Percolation Behavior of a Sulfide Electrolyte–Carbon Additive Matrix for Composite Cathodes in All-Solid-State Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Li6PS5Cl Powder and Separator
2.2. Preparation of the Conducting Matrix
2.3. Phase and Microstructural Analysis
2.4. Electrochemical Impedance Measurement
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rüdisüli, M.; Bach, C.; Bauer, C.; Beloin-Saint-Pierre, D.; Elber, U.; Georges, G.; Limpach, R.; Pareschi, G.; Kannan, R.; Teske, S.L. Prospective life-cycle assessment of greenhouse gas emissions of electricity-based mobility options. Appl. Energy 2022, 306, 118065. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 652. [Google Scholar] [CrossRef]
- Krauskopf, T.; Mogwitz, B.; Hartmann, H.; Singh, D.K.; Zeier, W.G.; Janek, J. The Fast Charge Transfer Kinetics of the Lithium Metal Anode on the Garnet-Type Solid Electrolyte Li6.25Al0.25La3Zr2O12. Adv. Energy Mater. 2020, 10, 2000945. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, Challenges, and Recent Progress of Inorganic Solid-State Electrolytes for All-Solid-State Lithium Batteries. Adv. Mater. 2018, 30, e1705702. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Adeli, P.; Bazak, J.D.; Huq, A.; Goward, G.R.; Nazar, L.F. Influence of Aliovalent Cation Substitution and Mechanical Compression on Li-Ion Conductivity and Diffusivity in Argyrodite Solid Electrolytes. Chem. Mater. 2021, 33, 146–157. [Google Scholar] [CrossRef]
- Li, Y.; Xu, B.; Xu, H.; Duan, H.; Lü, X.; Xin, S.; Zhou, W.; Xue, L.; Fu, G.; Manthiram, A.; et al. Hybrid Polymer/Garnet Electrolyte with a Small Interfacial Resistance for Lithium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2017, 56, 753–756. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Bielefeld, A.; Weber, D.A.; Janek, J. Modeling Effective Ionic Conductivity and Binder Influence in Composite Cathodes for All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2020, 12, 12821–12833. [Google Scholar] [CrossRef]
- Bielefeld, A.; Weber, D.A.; Rueß, R.; Glavas, V.; Janek, J. Influence of Lithium Ion Kinetics, Particle Morphology and Voids on the Electrochemical Performance of Composite Cathodes for All-Solid-State Batteries. J. Electrochem. Soc. 2022, 169, 20539. [Google Scholar] [CrossRef]
- Doux, J.-M.; Yang, Y.; Tan, D.H.S.; Nguyen, H.; Wu, E.A.; Wang, X.; Banerjee, A.; Meng, Y.S. Pressure effects on sulfide electrolytes for all solid-state batteries. J. Mater. Chem. A 2020, 8, 5049–5055. [Google Scholar] [CrossRef]
- Ruiz, A.G.; Sola, P.C.; Palmerola, N.M. Germanium: Current and Novel Recovery Processes. In Advanced Material and Device Applications with Germanium; Lee, S., Ed.; InTech: London, UK, 2018; ISBN 978-1-78984-031-5. [Google Scholar]
- Lee, J.; Lee, T.; Char, K.; Kim, K.J.; Choi, J.W. Issues and Advances in Scaling up Sulfide-Based All-Solid-State Batteries. Acc. Chem. Res. 2021, 54, 3390–3402. [Google Scholar] [CrossRef] [PubMed]
- Walther, F.; Koerver, R.; Fuchs, T.; Ohno, S.; Sann, J.; Rohnke, M.; Zeier, W.G.; Janek, J. Visualization of the Interfacial Decomposition of Composite Cathodes in Argyrodite-Based All-Solid-State Batteries Using Time-of-Flight Secondary-Ion Mass Spectrometry. Chem. Mater. 2019, 31, 3745–3755. [Google Scholar] [CrossRef]
- Jung, S.-K.; Gwon, H.; Lee, S.-S.; Kim, H.; Lee, J.C.; Chung, J.G.; Park, S.Y.; Aihara, Y.; Im, D. Understanding the effects of chemical reactions at the cathode–electrolyte interface in sulfide based all-solid-state batteries. J. Mater. Chem. A 2019, 7, 22967–22976. [Google Scholar] [CrossRef]
- Walther, F.; Randau, S.; Schneider, Y.; Sann, J.; Rohnke, M.; Richter, F.H.; Zeier, W.G.; Janek, J. Influence of Carbon Additives on the Decomposition Pathways in Cathodes of Lithium Thiophosphate-Based All-Solid-State Batteries. Chem. Mater. 2020, 32, 6123–6136. [Google Scholar] [CrossRef]
- Zhang, W.; Leichtweiß, T.; Culver, S.P.; Koerver, R.; Das, D.; Weber, D.A.; Zeier, W.G.; Janek, J. The Detrimental Effects of Carbon Additives in Li10GeP2S12-Based Solid-State Batteries. ACS Appl. Mater. Interfaces 2017, 9, 35888–35896. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Kim, J.-J.; Seong, W.M.; Lee, M.H.; Kang, K. Investigation on the interface between Li10GeP2S12 electrolyte and carbon conductive agents in all-solid-state lithium battery. Sci. Rep. 2018, 8, 8066. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Nichols, W.T.; Park, C.; Shin, D. Enhanced energy density and electrochemical performance of all-solid-state lithium batteries through microstructural distribution of solid electrolyte. Ceram. Int. 2017, 43, 15952–15958. [Google Scholar] [CrossRef]
- Ann, J.; Choi, S.; Do, J.; Lim, S.; Shin, D. Effects of binary conductive additives on electrochemical performance of a sheettype composite cathode with different weight ratios of LiNi0.6Co0.2Mn0.2O2 in allsolid-state lithium batteries. Ceram. Process. Res. 2018, 19, 413–418. [Google Scholar]
- Oh, G.; Hirayama, M.; Kwon, O.; Suzuki, K.; Kanno, R. Bulk-Type All Solid-State Batteries with 5 V Class LiNi0.5Mn1.5O4 Cathode and Li10GeP2S12 Solid Electrolyte. Chem. Mater. 2016, 28, 2634–2640. [Google Scholar] [CrossRef]
- Al-Salih, H.; Houache, M.S.E.; Baranova, E.A.; Abu-Lebdeh, Y. Composite Cathodes for Solid-State Lithium Batteries: “Catholytes” the Underrated Giants. Adv. Energy Sustain. Res. 2022, 3, 2200032. [Google Scholar] [CrossRef]
- Kato, Y.; Shiotani, S.; Morita, K.; Suzuki, K.; Hirayama, M.; Kanno, R. All-Solid-State Batteries with Thick Electrode Configurations. J. Phys. Chem. Lett. 2018, 9, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Randau, S.; Becker-Steinberger, K.; Danner, T.; Hein, S.; Ning, Z.; Marrow, J.; Richter, F.H.; Janek, J.; Latz, A. Analysis of Interfacial Effects in All-Solid-State Batteries with Thiophosphate Solid Electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 9277–9291. [Google Scholar] [CrossRef] [PubMed]
- Strauss, F.; Stepien, D.; Maibach, J.; Pfaffmann, L.; Indris, S.; Hartmann, P.; Brezesinski, T. Influence of electronically conductive additives on the cycling performance of argyrodite-based all-solid-state batteries. RSC Adv. 2020, 10, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Oh, G.; Park, J.-W.; Ha, Y.-C.; Lee, S.-M.; Yoon, S.Y.; Kim, B.G. Graphitic Hollow Nanocarbon as a Promising Conducting Agent for Solid-State Lithium Batteries. Small 2019, 15, e1900235. [Google Scholar] [CrossRef]
- Amin, R.; Chiang, Y.-M. Characterization of Electronic and Ionic Transport in Li1−xNi0.33Mn0.33Co0.33O2 (NMC333) and Li1−xNi0.50Mn0.20Co0.30O2 (NMC523) as a Function of Li Content. J. Electrochem. Soc. 2016, 163, A1512–A1517. [Google Scholar] [CrossRef]
- Asano, T.; Yubuchi, S.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Electronic and Ionic Conductivities of LiNi1/3Mn1/3Co1/3O2-Li3PS4 Positive Composite Electrodes for All-Solid-State Lithium Batteries. J. Electrochem. Soc. 2017, 164, A3960–A3963. [Google Scholar] [CrossRef]
- Conforto, G.; Ruess, R.; Schröder, D.; Trevisanello, E.; Fantin, R.; Richter, F.H.; Janek, J. Editors’ Choice—Quantification of the Impact of Chemo-Mechanical Degradation on the Performance and Cycling Stability of NCM-Based Cathodes in Solid-State Li-Ion Batteries. J. Electrochem. Soc. 2021, 168, 70546. [Google Scholar] [CrossRef]
- Han, Y.; Jung, S.H.; Kwak, H.; Jun, S.; Kwak, H.H.; Lee, J.H.; Hong, S.-T.; Jung, Y.S. Single- or Poly-Crystalline Ni-Rich Layered Cathode, Sulfide or Halide Solid Electrolyte: Which Will be the Winners for All-Solid-State Batteries? Adv. Energy Mater. 2021, 11, 2100126. [Google Scholar] [CrossRef]
- Nam, Y.J.; Oh, D.Y.; Jung, S.H.; Jung, Y.S. Toward practical all-solid-state lithium-ion batteries with high energy density and safety: Comparative study for electrodes fabricated by dry- and slurry-mixing processes. J. Power Sources 2018, 375, 93–101. [Google Scholar] [CrossRef]
- Froboese, L.; van der Sichel, J.F.; Loellhoeffel, T.; Helmers, L.; Kwade, A. Effect of Microstructure on the Ionic Conductivity of an All Solid-State Battery Electrode. J. Electrochem. Soc. 2019, 166, A318–A328. [Google Scholar] [CrossRef]
- Newman, M.E.; Ziff, R.M. Efficient Monte Carlo algorithm and high-precision results for percolation. Phys. Rev. Lett. 2000, 85, 4104–4107. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Su, X.; Jiao, Y. Continuum percolation of congruent overlapping spherocylinders. Phys. Rev. E 2016, 94, 32122. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, D.S.; Blaszkiewicz, M.; Newnham, R.E. Electrical Resistivity of Composites. J. Am. Ceram. Soc. 1990, 73, 2187–2203. [Google Scholar] [CrossRef]
- Minnmann, P.; Quillman, L.; Burkhardt, S.; Richter, F.H.; Janek, J. Editors’ Choice—Quantifying the Impact of Charge Transport Bottlenecks in Composite Cathodes of All-Solid-State Batteries. J. Electrochem. Soc. 2021, 168, 40537. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Yu, C.; Ganapathy, S.; Hageman, J.; van Eijck, L.; van Eck, E.R.H.; Zhang, L.; Schwietert, T.; Basak, S.; Kelder, E.M.; Wagemaker, M. Facile Synthesis toward the Optimal Structure-Conductivity Characteristics of the Argyrodite Li6PS5Cl Solid-State Electrolyte. ACS Appl. Mater. Interfaces 2018, 10, 33296–33306. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Liu, Y.; Yu, C.; Yan, X.; Xu, B.; Wang, L. Synthesis and characterization of argyrodite solid electrolytes for all-solid-state Li-ion batteries. J. Alloys Compd. 2018, 747, 227–235. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Gupta, P.K. Rigidity and conductivity percolation thresholds in particulate composites. Acta Metall. Mater. 1995, 43, 397–403. [Google Scholar] [CrossRef]
- Chen, D.; He, H.; Zhang, D.; Wang, H.; Ni, M. Percolation Theory in Solid Oxide Fuel Cell Composite Electrodes with a Mixed Electronic and Ionic Conductor. Energies 2013, 6, 1632–1656. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.C.; Chow, W.S.; To, C.K.; Tang, B.Z.; Kim, J.-K. Correlations between Percolation Threshold, Dispersion State, and Aspect Ratio of Carbon Nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.Y.; Shin, D.O.; Oh, J.; Kim, J.; Lee, M.J.; Lee, Y.-G.; Ryou, M.-H.; Lee, Y.M. Dimension-controlled solid oxide electrolytes for all-solid-state electrodes: Percolation pathways, specific contact area, and effective ionic conductivity. Chem. Eng. J. 2020, 391, 123528. [Google Scholar] [CrossRef]
- Bielefeld, A.; Weber, D.A.; Janek, J. Microstructural Modeling of Composite Cathodes for All-Solid-State Batteries. J. Phys. Chem. C 2019, 123, 1626–1634. [Google Scholar] [CrossRef]
- Guzmán, G.; Vazquez-Arenas, J.; Ramos-Sánchez, G.; Bautista-Ramírez, M.; González, I. Improved performance of LiFePO4 cathode for Li-ion batteries through percolation studies. Electrochim. Acta 2017, 247, 451–459. [Google Scholar] [CrossRef]


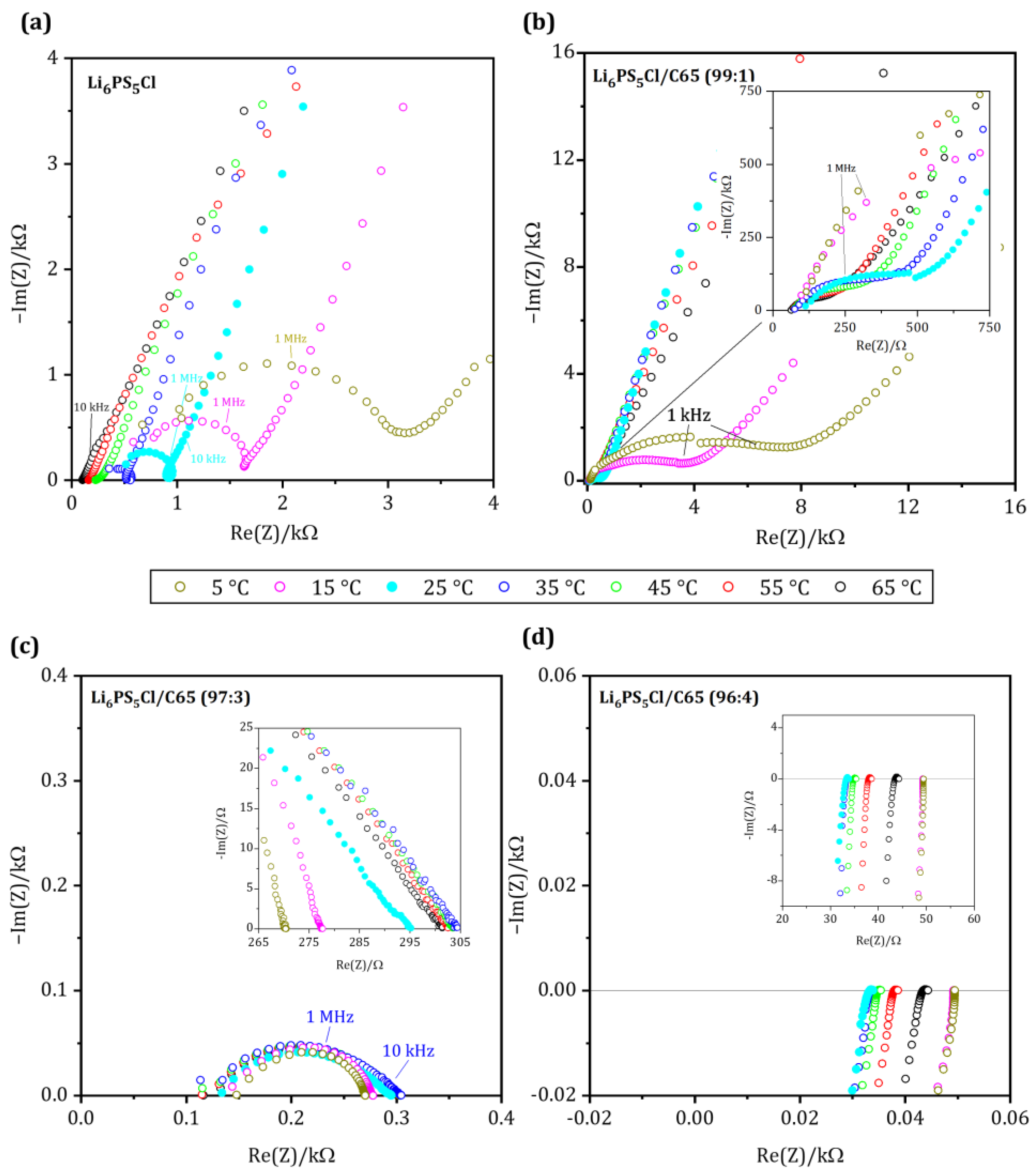
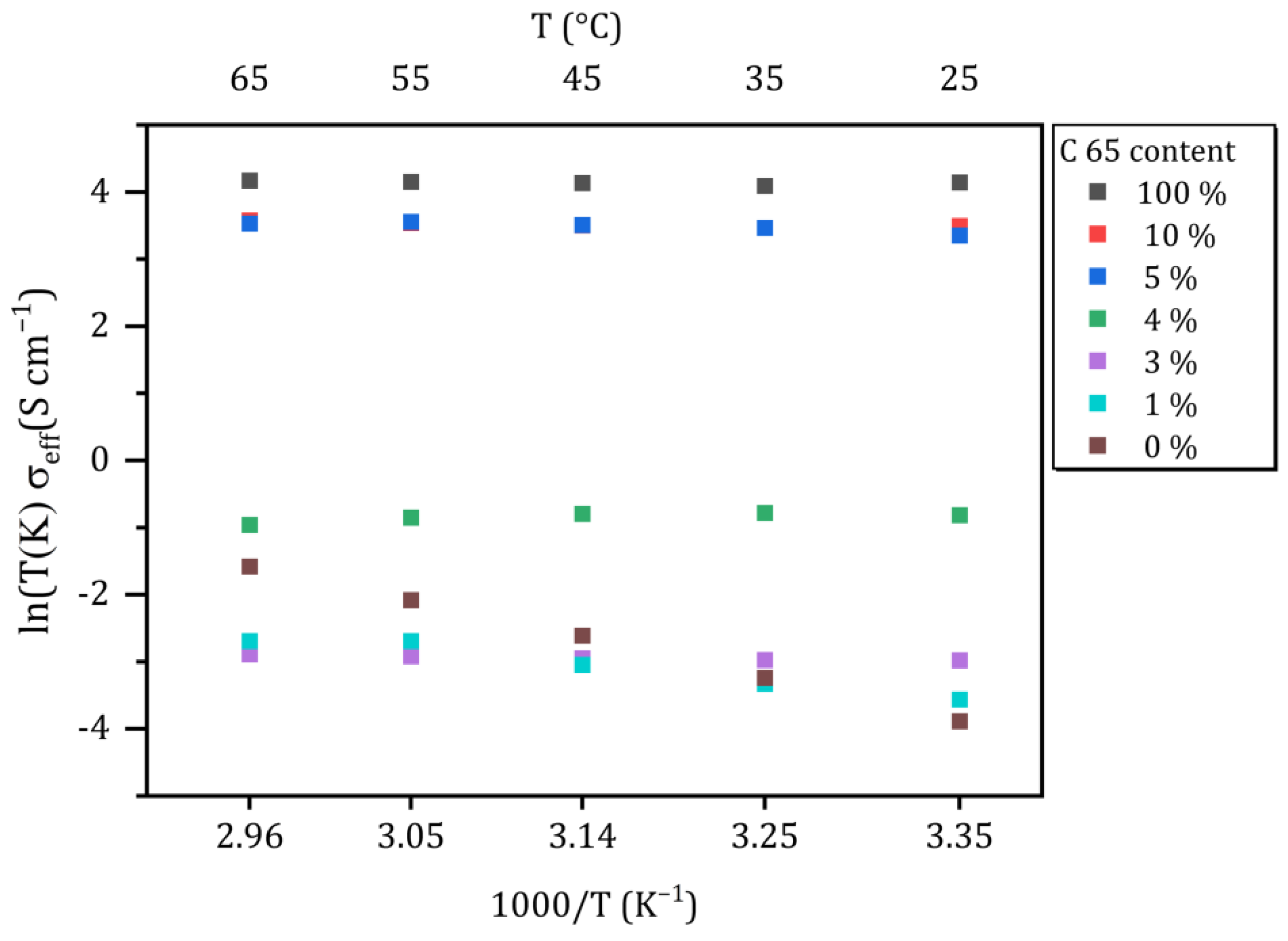


| Wt.-ratio [Li6PS5Cl:C65] | 100:0 | 90:10 | 95:5 | 96:4 | 97:3 | 99:1 | 0:100 |
| Fraction C65 [vol.] | 0.00% | 11.44% | 5.77% | 4.62% | 3.47% | 1.16% | 100.00% |
| Fraction C65 [wt.] | 0.00% | 10.00% | 5.00% | 4.00% | 3.00% | 1.00% | 100.00% |
| Li6PS5Cl [mg]/Pellet | 40.00 | 36.00 | 38.00 | 38.40 | 38.80 | 39.60 | 0.00 |
| C65 [mg]/Pellet | 0.00 | 4.00 | 2.00 | 1.60 | 1.20 | 0.40 | 40.00 |
| Measurement step | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Separator | |||||||
| Temperature [°C] | 25 | 65 | 55 | 45 | 35 | 15 | 5 |
| Conducting matrix | |||||||
| Temperature [°C] | 25 | 35 | 45 | 55 | 65 | 15 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reisacher, E.; Kaya, P.; Knoblauch, V. Percolation Behavior of a Sulfide Electrolyte–Carbon Additive Matrix for Composite Cathodes in All-Solid-State Batteries. Batteries 2023, 9, 595. https://doi.org/10.3390/batteries9120595
Reisacher E, Kaya P, Knoblauch V. Percolation Behavior of a Sulfide Electrolyte–Carbon Additive Matrix for Composite Cathodes in All-Solid-State Batteries. Batteries. 2023; 9(12):595. https://doi.org/10.3390/batteries9120595
Chicago/Turabian StyleReisacher, Elias, Pinar Kaya, and Volker Knoblauch. 2023. "Percolation Behavior of a Sulfide Electrolyte–Carbon Additive Matrix for Composite Cathodes in All-Solid-State Batteries" Batteries 9, no. 12: 595. https://doi.org/10.3390/batteries9120595
APA StyleReisacher, E., Kaya, P., & Knoblauch, V. (2023). Percolation Behavior of a Sulfide Electrolyte–Carbon Additive Matrix for Composite Cathodes in All-Solid-State Batteries. Batteries, 9(12), 595. https://doi.org/10.3390/batteries9120595











