, T.; Musa Obadia, P.; Kyanika Wa Mukoma, D.; Lunda Ilunga, J.M.; Nawrot, T.S.; et al. Sustainability of Artisanal Mining of Cobalt in DR Congo. Nat. Sustain. 2018, 1, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Gulley, A.L. One Hundred Years of Cobalt Production in the Democratic Republic of the Congo. Resour. Policy 2022, 79, 103007. [Google Scholar] [CrossRef]
- Secrist, E.S.; Fehring, T.K. Cobalt Mining in the Democratic Republic of the Congo for Orthopaedic Implants: A Complex Ethical Issue with No Simple Solutions. J. Bone Jt. Surg. 2023, 105, 167–171. [Google Scholar] [CrossRef]
- Dong, Q.; Guo, F.; Cheng, Z.; Mao, Y.; Huang, R.; Li, F.; Dong, H.; Zhang, Q.; Li, W.; Chen, H.; et al. Insights into the Dual Role of Lithium Difluoro(Oxalato)Borate Additive in Improving the Electrochemical Performance of NMC811||Graphite Cells. ACS Appl. Energy Mater. 2020, 3, 695–704. [Google Scholar] [CrossRef]
- Myung, S.T.; Maglia, F.; Park, K.J.; Yoon, C.S.; Lamp, P.; Kim, S.J.; Sun, Y.K. Nickel-Rich Layered Cathode Materials for Automotive Lithium-Ion Batteries: Achievements and Perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and Cost of Materials for Lithium-Based Rechargeable Automotive Batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes. J. Electrochem. Soc. 2017, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.J. Silicon Based Lithium-Ion Battery Anodes: A Chronicle Perspective Review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Müller, J.; Michalowski, P.; Kwade, A. Impact of Silicon Content and Particle Size in Lithium-Ion Battery Anodes on Particulate Properties and Electrochemical Performance. Batteries 2023, 9, 377. [Google Scholar] [CrossRef]
- Sakabe, J.; Ohta, N.; Ohnishi, T.; Mitsuishi, K.; Takada, K. Porous Amorphous Silicon Film Anodes for High-Capacity and Stable All-Solid-State Lithium Batteries. Commun. Chem. 2018, 1, 24. [Google Scholar] [CrossRef]
- Karkar, Z.; Jaouhari, T.; Tranchot, A.; Mazouzi, D.; Guyomard, D.; Lestriez, B.; Roué, L. How Silicon Electrodes Can Be Calendered without Altering Their Mechanical Strength and Cycle Life. J. Power Sources 2017, 371, 136–147. [Google Scholar] [CrossRef]
- Wang, B.; Wang, D.; Wang, F.; Li, J.; Wang, B.; Zhou, Y.; Liu, H.; Dou, S. Prelithiation: A Crucial Strategy for Boosting the Practical Application of next-Generation Lithium Ion Battery. ACS Nano 2021, 15, 2197–2218. [Google Scholar] [CrossRef] [PubMed]
- Holtstiege, F.; Bärmann, P.; Nölle, R.; Winter, M.; Placke, T. Pre-Lithiation Strategies for Rechargeable Energy Storage Technologies: Concepts, Promises and Challenges. Batteries 2018, 4, 4. [Google Scholar] [CrossRef]
- Jin, L.; Shen, C.; Shellikeri, A.; Wu, Q.; Zheng, J.; Andrei, P.; Zhang, J.G.; Zheng, J.P. Progress and Perspectives on Pre-Lithiation Technologies for Lithium Ion Capacitors. Energy Environ. Sci. 2020, 13, 2341–2362. [Google Scholar] [CrossRef]
- Cui, Y. Silicon Anodes. Nat. Energy 2021, 6, 995–996. [Google Scholar] [CrossRef]
- Günter, F.J.; Wassiliadis, N. State of the Art of Lithium-Ion Pouch Cells in Automotive Applications: Cell Teardown and Characterization. J. Electrochem. Soc. 2022, 169, 030515. [Google Scholar] [CrossRef]
- Yourey, W. Theoretical Impact of Manufacturing Tolerance on Lithium-Ion Electrode and Cell Physical Properties. Batteries 2020, 6, 23. [Google Scholar] [CrossRef]
- Moyassari, E.; Kücher, S.; Jobst, N.M.; Chang, C.-C.; Hou, S.-C.; Spingler, F.B.; Wohlfahrt-Mehrens, M.; Jossen, A. Influence of Initial Porosity on the Expansion Behavior of Electrodes in Lithium-Ion Batteries. J. Electrochem. Soc. 2023, 170, 050528. [Google Scholar] [CrossRef]
- Sun, P.; Bisschop, R.; Niu, H.; Huang, X. A Review of Battery Fires in Electric Vehicles. Fire Technol. 2020, 56, 1361–1410. [Google Scholar] [CrossRef]
- Yourey, W. Cell Design Considerations and Impact on Energy Density—A Practical Approach to EV Cell Design. World Electr. Veh. J. 2023, 14, 279. [Google Scholar] [CrossRef]
- Moyassari, E.; Roth, T.; Kücher, S.; Chang, C.-C.; Hou, S.-C.; Spingler, F.B.; Jossen, A. The Role of Silicon in Silicon-Graphite Composite Electrodes Regarding Specific Capacity, Cycle Stability, and Expansion. J. Electrochem. Soc. 2022, 169, 010504. [Google Scholar] [CrossRef]


| Positive Electrode (LCO) | ||
|---|---|---|
| Material | Weight Percent | Density (g/cm3) |
| LiCoO2 | 90 | 5.00 |
| Conductive Additive | 5 | 2.00 |
| PVDF Electrode Binder | 5 | 1.78 |
| Positive Mixture | - | 4.29 |
| Positive Electrode (NMC 811) | ||
| Material | Weight Percent | Density (g/cm3) |
| NMC 811 | 90 | 4.80 |
| Conductive Additive | 5 | 2.00 |
| PVDF Electrode Binder | 5 | 1.78 |
| Positive Mixture | - | 4.16 |
| Negative Electrode | ||
| Material | Weight Percent | Density (g/cm3) |
| Active Carbon | 90 → 0 | 2.20 |
| Silicon | 0 → 90 | 2.33 |
| Conductive Additive | 2 | 2.00 |
| PVDF Electrode Binder | 8 | 1.78 |
| Negative Mixture | - | 2.15 → 2.27 |
| Component | Dimensions | Material |
|---|---|---|
| Positive Current Collector | 20 µm | Al foil |
| Negative Current Collector | 10 µm | Cu foil |
| Negative Electrode | 200 mm × 120 mm | - |
| Positive Electrode | 199 mm × 119 mm | - |
| Separator 1 | 201.75 mm × 120 mm × 25 µm | Polypropylene/Polyethylene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yourey, W. Silicon Negative Electrodes—What Can Be Achieved for Commercial Cell Energy Densities. Batteries 2023, 9, 576. https://doi.org/10.3390/batteries9120576
Yourey W. Silicon Negative Electrodes—What Can Be Achieved for Commercial Cell Energy Densities. Batteries. 2023; 9(12):576. https://doi.org/10.3390/batteries9120576
Chicago/Turabian StyleYourey, William. 2023. "Silicon Negative Electrodes—What Can Be Achieved for Commercial Cell Energy Densities" Batteries 9, no. 12: 576. https://doi.org/10.3390/batteries9120576
APA StyleYourey, W. (2023). Silicon Negative Electrodes—What Can Be Achieved for Commercial Cell Energy Densities. Batteries, 9(12), 576. https://doi.org/10.3390/batteries9120576






