Effect of Zr4+ on Lithium-Ion Conductivity of Garnet-Type Li5+xLa3(Nb2−xZrx)O12 Solid Electrolytes
Abstract
:1. Introduction
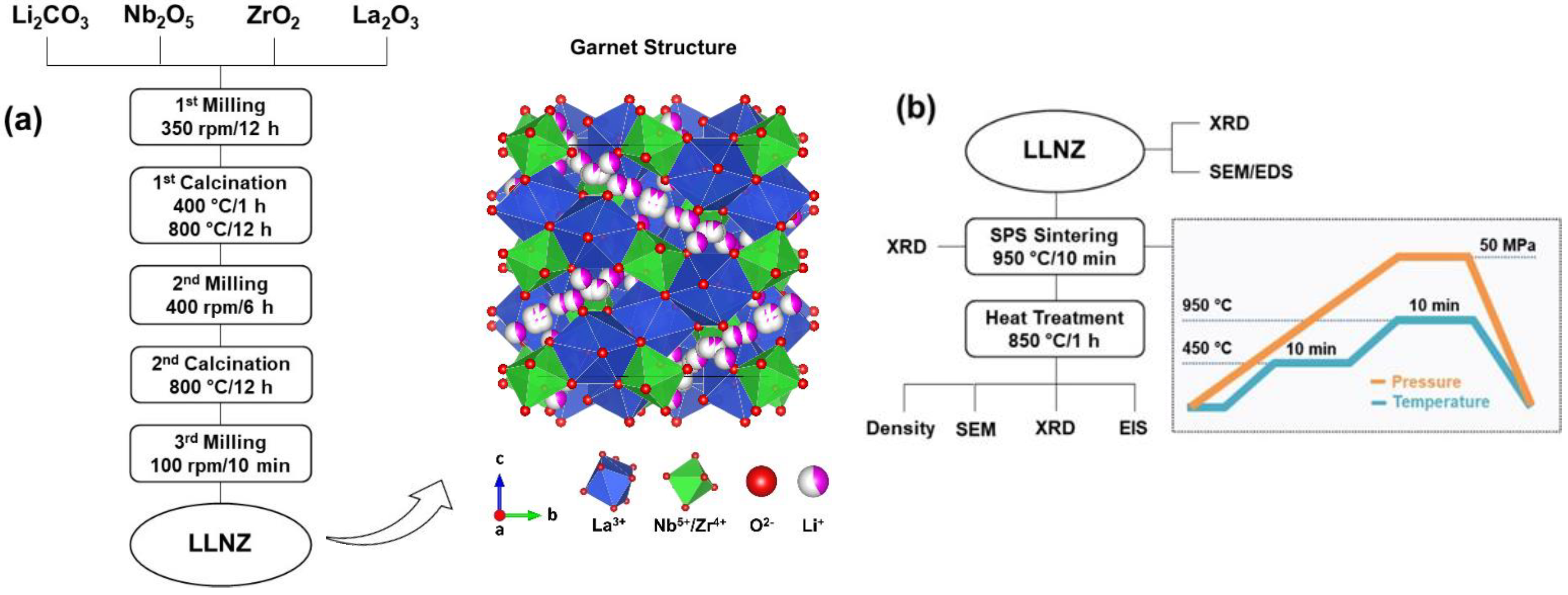
2. Materials and Methods
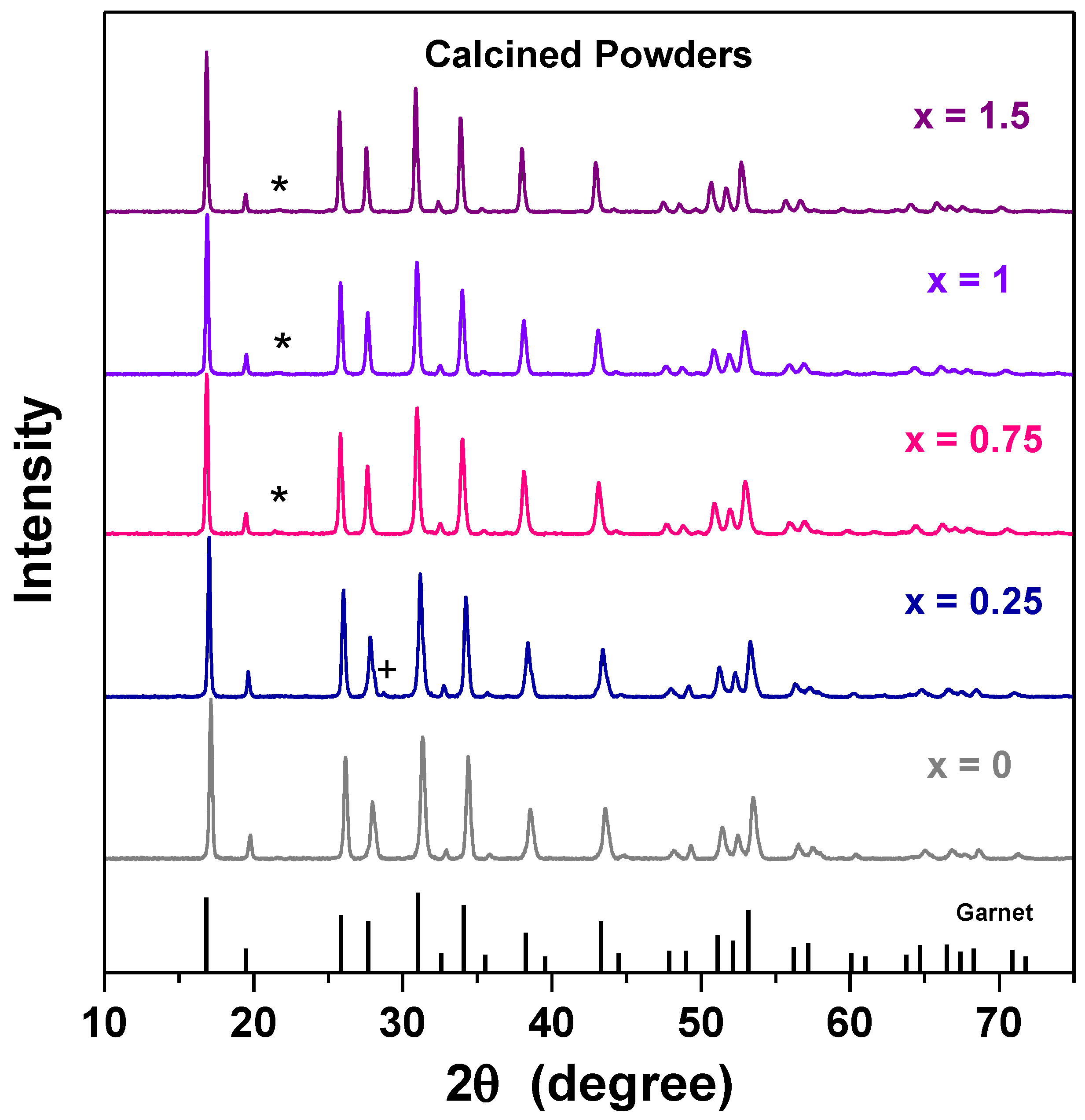
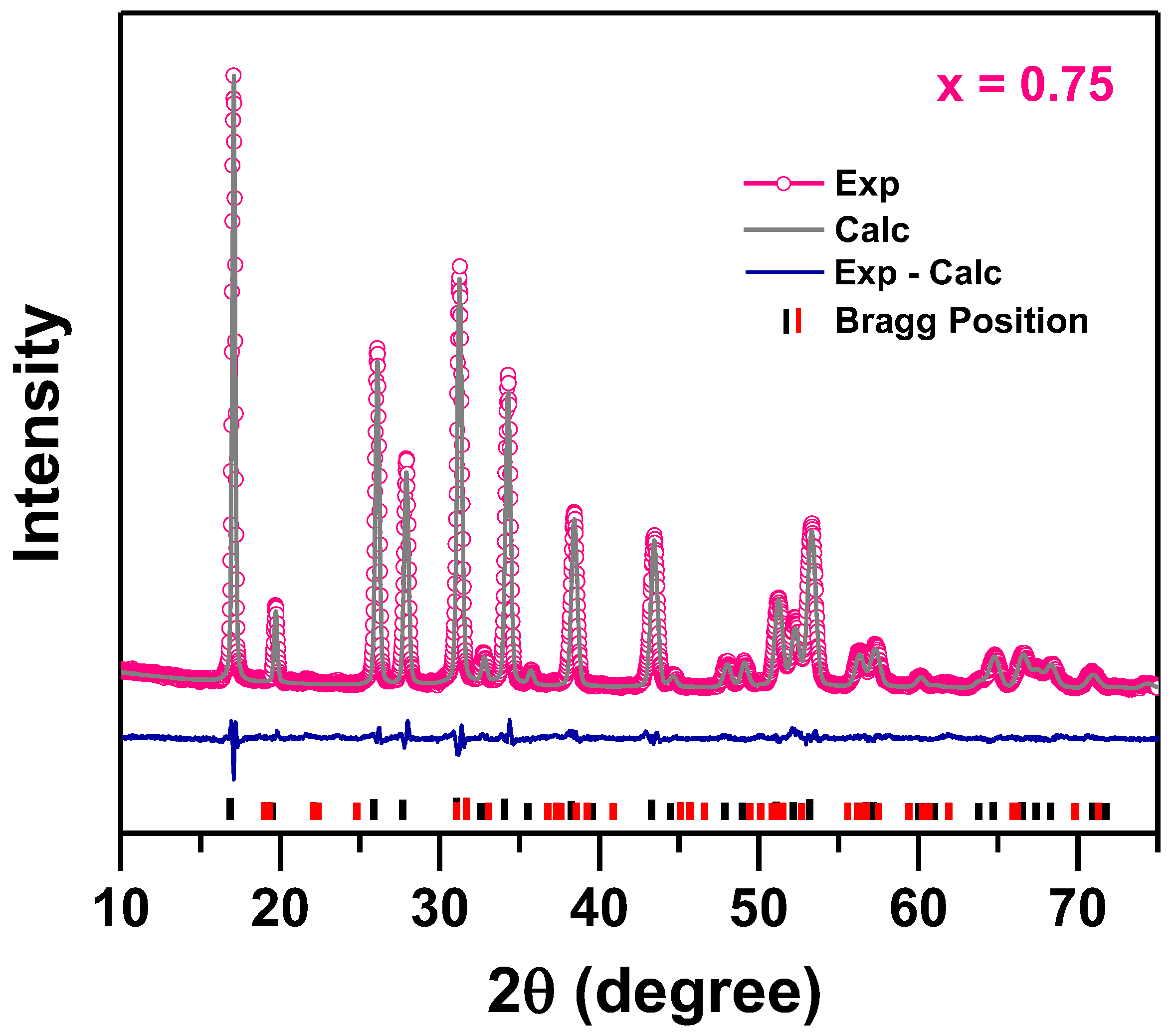
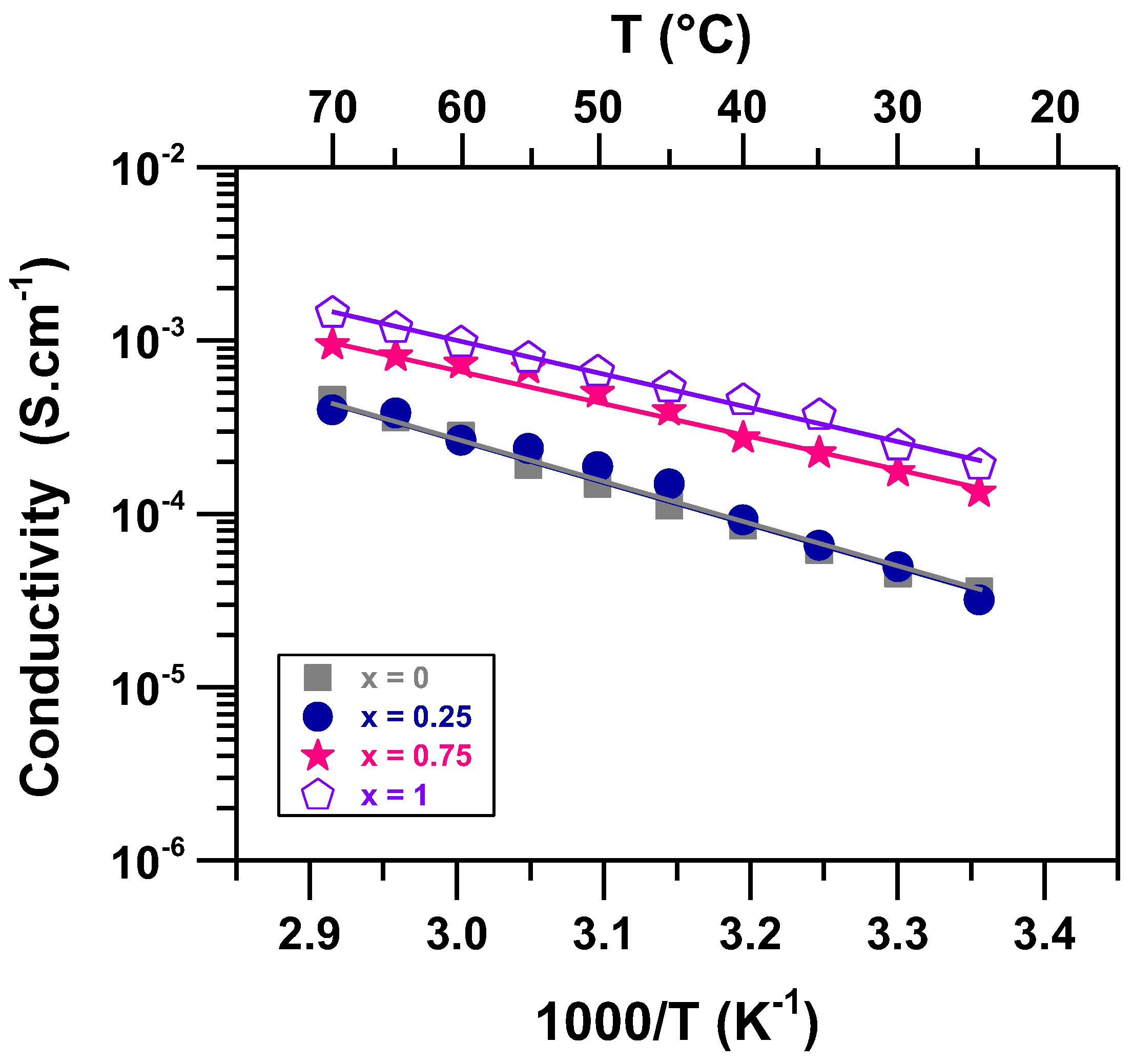
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Y.; Zhao, C.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.; Yu, D.; Liu, Y.; Titirici, M.; Chueh, Y.; et al. A Review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Lu, Y.-C. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef] [PubMed]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and challenges of lithium ion batteries in automotive applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Sun, P.; Bisschop, R.; Niu, H.; Huang, X. A Review of battery fires in electric vehicles. Fire Technol. 2020, 56, 1361–1410. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Campion, C.L.; Li, W.; Lucht, B.L. Thermal decomposition of LiPF6-based electrolytes for lithium-ion batteries. J. Electrochem. Soc. 2005, 152, A2327. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Sun, Y.-K. Promising all-solid-state batteries for future electric vehicles. ACS Energy Lett. 2020, 5, 3221–3223. [Google Scholar] [CrossRef]
- Ramakumar, S.; Deviannapoorani, C.; Dhivya, L.; Shankar, L.S.; Murugan, R. Lithium Garnets: Synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Prog. Mater. Sci. 2017, 88, 325–411. [Google Scholar] [CrossRef]
- Thangadurai, V.; Kaack, H.; Weppner, W.J.F. Novel fast lithium ion conduction in garnet-type Li5La3M2O12 (M = Nb, Ta). J. Am. Ceram. Soc. 2003, 86, 437–440. [Google Scholar] [CrossRef]
- Peng, H.; Wu, Q.; Xiao, L. Low temperature synthesis of Li5La3Nb2O12 with cubic garnet-type structure by sol-gel process. J. Sol-Gel Sci. Technol. 2013, 66, 175–179. [Google Scholar] [CrossRef]
- Dong, B.; Stockham, M.P.; Chater, P.A.; Slater, P.R. X-ray pair distribution function analysis and electrical and electrochemical properties of cerium doped Li5La3Nb2O12 garnet solid-state electrolyte. Dalt. Trans. 2020, 49, 11727–11735. [Google Scholar] [CrossRef]
- Ohta, S.; Kobayashi, T.; Asaoka, T. High lithium ionic conductivity in the garnet-type oxide Li7-xLa3(Zr2−x,Nbx)O12 (x = 0–2). J. Power Sources 2011, 196, 3342–3345. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chemie Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-type solid-state electrolytes: Materials, interfaces, and batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef]
- Teng, S.; Wang, W.; Tiwari, A. Novel low temperature molten salt synthesis of a Li5La3Nb2O12 solid state electrolyte and its properties. MRS Proc. 2014, 1679, 7–11. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Li6ALa2Nb2O12 (A = Ca, Sr, Ba): A new class of fast lithium ion conductors with garnet-like structure. J. Am. Ceram. Soc. 2005, 88, 411–418. [Google Scholar] [CrossRef]
- Truong, L.; Thangadurai, V. Soft-chemistry of garnet-type Li5+xBaxLa3−xNb2O12 (x = 0, 0.5, 1): Reversible H+ ↔ Li+ ion-exchange reaction and their X-ray, 7Li MAS NMR, IR, and AC impedance spectroscopy characterization. Chem. Mater. 2011, 23, 3970–3977. [Google Scholar] [CrossRef]
- Lustosa, G.M.M.M.; Franchetti, M.G.S.; de Souza, A.; Goulart, F.A.B.; da Conceição, L.; Berton, M.A.C. Fast synthesis and sintering of Li5La3Nb2O12 garnet ceramic. Mater. Chem. Phys. 2021, 257, 123848. [Google Scholar] [CrossRef]
- Ahmad, M.M. Enhanced lithium ionic conductivity and study of the relaxation and giant dielectric properties of spark plasma sintered Li5La3Nb2O12 nanomaterials. Ceram. Int. 2015, 41, 6398–6408. [Google Scholar] [CrossRef]
- Baek, S.-W.; Lee, J.-M.; Kim, T.Y.; Song, M.-S.; Park, Y. Garnet related lithium ion conductor processed by spark plasma sintering for all solid state batteries. J. Power Sources 2014, 249, 197–206. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, C.; Lin, F.; Li, B.; Yang, F.; Zhu, H.; Duan, J.; Chen, Z. Submicron-sized Nb-doped lithium garnet for high ionic conductivity solid electrolyte and performance of quasi-solid-state lithium battery. Materials 2020, 13, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, H.; Ito, T.; Hongahally Basappa, R. Sintering mechanisms of high-performance garnet-type solid electrolyte densified by spark plasma sintering. Electrochim. Acta 2016, 222, 648–656. [Google Scholar] [CrossRef] [Green Version]
- Nemori, H.; Matsuda, Y.; Matsui, M.; Yamamoto, O.; Takeda, Y.; Imanishi, N. Relationship between lithium content and ionic conductivity in the Li5+2xLa3Nb2-xScxO12 system. Solid State Ion. 2014, 266, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Stockham, M.P.; Dong, B.; Ding, Y.; Li, Y.; Slater, P.R. Evaluation of the effect of site substitution of Pr doping in the lithium garnet system Li5La3Nb2O12. Dalt. Trans. 2020, 49, 10349–10359. [Google Scholar] [CrossRef]
- Peng, H.; Feng, L.; Li, L.; Zhang, Y.; Zou, Y. Effect of Ge substitution for Nb on Li ion conductivity of Li5La3Nb2O12 solid state electrolyte. Electrochim. Acta 2017, 251, 482–487. [Google Scholar] [CrossRef]
- Peng, H.; Feng, L.; Li, L.; Zhang, Y.; Zou, Y. Synthesis of Li5+xLa3HfxNb2−xO12 (x = 0.2–1) ceramics with cubic garnet-type structure. Mater. Lett. 2017, 194, 138–141. [Google Scholar] [CrossRef]
- Pinzaru, D.; Thangadurai, V. Synthesis, structure and Li ion conductivity of garnet-like Li5+2xLa3Nb2−xSmxO12 (0 ≤ x ≤ 0.7). J. Electrochem. Soc. 2014, 161, A2060–A2067. [Google Scholar] [CrossRef]
- Kan, W.H.; Truong, L.; Thangadurai, V. Effect of V-doping on the structure and conductivity of garnet-type Li5La3Nb2O12. Ionics 2015, 21, 373–379. [Google Scholar] [CrossRef]
- Narayanan, S.; Baral, A.K.; Thangadurai, V. Dielectric characteristics of fast Li ion conducting garnet-type Li5+2xLa3Nb2xYxO12 (x = 0.25, 0.5 and 0.75). Phys. Chem. Chem. Phys. 2016, 18, 15418–15426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imagawa, H.; Ohta, S.; Kihira, Y.; Asaoka, T. Garnet-type Li6.75La3Zr1.75Nb0.25O12 synthesized by coprecipitation method and its lithium ion conductivity. Solid State Ion. 2014, 262, 609–612. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Cussen, E.J. The structure of lithium garnets: Cation disorder and clustering in a new family of fast Li+ conductors. Chem. Commun. 2006, 4, 412–413. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- German, R.M. Sintering: From Empirical Observations to Scientific Principles, 1st ed.; Elsevier: Woburn, MA, USA, 2014; ISBN 9780124016828. [Google Scholar]
- Paolella, A.; Zhu, W.; Bertoni, G.; Savoie, S.; Feng, Z.; Demers, H.; Gariepy, V.; Girard, G.; Rivard, E.; Delaporte, N.; et al. Discovering the influence of lithium loss on garnet Li7La3Zr2O12 electrolyte phase stability. ACS Appl. Energy Mater. 2020, 3, 3415–3424. [Google Scholar] [CrossRef]
- Chen, F.; Xu, L.; Li, J.; Yang, Y.; Shen, Q. Effect of bottleneck size on lithium migration in lithium garnets Li7La3Zr2O12 (LLZO). Ionics 2020, 26, 3193–3198. [Google Scholar] [CrossRef]
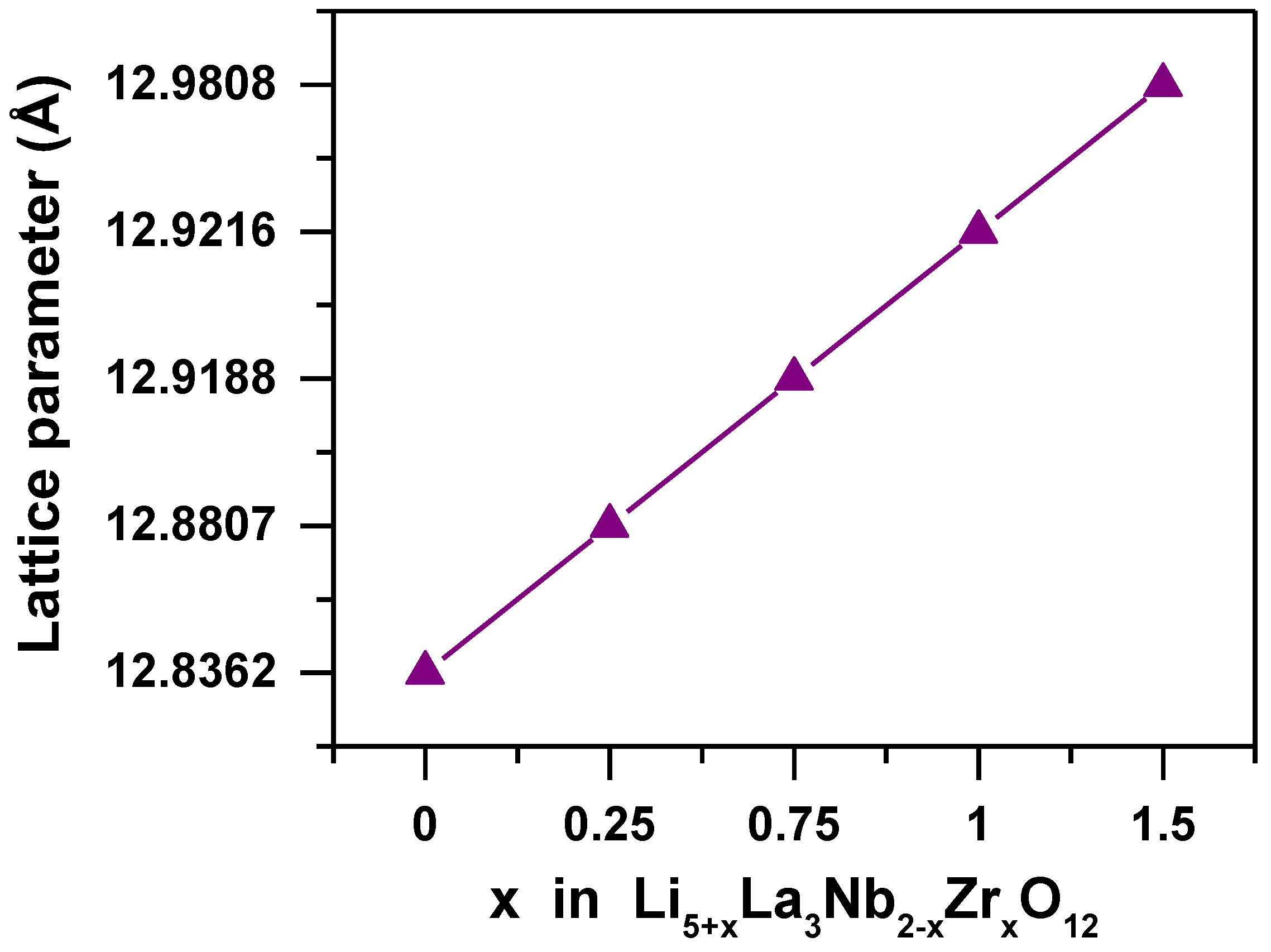
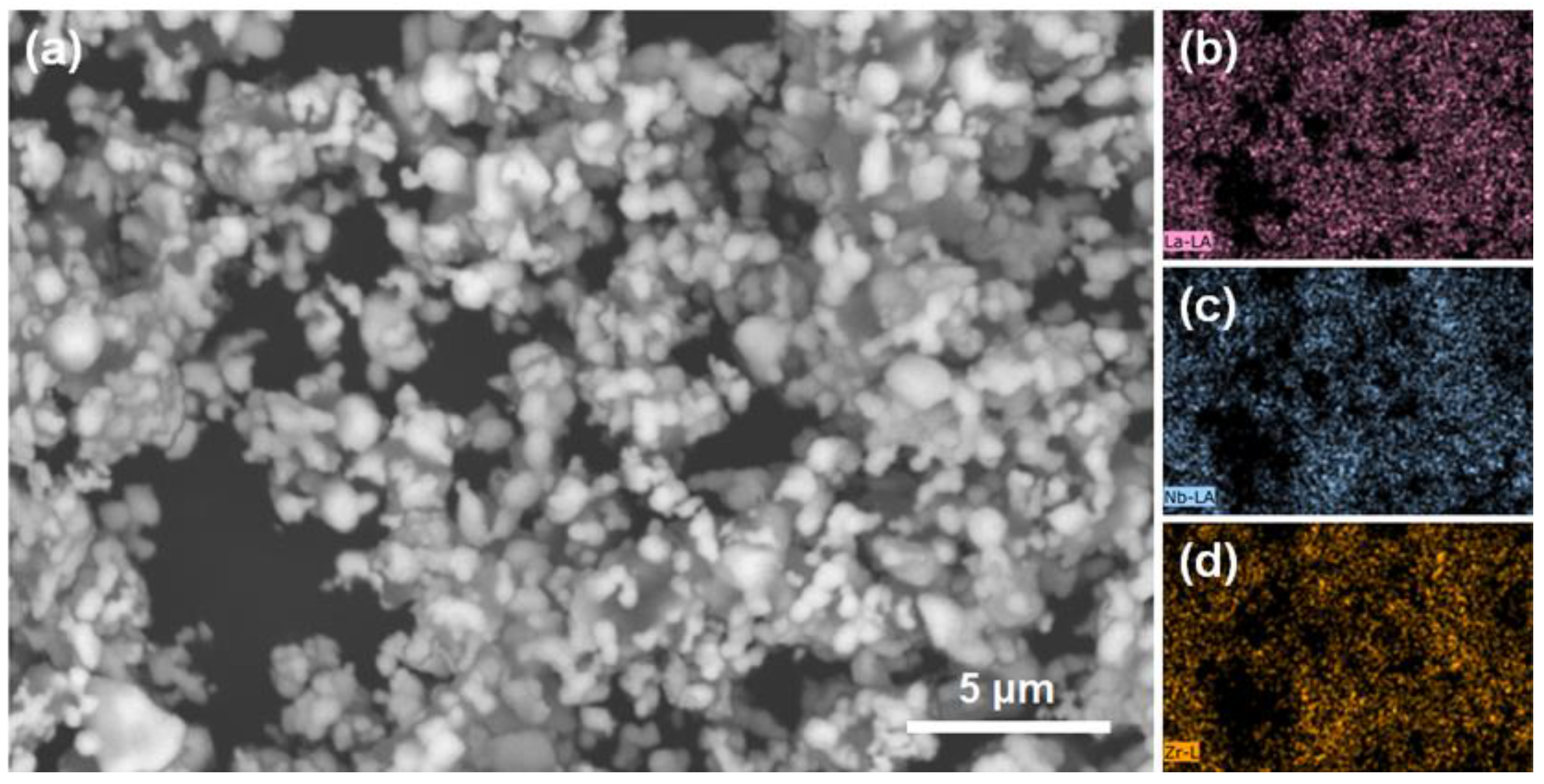
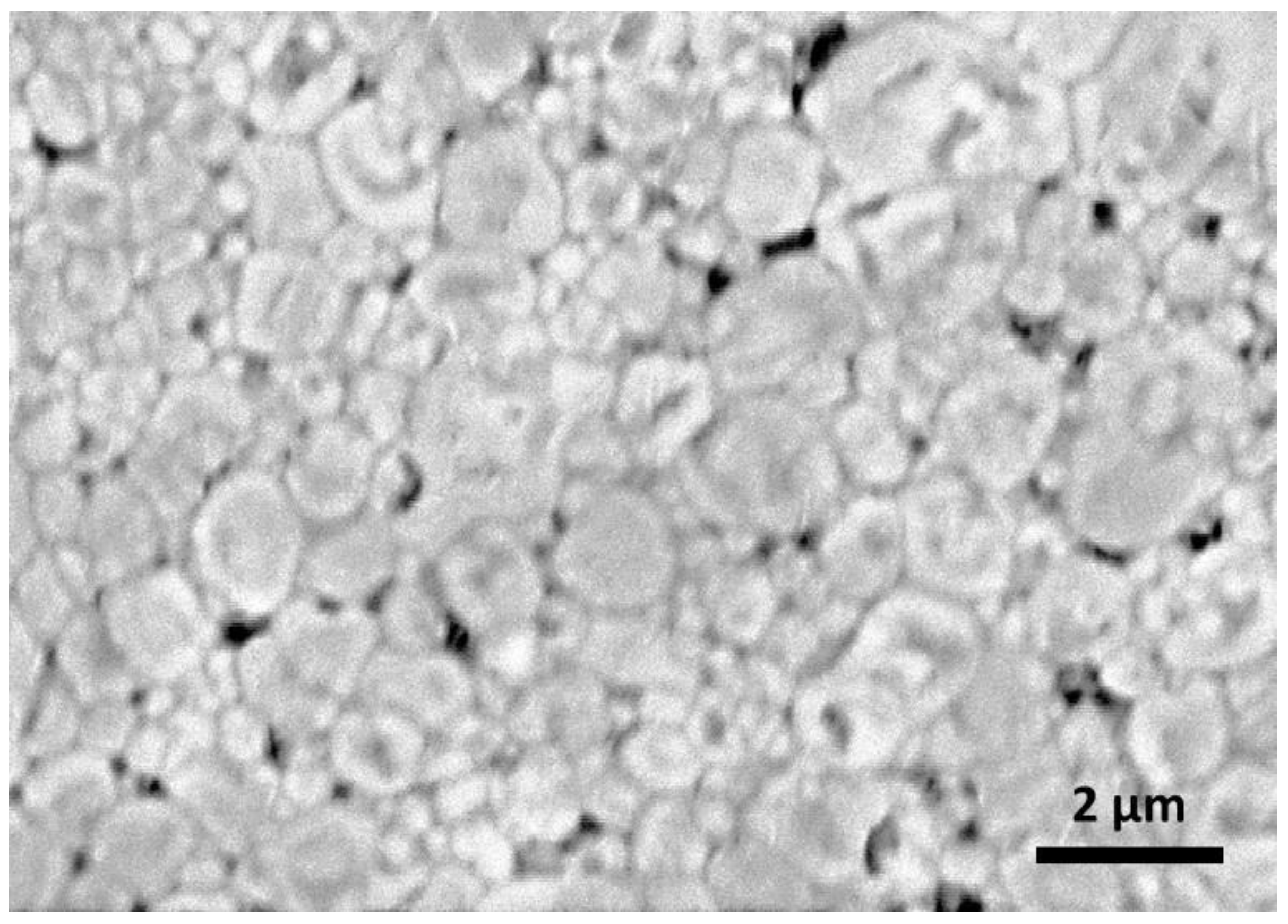
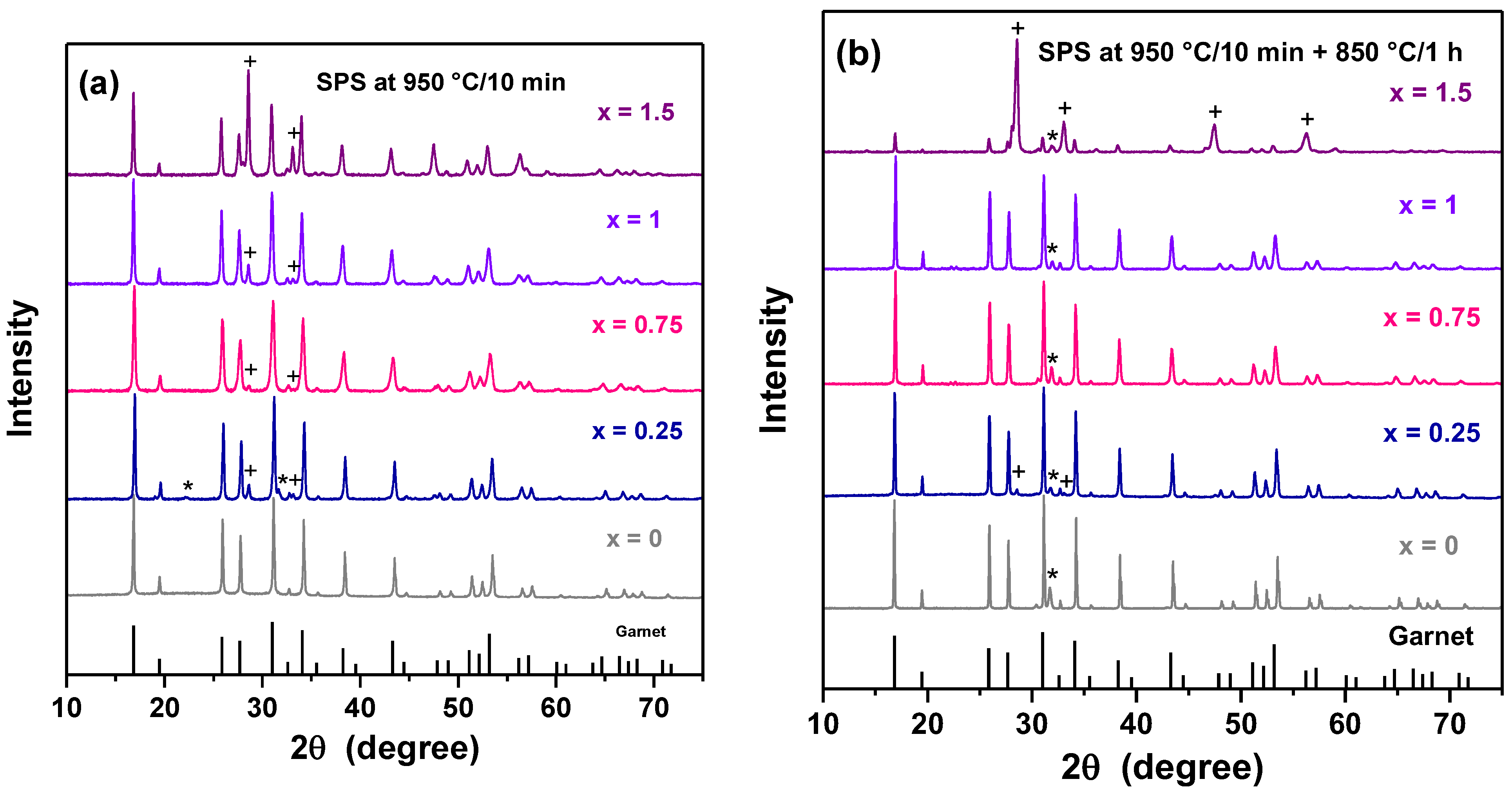
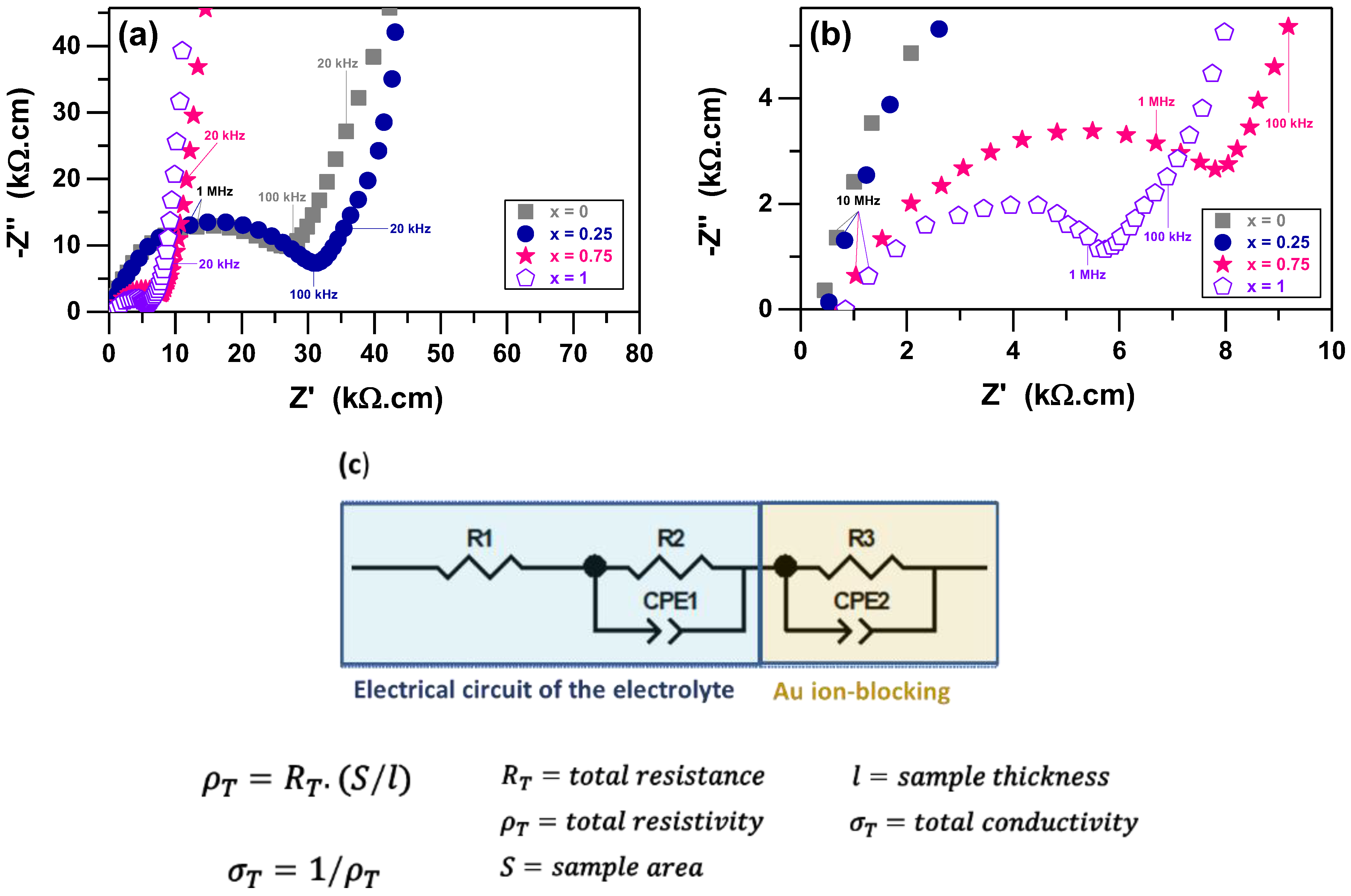
| Formula | x | Sintering | ρR (%) | σ25 °C (S.cm−1) | Ea (eV) | Ref. |
|---|---|---|---|---|---|---|
| LLN | ||||||
| Li5La3Nb2O12 | - | CS | 93.7 | 2.5 × 10−5 | - | [17] |
| Li5La3Nb2O12 | - | CS | - | 8.0 × 10−6 | 0.43 | [18] |
| Li5La3Nb2O12 | - | CS | - | 2.54 × 10−7 | 0.51 | [19] |
| Li5La3Nb2O12 | - | CS | 89–92 | 3.73 × 10−5 | 0.43 | [14] |
| Li5La3Nb2O12 | - | SPS | 89 | 2.7 × 10−6 | - | [20] |
| Li5La3Nb2O12 | - | SPS | - | 6.99 × 10−6 | 0.59 | [21] |
| LLZ | ||||||
| Li7La3Zr2O12 | - | CS | - | 7.74 × 10−4 | - | [15] |
| Li7La3Zr2O12 | - | CS | - | 1.68 × 10−4 | 0.36 | [22] |
| Li7La3Zr2O12 | - | CS | 89–92 | 3.01 × 10−4 | 0.33 | [14] |
| Doped LLZ | ||||||
| Li7−xLa3(Zr2 − xNbx)O12 | 0.25 | CS | 89–92 | 8.19 × 10−4 | 0.30 | [14] |
| Li7−xLa3(Zr2−xNbx)O12 | 0.5 | CS | 89–92 | 3.74 × 10−4 | 0.32 | [14] |
| Li7−xLa3(Zr2−xNbx)O12 | 0.4 | CS | 87.3 | 5.09 × 10−4 | 0.31 | [23] |
| Li7−xLa3(Zr2−xTax)O12 | 0.5 | CS | - | 5.22 × 10−4 | 0.32 | [22] |
| Li7−xLa3(Zr2−xTax)O12 | 0.56 | SPS | - | 1.35 × 10−3 | 0.41 | [22] |
| Li7−xLa3(Zr2−xTax)O12 | 0.5 | SPS | 95.5 | 6.9 × 10−4 | 0.42 | [24] |
| Doped LLN | ||||||
| Li5+xLa3(Nb2−xCex)O12 | 0.75 | CS | 75.1 | 1.4 × 10−4 | 0.35 | [13] |
| Li5+xLa3(Nb2−xGex)O12 | 0.75 | CS | 92.0 | 1.2 × 10−4 | - | [27] |
| Li5+xLa3(Nb2−xHfx)O12 | 1 | CS | - | 5.0 × 10−5 | - | [28] |
| Li5+2xLa3(Nb2−xScx)O12 | 0.625 | CS | 92–95 | 1.38 × 10−4 | 0.36 | [25] |
| Li5+2xLa3(Nb2−xSmx)O12 | 0.30 | CS | 78 | 5.84 × 10−5 | 0.38 | [29] |
| Li5La3(Nb2−xVx)O12 | 0.15 | CS | - | 6.0 × 10−6 | 0.37 | [30] |
| Li5+2xLa3(Nb2−xYx)O12 | 0.75 | CS | - | 2.99 × 10−4 | 0.42 | [31] |
| x | Rwp | Rp | a (Å) | ρ (g/cm3) |
|---|---|---|---|---|
| 0 | 9.18 | 6.75 | 12.8362(2) | 5.208 |
| 0.25 | 11.8 | 8.83 | 12.8807(5) | 5.152 |
| 0.75 | 4.92 | 3.81 | 12.91889(4) | 5.101 |
| 1 | 8.82 | 6.63 | 12.9216(3) | 5.095 |
| 1.5 | 8.46 | 6.70 | 12.9808(3) | 5.020 |
| x | ρR (%) | σ25 °C (S.cm−1) | Ea (eV) |
|---|---|---|---|
| 0 | 99 | 3.6 × 10−5 | 0.51 ± 0.01 |
| 0.25 | 98 | 3.2 × 10−5 | 0.49 ± 0.02 |
| 0.75 | 98 | 1.3 × 10−4 | 0.40 ± 0.02 |
| 1 | 98 | 1.9 × 10−4 | 0.38 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, S.; Grosso, R.; Kosctiuk, J.; Franchetti, M.; Oliveira, F.; Souza, A.; Gonin, C.; Freitas, H.; Monteiro, R.; Parreira, L.; et al. Effect of Zr4+ on Lithium-Ion Conductivity of Garnet-Type Li5+xLa3(Nb2−xZrx)O12 Solid Electrolytes. Batteries 2023, 9, 137. https://doi.org/10.3390/batteries9020137
Reis S, Grosso R, Kosctiuk J, Franchetti M, Oliveira F, Souza A, Gonin C, Freitas H, Monteiro R, Parreira L, et al. Effect of Zr4+ on Lithium-Ion Conductivity of Garnet-Type Li5+xLa3(Nb2−xZrx)O12 Solid Electrolytes. Batteries. 2023; 9(2):137. https://doi.org/10.3390/batteries9020137
Chicago/Turabian StyleReis, Shirley, Robson Grosso, Juliane Kosctiuk, Marianne Franchetti, Francisca Oliveira, Adler Souza, Cyrille Gonin, Heverson Freitas, Robson Monteiro, Luanna Parreira, and et al. 2023. "Effect of Zr4+ on Lithium-Ion Conductivity of Garnet-Type Li5+xLa3(Nb2−xZrx)O12 Solid Electrolytes" Batteries 9, no. 2: 137. https://doi.org/10.3390/batteries9020137
APA StyleReis, S., Grosso, R., Kosctiuk, J., Franchetti, M., Oliveira, F., Souza, A., Gonin, C., Freitas, H., Monteiro, R., Parreira, L., & Berton, M. (2023). Effect of Zr4+ on Lithium-Ion Conductivity of Garnet-Type Li5+xLa3(Nb2−xZrx)O12 Solid Electrolytes. Batteries, 9(2), 137. https://doi.org/10.3390/batteries9020137






