Traditional and Iterative Group-IV Material Batteries through Ion Migration
Abstract
:1. Introduction
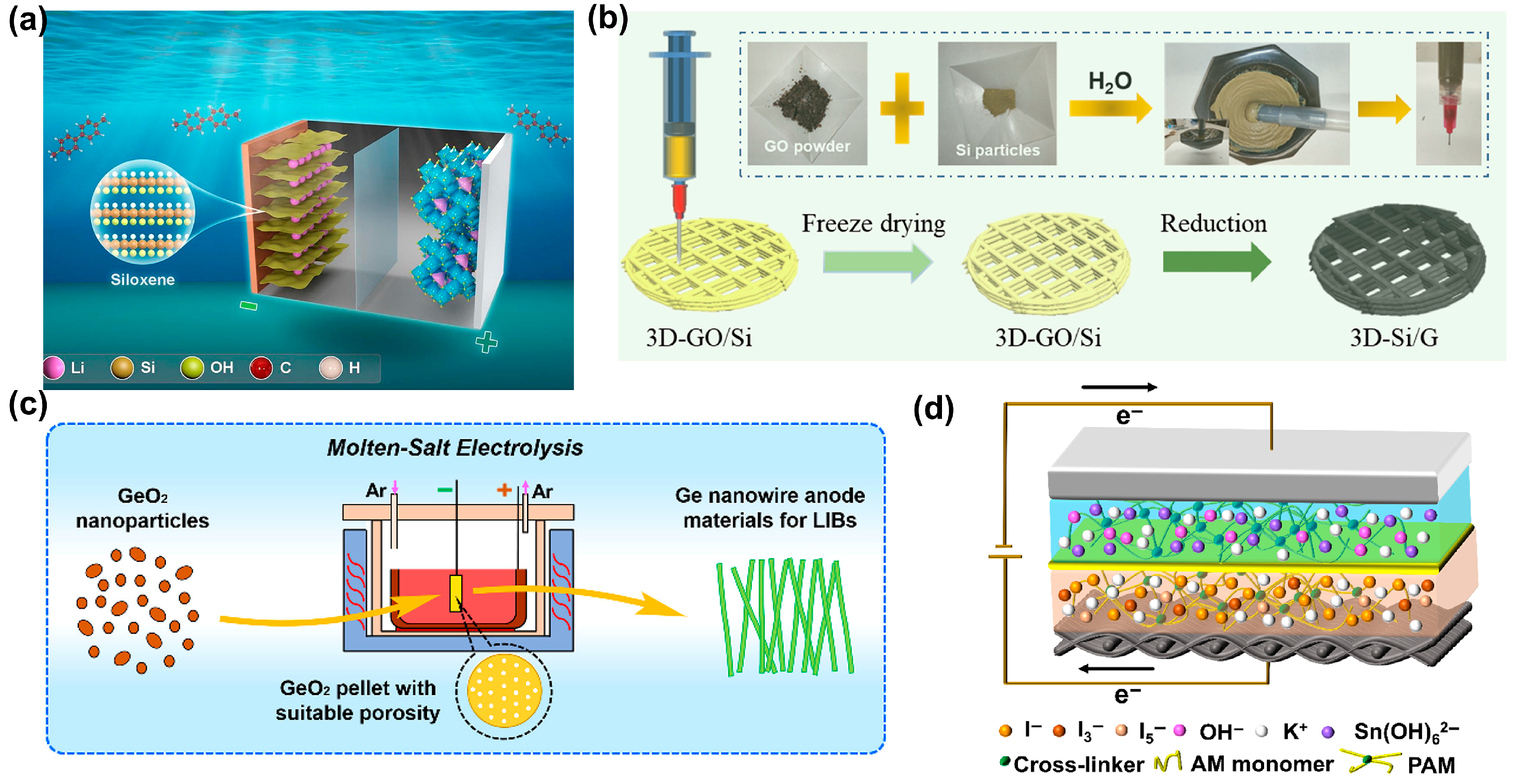
2. Traditional Group-IV Material Ion Batteries
2.1. Monovalent Alkali Metal Ion Batteries Assembled with Carbon Electrodes
2.2. Multivalent Metal-Ion Batteries Assembled with Carbon Materials
2.3. Metal-Ion Batteries Assembled with Other Group-IV Materials
3. Iterative Group-IV Materials Ion Batteries
3.1. Moisture Hydrogen Ion Batteries
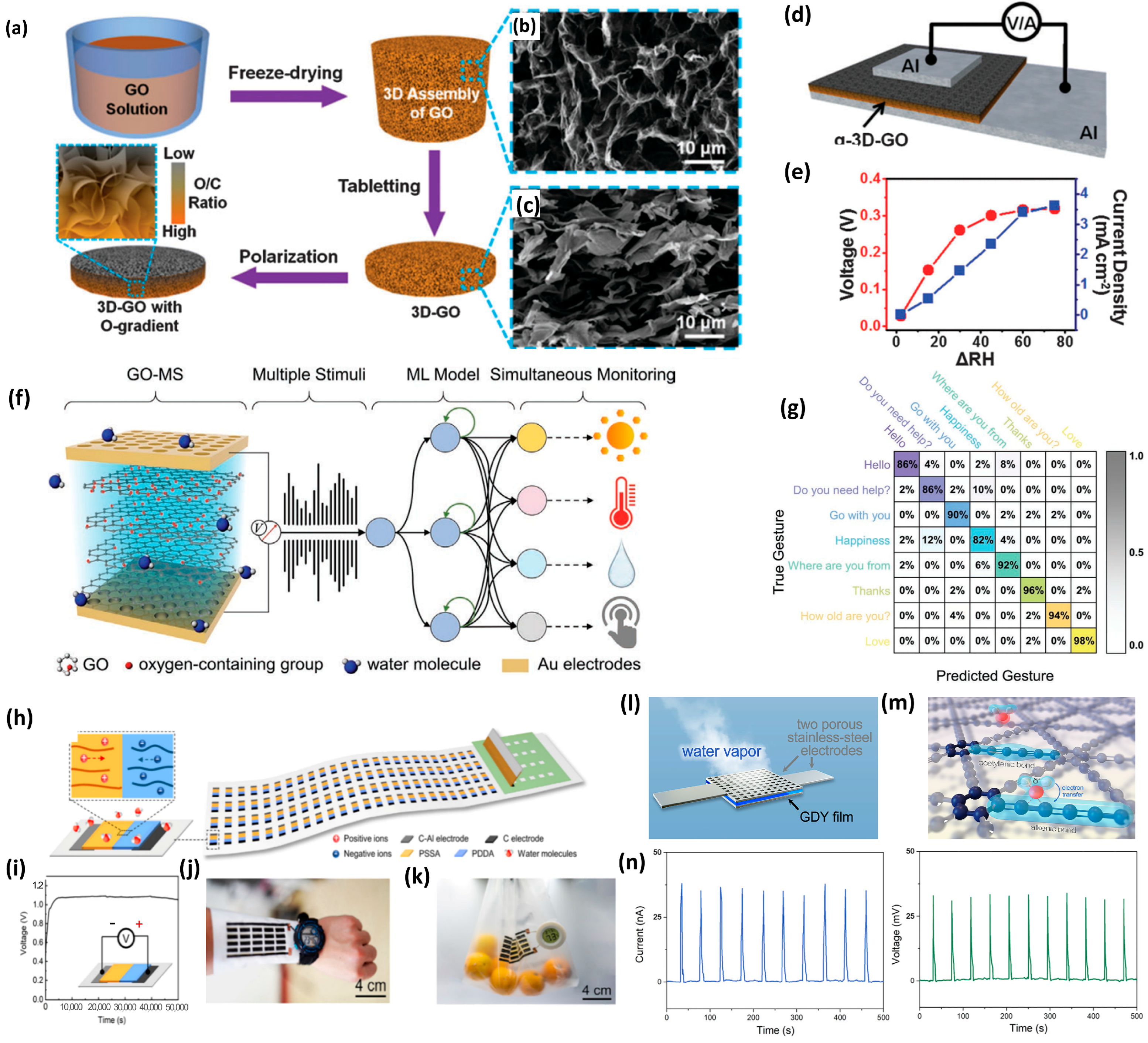
3.2. Moisture Metal-Ion Batteries
3.3. Moisture Ion Batteries with Complex Structure
4. Summary and Prospects
Funding
Conflicts of Interest
References
- Song, Y.-C.; Woo, J.-H.; Oh, G.-G.; Kim, D.-H.; Lee, C.-Y.; Kim, H.-W. External electric field promotes ammonia stripping from wastewater. Water Res. 2021, 203, 117518. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wang, H.; Li, F.; Jiang, B.; Tang, D.; Li, L. Ion engines in hydrogels boosting hydrovoltaic electricity generation. Energy Environ. Sci. 2023, 16, 2494–2504. [Google Scholar] [CrossRef]
- Jacobs, D.A.; Wolff, C.M.; Chin, X.-Y.; Artuk, K.; Ballif, C.; Jeangros, Q. Lateral ion migration accelerates degradation in halide perovskite devices. Energy Environ. Sci. 2022, 15, 5324–5339. [Google Scholar] [CrossRef]
- Lu, X.; Sun, Y.; Hu, W. The external electric field effect on the charge transport performance of organic semiconductors: A theoretical investigation. J. Mater. Chem. A 2021, 9, 21044–21050. [Google Scholar] [CrossRef]
- Zhu, X.; Hao, J.; Bao, B.; Zhou, Y.; Zhang, H.; Pang, J.; Jiang, Z.; Jiang, L. Unique ion rectification in hypersaline environment: A high-performance and sustainable power generator system. Sci. Adv. 2018, 4, eaau1665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, M.; Yin, J.; Abou-Hamad, E.; Schwingenschlögl, U.; Costa, P.M.F.J.; Alshareef, H.N. A Cyclized Polyacrylonitrile Anode for Alkali Metal Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 1355–1363. [Google Scholar] [CrossRef]
- Li, Y.; Song, S.; Kim, H.; Nomoto, K.; Kim, H.; Sun, X.; Hori, S.; Suzuki, K.; Matsui, N.; Hirayama, M.; et al. A lithium superionic conductor for millimeter-thick battery electrode. Science 2023, 381, 50–53. [Google Scholar] [CrossRef]
- Setiawan, D.; Kim, H.J.; Lyoo, J.; Hong, S.-T.; Chae, M.S. Novel layered iron vanadate as a stable high-voltage cathode material for nonaqueous magnesium-ion batteries. Chem. Eng. J. 2023, 474, 145596. [Google Scholar] [CrossRef]
- Li, Y.; Jia, H.; Ali, U.; Wang, H.; Liu, B.; Li, L.; Zhang, L.; Wang, C. Successive Gradient Internal Electric Field Strategy toward Dendrite-Free Zinc Metal Anode. Adv. Energy Mater. 2023, 13, 2301643. [Google Scholar] [CrossRef]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.-L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 2016, 1, 16070. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Chen, G.Z. Supercapacitor and supercapattery as emerging electrochemical energy stores. Int. Mater. Rev. 2017, 62, 173–202. [Google Scholar] [CrossRef]
- Liu, T.; Yan, R.; Huang, H.; Pan, L.; Cao, X.; deMello, A.; Niederberger, M. A Micromolding Method for Transparent and Flexible Thin-Film Supercapacitors and Hybrid Supercapacitors. Adv. Funct. Mater. 2020, 30, 2004410. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Huang, Y.; Pei, Z.; Xue, Q.; Li, H.; Geng, H.; Zhi, C. Capacitance Enhancement in a Semiconductor Nanostructure-Based Supercapacitor by Solar Light and a Self-Powered Supercapacitor–Photodetector System. Adv. Funct. Mater. 2016, 26, 4481–4490. [Google Scholar] [CrossRef]
- Qin, H.; Liu, P.; Chen, C.; Cong, H.-P.; Yu, S.-H. A multi-responsive healable supercapacitor. Nat. Commun. 2021, 12, 4297. [Google Scholar] [CrossRef] [PubMed]
- Flores-Diaz, N.; De Rossi, F.; Das, A.; Deepa, M.; Brunetti, F.; Freitag, M. Progress of Photocapacitors. Chem. Rev. 2023, 123, 9327–9355. [Google Scholar] [CrossRef]
- Yi, F.; Ren, H.; Dai, K.; Wang, X.; Han, Y.; Wang, K.; Li, K.; Guan, B.; Wang, J.; Tang, M.; et al. Solar thermal-driven capacitance enhancement of supercapacitors. Energy Environ. Sci. 2018, 11, 2016–2024. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, H.; Khan, Z.U.; Chen, J.C.; Gabrielsson, R.; Jonsson, M.P.; Berggren, M.; Crispin, X. Ionic thermoelectric supercapacitors. Energy Environ. Sci. 2016, 9, 1450–1457. [Google Scholar] [CrossRef]
- Hoffmann, M.; Fengler, F.P.G.; Max, B.; Schroeder, U.; Slesazeck, S.; Mikolajick, T. Negative Capacitance for Electrostatic Supercapacitors. Adv. Energy Mater. 2019, 9, 1901154. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Zheng, X.; Sun, W.; Dou, S.X.; Ma, T.; Pan, H. Anion-exchange membrane water electrolyzers and fuel cells. Chem. Soc. Rev. 2022, 51, 9620–9693. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Graphene oxide: A promising membrane material for fuel cells. Renew. Sustain. Energy Rev. 2018, 82, 714–733. [Google Scholar] [CrossRef]
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Yurko, Y.; Elbaz, L. Direct Quinone Fuel Cells. J. Am. Chem. Soc. 2023, 145, 2653–2660. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.J. Microbial fuel cells: Running on gas. Nat. Energy 2017, 2, 17093. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.; Wu, Z.; Chen, G.; Yang, F.; Zhu, S.; Siddharth, K.; Kong, Z.; Lu, A.; Li, J.; et al. Recent Advances in Electrocatalysts for Proton Exchange Membrane Fuel Cells and Alkaline Membrane Fuel Cells. Adv. Mater. 2021, 33, 2006292. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Zhang, Y.; Tao, L.; Wang, T.; Zou, Y.; Wang, Y.; Chen, R.; Wang, S. Defect Engineering for Fuel-Cell Electrocatalysts. Adv. Mater. 2020, 32, 1907879. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Kee, R.J.; Zhu, H.; Karakaya, C.; Chen, Y.; Ricote, S.; Jarry, A.; Crumlin, E.J.; Hook, D.; Braun, R.; et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018, 557, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, S. Production of Liquid Solar Fuels and Their Use in Fuel Cells. Joule 2017, 1, 689–738. [Google Scholar] [CrossRef]
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 17832–17852. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Lu, S.; Xiang, Y. Carbon Anode Materials: A Detailed Comparison between Na-ion and K-ion Batteries. Adv. Energy Mater. 2021, 11, 2003640. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Z.; Lai, W.; Tao, Y.; Peng, J.; Miao, Z.; Wang, Y.; Chou, S.; Liu, H.; Dou, S. Hard Carbon Anodes: Fundamental Understanding and Commercial Perspectives for Na-Ion Batteries beyond Li-Ion and K-Ion Counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Wang, G.; Yu, M.; Feng, X. Carbon materials for ion-intercalation involved rechargeable battery technologies. Chem. Soc. Rev. 2021, 50, 2388–2443. [Google Scholar] [CrossRef] [PubMed]
- Quertite, K.; Enriquez, H.; Trcera, N.; Tong, Y.; Bendounan, A.; Mayne, A.J.; Dujardin, G.; Lagarde, P.; El Kenz, A.; Benyoussef, A.; et al. Silicene Nanoribbons on an Insulating Thin Film. Adv. Funct. Mater. 2021, 31, 2007013. [Google Scholar] [CrossRef]
- Martella, C.; Massetti, C.; Dhungana, D.S.; Bonera, E.; Grazianetti, C.; Molle, A. Bendable Silicene Membranes. Adv. Mater. 2023, 35, 2211419. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Gao, N.; Li, Z.; Xu, X.; Wang, J.; Zhao, J.; Dou, S.X.; Du, Y. Cooperative Electron–Phonon Coupling and Buckled Structure in Germanene on Au(111). ACS Nano 2017, 11, 3553–3559. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, F.; Liu, Y.; Huang, T.; Zheng, B.; Ananth, N.; Xu, Z.; Gao, W.; Gao, C. A Defect-Free Principle for Advanced Graphene Cathode of Aluminum-Ion Battery. Adv. Mater. 2017, 29, 1605958. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Liu, H.; You, Z.; Li, Z.; Kang, F.; Wei, B. A Highly Flexible and Lightweight MnO2/Graphene Membrane for Superior Zinc-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2007397. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, B.; Ye, D.; Zhu, X.; Lyu, M.; Wang, L. An Innovative Freeze-Dried Reduced Graphene Oxide Supported SnS2 Cathode Active Material for Aluminum-Ion Batteries. Adv. Mater. 2017, 29, 1606132. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Y.; Xu, B.; Zhang, X.; Al-Ghanim, K.A.; Mahboob, S. Cobalt Sulfide Confined in N-Doped Porous Branched Carbon Nanotubes for Lithium-Ion Batteries. Nano-Micro Lett. 2019, 11, 29. [Google Scholar] [CrossRef]
- Fan, L.; Ma, R.; Zhang, Q.; Jia, X.; Lu, B. Graphite Anode for a Potassium-Ion Battery with Unprecedented Performance. Angew. Chem. Int. Ed. 2019, 58, 10500–10505. [Google Scholar] [CrossRef]
- Pang, Q.; Sun, C.; Yu, Y.; Zhao, K.; Zhang, Z.; Voyles, P.M.; Chen, G.; Wei, Y.; Wang, X. H2V3O8 Nanowire/Graphene Electrodes for Aqueous Rechargeable Zinc Ion Batteries with High Rate Capability and Large Capacity. Adv. Energy Mater. 2018, 8, 1800144. [Google Scholar] [CrossRef]
- Share, K.; Cohn, A.P.; Carter, R.; Rogers, B.; Pint, C.L. Role of Nitrogen-Doped Graphene for Improved High-Capacity Potassium Ion Battery Anodes. ACS Nano 2016, 10, 9738–9744. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Duley, W.W.; Peng, P.; Xiao, M.; Feng, J.; Liu, L.; Zou, G.; Zhou, Y.N. Moisture-Enabled Electricity Generation: From Physics and Materials to Self-Powered Applications. Adv. Mater. 2020, 32, 2003722. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, T.; Yan, T.; Wu, S.; Wu, M.; Chao, J.; Huo, X.; Wang, P.; Wang, R. Ultrahigh solar-driven atmospheric water production enabled by scalable rapid-cycling water harvester with vertically aligned nanocomposite sorbent. Energy Environ. Sci. 2021, 14, 5979–5994. [Google Scholar] [CrossRef]
- Zhao, F.; Cheng, H.; Zhang, Z.; Jiang, L.; Qu, L. Direct Power Generation from a Graphene Oxide Film under Moisture. Adv. Mater. 2015, 27, 4351–4357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Das, S.; Xing, G.; Fayon, P.; Heasman, P.; Jay, M.; Bailey, S.; Lambert, C.; Yamada, H.; Wakihara, T.; et al. A 3D Organically Synthesized Porous Carbon Material for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2018, 57, 11952–11956. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liang, J.; Dou, M.; Li, Z.; Huang, Y.; Wang, F. Biomass-derived mesopore-dominant porous carbons with large specific surface area and high defect density as high performance electrode materials for Li-ion batteries and supercapacitors. Nano Energy 2017, 36, 322–330. [Google Scholar] [CrossRef]
- Gao, F.; Geng, C.; Xiao, N.; Qu, J.; Qiu, J. Hierarchical porous carbon sheets derived from biomass containing an activation agent and in-built template for lithium ion batteries. Carbon 2018, 139, 1085–1092. [Google Scholar] [CrossRef]
- Liu, S.; Lin, Z.; Xiao, F.; Zhang, J.; Wang, D.; Chen, X.; Zhao, Y.; Xu, J. Co-N-C in porous carbon with enhanced lithium ion storage properties. Chem. Eng. J. 2020, 389, 124377. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Li, Y.; Shen, C.; Zhou, C.; Tan, X.; Yan, K.; Zhang, G.; Xu, X. Carbon Nanotube Interwoven Polyhedrons with Inside-out Lithiophilic Gradients toward Stable Lithium Metal Battery. Chem. Eng. J. 2022, 442, 136256. [Google Scholar] [CrossRef]
- Abdul Razzaq, A.; Yao, Y.; Shah, R.; Qi, P.; Miao, L.; Chen, M.; Zhao, X.; Peng, Y.; Deng, Z. High-performance lithium sulfur batteries enabled by a synergy between sulfur and carbon nanotubes. Energy Storage Mater. 2019, 16, 194–202. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Z.; Weng, S.; Zhang, Q.; Wang, X.; Wang, Z.; Gu, L.; Chen, L. Iron carbide allured lithium metal storage in carbon nanotube cavities. Energy Storage Mater. 2021, 36, 459–465. [Google Scholar] [CrossRef]
- Pan, Z.; Ren, J.; Guan, G.; Fang, X.; Wang, B.; Doo, S.-G.; Son, I.H.; Huang, X.; Peng, H. Synthesizing Nitrogen-Doped Core-Sheath Carbon Nanotube Films for Flexible Lithium Ion Batteries. Adv. Energy Mater. 2016, 6, 1600271. [Google Scholar] [CrossRef]
- Kühne, M.; Börrnert, F.; Fecher, S.; Ghorbani-Asl, M.; Biskupek, J.; Samuelis, D.; Krasheninnikov, A.V.; Kaiser, U.; Smet, J.H. Reversible superdense ordering of lithium between two graphene sheets. Nature 2018, 564, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Li, F.; Lu, X.; Liu, F.; Xu, J.; Kong, D.; Zhang, C.; Tan, X.; Ma, S.; Shi, W.; et al. High-Conductivity–Dispersibility Graphene Made by Catalytic Exfoliation of Graphite for Lithium-Ion Battery. Adv. Funct. Mater. 2021, 31, 2007630. [Google Scholar] [CrossRef]
- Fan, Q.; Noh, H.-J.; Wei, Z.; Zhang, J.; Lian, X.; Ma, J.; Jung, S.-M.; Jeon, I.-Y.; Xu, J.; Baek, J.-B. Edge-thionic acid-functionalized graphene nanoplatelets as anode materials for high-rate lithium ion batteries. Nano Energy 2019, 62, 419–425. [Google Scholar] [CrossRef]
- Zhang, J.; Ai, Y.; Wu, J.; Zhang, D.; Wang, Y.; Feng, Z.; Sun, H.; Liang, Q.; Sun, T.; Yang, Y. Nickel-Catalyzed Synthesis of 3D Edge-Curled Graphene for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2020, 30, 1904645. [Google Scholar] [CrossRef]
- Wang, N.; He, J.; Tu, Z.; Yang, Z.; Zhao, F.; Li, X.; Huang, C.; Wang, K.; Jiu, T.; Yi, Y.; et al. Synthesis of Chlorine-Substituted Graphdiyne and Applications for Lithium-Ion Storage. Angew. Chem. Int. Ed. 2017, 56, 10740–10745. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, J.; Ong, S.J.H.; Yao, Q.; Shi, X.; Hou, K.; Xu, Z.J.; Guan, L. High-Rate and Ultralong Cycle-Life Potassium Ion Batteries Enabled by In Situ Engineering of Yolk–Shell FeS2@C Structure on Graphene Matrix. Adv. Energy Mater. 2018, 8, 1802565. [Google Scholar] [CrossRef]
- Sun, J.; Sadd, M.; Edenborg, P.; Grönbeck, H.; Thiesen, P.H.; Xia, Z.; Quintano, V.; Qiu, R.; Matic, A.; Palermo, V. Real-time imaging of Na+ reversible intercalation in “Janus” graphene stacks for battery applications. Sci. Adv. 2021, 7, eabf0812. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Z.; Ren, J.; Xu, Y.; Xu, X.; Zhou, J.; Gao, F.; Tang, H.; Liu, S.; Wang, Z.; et al. Fe2VO4 nanoparticles on rGO as anode material for high-rate and durable lithium and sodium ion batteries. Chem. Eng. J. 2023, 451, 138882. [Google Scholar] [CrossRef]
- Yi, Y.; Li, J.; Zhao, W.; Zeng, Z.; Lu, C.; Ren, H.; Sun, J.; Zhang, J.; Liu, Z. Temperature-Mediated Engineering of Graphdiyne Framework Enabling High-Performance Potassium Storage. Adv. Funct. Mater. 2020, 30, 2003039. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Tu, Z.; Zhao, F.; He, J.; Guan, Z.; Huang, C.; Yi, Y.; Li, Y. Synthesis and Electronic Structure of Boron-Graphdiyne with an sp-Hybridized Carbon Skeleton and Its Application in Sodium Storage. Angew. Chem. Int. Ed. 2018, 57, 3968–3973. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, B.; Zhou, J.; Liang, S.; Rao, A.M.; Lu, B. Hierarchically Structured Nitrogen-Doped Carbon Microspheres for Advanced Potassium Ion Batteries. ACS Mater. Lett. 2020, 2, 853–860. [Google Scholar] [CrossRef]
- Ge, J.; Fan, L.; Wang, J.; Zhang, Q.; Liu, Z.; Zhang, E.; Liu, Q.; Yu, X.; Lu, B. MoSe2/N-Doped Carbon as Anodes for Potassium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1801477. [Google Scholar] [CrossRef]
- Xie, J.; Li, C.; Cui, Z.; Guo, X. Transition-Metal-Free Magnesium-Based Batteries Activated by Anionic Insertion into Fluorinated Graphene Nanosheets. Adv. Funct. Mater. 2015, 25, 6519–6526. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Liu, G.; Zhang, T.; Zhang, C.; Zhang, Y.; Feng, Y.; Chi, Q. Improvements in the Magnesium Ion Transport Properties of Graphene/CNT-Wrapped TiO2-B Nanoflowers by Nickel Doping. Small 2023, 19, 2304969. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Xiao, Y.; Yang, J.; Zuo, C.; Xiong, F.; Tang, C.; Liu, G.; Zhang, W.; Tang, W.; Wang, S.; et al. Polyaniline nanoarrays/carbon cloth as binder-free and flexible cathode for magnesium ion batteries. Chem. Eng. J. 2022, 433, 133772. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; He, P.; Zhang, Z.; Chen, T. Edge-rich vertical graphene nanosheets templating V2O5 for highly durable zinc ion battery. Carbon 2021, 172, 207–213. [Google Scholar] [CrossRef]
- Qiu, N.; Yang, Z.; Xue, R.; Wang, Y.; Zhu, Y.; Liu, W. Toward a High-Performance Aqueous Zinc Ion Battery: Potassium Vanadate Nanobelts and Carbon Enhanced Zinc Foil. Nano Lett. 2021, 21, 2738–2744. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Zhang, B.; Feng, J.; Zhang, J.; Ou, X.; Hou, F.; Liang, J. A flexible carbon nanotube@V2O5 film as a high-capacity and durable cathode for zinc ion batteries. J. Energy Chem. 2021, 59, 126–133. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, H.; Sun, X.; Yang, Y.; Chen, N.; Qu, L. In Situ Synthesis of Cathode Materials for Aqueous High-Rate and Durable Zn–I2 Batteries. ACS Mater. Lett. 2022, 4, 1872–1881. [Google Scholar] [CrossRef]
- Wang, X.; Gao, J.; Cheng, Z.; Chen, N.; Qu, L. A Responsive Battery with Controlled Energy Release. Angew. Chem. Int. Ed. 2016, 55, 14643–14647. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, F.; Shi, X.; Qin, J.; Zhang, Z.; Bao, X.; Wu, Z.-S. Graphene aerogel derived compact films for ultrafast and high-capacity aluminum ion batteries. Energy Storage Mater. 2019, 23, 664–669. [Google Scholar] [CrossRef]
- Kong, Y.; Tang, C.; Huang, X.; Nanjundan, A.K.; Zou, J.; Du, A.; Yu, C. Thermal Reductive Perforation of Graphene Cathode for High-Performance Aluminum-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2010569. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Zhao, Y.; Meng, J.; Wen, B.; Muttaqi, K.M.; Islam, M.R.; Cai, Q.; Zhang, S. High-Performance Rechargeable Aluminum-Ion Batteries Enabled by Composite FeF3 @ Expanded Graphite Cathode and Carbon Nanotube-Modified Separator. Adv. Energy Mater. 2022, 12, 2200959. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, G.; Huang, H.; Liu, C.; Lyu, P.; Zhang, N. Interlayer-expanded MoS2 nanoflowers anchored on the graphene: A high-performance Li+/Mg2+ co-intercalation cathode material. Chem. Eng. J. 2022, 428, 131214. [Google Scholar] [CrossRef]
- Shen, H.; An, Y.; Man, Q.; Wang, J.; Liu, C.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Controlled prelithiation of siloxene nanosheet anodes enables high performance 5 V-class lithium-ion batteries. Chem. Eng. J. 2023, 454, 140136. [Google Scholar] [CrossRef]
- Mu, T.; Xiang, L.; Wan, X.; Lou, S.; Du, C.; Zuo, P.; Yin, G. Ultrahigh areal capacity silicon anodes realized via manipulating electrode structure. Energy Storage Mater. 2022, 53, 958–968. [Google Scholar] [CrossRef]
- Liu, H.; Wu, T.; Zhang, L.; Wang, X.; Li, H.; Liu, S.; Zhang, Q.; Zhang, X.; Yu, H. Germanium Nanowires via Molten-Salt Electrolysis for Lithium Battery Anode. ACS Nano 2022, 16, 14402–14411. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yang, Y.; Wang, L.; Chen, N.; Qu, L. The Feasible Design of Quasi-Solid-State Aqueous Tin-Iodine Batteries. Renewables 2023, 1, 474–483. [Google Scholar] [CrossRef]
- Yuan, L.; Xiao, X.; Ding, T.; Zhong, J.; Zhang, X.; Shen, Y.; Hu, B.; Huang, Y.; Zhou, J.; Wang, Z.L. Paper-Based Supercapacitors for Self-Powered Nanosystems. Angew. Chem. 2012, 124, 5018–5022. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-Based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2021, 33, 2000619. [Google Scholar] [CrossRef]
- Zhao, F.; Liang, Y.; Cheng, H.; Jiang, L.; Qu, L. Highly efficient moisture-enabled electricity generation from graphene oxide frameworks. Energy Environ. Sci. 2016, 9, 912–916. [Google Scholar] [CrossRef]
- Yang, C.; Wang, H.; Yang, J.; Yao, H.; He, T.; Bai, J.; Guang, T.; Cheng, H.; Yan, J.; Qu, L. A Machine-Learning-Enhanced Simultaneous and Multimodal Sensor Based on Moist-Electric Powered Graphene Oxide. Adv. Mater. 2022, 34, 2205249. [Google Scholar] [CrossRef]
- He, T.; Wang, H.; Lu, B.; Guang, T.; Yang, C.; Huang, Y.; Cheng, H.; Qu, L. Fully printed planar moisture-enabled electric generator arrays for scalable function integration. Joule 2023, 7, 935–951. [Google Scholar] [CrossRef]
- Chen, N.; Yang, Y.; He, F.; Li, Y.; Liu, Q.; Li, Y. Chemical bond conversion directly drives power generation on the surface of graphdiyne. Matter 2022, 5, 2933–2945. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, F.; Cheng, Z.; Zhou, Q.; Shao, H.; Jiang, L.; Qu, L. Self-powered wearable graphene fiber for information expression. Nano Energy 2017, 32, 329–335. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, F.; Cheng, Z.; Deng, Y.; Xiao, Y.; Cheng, H.; Zhang, P.; Huang, Y.; Shao, H.; Qu, L. Electric power generation via asymmetric moisturizing of graphene oxide for flexible, printable and portable electronics. Energy Environ. Sci. 2018, 11, 1730–1735. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, Y.; Zhao, F.; Yang, C.; Zhang, P.; Jiang, L.; Shi, G.; Qu, L. Spontaneous power source in ambient air of a well-directionally reduced graphene oxide bulk. Energy Environ. Sci. 2018, 11, 2839–2845. [Google Scholar] [CrossRef]
- Xu, T.; Ding, X.; Huang, Y.; Shao, C.; Song, L.; Gao, X.; Zhang, Z.; Qu, L. An efficient polymer moist-electric generator. Energy Environ. Sci. 2019, 12, 972–978. [Google Scholar] [CrossRef]
- Chen, N.; Liu, Q.; Liu, C.; Zhang, G.; Jing, J.; Shao, C.; Han, Y.; Qu, L. MEG actualized by high-valent metal carrier transport. Nano Energy 2019, 65, 104047. [Google Scholar] [CrossRef]
- Sun, Z.; Wen, X.; Guo, S.; Zhou, M.; Wang, L.; Qin, X.; Tan, S.C. Weavable yarn-shaped moisture-induced electric generator. Nano Energy 2023, 116, 108748. [Google Scholar] [CrossRef]
- Fu, X.; He, F.; Gao, J.; Yan, X.; Chang, Q.; Zhang, Z.; Huang, C.; Li, Y. Directly Growing Graphdiyne Nanoarray Cathode to Integrate an Intelligent Solid Mg-Moisture Battery. J. Am. Chem. Soc. 2023, 145, 2759–2764. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; He, T.; Huang, Y.; Cheng, H.; Li, C.; Xie, D.; Yang, P.; Zhang, Y.; Qu, L. Bilayer of polyelectrolyte films for spontaneous power generation in air up to an integrated 1000 V output. Nat. Nanotechnol. 2021, 16, 811–819. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Wei, X.; Jin, Z.; Wang, Z.; Yang, Y.; Lv, J.; Chen, N. Traditional and Iterative Group-IV Material Batteries through Ion Migration. Batteries 2023, 9, 591. https://doi.org/10.3390/batteries9120591
He X, Wei X, Jin Z, Wang Z, Yang Y, Lv J, Chen N. Traditional and Iterative Group-IV Material Batteries through Ion Migration. Batteries. 2023; 9(12):591. https://doi.org/10.3390/batteries9120591
Chicago/Turabian StyleHe, Xiaojun, Xiaoyan Wei, Zifeng Jin, Zhenglin Wang, Ya’nan Yang, Jinsheng Lv, and Nan Chen. 2023. "Traditional and Iterative Group-IV Material Batteries through Ion Migration" Batteries 9, no. 12: 591. https://doi.org/10.3390/batteries9120591





