Mini-Review on the Regulation of Electrolyte Solvation Structure for Aqueous Zinc Ion Batteries
Abstract
1. Introduction
1.1. Zn2+ Insertion/Extraction Mechanism
1.2. H+ and Zn2+ Co-Insertion/Extraction Mechanism
1.3. Chemical Conversion Reaction Mechanism
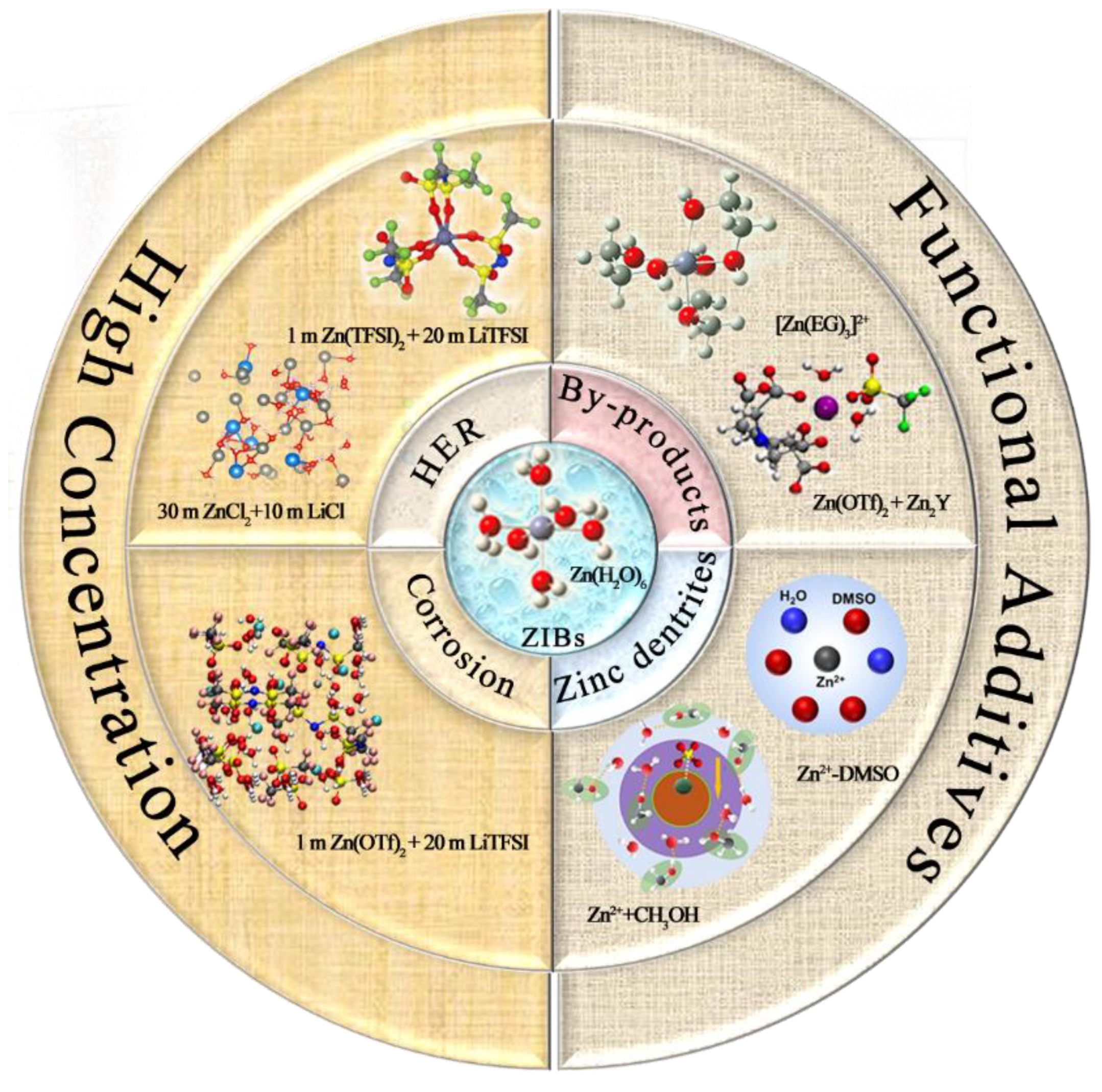
2. Solvation Structure
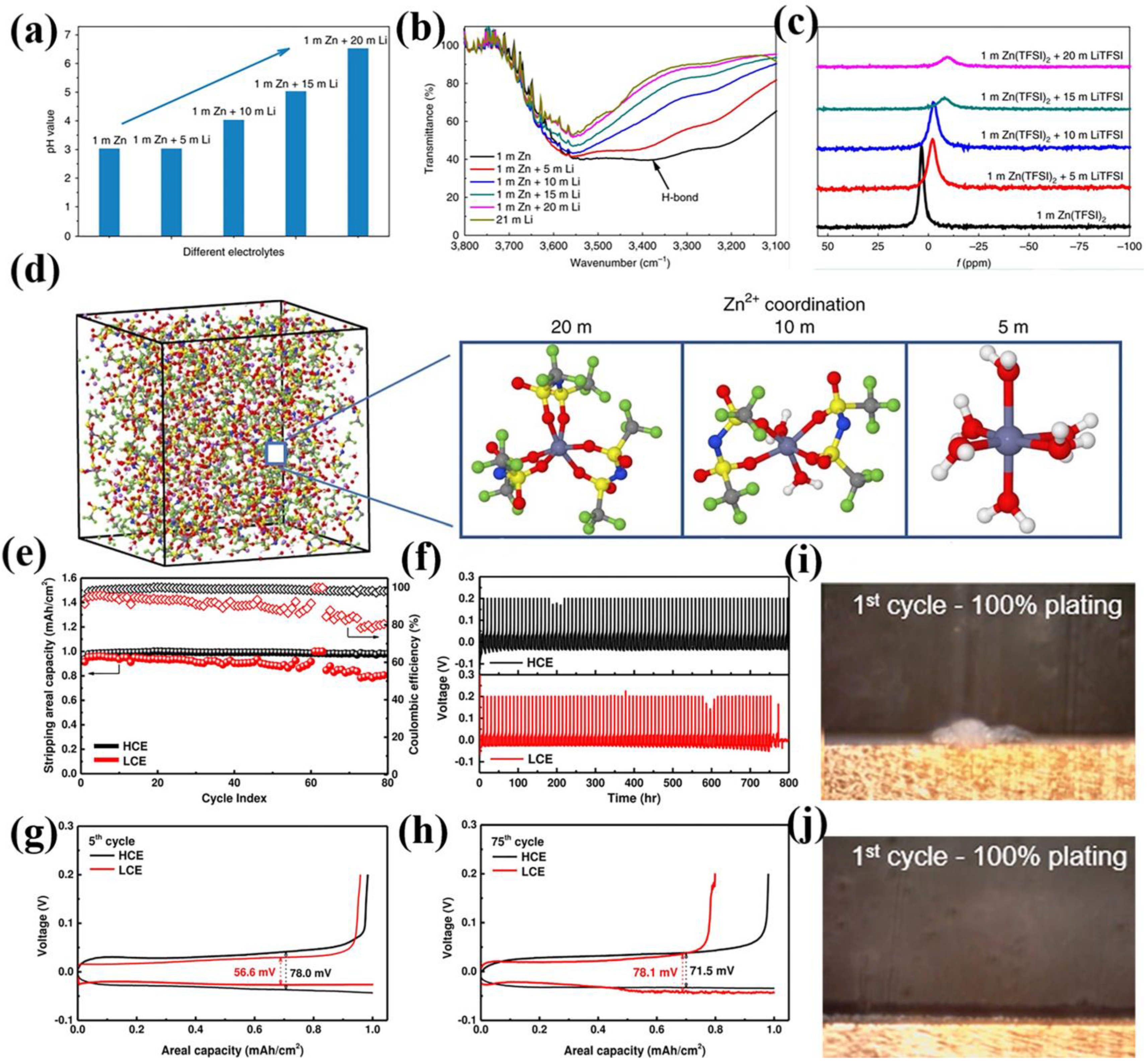
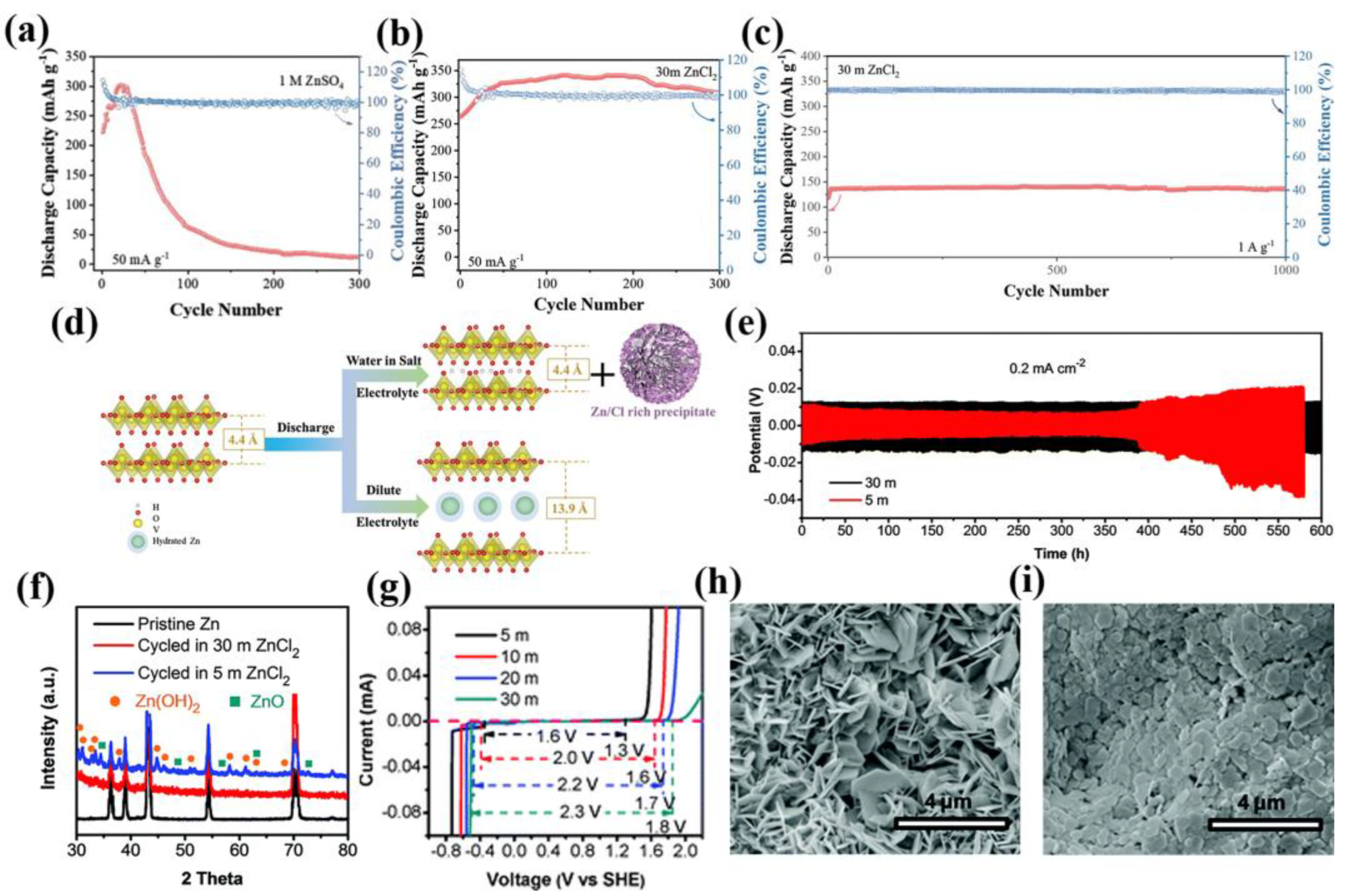
2.1. High-Concentration “Water-in-Salt” Strategy
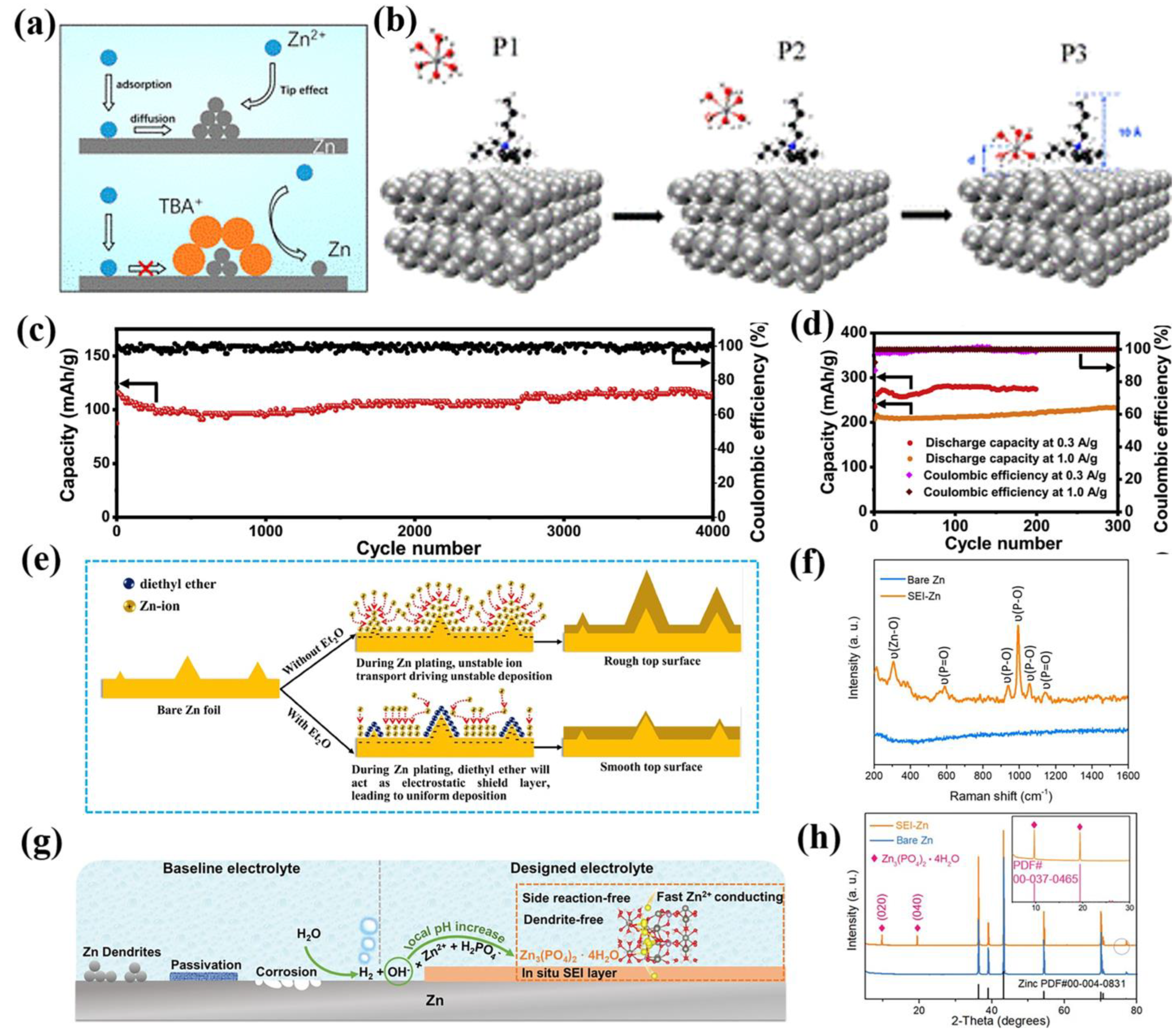
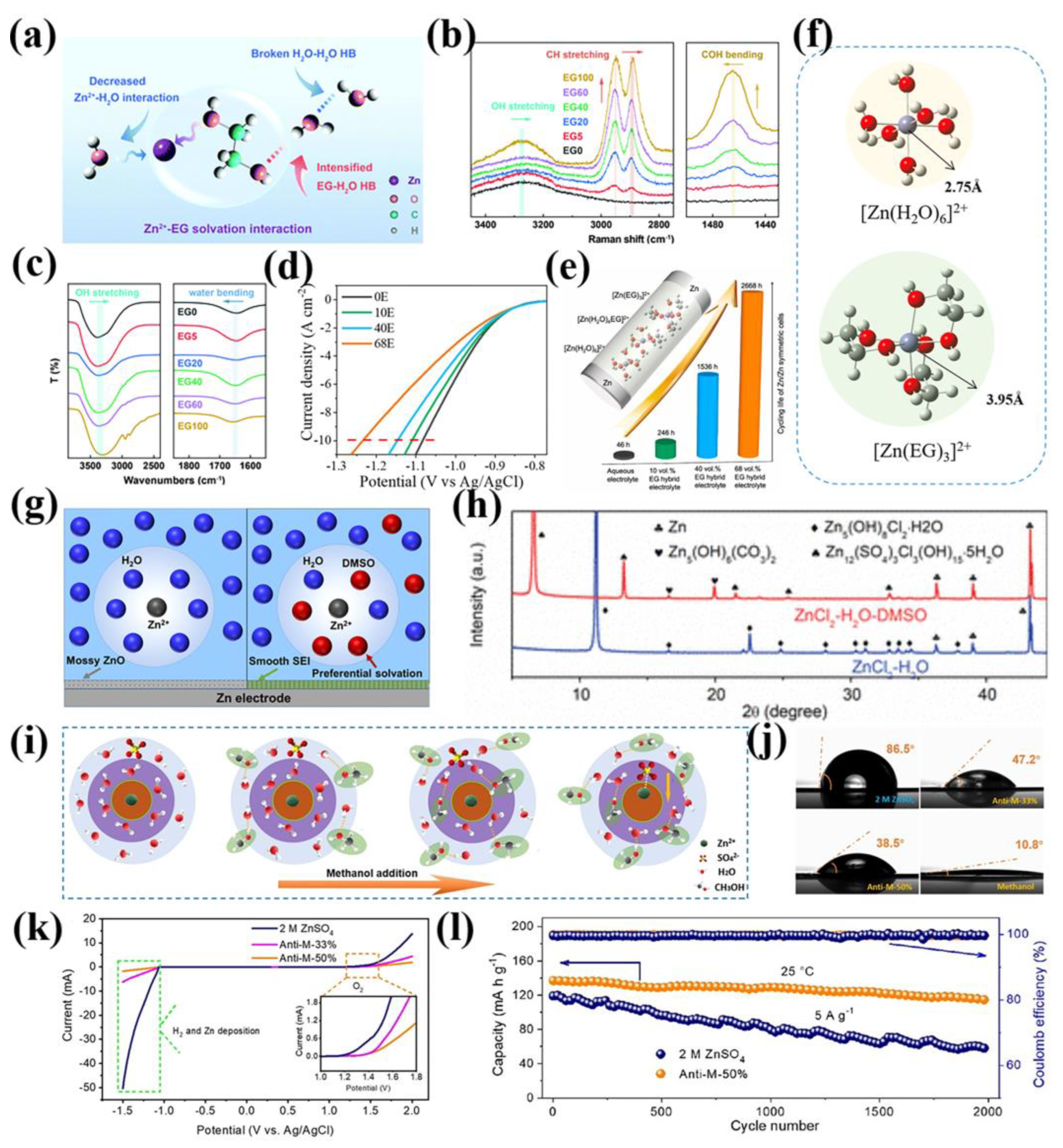
2.2. Functional Additives
3. Challenges and Future Development Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, C.; Du, J.; Zhu, Y.; Qin, W.; Wang, X.; Jia, C.; Zhang, K. Highly stable 3D hierarchical manganese sulfide multi-layer nanoflakes with excellent electrochemical performances for supercapacitor electrodes. J. Alloys Compd. 2022, 894, 162390. [Google Scholar] [CrossRef]
- Xing, T.; Ouyang, Y.; Chen, Y.; Zheng, L.; Wu, C.; Wang, X. P-doped ternary transition metal oxide as electrode material of asymmetric supercapacitor. J. Energy Storage 2020, 28, 101248. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, Y.; Guan, C.; Jia, C.; Qin, W.; Wang, X.; Zhang, K. Mesoporous aluminium manganese cobalt oxide with pentahedron structures for energy storage devices. J. Mater. Chem. A 2019, 7, 18417–18427. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Liu, N.; Li, B.; He, Z.; Dai, L.; Wang, H.; Wang, L. Recent advances and perspectives on vanadium- and manganese-based cathode materials for aqueous zinc ion batteries. J. Energy Chem. 2021, 59, 134–159. [Google Scholar] [CrossRef]
- Wu, Y.; Song, T.-Y.; Chen, L.-N. A review on recent developments of vanadium-based cathode for rechargeable zinc-ion batteries. Tungsten 2021, 3, 289–304. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shoji, T. Rechargeable Zn∣ZnSO4∣MnO2-type cells. Inorg. Chim. Acta 1986, 117, L27–L28. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic Zinc Ion Chemistry: The Rechargeable Zinc Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef]
- Chen, D.; Lu, M.; Cai, D.; Yang, H.; Han, W. Recent advances in energy storage mechanism of aqueous zinc-ion batteries. J. Energy Chem. 2021, 54, 712–726. [Google Scholar] [CrossRef]
- Chen, L.; Ruan, Y.; Zhang, G.; Wei, Q.; Jiang, Y.; Xiong, T.; He, P.; Yang, W.; Yan, M.; An, Q.; et al. Ultrastable and High-Performance Zn/VO2 Battery Based on a Reversible Single-Phase Reaction. Chem. Mater. 2019, 31, 699–706. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry With H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pulletikurthi, G.; Endres, F. A Prussian Blue/Zinc Secondary Battery with a Bio-Ionic Liquid–Water Mixture as Electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 12158–12164. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent Advances in Zn-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.L.; Bockris, J.O.M. The Electrolytic Growth of Dendrites from Ionic Solutions. Proc. R. Soc. Lond. A Math. Phys. Sci. 1962, 268, 485–505. [Google Scholar]
- Yang, Q.; Liang, G.; Guo, Y.; Liu, Z.; Yan, B.; Wang, D.; Huang, Z.; Li, X.; Fan, J.; Zhi, C. Do Zinc Dendrites Exist in Neutral Zinc Batteries: A Developed Electrohealing Strategy to In Situ Rescue In-Service Batteries. Adv. Mater. 2019, 31, 1903778. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuang, H.L.; Fan, L.; Gao, L.; Lu, Y. A “cation-anion regulation” synergistic anode host for dendrite-free lithium metal batteries. Sci. Adv. 2018, 4, eaar4410. [Google Scholar] [CrossRef]
- Xie, C.; Li, Y.; Wang, Q.; Sun, D.; Tang, Y.; Wang, H. Issues and solutions toward zinc anode in aqueous zinc-ion batteries: A mini review. Carbon Energy 2020, 2, 540–560. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; Wang, Y.; Cao, Z.; Cavallo, L.; Hedhili, M.N.; Alshareef, H.N. Organic Acid Etching Strategy for Dendrite Suppression in Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2022, 12, 2102797. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.; Liu, K. Rational modulation of emerging MXene materials for zinc-ion storage. Carbon Energy 2022, 4, 60–76. [Google Scholar] [CrossRef]
- Zhang, Z.; Said, S.; Smith, K.; Zhang, Y.S.; He, G.; Jervis, R.; Shearing, P.R.; Miller, T.S.; Brett, D.J.L. Dendrite suppression by anode polishing in zinc-ion batteries. J. Mater. Chem. A 2021, 9, 15355–15362. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Du, H.; Liu, Y.; Xiang, Y.; Xiong, L.; Wu, X.; Wu, X. Copper Nanoparticle-Modified Carbon Nanofiber for Seeded Zinc Deposition Enables Stable Zn Metal Anode. ACS Sustain. Chem. Eng. 2022, 10, 12630–12641. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Ghosh, M.; Kurian, M.; Torris, A.; Dilwale, S.; Badiger, M.V.; Winter, M.; Nair, J.R.; Kurungot, S. An In Situ Cross-Linked Nonaqueous Polymer Electrolyte for Zinc-Metal Polymer Batteries and Hybrid Supercapacitors. Small 2020, 16, 2002528. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Huang, H.; Li, J.; Zhang, X.; Yang, Z.; Xu, M.; Pan, L. A novel redox bromide-ion additive hydrogel electrolyte for flexible Zn-ion hybrid supercapacitors with boosted energy density and controllable zinc deposition. J. Mater. Chem. A 2020, 8, 15042–15050. [Google Scholar] [CrossRef]
- Fang, Y.; Xie, X.; Zhang, B.; Chai, Y.; Lu, B.; Liu, M.; Zhou, J.; Liang, S. Regulating Zinc Deposition Behaviors by the Conditioner of PAN Separator for Zinc-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2109671. [Google Scholar] [CrossRef]
- Yang, X.; Wu, W.; Liu, Y.; Lin, Z.; Sun, X. Chitosan modified filter paper separators with specific ion adsorption to inhibit side reactions and induce uniform Zn deposition for aqueous Zn batteries. Chem. Eng. J. 2022, 450, 137902. [Google Scholar] [CrossRef]
- Yu, H.; Zeng, Y.; Li, N.W.; Luan, D.; Yu, L.; Lou, X.W. Confining Sn nanoparticles in interconnected N-doped hollow carbon spheres as hierarchical zincophilic fibers for dendrite-free Zn metal anodes. Sci. Adv. 2022, 8, eabm5766. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Yu, H.; Liu, W.; Kuang, G.; Mei, L.; Wu, Z.; Wei, W.; Ji, X.; Qu, B.; et al. A Multifunctional Artificial Interphase with Fluorine-Doped Amorphous Carbon layer for Ultra-Stable Zn Anode. Adv. Funct. Mater. 2022, 32, 2205600. [Google Scholar] [CrossRef]
- Kang, L.; Cui, M.; Jiang, F.; Gao, Y.; Luo, H.; Liu, J.; Liang, W.; Zhi, C. Nanoporous CaCO3 Coatings Enabled Uniform Zn Stripping/Plating for Long-Life Zinc Rechargeable Aqueous Batteries. Adv. Energy Mater. 2018, 8, 1801090. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Sun, J.; Liu, Y.; Hou, L. Recent Progress in “Water-in-Salt” Electrolytes Toward Non-lithium Based Rechargeable Batteries. Front. Chem. 2020, 8, 595. [Google Scholar] [CrossRef]
- Zhai, C.; Zhao, D.; He, Y.; Huang, H.; Chen, B.; Wang, X.; Guo, Z. Electrolyte Additive Strategies for Suppression of Zinc Dendrites in Aqueous Zinc-Ion Batteries. Batteries 2022, 8, 153. [Google Scholar] [CrossRef]
- Peled, E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126, 2047–2051. [Google Scholar] [CrossRef]
- Jian, Q.; Wang, T.; Sun, J.; Wu, M.; Zhao, T. In-situ construction of fluorinated solid-electrolyte interphase for highly reversible zinc anodes. Energy Storage Mater. 2022, 53, 559–568. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Li, Z.; Xu, X.; Su, X.; Lai, J.; Liu, Y.; Ding, K.; Chen, L.; Cai, Y.-P.; et al. Three Birds with One Stone: Tetramethylurea as Electrolyte Additive for Highly Reversible Zn-Metal Anode. Adv. Funct. Mater. 2022, 32, 2209642. [Google Scholar] [CrossRef]
- Cheng, H.; Sun, Q.; Li, L.; Zou, Y.; Wang, Y.; Cai, T.; Zhao, F.; Liu, G.; Ma, Z.; Wahyudi, W.; et al. Emerging Era of Electrolyte Solvation Structure and Interfacial Model in Batteries. ACS Energy Lett. 2022, 7, 490–513. [Google Scholar] [CrossRef]
- Alvarado, J.; Schroeder, M.A.; Pollard, T.P.; Wang, X.; Lee, J.Z.; Zhang, M.; Wynn, T.; Ding, M.; Borodin, O.; Meng, Y.S.; et al. Bisalt ether electrolytes: A pathway towards lithium metal batteries with Ni-rich cathodes. Energy Environ. Sci. 2019, 12, 780–794. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. Npj Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Ming, J.; Cao, Z.; Wahyudi, W.; Li, M.; Kumar, P.; Wu, Y.; Hwang, J.-Y.; Hedhili, M.N.; Cavallo, L.; Sun, Y.-K.; et al. New Insights on Graphite Anode Stability in Rechargeable Batteries: Li Ion Coordination Structures Prevail Over Solid Electrolyte Interphases. ACS Energy Lett. 2018, 3, 335–340. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Ma, L.; Huang, W. Solvation Structures in Aqueous Metal-Ion Batteries. Adv. Energy Mater. 2022, 12, 2202068. [Google Scholar] [CrossRef]
- von Wald Cresce, A.; Gobet, M.; Borodin, O.; Peng, J.; Russell, S.M.; Wikner, E.; Fu, A.; Hu, L.; Lee, H.-S.; Zhang, z.; et al. Anion Solvation in Carbonate-Based Electrolytes. J. Phys. Chem. C 2015, 119, 27255–27264. [Google Scholar] [CrossRef]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active Materials for Aqueous Zinc Ion Batteries: Synthesis, Crystal Structure, Morphology, and Electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Wang, X.; Meng, J.; Liu, X.; Wu, B.; Han, C.; Mai, L. Comprehensive understanding of the roles of water molecules in aqueous Zn-ion batteries: From electrolytes to electrode materials. Energy Environ. Sci. 2021, 14, 3796–3839. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Zhang, X.; Zeng, Z.; Qin, J.; Huang, Y. Strategies of regulating Zn2+ solvation structures for dendrite-free and side reaction-suppressed zinc-ion batteries. Energy Environ. Sci. 2022, 15, 499–528. [Google Scholar] [CrossRef]
- Dou, Q.; Yao, N.; Pang, W.K.; Park, Y.; Xiong, P.; Han, X.; Rana, H.H.; Chen, X.; Fu, Z.-H.; Thomsen, L.; et al. Unveiling solvation structure and desolvation dynamics of hybrid electrolytes for ultralong cyclability and facile kinetics of Zn–Al alloy anodes. Energy Environ. Sci. 2022, 15, 4572–4583. [Google Scholar] [CrossRef]
- Smith, L.; Dunn, B. Opening the window for aqueous electrolytes. Science 2015, 350, 918. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, L.; Yue, J.; Zhang, Q.; Zhou, A.; Borodin, O.; Suo, L.; Li, H.; Chen, L.; Xu, K.; et al. High-Voltage Aqueous Na-Ion Battery Enabled by Inert-Cation-Assisted Water-in-Salt Electrolyte. Adv. Mater. 2020, 32, 1904427. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Hao, J.; Kao, C.-C.; Wu, C.; Liu, H.-K.; Dou, S.-X.; Qiao, S.-Z. Regulation methods for the Zn/electrolyte interphase and the effectiveness evaluation in aqueous Zn-ion batteries. Energy Environ. Sci. 2021, 14, 5669–5689. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J. Reshaping Electrolyte Solvation Structure for High-Energy Aqueous Batteries. Energy Environ. Mater. 2022, 5, 686–687. [Google Scholar] [CrossRef]
- Olbasa, B.W.; Fenta, F.W.; Chiu, S.-F.; Tsai, M.-C.; Huang, C.-J.; Jote, B.A.; Beyene, T.T.; Liao, Y.-F.; Wang, C.-H.; Su, W.-N.; et al. High-Rate and Long-Cycle Stability with a Dendrite-Free Zinc Anode in an Aqueous Zn-Ion Battery Using Concentrated Electrolytes. ACS Appl. Energy Mater. 2020, 3, 4499–4508. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, J.; Wang, L.; Long, X.; Liu, X.; Li, F.; Chen, J. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Glatz, H.; Tervoort, E.; Kundu, D. Unveiling Critical Insight into the Zn Metal Anode Cyclability in Mildly Acidic Aqueous Electrolytes: Implications for Aqueous Zinc Batteries. ACS Appl. Mater. Interfaces 2020, 12, 3522–3530. [Google Scholar] [CrossRef]
- Clarisza, A.; Bezabh, H.K.; Jiang, S.-K.; Huang, C.-J.; Olbasa, B.W.; Wu, S.-H.; Su, W.-N.; Hwang, B.J. Highly Concentrated Salt Electrolyte for a Highly Stable Aqueous Dual-Ion Zinc Battery. ACS Appl. Mater. Interfaces 2022, 14, 36644–36655. [Google Scholar] [CrossRef]
- Patil, N.; de la Cruz, C.; Ciurduc, D.; Mavrandonakis, A.; Palma, J.; Marcilla, R. An Ultrahigh Performance Zinc-Organic Battery using Poly(catechol) Cathode in Zn(TFSI)2-Based Concentrated Aqueous Electrolytes. Adv. Energy Mater. 2021, 11, 2100939. [Google Scholar] [CrossRef]
- Tang, X.; Wang, P.; Bai, M.; Wang, Z.; Wang, H.; Zhang, M.; Ma, Y. Unveiling the Reversibility and Stability Origin of the Aqueous V2O5–Zn Batteries with a ZnCl2 “Water-in-Salt” Electrolyte. Adv. Sci. 2021, 8, 2102053. [Google Scholar] [CrossRef]
- Zhang, C.; Shin, W.; Zhu, L.; Chen, C.; Neuefeind, J.C.; Xu, Y.; Allec, S.I.; Liu, C.; Wei, Z.; Daniyar, A.; et al. The electrolyte comprising more robust water and superhalides transforms Zn-metal anode reversibly and dendrite-free. Carbon Energy 2021, 3, 339–348. [Google Scholar] [CrossRef]
- Zhang, C.; Holoubek, J.; Wu, X.; Daniyar, A.; Zhu, L.; Chen, C.; Leonard, D.P.; Rodríguez-Pérez, I.A.; Jiang, J.-X.; Fang, C.; et al. A ZnCl2 water-in-salt electrolyte for a reversible Zn metal anode. Chem. Commun. 2018, 54, 14097–14099. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, K.; Huo, W.; Wang, Y.; Yao, G.; Gu, X.; Cheng, H.; Mai, L.; Hu, C.; Wang, X. Diethyl ether as self-healing electrolyte additive enabled long-life rechargeable aqueous zinc ion batteries. Nano Energy 2019, 62, 275–281. [Google Scholar] [CrossRef]
- Zeng, X.; Mao, J.; Hao, J.; Liu, J.; Liu, S.; Wang, Z.; Wang, Y.; Zhang, S.; Zheng, T.; Liu, J.; et al. Electrolyte Design for In Situ Construction of Highly Zn2+-Conductive Solid Electrolyte Interphase to Enable High-Performance Aqueous Zn-Ion Batteries under Practical Conditions. Adv. Mater. 2021, 33, 2007416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Cheng, F.; Liu, Y.; Zhao, Q.; Lei, K.; Chen, C.; Liu, X.; Chen, J. Cation-Deficient Spinel ZnMn2O4 Cathode in Zn(CF3SO3)2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery. J. Am. Chem. Soc. 2016, 138, 12894–12901. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Wang, G.; Feng, J.; Ma, Q. Developing high voltage Zn(TFSI)2/Pyr14TFSI/AN hybrid electrolyte for a carbon-based Zn-ion hybrid capacitor. Nanoscale 2021, 13, 17068–17076. [Google Scholar] [CrossRef]
- Shah, D.; Mjalli, F.S. Effect of water on the thermo-physical properties of Reline: An experimental and molecular simulation based approach. Phys. Chem. Chem. Phys. 2014, 16, 23900–23907. [Google Scholar] [CrossRef]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Owusu, K.A.; Pan, X.; Yu, R.; Qu, L.; Liu, Z.; Wang, Z.; Tahir, M.; Haider, W.A.; Zhou, L.; Mai, L. Introducing Na2SO4 in aqueous ZnSO4 electrolyte realizes superior electrochemical performance in zinc-ion hybrid capacitor. Mater. Today Energy 2020, 18, 100529. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Hu, H.; Shi, H.-Y.; Song, Y.; Guo, D.; Liu, X.-X.; Sun, X. A Zn(ClO4)2 Electrolyte Enabling Long-Life Zinc Metal Electrodes for Rechargeable Aqueous Zinc Batteries. ACS Appl. Mater. Interfaces 2019, 11, 42000–42005. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Zhou, M.; Zhan, H.; Cheng, S.; Jiang, K. Advanced Low-Cost, High-Voltage, Long-Life Aqueous Hybrid Sodium/Zinc Batteries Enabled by a Dendrite-Free Zinc Anode and Concentrated Electrolyte. ACS Appl. Mater. Interfaces 2018, 10, 22059–22066. [Google Scholar] [CrossRef]
- Bayaguud, A.; Luo, X.; Fu, Y.; Zhu, C. Cationic Surfactant-Type Electrolyte Additive Enables Three-Dimensional Dendrite-Free Zinc Anode for Stable Zinc-Ion Batteries. ACS Energy Lett. 2020, 5, 3012–3020. [Google Scholar] [CrossRef]
- Chang, N.; Li, T.; Li, R.; Wang, S.; Yin, Y.; Zhang, H.; Li, X. An aqueous hybrid electrolyte for low-temperature zinc-based energy storage devices. Energy Environ. Sci. 2020, 13, 3527–3535. [Google Scholar] [CrossRef]
- Kumar, R.M.; Baskar, P.; Balamurugan, K.; Das, S.; Subramanian, V. On the Perturbation of the H-Bonding Interaction in Ethylene Glycol Clusters upon Hydration. J. Phys. Chem. A 2012, 116, 4239–4247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, P.; Ma, K.; Han, F.; Chen, G.; Wei, X. Hydrogen bonding interactions between ethylene glycol and water: Density, excess molar volume, and spectral study. Sci. China Ser. B Chem. 2008, 51, 420–426. [Google Scholar] [CrossRef]
- Qin, R.; Wang, Y.; Zhang, M.; Wang, Y.; Ding, S.; Song, A.; Yi, H.; Yang, L.; Song, Y.; Cui, Y.; et al. Tuning Zn2+ coordination environment to suppress dendrite formation for high-performance Zn-ion batteries. Nano Energy 2021, 80, 105478. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Chi, S.-S.; Wang, Q.; Han, B.; Luo, C.; Jiang, Y.; Wang, J.; Wang, C.; Yu, Y.; Deng, Y. Lithiophilic Zn Sites in Porous CuZn Alloy Induced Uniform Li Nucleation and Dendrite-free Li Metal Deposition. Nano Lett. 2020, 20, 2724–2732. [Google Scholar] [CrossRef]
- Cao, L.; Li, D.; Hu, E.; Xu, J.; Deng, T.; Ma, L.; Wang, Y.; Yang, X.-Q.; Wang, C. Solvation Structure Design for Aqueous Zn Metal Batteries. J. Am. Chem. Soc. 2020, 142, 21404–21409. [Google Scholar] [CrossRef]
- Kamieńska-Piotrowicz, E. Solvation of cobalt(II) and perchlorate ions in binary mixtures of donor solvents. J. Chem. Soc. Faraday Trans. 1995, 91, 71–75. [Google Scholar] [CrossRef]
- Senanayake, G.; Muir, D.M. Competitive solvation and complexation of Cu(I), Cu(II), Pb(II), Zn(II), and Ag(I) in aqueous ethanol, acetonitrile, and dimethylsulfoxide solutions containing chloride ion with applications to hydrometallurgy. Metall. Trans. B 1990, 21, 439–448. [Google Scholar] [CrossRef]
- Hao, J.; Yuan, L.; Ye, C.; Chao, D.; Davey, K.; Guo, Z.; Qiao, S.-Z. Boosting Zinc Electrode Reversibility in Aqueous Electrolytes by Using Low-Cost Antisolvents. Angew. Chem. Int. Ed. 2021, 60, 7366–7375. [Google Scholar] [CrossRef] [PubMed]
- Mohsen-Nia, M.; Amiri, H.; Jazi, B. Dielectric Constants of Water, Methanol, Ethanol, Butanol and Acetone: Measurement and Computational Study. J. Solution Chem. 2010, 39, 701–708. [Google Scholar] [CrossRef]
- Aguayo, A.T.; Gayubo, A.G.; Vivanco, R.; Olazar, M.; Bilbao, J. Role of acidity and microporous structure in alternative catalysts for the transformation of methanol into olefins. Appl. Catal. A 2005, 283, 197–207. [Google Scholar] [CrossRef]
- Li, T.C.; Lim, Y.; Li, X.L.; Luo, S.; Lin, C.; Fang, D.; Xia, S.; Wang, Y.; Yang, H.Y. A Universal Additive Strategy to Reshape Electrolyte Solvation Structure toward Reversible Zn Storage. Adv. Energy Mater. 2022, 12, 2103231. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, Y.; Zhang, Q.; Miao, L.; Zhang, K.; Yan, Z.; Li, F.; Chen, J. Exploring the Interfacial Chemistry between Zinc Anodes and Aqueous Electrolytes via an In Situ Visualized Characterization System. ACS Appl. Mater. Interfaces 2020, 12, 55476–55482. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Cao, F.; Hou, L.; Li, T.; Jiao, Y.; Wu, P. Immunizing Aqueous Zn Batteries against Dendrite Formation and Side Reactions at Various Temperatures via Electrolyte Additives. Small 2021, 17, 2103195. [Google Scholar] [CrossRef]
- Yan, M.; Xu, C.; Sun, Y.; Pan, H.; Li, H. Manipulating Zn anode reactions through salt anion involving hydrogen bonding network in aqueous electrolytes with PEO additive. Nano Energy 2021, 82, 105739. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, W.; Huang, A.; Chen, M.; Tian, Q.; Chen, J. Developing improved electrolytes for aqueous zinc-ion batteries to achieve excellent cyclability and antifreezing ability. J. Colloid Interface Sci. 2021, 586, 362–370. [Google Scholar] [CrossRef]
- Wei, T.; Peng, Y.; Mo, L.E.; Chen, S.; Ghadari, R.; Li, Z.; Hu, L. Modulated bonding interaction in propanediol electrolytes toward stable aqueous zinc-ion batteries. Sci. China Mater. 2022, 65, 1156–1164. [Google Scholar] [CrossRef]
- Di, S.; Miao, L.; Wang, Y.; Ma, G.; Wang, Y.; Yuan, W.; Qiu, K.; Nie, X.; Zhang, N. Dual-anion-coordinated solvation sheath for stable aqueous zinc batteries. J. Power Sources 2022, 535, 231452. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Y.; Lu, Y.; Ni, Y.; Lin, L.; Hao, Z.; Yan, Z.; Zhao, Q.; Chen, J. Halogenated Zn2+ Solvation Structure for Reversible Zn Metal Batteries. J. Am. Chem. Soc. 2022, 144, 18435–18443. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Xu, Z.; Wang, X. High-donor electrolyte additive enabling stable aqueous zinc-ion batteries. Energy Storage Mater. 2022, 52, 52–60. [Google Scholar] [CrossRef]
- Liu, S.; Mao, J.; Pang, W.K.; Vongsvivut, J.; Zeng, X.; Thomsen, L.; Wang, Y.; Liu, J.; Li, D.; Guo, Z. Tuning the Electrolyte Solvation Structure to Suppress Cathode Dissolution, Water Reactivity, and Zn Dendrite Growth in Zinc-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2104281. [Google Scholar] [CrossRef]



| Electrolyte | Current Density | CE | Lifespan | Ref. |
|---|---|---|---|---|
| 3 M ZnSO4 | 20 mA cm−2, 1 mAh cm−2 | 100% | 800 h | [56] |
| 3 M ZnSO4 + 2 M LiCl | 0.2 mA cm−2, 2 mAh cm−2 | __ | 170 h | [68] |
| 4.2 M ZnSO4 + 0.1 M MnSO4 | 0.5 mA cm−2, 1 mAh cm−2 | 99.21% | 1000 h | [54] |
| 3 M Zn(CF3SO3)2 | 0.1 mA cm−2, 0.1 mAh cm−2 | 100% | 800 h | [64] |
| 1 m Zn(TFSI)2 + 20 m LiTFSI | 0.2 mA cm−2, 0.033 mAh cm−2 | 100% | 170 h | [15] |
| 30 m ZnCl2 | 0.2 mA cm−2,0.035 mAh cm−2 | 95.4% | 600 h | [61] |
| 30 m ZnCl2 + 5 m LiCl | 2 mA cm−2, 4 mAh cm−2 | 99.7% | 4000 h | [60] |
| 2.4 m Zn(ClO4)2 | 1 mA cm−2, 1 mAh cm−2 | 99% | 3000 h | [69] |
| 8 M NaClO4 + 0.4 M Zn(CF3SO3)2 | 1 mA cm−2, 1 mAh cm−2 | __ | 200 h | [70] |
| Electrolyte Addictive | Current Density | CE | Lifespan | Ref. |
|---|---|---|---|---|
| 68 vol.% Ethylene glycol | 0.5 mA cm−2, 0.5 mAh cm−2 | __ | 2668 h | [75] |
| 50 vol.% Methanol | 1 mA cm−2, 0.5 mAh cm−2 | 99.7% | __ | [81] |
| Polyethylene glycol | 1 mA cm−2, 1 mAh cm−2 | 99.6% | 650 h | [85] |
| Dimethyl sulfoxide | 1 mA cm−2, 1 mAh cm−2 | 99.73% | 2100 h | [86] |
| PEO | 1 mA cm−2, 1 mAh cm−2 | 98.7 | 700 h | [87] |
| Diethyl ether + Ethylene glycol | 20 mA cm−2, 1 mAh cm−2 | 98% | 700 h | [88] |
| Propanediol | 0.2 mA cm−2, 0.2 mAh cm−2 | 98.9% | 1000 h | [89] |
| Diethyl ether | 20 mA cm−2, 1 mAh cm−2 | __ | 250 h | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Xu, H.; Hao, J.; Du, J.; Wu, C.; Ma, Z.; Qin, W. Mini-Review on the Regulation of Electrolyte Solvation Structure for Aqueous Zinc Ion Batteries. Batteries 2023, 9, 73. https://doi.org/10.3390/batteries9020073
Wang B, Xu H, Hao J, Du J, Wu C, Ma Z, Qin W. Mini-Review on the Regulation of Electrolyte Solvation Structure for Aqueous Zinc Ion Batteries. Batteries. 2023; 9(2):73. https://doi.org/10.3390/batteries9020073
Chicago/Turabian StyleWang, Bixia, Hui Xu, Jiayi Hao, Jinchao Du, Chun Wu, Zhen Ma, and Wei Qin. 2023. "Mini-Review on the Regulation of Electrolyte Solvation Structure for Aqueous Zinc Ion Batteries" Batteries 9, no. 2: 73. https://doi.org/10.3390/batteries9020073
APA StyleWang, B., Xu, H., Hao, J., Du, J., Wu, C., Ma, Z., & Qin, W. (2023). Mini-Review on the Regulation of Electrolyte Solvation Structure for Aqueous Zinc Ion Batteries. Batteries, 9(2), 73. https://doi.org/10.3390/batteries9020073






