Rational Design of a Cost-Effective Biomass Carbon Framework for High-Performance Lithium Sulfur Batteries
Abstract
:1. Introduction
2. Experimental Section
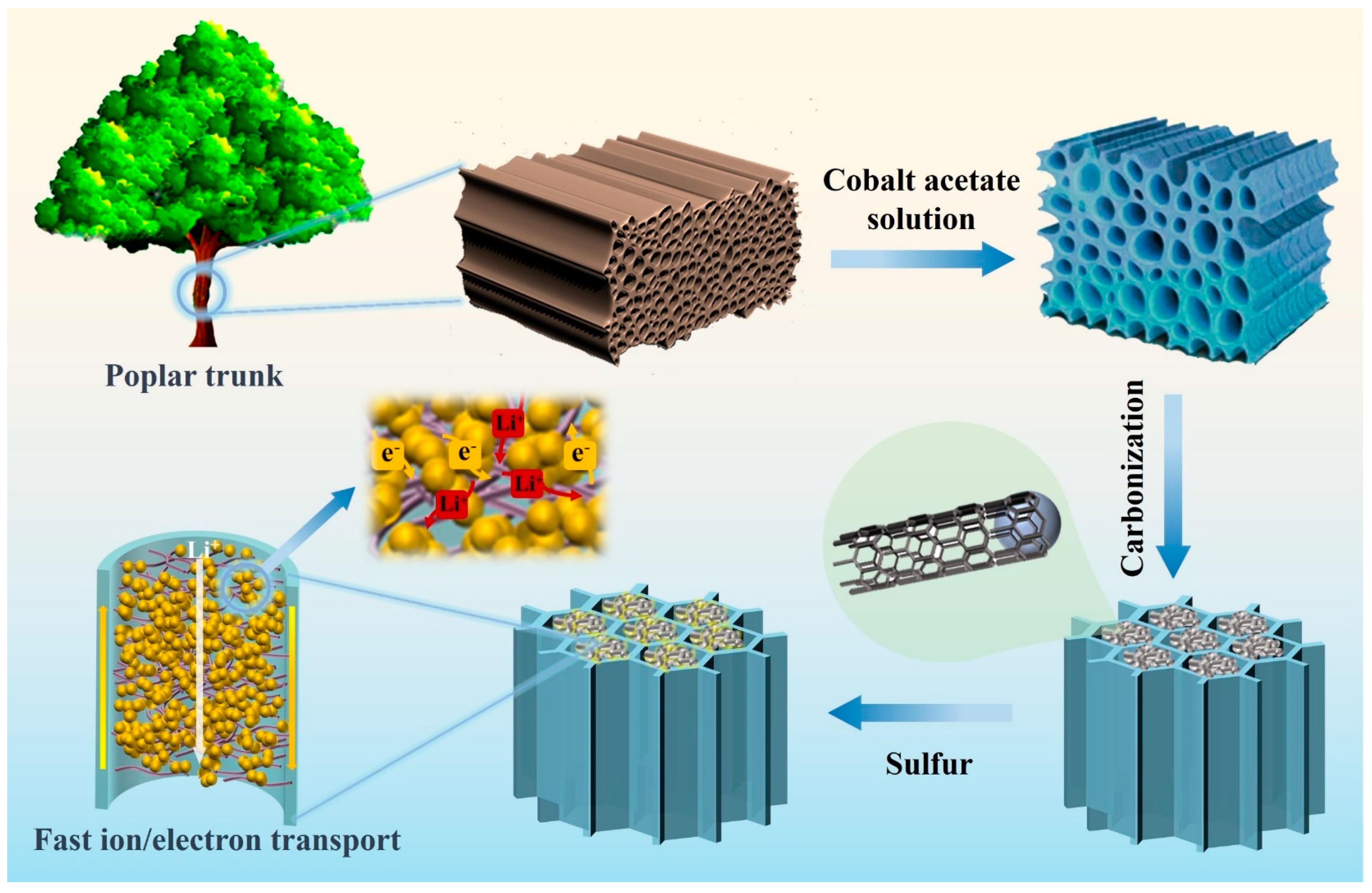
2.1. Preparation of WF-CNT
2.2. Preparation of S@WF-CNT
2.3. Characterization
2.4. Electrochemical Measurement
3. Results and Discussion
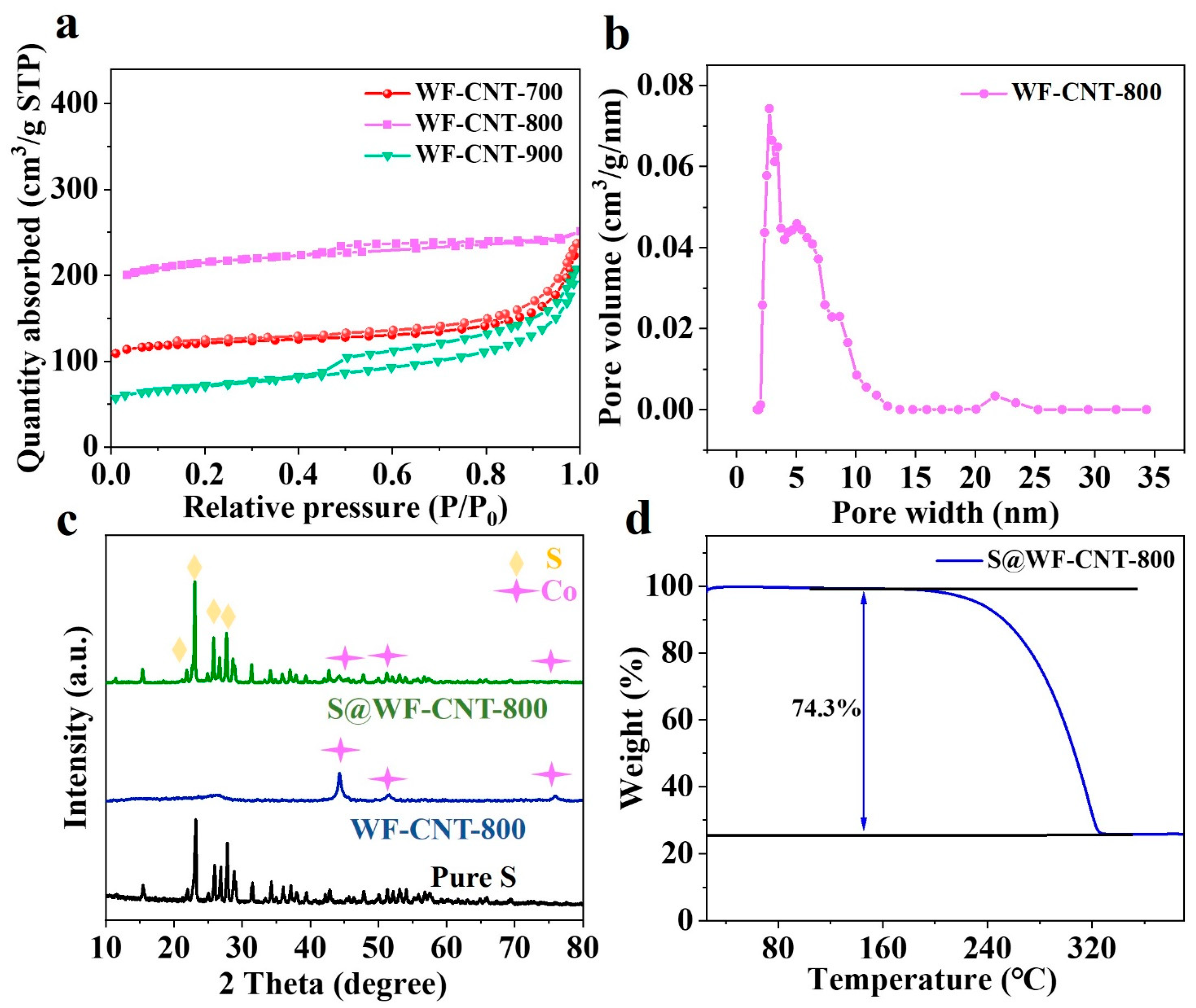
3.1. Morphology and Structure Characterization
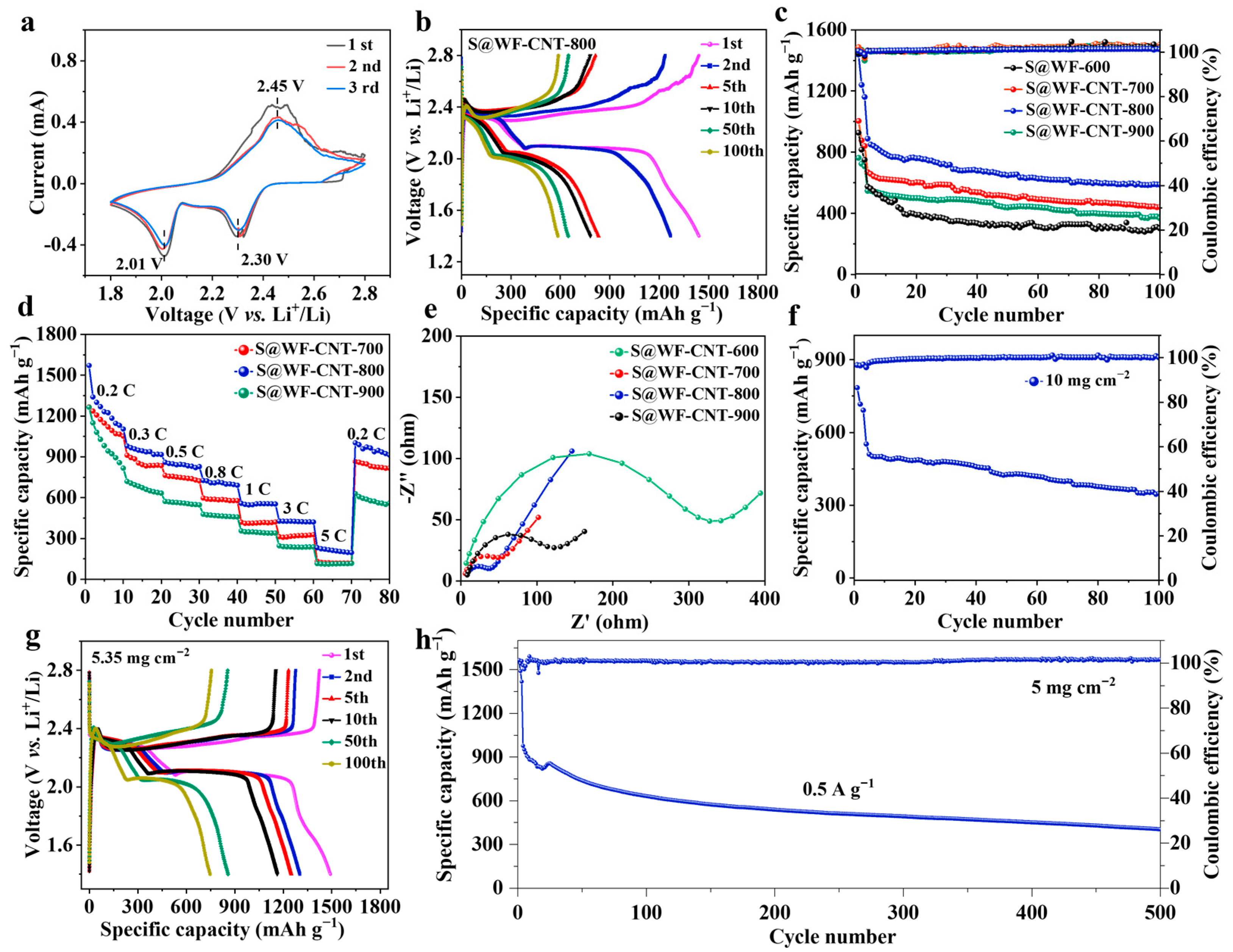
3.2. Electrochemical Performance of Li–S Batteries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wang, N.; Sun, C.; Lu, Z.; Xue, P.; Tang, B.; Bai, Z.; Dou, S. 3D spongy CoS2 nanoparticles/carbon composite as high-performance anode material for lithium/sodium ion batteries. Chem. Eng. J. 2018, 332, 370–376. [Google Scholar] [CrossRef]
- Xue, P.; Wang, N.; Fang, Z.; Lu, Z.; Xu, X.; Wang, L.; Du, Y.; Ren, X.; Bai, Z.; Dou, S. Rayleigh-instability-induced bismuth nanorod@nitrogen-doped carbon nanotubes as a long cycling and high rate anode for sodium-ion batteries. Nano Lett. 2019, 19, 1998–2004. [Google Scholar] [CrossRef]
- Parekh, M.H.; Palanisamy, M.; Pol, V.G. Reserve lithium-ion batteries: Deciphering in situ lithiation of lithium-ion free vanadium pentoxide cathode with graphitic anode. Carbon 2023, 203, 561–570. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Lei, J.; Liu, T.; Chen, J.; Zheng, M.; Zhang, Q.; Mao, B.; Dong, Q. Exploring and understanding the roles of Li2Sn and the strategies to beyond present Li-S batteries. Chem 2020, 6, 2533–2557. [Google Scholar] [CrossRef]
- Pope, M.A.; Aksay, I.A. Structural design of cathodes for Li-S batteries. Adv. Energy Mater. 2015, 5, 1500124. [Google Scholar] [CrossRef]
- Bradbury, R.; Dewald, G.F.; Kraft, M.A.; Arlt, T.; Kardjilov, N.; Janek, J.; Manke, I.; Zeier, W.G.; Ohno, S. Visualizing Reaction Fronts and Transport Limitations in Solid-State Li–S Batteries via Operando Neutron Imaging. Adv. Energy Mater. 2023, 13, 2203426. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable lithium–sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Kim, J.T.; Hao, X.; Wang, C.; Sun, X. Cathode materials for single-phase solid-solid conversion Li-S batteries. Matter 2023, 6, 316–343. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Morcrette, M.; Nouar, F.; Davoisne, C.; Devic, T.; Gonbeau, D.; Dominko, R.; Serre, C.; Férey, G.; Tarascon, J.-M. Cathode composites for Li–S batteries via the use of oxygenated porous architectures. J. Am. Chem. Soc. 2011, 133, 16154–16160. [Google Scholar] [CrossRef]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium–sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Ji, X.; Nazar, L.F. Advances in Li–S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Shaibani, M.; Akbari, A.; Sheath, P.; Easton, C.D.; Banerjee, P.C.; Konstas, K.; Fakhfouri, A.; Barghamadi, M.; Musameh, M.M.; Best, A.S. Suppressed polysulfide crossover in Li–S batteries through a high-flux graphene oxide membrane supported on a sulfur cathode. ACS Nano 2016, 10, 7768–7779. [Google Scholar] [CrossRef]
- Kim, H.M.; Sun, H.-H.; Belharouak, I.; Manthiram, A.; Sun, Y.-K. An alternative approach to enhance the performance of high sulfur-loading electrodes for Li–S batteries. ACS Energy Lett. 2016, 1, 136–141. [Google Scholar] [CrossRef]
- Wu, S.; Wang, W.; Shan, J.; Wang, X.; Lu, D.; Zhu, J.; Liu, Z.; Yue, L.; Li, Y. Conductive 1T-VS2−MXene heterostructured bidirectional electrocatalyst enabling compact Li-S batteries with high volumetric and areal capacity. Energy Storage Mater. 2022, 49, 153–163. [Google Scholar] [CrossRef]
- Elazari, R.; Salitra, G.; Garsuch, A.; Panchenko, A.; Aurbach, D. Sulfur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li-S batteries. Adv. Mater. 2011, 23, 5641–5644. [Google Scholar] [CrossRef]
- Senthil, C.; Jung, H.Y. Molecular polysulfide-scavenging sulfurized–triazine polymer enable high energy density Li-S battery under lean electrolyte. Energy Storage Mater. 2023, 55, 225–235. [Google Scholar] [CrossRef]
- Yan, R.; Mishra, B.; Traxler, M.; Roeser, J.; Chaoui, N.; Kumbhakar, B.; Schmidt, J.; Li, S.; Thomas, A.; Pachfule, P. A Thiazole-linked Covalent Organic Framework for Lithium-Sulphur Batteries. Angew. Chem. Int. Ed. 2023, 62, e202302276. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. A polyethylene glycol-supported microporous carbon coating as a polysulfide trap for utilizing pure sulfur cathodes in lithium–sulfur batteries. Adv. Mater. 2014, 26, 7352–7357. [Google Scholar] [CrossRef]
- Zhuang, T.Z.; Huang, J.Q.; Peng, H.J.; He, L.Y.; Cheng, X.B.; Chen, C.M.; Zhang, Q. Rational integration of polypropylene/graphene oxide/nafion as ternary-layered separator to retard the shuttle of polysulfides for lithium–sulfur batteries. Small 2016, 12, 381–389. [Google Scholar] [CrossRef]
- Luo, L.; Chung, S.-H.; Manthiram, A. A trifunctional multi-walled carbon nanotubes/polyethylene glycol (MWCNT/PEG)-coated separator through a layer-by-layer coating strategy for high-energy Li–S batteries. J. Mater. Chem. A 2016, 4, 16805–16811. [Google Scholar] [CrossRef]
- Choi, J.M.; Saroha, R.; Kim, J.S.; Jang, M.R.; Cho, J.S. Porous nanofibers comprising VN nanodots and densified N-doped CNTs as redox-active interlayers for Li–S batteries. J. Power Sources 2023, 559, 232632. [Google Scholar] [CrossRef]
- Cheng, L.; Curtiss, L.A.; Zavadil, K.R.; Gewirth, A.A.; Shao, Y.; Gallagher, K.G. Sparingly solvating electrolytes for high energy density lithium–sulfur batteries. ACS Energy Lett. 2016, 1, 503–509. [Google Scholar] [CrossRef]
- Xing, C.; Chen, H.; Qian, S.; Wu, Z.; Nizami, A.; Li, X.; Zhang, S.; Lai, C. Regulating liquid and solid-state electrolytes for solid-phase conversion in Li–S batteries. Chem 2022, 8, 1201–1230. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, K.; Fu, Y.; Guo, W. Benzoselenol as an organic electrolyte additive in Li-S battery. Nano Res. 2023, 16, 3814–3822. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Shi, C.; Alexander, G.V.; O’Neill, J.; Duncan, K.; Godbey, G.; Wachsman, E.D. All-Solid-State Garnet Type Sulfurized Polyacrylonitrile/Lithium-Metal Battery Enabled by an Inorganic Lithium Conductive Salt and a Bilayer Electrolyte Architecture. ACS Energy Lett. 2023, 8, 1803–1810. [Google Scholar] [CrossRef]
- Chi, S.S.; Liu, Y.; Song, W.L.; Fan, L.Z.; Zhang, Q. Prestoring lithium into stable 3D nickel foam host as dendrite-free lithium metal anode. Adv. Funct. Mater. 2017, 27, 1700348. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, X.; Liang, C.; Yu, Y.; Liu, W. Stabilized lithium metal anode by an efficient coating for high-performance Li–S batteries. Energy Storage Mater. 2020, 24, 329–335. [Google Scholar] [CrossRef]
- Yao, Y.X.; Zhang, X.Q.; Li, B.Q.; Yan, C.; Chen, P.Y.; Huang, J.Q.; Zhang, Q. A compact inorganic layer for robust anode protection in lithium-sulfur batteries. InfoMat 2020, 2, 379–388. [Google Scholar] [CrossRef]
- Chung, S.-H.; Chang, C.-H.; Manthiram, A. A core–shell electrode for dynamically and statically stable Li–S battery chemistry. Energy Environ. Sci. 2016, 9, 3188–3200. [Google Scholar] [CrossRef]
- Xiao, J. Understanding the Lithium Sulfur Battery System at Relevant Scales. Adv. Energy Mater. 2015, 5, 1501102. [Google Scholar] [CrossRef]
- Wang, M.; Bai, Z.; Yang, T.; Nie, C.; Xu, X.; Wang, Y.; Yang, J.; Dou, S.; Wang, N. Advances in High Sulfur Loading Cathodes for Practical Lithium-Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2201585. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, L.; Zhang, C.; Liu, L.; Li, Y.; Qiao, Z.; Lin, J.; Wei, Q.; Wang, L.; Xie, Q. Recent advances and strategies toward polysulfides shuttle inhibition for high-performance Li–S batteries. Adv. Sci. 2022, 9, 2106004. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Huang, J.; Tu, T.; Li, L. Cost-trivial material contributes greatly: A review of the application of starch in energy storage systems. J. Energy Storage 2023, 73, 109060. [Google Scholar] [CrossRef]
- Jung, D.S.; Ryou, M.H.; Sung, Y.J.; Park, S.B.; Choi, J.W. Recycling rice husks for high-capacity lithium battery anodes. Proc. Natl. Acad. Sci. USA 2013, 110, 12229–12234. [Google Scholar] [CrossRef]
- Xia, Y.; Xiao, Z.; Dou, X.; Huang, H.; Lu, X.; Yan, R.; Gan, Y.; Zhu, W.; Tu, J.; Zhang, W. Green and facile fabrication of hollow porous MnO/C microspheres from microalgaes for lithium-ion batteries. ACS Nano 2013, 7, 7083–7092. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Zhang, Q.; Yang, J.; Duan, G.; Xu, W.; Yang, F.; Jiang, S. Electrode thickness design toward bulk energy storage devices with high areal/volumetric energy density. Appl. Energy 2021, 289, 116734. [Google Scholar] [CrossRef]
- Yu, Z.-L.; Yang, N.; Zhou, L.-C.; Ma, Z.-Y.; Zhu, Y.-B.; Lu, Y.-Y.; Qin, B.; Xing, W.-Y.; Ma, T.; Li, S.-C. Bioinspired polymeric woods. Sci. Adv. 2018, 4, eaat7223. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Li, Y.; Kuang, Y.; Song, J.; Luo, W.; Wang, Y.; Yao, Y.; Pastel, G.; Xie, J. Highly conductive, lightweight, low-tortuosity carbon frameworks as ultrathick 3D current collectors. Adv. Energy Mater. 2017, 7, 1700595. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Ju, Z.; Yu, X.; Wang, Y.; Du, Y.; Bai, Z.; Dou, S.; Yu, G. Thickness-independent scalable high-performance Li-S batteries with high areal sulfur loading via electron-enriched carbon framework. Nat. Commun. 2021, 12, 4519. [Google Scholar] [CrossRef]
- He, J.; Lv, W.; Chen, Y.; Xiong, J.; Wen, K.; Xu, C.; Zhang, W.; Li, Y.; Qin, W.; He, W. Direct impregnation of SeS2 into a MOF-derived 3D nanoporous Co–N–C architecture towards superior rechargeable lithium batteries. J. Mater. Chem. A 2018, 6, 10466–10473. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, J.; Yanzhang, R.; Du, M.; Wang, Q.; Gao, G.; Wu, J.; Wu, G.; Zhang, M.; Liu, B. When cubic cobalt sulfide meets layered molybdenum disulfide: A core–shell system toward synergetic electrocatalytic water splitting. Adv. Mater. 2015, 27, 4752–4759. [Google Scholar] [CrossRef]
- Lu, Z.; Zhai, Y.; Wang, N.; Zhang, Y.; Xue, P.; Guo, M.; Tang, B.; Huang, D.; Wang, W.; Bai, Z. FeS2 nanoparticles embedded in N/S co-doped porous carbon fibers as anode for sodium-ion batteries. Chem. Eng. J. 2020, 380, 122455. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, Z.; Yao, Y.; Jiang, Y.; Rui, X.; Liu, J.; Yu, Y. A High-Efficiency Mo2C Electrocatalyst Promoting the Polysulfide Redox Kinetics for Na-S Batteries. Adv. Mater. 2022, 34, e2200479. [Google Scholar] [CrossRef]
- Song, J.; Xu, T.; Gordin, M.L.; Zhu, P.; Lv, D.; Jiang, Y.B.; Chen, Y.; Duan, Y.; Wang, D. Nitrogen-doped mesoporous carbon promoted chemical adsorption of sulfur and fabrication of high-areal-capacity sulfur cathode with exceptional cycling stability for lithium-sulfur batteries. Adv. Funct. Mater. 2014, 24, 1243–1250. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. Current status and future prospects of metal–sulfur batteries. Adv. Mater. 2019, 31, 1901125. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Zhang, Q.; Peng, H.-J.; Liu, X.-Y.; Qian, W.-Z.; Wei, F. Ionic shield for polysulfides towards highly-stable lithium–sulfur batteries. Energy Environ. Sci. 2014, 7, 347–353. [Google Scholar] [CrossRef]
- Xu, R.; Lu, J.; Amine, K. Progress in mechanistic understanding and characterization techniques of Li-S batteries. Adv. Energy Mater. 2015, 5, 1500408. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Chen, Z.; Peng, X. CNT-threaded N-doped porous carbon film as binder-free electrode for high-capacity supercapacitor and Li–S battery. J. Mater. Chem. A 2017, 5, 9775–9784. [Google Scholar] [CrossRef]
- Ma, L.; Zhuang, H.L.; Wei, S.; Hendrickson, K.E.; Kim, M.S.; Cohn, G.; Hennig, R.G.; Archer, L.A. Enhanced Li–S batteries using amine-functionalized carbon nanotubes in the cathode. ACS Nano 2016, 10, 1050–1059. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.-W.; Li, F.; Hou, P.-X.; Yin, L.; Liu, C.; Lu, G.Q.M.; Gentle, I.R.; Cheng, H.-M. A flexible nanostructured sulphur–carbon nanotube cathode with high rate performance for Li-S batteries. Energy Environ. Sci. 2012, 5, 8901–8906. [Google Scholar] [CrossRef]
- Lee, J.S.; Jun, J.; Jang, J.; Manthiram, A. Sulfur-immobilized, activated porous carbon nanotube composite based cathodes for lithium–sulfur batteries. Small 2017, 13, 1602984. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Wang, J.; Luo, D.; Sun, Z.; Zhao, Y.; Yu, A.; Wang, X.; Chen, Z. “Sauna” Activation toward Intrinsic Lattice Deficiency in Carbon Nanotube Microspheres for High-Energy and Long-Lasting Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2100497. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, L.; Wang, J.; Zhu, J.; Shen, P.K. In situ carbon nanotube clusters grown from three-dimensional porous graphene networks as efficient sulfur hosts for high-rate ultra-stable Li–S batteries. Nano Res. 2018, 11, 1731–1743. [Google Scholar] [CrossRef]
- Xu, D.W.; Xin, S.; You, Y.; Li, Y.; Cong, H.P.; Yu, S.H. Built-in Carbon Nanotube Network inside a Biomass-Derived Hierarchically Porous Carbon to Enhance the Performance of the Sulfur Cathode in a Li-S Battery. ChemNanoMat 2016, 2, 712–718. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, Y.; Huang, Y.; Yue, H.; Cao, Z.; Dong, H.; Yin, Y.; Yang, S. Organic alkali metal salt derived three-dimensional N-doped porous carbon/carbon nanotubes composites with superior Li–S battery performance. ACS Sustain. Chem. Eng. 2019, 7, 3995–4003. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Z.; Fan, K.; Guo, M.; Wang, M.; Yang, T.; Wang, N. Rational Design of a Cost-Effective Biomass Carbon Framework for High-Performance Lithium Sulfur Batteries. Batteries 2023, 9, 594. https://doi.org/10.3390/batteries9120594
Bai Z, Fan K, Guo M, Wang M, Yang T, Wang N. Rational Design of a Cost-Effective Biomass Carbon Framework for High-Performance Lithium Sulfur Batteries. Batteries. 2023; 9(12):594. https://doi.org/10.3390/batteries9120594
Chicago/Turabian StyleBai, Zhongchao, Kai Fan, Meiqing Guo, Mingyue Wang, Ting Yang, and Nana Wang. 2023. "Rational Design of a Cost-Effective Biomass Carbon Framework for High-Performance Lithium Sulfur Batteries" Batteries 9, no. 12: 594. https://doi.org/10.3390/batteries9120594
APA StyleBai, Z., Fan, K., Guo, M., Wang, M., Yang, T., & Wang, N. (2023). Rational Design of a Cost-Effective Biomass Carbon Framework for High-Performance Lithium Sulfur Batteries. Batteries, 9(12), 594. https://doi.org/10.3390/batteries9120594







