Battery-Type Lithium-Ion Hybrid Capacitors: Current Status and Future Perspectives
Abstract
:1. Introduction
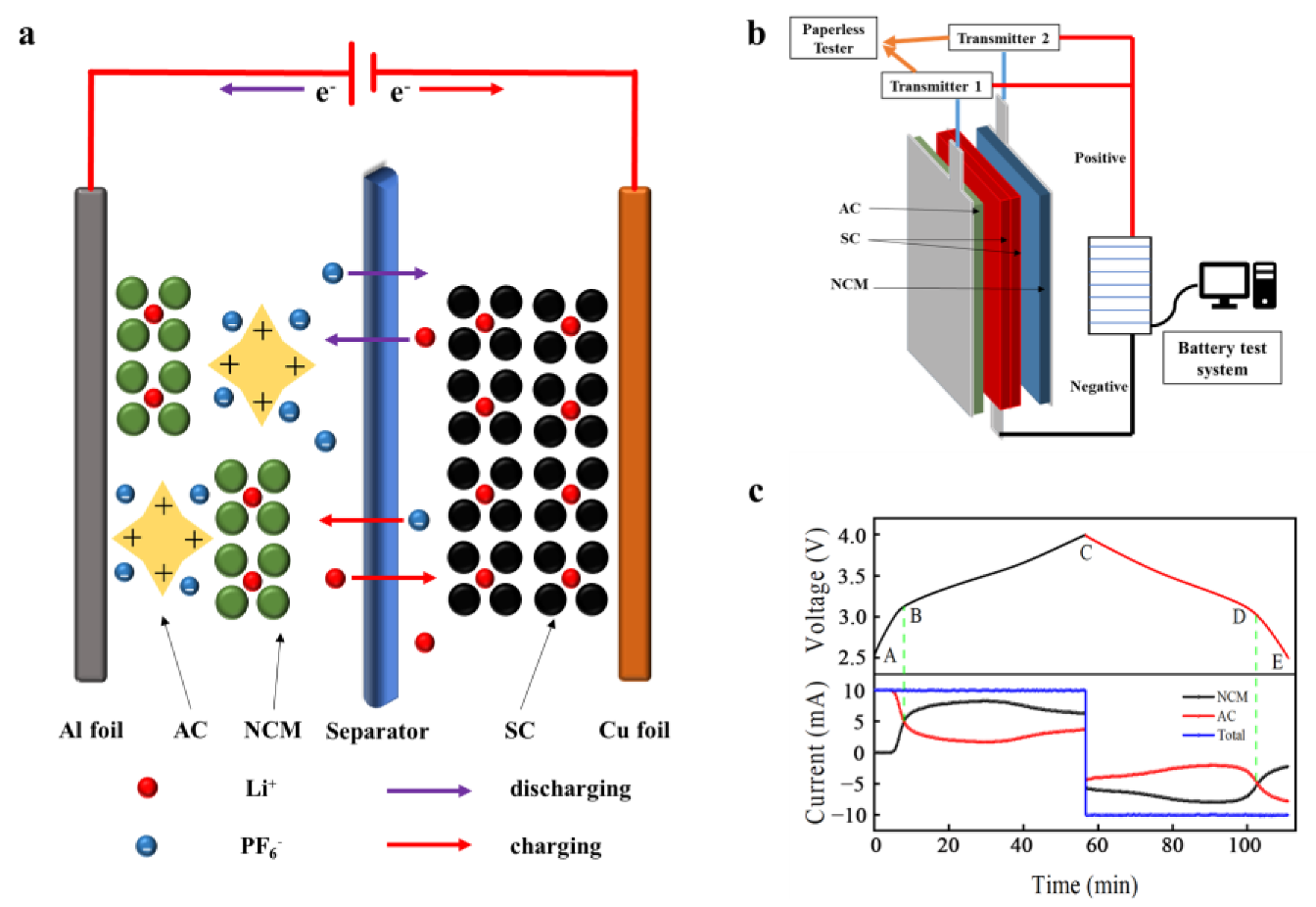
2. Structure and Working Principle
3. Advanced LIBCs
3.1. Anode Material
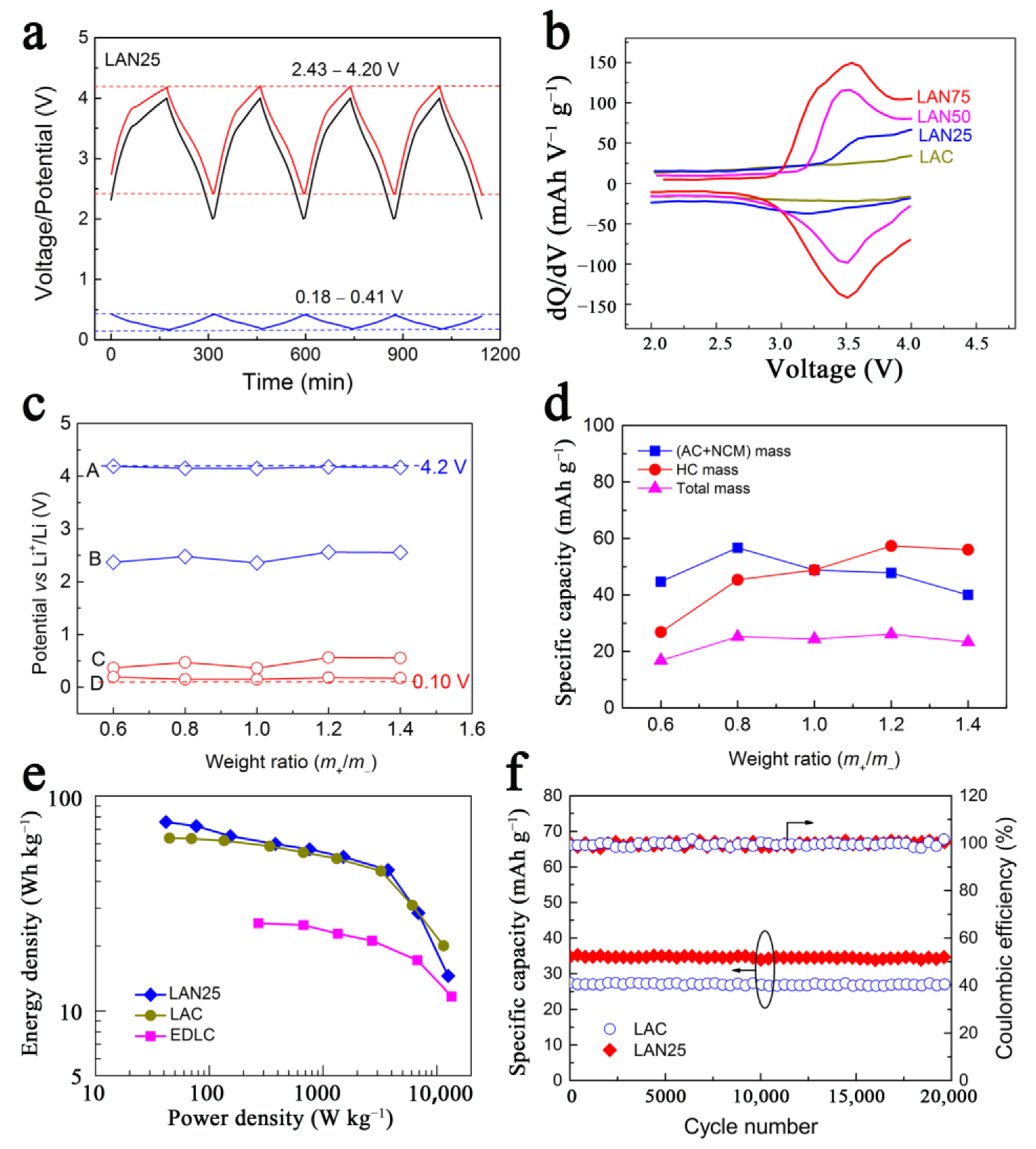
3.1.1. Non-Lithiated Anode Material
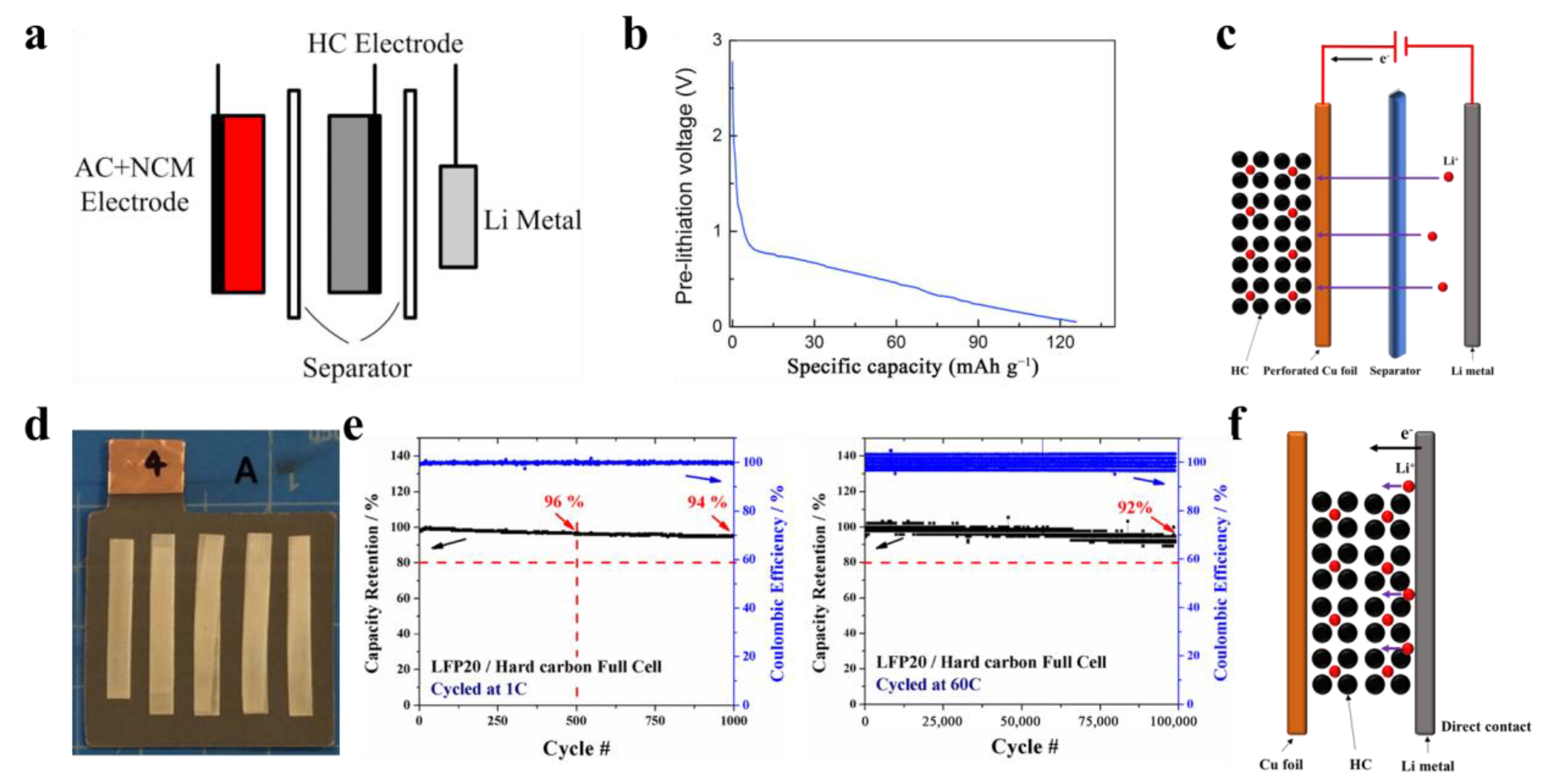
3.1.2. Pre-Lithiated Anode Material
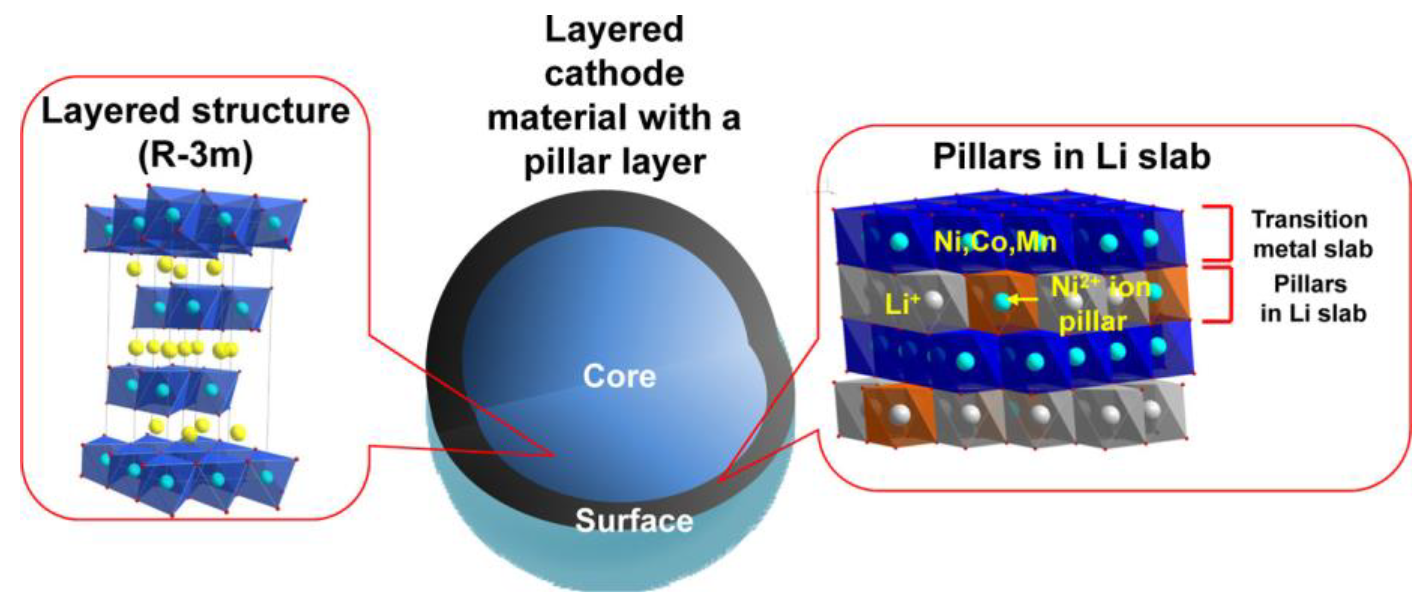
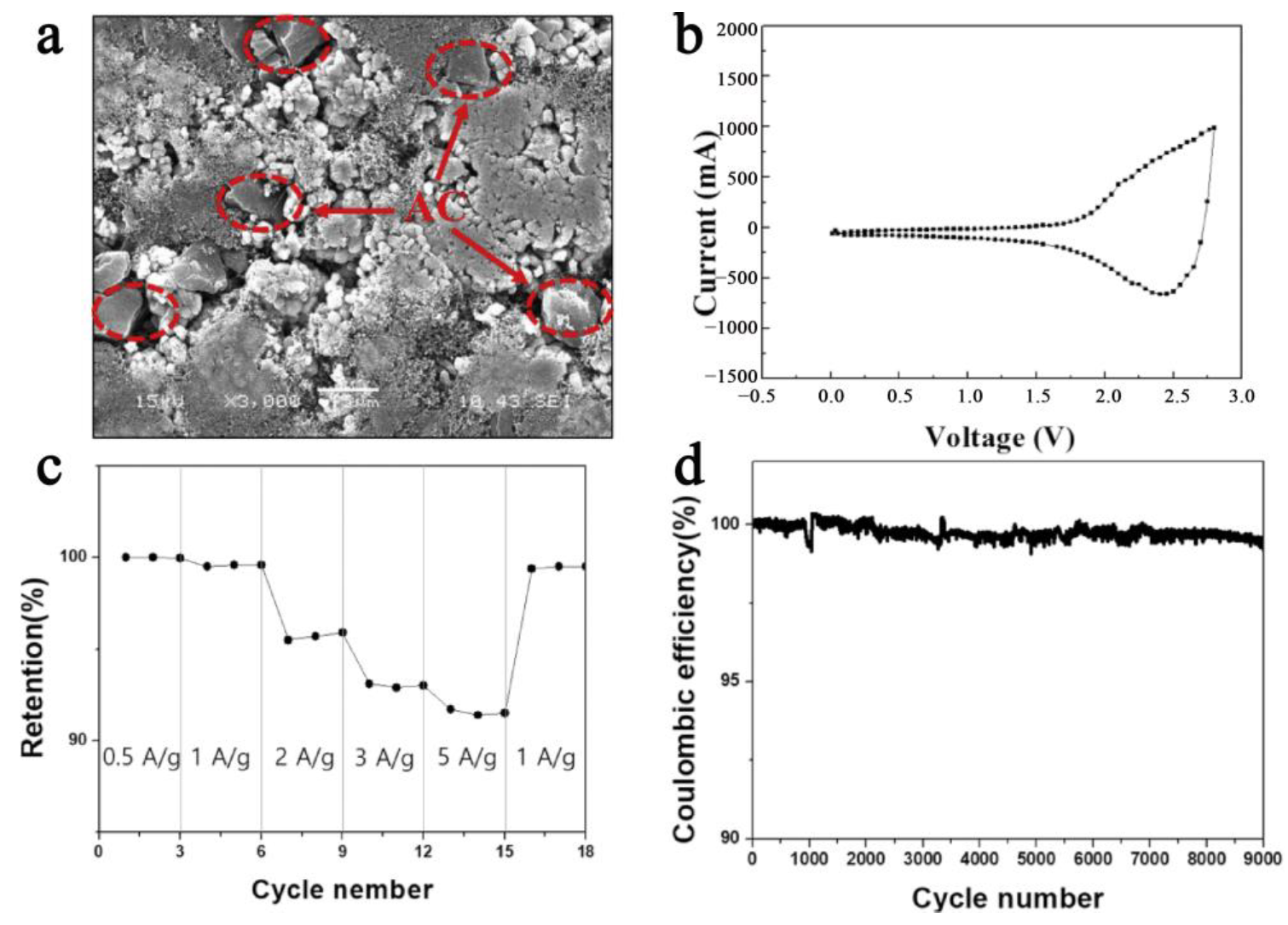
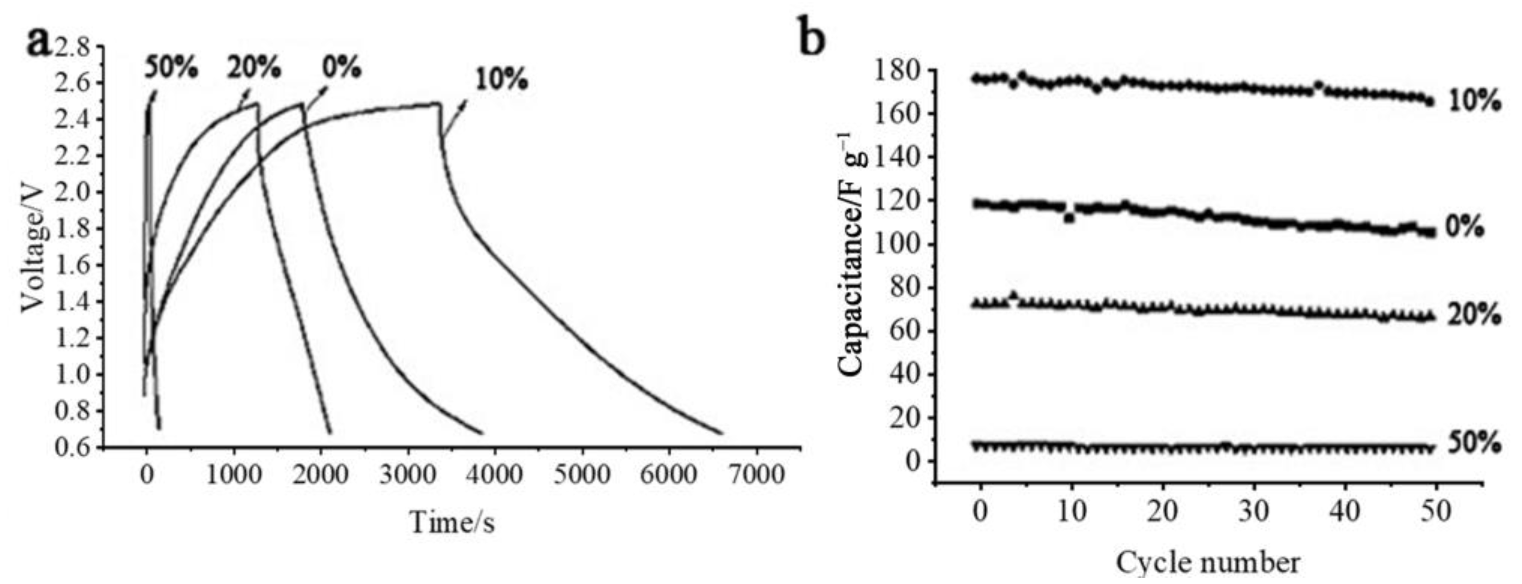
3.2. Capacitor Material in Cathode
3.3. Separator
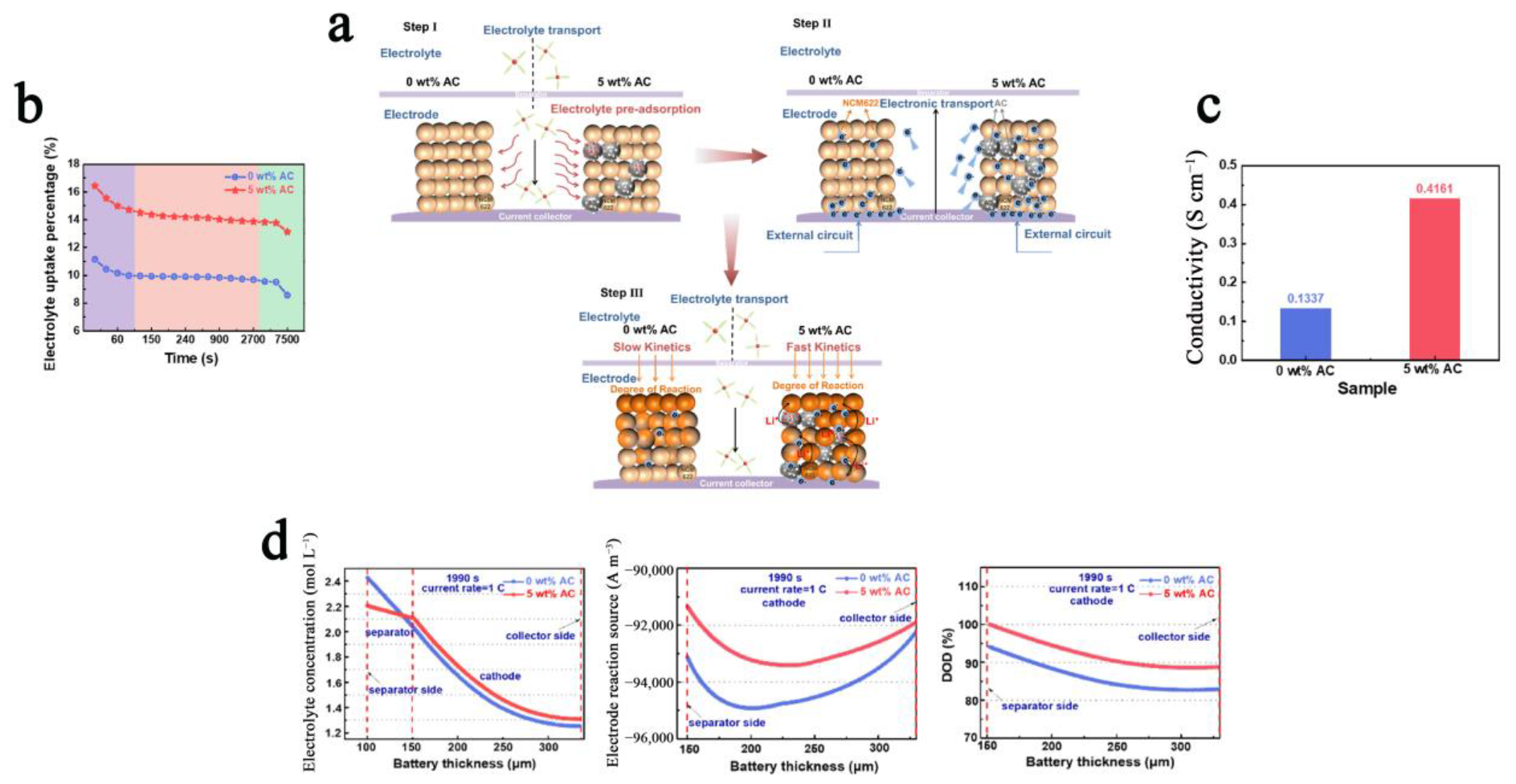
3.4. Polarization Phenomenon
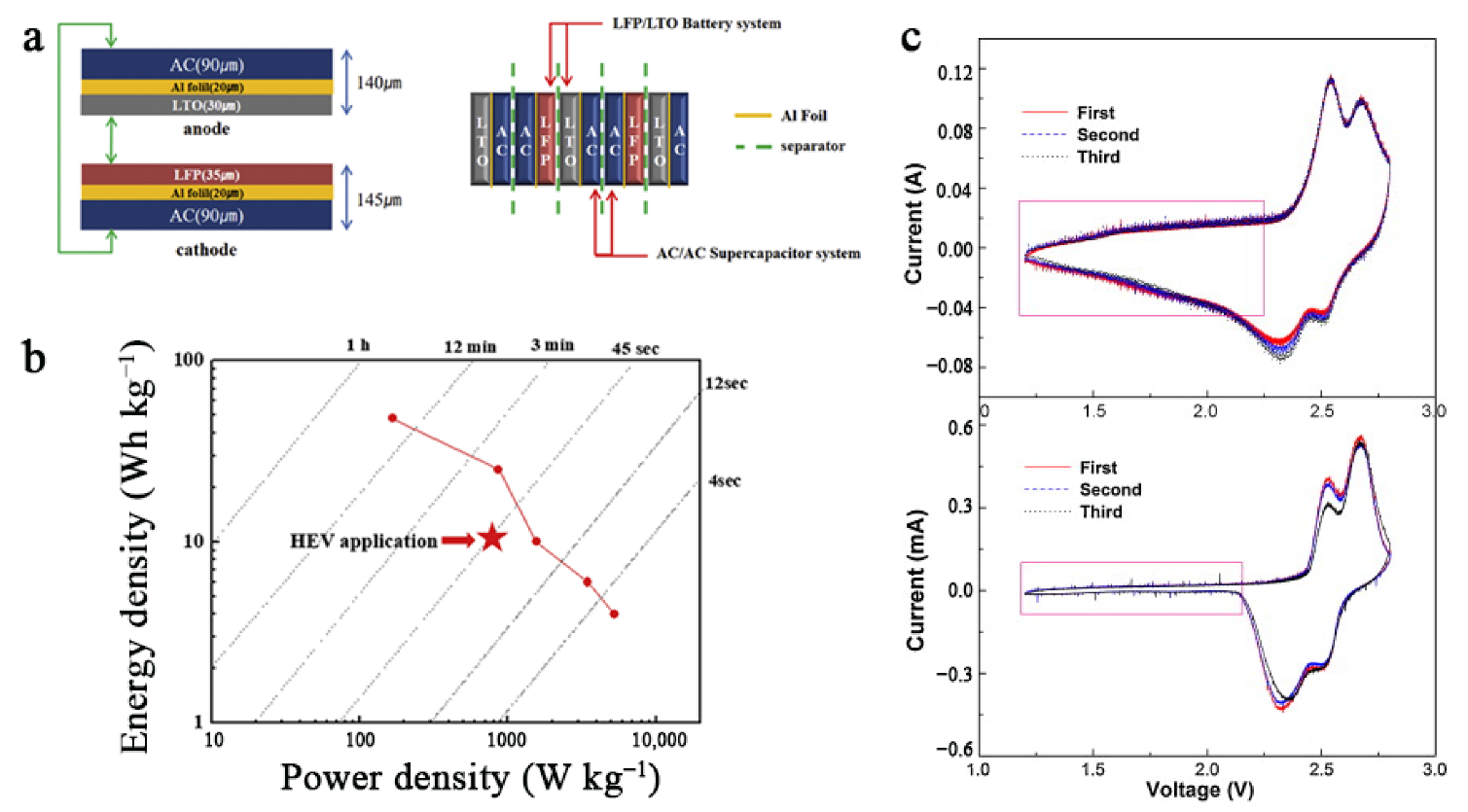
3.5. Pulsed Performance
3.6. Safety Issues
3.7. Electrode Engineering
4. Summary and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hannan, M.; Hoque, M.M.; Mohamed, A.; Ayob, A. Review of energy storage systems for electric vehicle applications: Issues and challenges. Renew. Sustain. Energy Rev. 2017, 69, 771–789. [Google Scholar] [CrossRef]
- Zame, K.K.; Brehm, C.A.; Nitica, A.T.; Richard, C.L.; Schweitzer, G.D., III. Smart grid and energy storage: Policy recommendations. Renew. Sustain. Energy Rev. 2018, 82, 1646–1654. [Google Scholar] [CrossRef]
- Cho, H.; Park, K.J. Energy density improvement by controlling the properties of conductive agents in Ni-rich cathodes. Int. J. Energy Res. 2022, 46, 2073–2080. [Google Scholar] [CrossRef]
- Flandrois, S.; Simon, B. Carbon materials for lithium-ion rechargeable batteries. Carbon 1999, 37, 165–180. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar]
- Zhang, J.-N.; Li, Q.; Ouyang, C.; Yu, X.; Ge, M.; Huang, X.; Hu, E.; Ma, C.; Li, S.; Xiao, R. Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V. Nat. Energy 2019, 4, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Kuo, T.-R.; Yougbaré, S.; Lin, L.-Y. Design of LiFePO4 and porous carbon composites with excellent High-Rate charging performance for Lithium-Ion secondary battery. J. Colloid Interface Sci. 2022, 607, 1457–1465. [Google Scholar] [CrossRef]
- Chae, C.; Park, H.; Kim, D.; Kim, J.; Oh, E.-S.; Lee, J.K. A Li-ion battery using LiMn2O4 cathode and MnOx/C anode. J. Power Sources 2013, 244, 214–221. [Google Scholar] [CrossRef]
- Kondrakov, A.O.; Schmidt, A.; Xu, J.; GeßWein, H.; MöNig, R.; Hartmann, P.; Sommer, H.; Brezesinski, T.; Janek, J. Anisotropic Lattice Strain and Mechanical Degradation of High- and Low-Nickel NCM Cathode Materials for Li-Ion Batteries. J. Phys. Chem. C 2017, 121, 3286–3294. [Google Scholar] [CrossRef]
- Cho, Y.; Oh, P.; Cho, J. A new type of protective surface layer for high-capacity Ni-based cathode materials: Nanoscaled surface pillaring layer. Nano Lett. 2013, 13, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Tanaka, I.; Adachi, H.; Makimura, Y.; Ohzuku, T. Crystal and electronic structures of superstructural Li1-x[Co1/3Ni1/3Mn1/3]O2 (0 ≤ x ≤ 1). J. Power Sources 2003, 119–121, 644–648. [Google Scholar] [CrossRef]
- Jung, C.H.; Shim, H.; Eum, D.; Hong, S.H. Challenges and recent progress in LiNixCoyMn1-x-yO2 (NCM) cathodes for lithium ion batteries. J. Korean Ceram. Soc. 2021, 58, 1–27. [Google Scholar] [CrossRef]
- Liao, P.; Duh, J.; Sheen, S. Microstructure and electrochemical performance of LiNi0. 6Co0. 4− xMnxO2 cathode materials. J. Power Sources 2005, 143, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-H.; Shin, H.-S.; Shin, D.; Sun, Y.-K. Synthesis and electrochemical properties of Li [Ni0.8Co0.1Mn0.1]O2 and Li[Ni0.8Co0.2]O2 via co-precipitation. J. Power Sources 2006, 159, 1328–1333. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Q.; Liang, Q. Carbon-Coatings Improve Performance of Li-Ion Battery. Nanomaterials 2022, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: A review. Energy Storage Mater. 2020, 24, 85–112. [Google Scholar]
- Jin, X.; Xu, Q.; Liu, H.; Yuan, X.; Xia, Y. Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery. Electrochim. Acta 2014, 136, 19–26. [Google Scholar] [CrossRef]
- Li, Q.; Li, G.; Fu, C.; Luo, D.; Fan, J.; Li, L. K+-doped Li1. 2Mn0. 54Co0. 13Ni0. 13O2: A novel cathode material with an enhanced cycling stability for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 10330–10341. [Google Scholar] [CrossRef]
- Wilcox, J.; Patoux, S.; Doeff, M. Structure and electrochemistry of LiNi1/3Co1/3−yMyMn1/3O2 (M = Ti, Al, Fe) positive electrode materials. J. Electrochem. Soc. 2009, 156, A192–A198. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.S.; Martin, S.W. Synthesis and electrochemical characteristics of Al2O3-coated LiCo1/3Ni1/3Mn1/3O2 cathode materials for lithium ion batteries. Electrochim. Acta 2006, 52, 1316–1322. [Google Scholar] [CrossRef]
- Feng, W.; Meng, W.; Su, Y.; Shi, C.; Xu, B. Effect of TiO2-coating on the electrochemical performances of LiCo1/3Ni1/3Mn1/3O2. J. Power Sources 2009, 191, 628–632. [Google Scholar]
- Hu, S.-K.; Cheng, G.-H.; Cheng, M.-Y.; Hwang, B.-J.; Santhanam, R. Cycle life improvement of ZrO2-coated spherical LiCo1/3Ni1/3Mn1/3O2 cathode material for lithium ion batteries. J. Power Sources 2009, 188, 564–569. [Google Scholar] [CrossRef]
- Wang, J.-H.; Wang, Y.; Guo, Y.-Z.; Ren, Z.-Y.; Liu, C.-W. Effect of heat-treatment on the surface structure and electrochemical behavior of AIPO4-coated LiNi1/3Mn1/3Co1/3O2 cathode materials. J. Mater. Chem. A 2013, 1, 4879–4884. [Google Scholar] [CrossRef]
- Bai, Y.; Chang, Q.; Yu, Q.; Zhao, S.; Jiang, K. A novel approach to improve the electrochemical performances of layered LiNi1/3Co1/3Mn1/3O2 cathode by YPO4 surface coating. Electrochim. Acta 2013, 112, 414–421. [Google Scholar] [CrossRef]
- Fan, X.; Hu, G.; Zhang, B.; Ou, X.; Yang, Y. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 2020, 70, 104450. [Google Scholar] [CrossRef]
- Xu, X.; Huo, H.; Jian, J.; Wang, L.; Zhu, H.; Xu, S.; He, X.; Yin, G.; Du, C.; Sun, X. Radially Oriented Single-Crystal Primary Nanosheets Enable Ultrahigh Rate and Cycling Properties of LiNi0.8Co0.1Mn0.1O2 Cathode Material for Lithium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1803963. [Google Scholar] [CrossRef]
- Liu, Y.; Harlow, J.; Dahn, J. Microstructural Observations of "Single Crystal" Positive Electrode Materials Before and After Long Term Cycling by Cross-section Scanning Electron Microscopy. J. Electrochem. Soc. 2020, 167, 020512. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A comprehensive understanding of electrode thickness effects on the electrochemical performances of Li-ion battery cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- Dong, W.K.; Hwang, S.M.; Ji, B.Y.; Kim, Y. Electrode Engineering with CNTs to Enhance the Electrochemical Performance of LiNi0.6Co0.2Mn0.2O2 Cathodes with Commercial Level Design Parameters. ChemElectroChem 2020, 7, 2621–2628. [Google Scholar]
- Liu, W.; Li, C.; Sun, X.; Zhang, X.; Wang, K.; Li, Z.; Hao, Q.; Ma, Y. Improvement of the high-rate capability of LiNi1/3Mn1/3Co1/3O2 cathode by adding highly electroconductive and mesoporous graphene. J. Alloy. Compd. 2018, 758, 206–213. [Google Scholar] [CrossRef]
- Li, M.; Sheng, L.; Xu, R.; Yang, Y.; Bai, Y.; Song, S.; Liu, G.; Wang, T.; Huang, X.; He, J. Enhanced the mechanical strength of polyimide (PI) nanofiber separator via PAALi binder for lithium ion battery. Compos. Commun. 2021, 24, 100607. [Google Scholar] [CrossRef]
- Plitz, I.; DuPasquier, A.; Badway, F.; Gural, J.; Pereira, N.; Gmitter, A.; Amatucci, G. The design of alternative nonaqueous high power chemistries. Appl. Phys. A 2006, 82, 615–626. [Google Scholar] [CrossRef]
- Hu, X.; Huai, Y.; Lin, Z.; Suo, J.; Deng, Z. A (LiFePO4–AC)/Li4Ti5O12 hybrid battery capacitor. J. Electrochem. Soc. 2007, 154, A1026–A1030. [Google Scholar] [CrossRef]
- Cericola, D.; Novák, P.; Wokaun, A.; Kötz, R. Segmented bi-material electrodes of activated carbon and LiMn2O4 for electrochemical hybrid storage devices: Effect of mass ratio and C-rate on current sharing. Electrochim. Acta 2011, 56, 1288–1293. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Huang, B.; Zhang, H.; Zhang, D.; Ma, Y. (LiNi0.5Co0.2Mn0.3O2 + AC)/graphite hybrid energy storage device with high specific energy and high rate capability. J. Power Sources 2013, 243, 361–368. [Google Scholar] [CrossRef]
- Cericola, D.; Novák, P.; Wokaun, A.; Kötz, R. Hybridization of electrochemical capacitors and rechargeable batteries: An experimental analysis of the different possible approaches utilizing activated carbon, Li4Ti5O12 and LiMn2O4. J. Power Sources 2011, 196, 10305–10313. [Google Scholar] [CrossRef]
- Naoi, K.; Ishimoto, S.; Miyamoto, J.-I.; Naoi, W. Second generation ‘nanohybrid supercapacitor’: Evolution of capacitive energy storage devices. Energy Environ. Sci. 2012, 5, 9363–9373. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Q.; Xu, B.; Liu, T.; Wang, D.; Zhao, G. The synergy effect on Li storage of LiFePO4 with activated carbon modifications. RSC Adv. 2013, 3, 20024–20033. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Liu, Z.; Sun, X.; Du, T.; Zhang, D.; An, Y.; Zhang, X.; Zhang, H.; Zhang, X.; Wang, K.; et al. Probing current contribution of lithium-ion battery/lithium-ion capacitor multi-structure hybrid systems. J. Power Sources 2022, 548, 232016. [Google Scholar] [CrossRef]
- Lorca, S.; Santos, F.; Padilla, J.; López Cascales, J.; Fernández Romero, A.J. Importance of Continuous and Simultaneous Monitoring of Both Electrode Voltages during Discharge/Charge Battery Tests: Application to Zn-Based Batteries. Batteries 2022, 8, 221. [Google Scholar] [CrossRef]
- Sahin, M.E.; Blaabjerg, F.; Sangwongwanich, A. A review on supercapacitor materials and developments. Turk. J. Mater. 2020, 5, 10–24. [Google Scholar]
- Dou, Q.; Wu, N.; Yuan, H.; Kang, H.S.; Tang, Y.; Mitlin, D.; Park, H.S. Emerging trends in anion storage materials for the capacitive and hybrid energy storage and beyond. Chem. Soc. Rev. 2021, 50, 6734–6789. [Google Scholar]
- Han, Y.-L.; Wang, Z.-F.; Xie, L.-J.; Wang, H.; Yi, Z.L.; Li, J.-X.; Song, G.; Yan, C.; Su, F.-Y.; Chen, C.-M. Revealing the accelerated reaction kinetic of Ni-rich cathodes by activated carbons for high performance lithium-ion batteries. Carbon 2023, 203, 445–454. [Google Scholar] [CrossRef]
- Du Pasquier, A.; Plitz, I.; Gural, J.; Badway, F.; Amatucci, G. Power-ion battery: Bridging the gap between Li-ion and supercapacitor chemistries. J. Power Sources 2004, 136, 160–170. [Google Scholar] [CrossRef]
- Xia, H.; An, Z.; Huang, T.; Fang, W.; Du, L.; Wu, M.; Suo, L.; Xu, J.; Hua, L. Construction of Li-ion supercapacitor-type battery using active carbon/LiNi0.5Co0.2Mn0.3O2 composite as cathode and its electrochemical performances. Energy Storage Sci. Technol. 2018, 7, 1233. [Google Scholar]
- Lee, S.-H.; Lee, T.-H. High performance hybrid supercapacitors with LiNi1/3Mn1/3Co1/3O2/activated carbon cathode and activated carbon anode. Int. J. Hydrog. Energy 2018, 43, 15365–15369. [Google Scholar] [CrossRef]
- Ping, L.; Zheng, J.; Shi, Z.; Qi, J.; Wang, C. Electrochemical performance of MCMB/(AC + LiFePO4) lithium-ion capacitors. Chin. Sci. Bull. 2013, 58, 689–695. [Google Scholar] [CrossRef]
- Cai, R.; Yu, X.; Liu, X.; Shao, Z. Li4Ti5O12/Sn composite anodes for lithium-ion batteries: Synthesis and electrochemical performance. J. Power Sources 2010, 195, 8244–8250. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Jalbout, A.; Yan, X.; Pan, X.; Xie, H.; Wang, R. High-rate characteristics of novel anode Li4Ti5O12/polyacene materials for Li-ion secondary batteries. Electrochim. Acta 2008, 53, 4200–4204. [Google Scholar] [CrossRef]
- Zhu, J.; Zu, W.; Yang, G.; Song, Q. A novel electrochemical supercapacitor based on Li4Ti5O12 and LiNi1/3Mn1/3Co1/3O2. Mater. Lett. 2014, 115, 237–240. [Google Scholar] [CrossRef]
- Chen, W.; Sun, X.; Hu, H.; Wang, J.; Li, X.; Liang, G.; Huang, Y.; Wei, C. AC + Li(NiCoMn)O2/Li4Ti5O12 + MWCNTs hybrid capacitors. J. Mater. Eng. 2020, 48, 128–135. [Google Scholar]
- Chen, S.; Hu, H.; Wang, C.; Wang, G.; Yin, J.; Cao, D. (LiFePO4-AC)/Li4Ti5O12 hybrid supercapacitor: The effect of LiFePO4 content on its performance. J. Renew. Sustain. Energy 2012, 4, 033114. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jin, B.-S.; Kim, H.-S.; Ahn, H.-J.; Lee, B.-G. A novel battery-supercapacitor system with extraordinarily high performance. Int. J. Hydrog. Energy 2019, 44, 3013–3020. [Google Scholar] [CrossRef]
- Hu, X.; Deng, Z.; Suo, J.; Pan, Z. A high rate, high capacity and long life (LiMn2O4 + AC)/Li4Ti5O12 hybrid battery–supercapacitor. J. Power Sources 2009, 187, 635–639. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, X.; Li, C.; Wang, K.; Sun, X.; Ma, Y. High-efficiency sacrificial prelithiation of lithium-ion capacitors with superior energy-storage performance. Energy Storage Mater. 2020, 24, 160–166. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Zhang, H.; Xu, N.; Wang, K.; Ma, Y. High performance lithium-ion hybrid capacitors with pre-lithiated hard carbon anodes and bifunctional cathode electrodes. J. Power Sources 2014, 270, 318–325. [Google Scholar] [CrossRef]
- Kim, M.; Xu, F.; Lee, J.H.; Jung, C.; Hong, S.M.; Zhang, Q.; Koo, C.M. A fast and efficient pre-doping approach to high energy density lithium-ion hybrid capacitors. J. Mater. Chem. A 2014, 2, 10029–10033. [Google Scholar] [CrossRef]
- Shellikeri, A.; Yturriaga, S.; Zheng, J.; Cao, W.; Hagen, M.; Read, J.; Jow, T.; Zheng, J. Hybrid lithium-ion capacitor with LiFePO4/AC composite cathode–Long term cycle life study, rate effect and charge sharing analysis. J. Power Sources 2018, 392, 285–295. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Zhang, L.; Shellikeri, A.; Cao, W.; Wu, Q.; Zheng, J.P. A hybrid electrochemical device based on a synergetic inner combination of Li ion battery and Li ion capacitor for energy storage. Sci. Rep. 2017, 7, 41910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, M.; Winter, M.; Passerini, S.; Balducci, A. On the cycling stability of lithium-ion capacitors containing soft carbon as anodic material. J. Power Sources 2013, 238, 388–394. [Google Scholar] [CrossRef]
- Du, T.; Liu, Z.; Sun, X.; Geng, L.; Zhang, X.; An, Y.; Zhang, X.; Wang, K.; Ma, Y. Segmented bi-material cathodes to boost the lithium-ion battery-capacitors. J. Power Sources 2020, 478, 228994. [Google Scholar] [CrossRef]
- Chen, X.; Mu, Y.; Cao, G.; Qiu, J.; Zhang, W.; Zhang, Q.; Ming, H. Structure–Activity Relationship of Carbon Additives in Cathodes for Advanced Capacitor Batteries. Electrochim. Acta 2022, 413, 140165. [Google Scholar] [CrossRef]
- Yan, D.; Lu, A.-H.; Chen, Z.-Y.; He, L.; Li, W.-C. Pseudocapacitance-Dominated Li-Ion Capacitors Showing Remarkable Energy Efficiency by Introducing Amorphous LiFePO4 in the Cathode. ACS Appl. Energy Mater. 2021, 4, 1824–1832. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Niu, Z. Carbon-based materials for lithium-ion capacitors. Mater. Chem. Front. 2019, 3, 1265–1279. [Google Scholar] [CrossRef]
- Xing, F.; Bi, Z.; Su, F.; Liu, F.; Wu, Z.S. Unraveling the Design Principles of Battery-Supercapacitor Hybrid Devices: From Fundamental Mechanisms to Microstructure Engineering and Challenging Perspectives. Adv. Energy Mater. 2022, 12, 2200594. [Google Scholar] [CrossRef]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gomez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, X.; Sun, C.; Wang, K.; Sun, X.; Ma, Y. Recent progress of graphene-based materials in lithium-ion capacitors. J. Phys. D Appl. Phys. 2019, 52, 143001. [Google Scholar] [CrossRef]
- Liu, W.-J.; Sun, X.-Z.; Zhang, X.; Li, C.; Wang, K.; Wen, W.; Ma, Y.-W. Structural evolution of mesoporous graphene/LiNi1/3Mn1/3Co1/3O2 composite cathode for Li–ion battery. Rare Met. 2021, 40, 521–528. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Wang, M.-S.; Zhang, K.-J.; Huang, Y.; Qu, M.-Z.; Yu, Z.-L.; Geng, D.-S.; Zhao, W.-G.; Zheng, J.-M. Improved rate capability of a LiNi1/3Mn1/3Co1/3O2/CNT/graphene hybrid material for Li-ion batteries. RSC Adv. 2017, 7, 24359–24367. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Cheng, S.; Zhao, J.; Zhang, C.; Sun, S.; Zhou, N.; Qiu, Y.; Zhang, X. Heat treatment of electrospun Polyvinylidene fluoride fibrous membrane separators for rechargeable lithium-ion batteries. J. Power Sources 2013, 240, 204–211. [Google Scholar] [CrossRef]
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and performance evaluation of lithium-ion battery separators. Nat. Energy 2019, 4, 16–25. [Google Scholar]
- Zhang, H.; Zhao, H.; Khan, M.A.; Zou, W.; Xu, J.; Zhang, L.; Zhang, J. Recent progress in advanced electrode materials, separators and electrolytes for lithium batteries. J. Mater. Chem. A 2018, 6, 20564–20620. [Google Scholar]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Li, Y.; Pu, H.; Wei, Y. Polypropylene/polyethylene multilayer separators with enhanced thermal stability for lithium-ion battery via multilayer coextrusion. Electrochim. Acta 2018, 264, 140–149. [Google Scholar] [CrossRef]
- Rajan, R.; Krishna, T.P.; Moss, P.L.; Weatherspoon, M.H. Analysis of the Separator Thickness and Porosity on the Performance of Lithium-Ion Batteries. Int. J. Electrochem. 2018, 2018, 1925708. [Google Scholar]
- Xiong, M.; Tang, H.; Wang, Y.; Pan, M. Ethylcellulose-coated polyolefin separators for lithium-ion batteries with improved safety performance. Carbohydr. Polym. 2014, 101, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhan, H.; Hu, J.; Liang, Y.; Zeng, S. Wet-laid non-woven fabric for separator of lithium-ion battery. J. Power Sources 2009, 189, 616–619. [Google Scholar]
- Prosini, P.P.; Villano, P.; Carewska, M. A novel intrinsically porous separator for self-standing lithium-ion batteries. Electrochim. Acta 2002, 48, 227–233. [Google Scholar] [CrossRef]
- Augustin, S.; Hennige, V.; Hörpel, G.; Hying, C. Ceramic but flexible: New ceramic membrane foils for fuel cells and batteries. Desalination 2002, 146, 23–28. [Google Scholar] [CrossRef]
- Appetecchi, G.; Romagnoli, P.; Scrosati, B. Composite gel membranes: A new class of improved polymer electrolytes for lithium batteries. Electrochem. Commun. 2001, 3, 281–284. [Google Scholar] [CrossRef]
- Lv, D.; Chai, J.; Wang, P.; Zhu, L.; Liu, C.; Nie, S.; Li, B.; Cui, G. Pure cellulose lithium-ion battery separator with tunable pore size and improved working stability by cellulose nanofibrils. Carbohydr. Polym. 2021, 251, 116975. [Google Scholar] [CrossRef] [PubMed]
- Zolin, L.; Destro, M.; Chaussy, D.; Penazzi, N.; Gerbaldi, C.; Beneventi, D. Aqueous processing of paper separators by filtration dewatering: Towards Li-ion paper batteries. J. Mater. Chem. A 2015, 3, 14894–14901. [Google Scholar] [CrossRef]
- Sun, X.-Z.; Zhang, X.; Huang, B.; MA, Y.-W. Effects of separator on the electrochemical performance of electrical double-layer capacitor and hybrid battery-supercapacitor. Acta Phys. Chim. Sin. 2014, 30, 485–491. [Google Scholar]
- Kasnatscheew, J.; Rodehorst, U.; Streipert, B.; Wiemers-Meyer, S.; Jakelski, R.; Wagner, R.; Laskovic, I.C.; Winter, M. Learning from overpotentials in lithium ion batteries: A case study on the LiCo1/3Ni1/3Mn1/3O2 (NCM) cathode. J. Electrochem. Soc. 2016, 163, A2943–A2950. [Google Scholar] [CrossRef]
- Shi, Y.; Wen, L.; Li, F.; Cheng, H.-M. Nanosized Li4Ti5O12/graphene hybrid materials with low polarization for high rate lithium ion batteries. J. Power Sources 2011, 196, 8610–8617. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, J.; Amine, K.; Pan, F. Depolarization effect to enhance the performance of lithium ions batteries. Nano Energy 2017, 33, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Hagen, M.; Yan, J.; Cao, W.; Chen, X.; Shellikeri, A.; Du, T.; Read, J.; Jow, T.; Zheng, J. Hybrid lithium-ion battery-capacitor energy storage device with hybrid composite cathode based on activated carbon/LiNi0.5Co0.2Mn0.3O2. J. Power Sources 2019, 433, 126689. [Google Scholar] [CrossRef]
- Yu, H.; Qian, Y.; Otani, M.; Tang, D.; Guo, S.; Zhu, Y.; Zhou, H. Study of the lithium/nickel ions exchange in the layered LiNi0.42Mn0.42Co0.16O2 cathode material for lithium ion batteries: Experimental and first-principles calculations. Energy Environ. Sci. 2014, 7, 1068–1078. [Google Scholar] [CrossRef]
- Nam, G.W.; Park, N.-Y.; Park, K.-J.; Yang, J.; Liu, J.; Yoon, C.S.; Sun, Y.-K. Capacity fading of Ni-rich NCA cathodes: Effect of microcracking extent. ACS Energy Lett. 2019, 4, 2995–3001. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef]
- Wu, L.; Nam, K.-W.; Wang, X.; Zhou, Y.; Zheng, J.-C.; Yang, X.-Q.; Zhu, Y. Structural origin of overcharge-induced thermal instability of Ni-containing layered-cathodes for high-energy-density lithium batteries. Chem. Mater. 2011, 23, 3953–3960. [Google Scholar] [CrossRef]
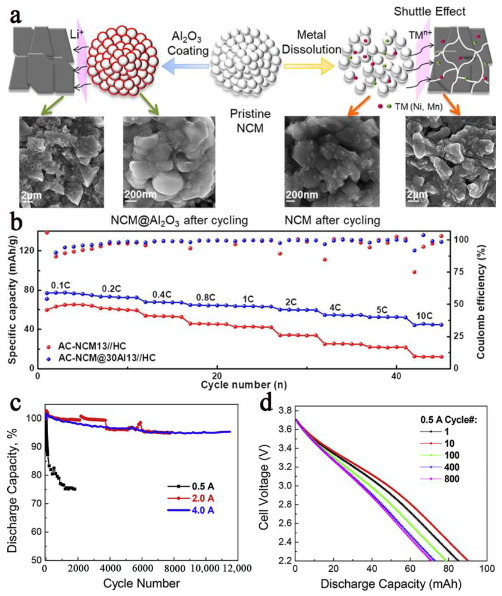
- Zhao, L.; Chen, G.; Weng, Y.; Yan, T.; Shi, L.; An, Z.; Zhang, D. Precise Al2O3 coating on LiNi0. 5Co0. 2Mn0. 3O2 by atomic layer deposition restrains the shuttle effect of transition metals in Li-ion capacitors. Chem. Eng. J. 2020, 401, 126138. [Google Scholar] [CrossRef]
- Hagen, M.; Cao, W.; Shellikeri, A.; Adams, D.; Chen, X.; Brandt, W.; Yturriaga, S.; Wu, Q.; Read, J.; Jow, T. Improving the specific energy of Li-Ion capacitor laminate cell using hybrid activated Carbon/LiNi0. 5Co0. 2Mn0. 3O2 as positive electrodes. J. Power Sources 2018, 379, 212–218. [Google Scholar] [CrossRef]
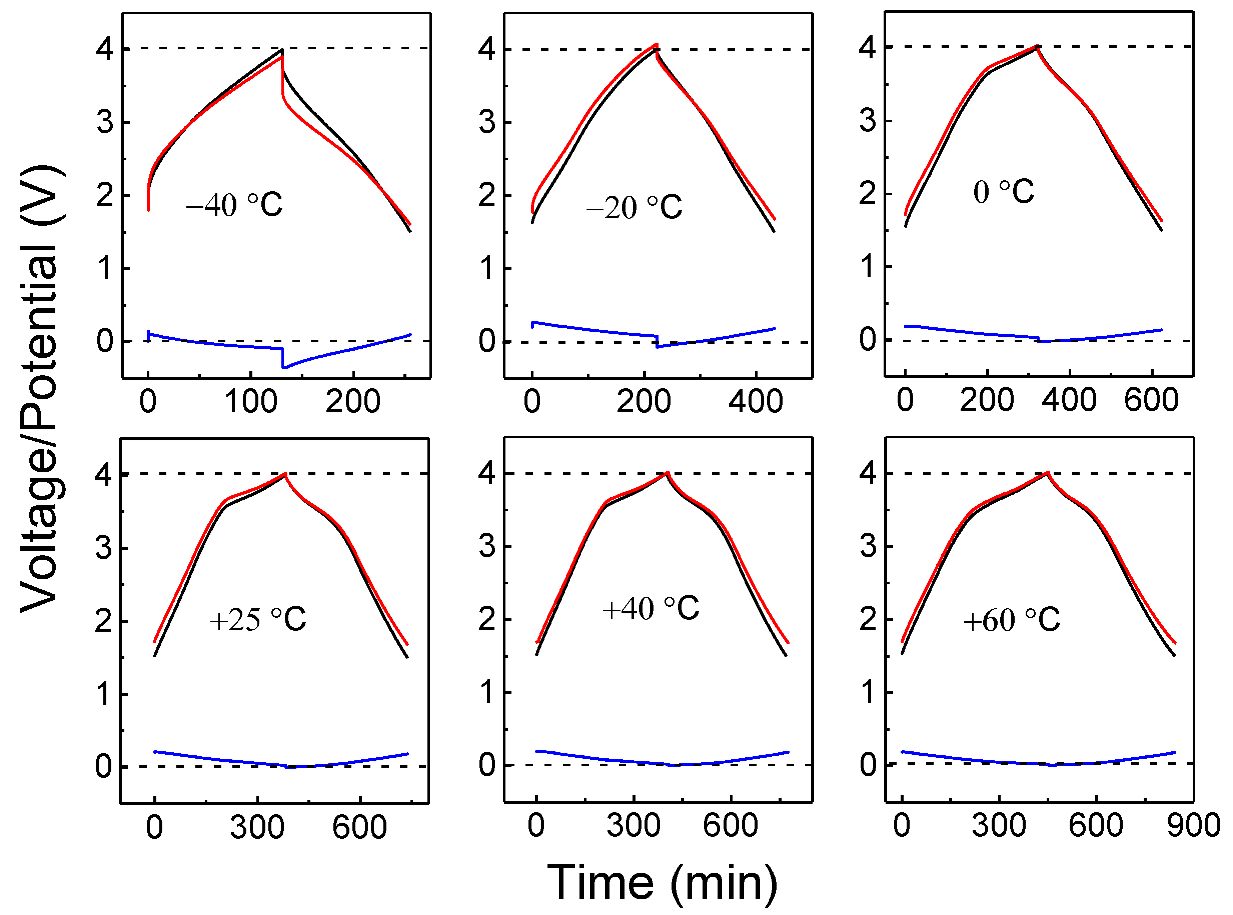
- Sun, X.; Zhang, X.; Wang, K.; Xu, N.; Ma, Y. Temperature effect on electrochemical performances of Li-ion hybrid capacitors. J. Solid State Electrochem. 2015, 19, 2501–2506. [Google Scholar] [CrossRef]
- Escobar-Hernandez, H.U.; Gustafson, R.M.; Papadaki, M.I.; Sachdeva, S.; Mannan, M.S. Thermal runaway in lithium-ion batteries: Incidents, kinetics of the runaway and assessment of factors affecting its initiation. J. Electrochem. Soc. 2016, 163, A2691–A2701. [Google Scholar] [CrossRef]
- Mao, B.; Huang, P.; Chen, H.; Wang, Q.; Sun, J. Self-heating reaction and thermal runaway criticality of the lithium ion battery. Int. J. Heat Mass Transf. 2020, 149, 119178. [Google Scholar] [CrossRef]
- Dong, T.; Peng, P.; Jiang, F. Numerical modeling and analysis of the thermal behavior of NCM lithium-ion batteries subjected to very high C-rate discharge/charge operations. Int. J. Heat Mass Transf. 2018, 117, 261–272. [Google Scholar] [CrossRef]
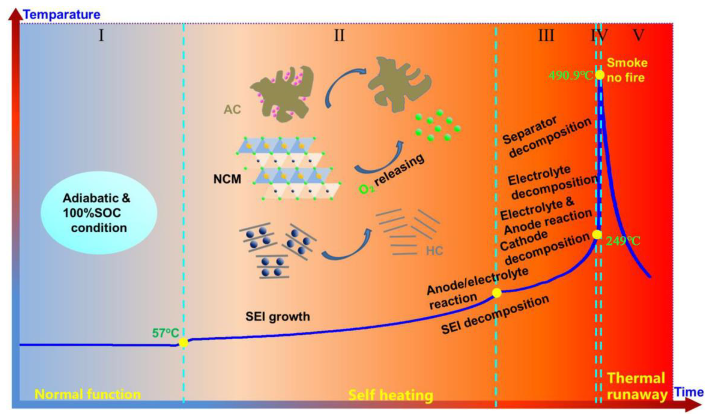
- Wu, M.; Zhang, C.; Yang, C.; An, Z.; Xu, J. Over-heating triggered thermal runaway characteristic of lithium ion hybrid supercapacitor based lithium nickel cobalt manganate oxide/activated carbon cathode and hard carbon anode. Solid State Ion. 2022, 377, 115883. [Google Scholar] [CrossRef]
- Qi, X.; Liu, B.; Yun, F.; Wang, C.; Wang, R.; Pang, J.; Tang, H.; Quan, W.; Zhang, Q.; Yang, M. Probing heat generation and release in a 57.5 A h high-energy-density Li-ion pouch cell with a nickel-rich cathode and SiOx/graphite anode. J. Mater. Chem. A 2022, 10, 1227–1235. [Google Scholar] [CrossRef]
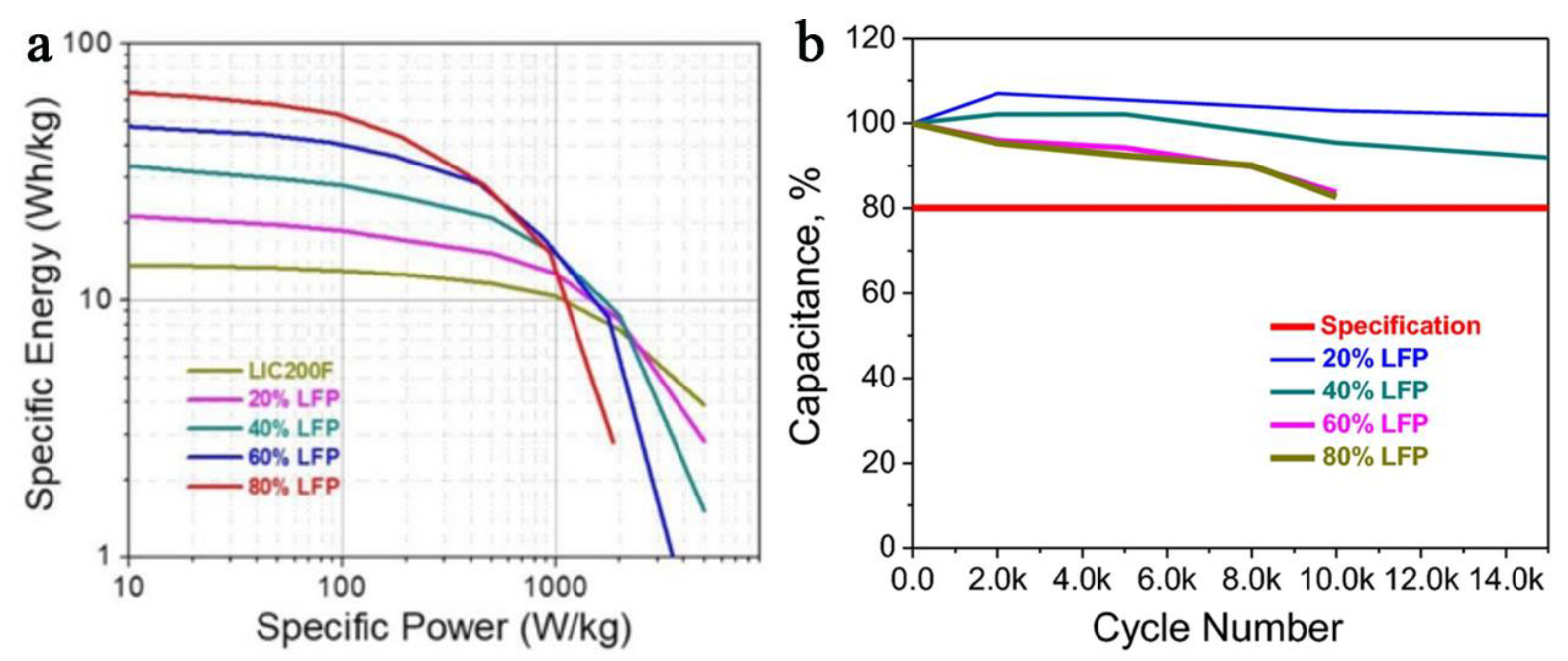
- Yan, J.; Chen, X.; Shellikeri, A.; Du, T.; Hagen, M.; Zheng, J.; Cao, W. Influence of lithium iron phosphate positive electrode material to hybrid lithium-ion battery capacitor (H-LiBC) energy storage devices. J. Electrochem. Soc. 2018, 165, A2774–A2780. [Google Scholar] [CrossRef]
- Guan, Y.; Shen, J.; Wei, X.; Zhu, Q.; Zheng, X.; Zhou, S.; Xu, B. LiFePO4/activated carbon/graphene composite with capacitive-battery characteristics for superior high-rate lithium-ion storage. Electrochim. Acta 2019, 294, 148–155. [Google Scholar] [CrossRef]
- Wood, M.; Li, J.; Du, Z.; Daniel, C.; Dunlop, A.R.; Polzin, B.J.; Jansen, A.N.; Krumdick, G.K.; Wood Iii, D.L. Impact of secondary particle size and two-layer architectures on the high-rate performance of thick electrodes in lithium-ion battery pouch cells. J. Power Sources 2021, 515, 230429. [Google Scholar] [CrossRef]
- He, F.; Zhang, Q.; Guo, D.; Guo, Y.; Guo, X. Influences of electrode structure on the electrical properties of (NMC + AC)/HC hybrid capacitor. Energy Storage Sci. Technol. 2022, 11, 2051–2058. [Google Scholar]
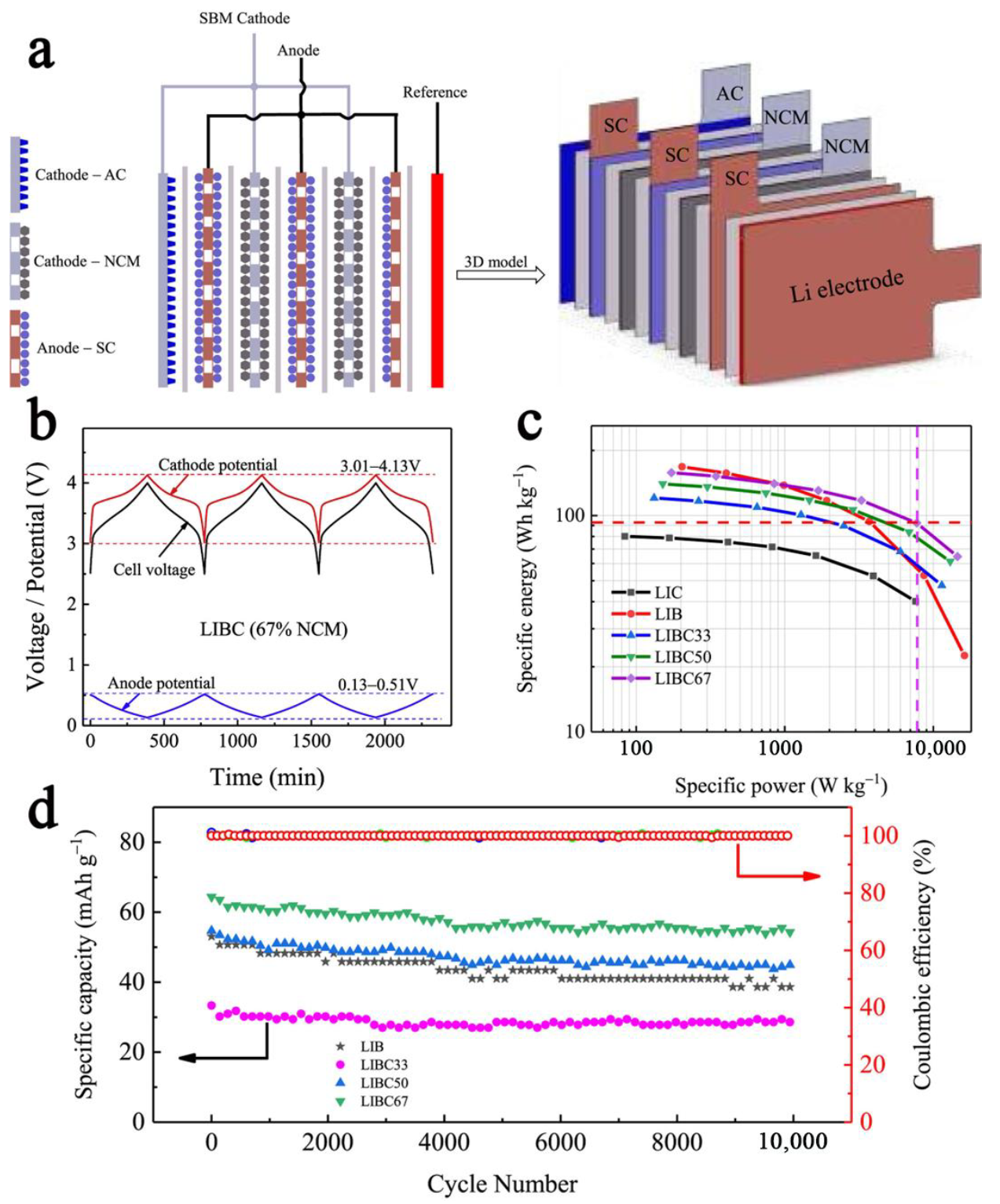
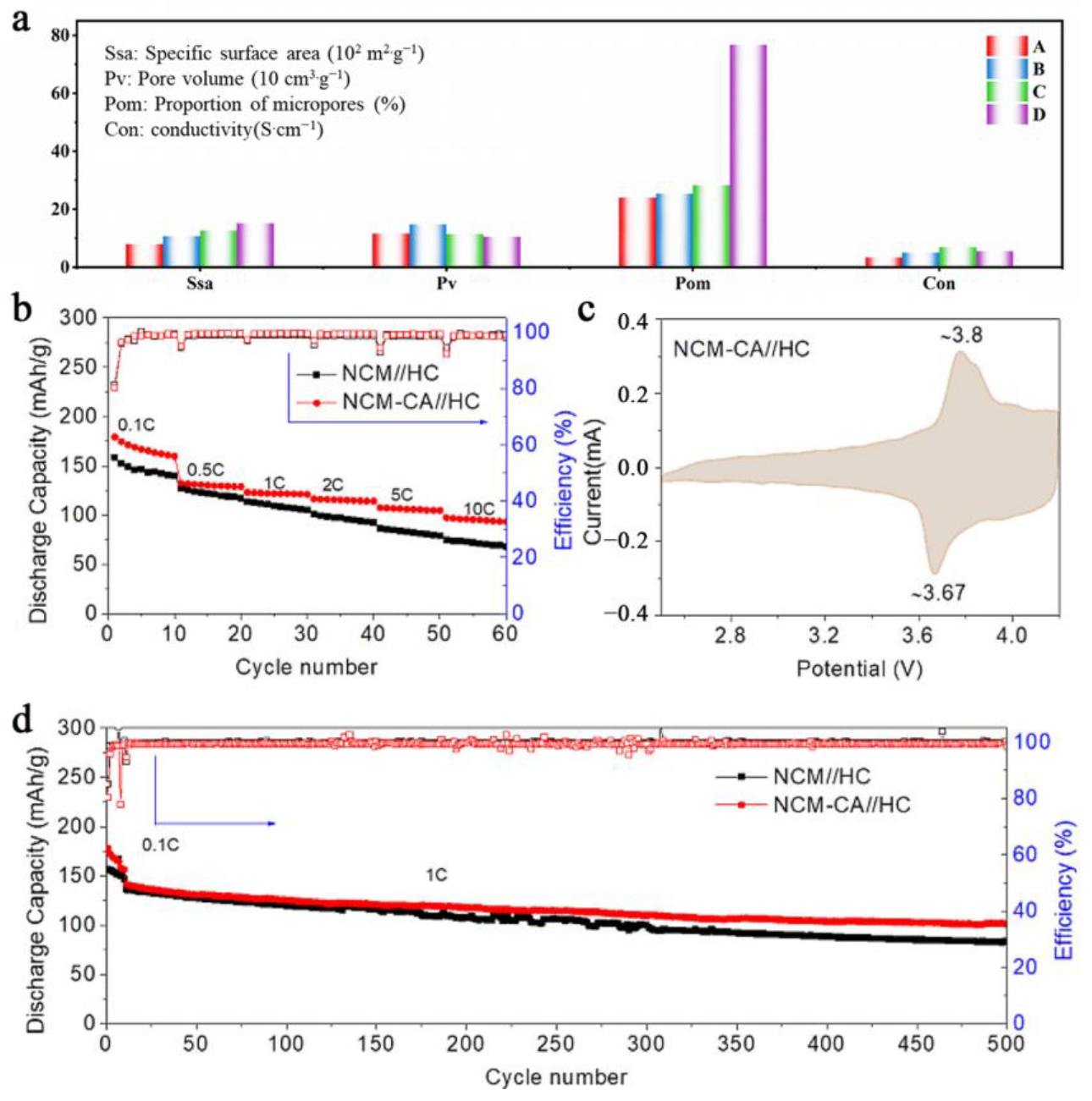
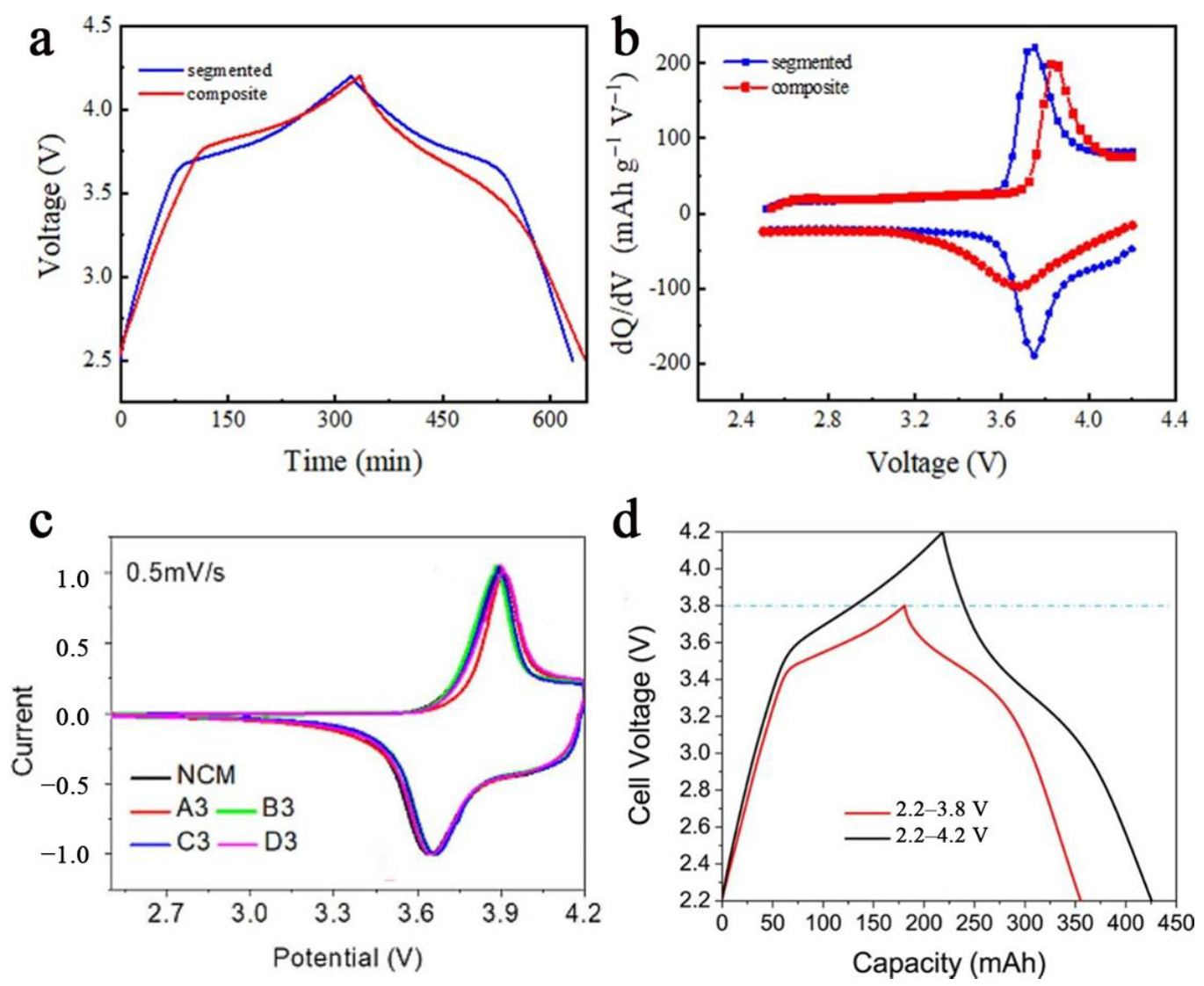
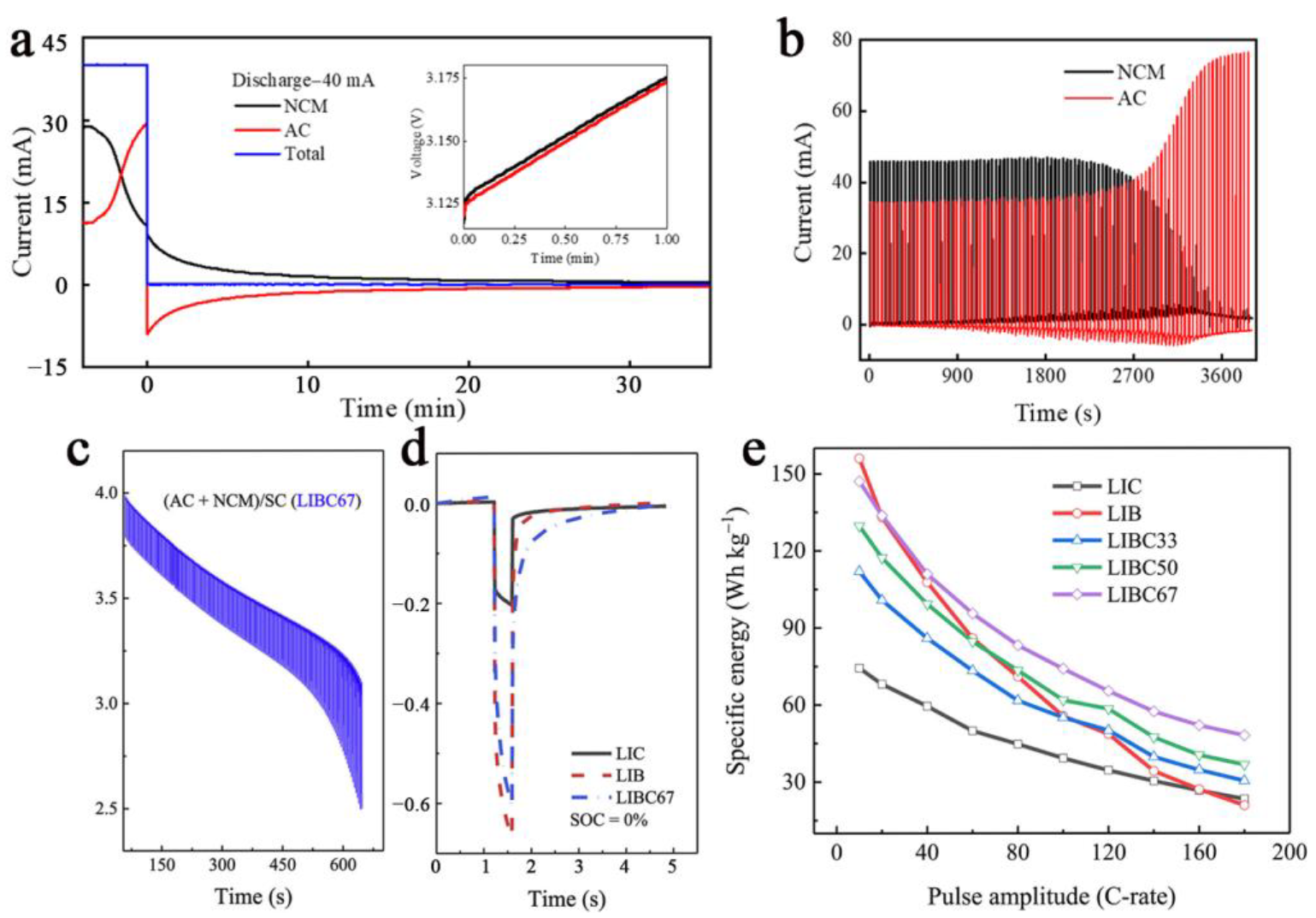

| Cell | Voltage/V | Scan Rate or C-Rate | Voltage Plateau Difference/mV | Cyclability | Ref. |
|---|---|---|---|---|---|
| (75% NCM523 + 25% AC)//graphite | 2.5–4.0 | 0.5 mV s−1 | 300 | / | [35] |
| (33% NCM111 + 67% AC)//Li | 2.5–4.2 | 0.2C | 280 | / | [61] |
| Segmented (33% NCM111 + 67% AC)//Li | 2.5–4.2 | 0.2C | 40 | / | [61] |
| Segmented (NCM111 + 67% AC)//pre-lithiated SC | 2.5–4.0 | / | / | More than 80% at 10C after 10,000 cycles | [61] |
| (96% NCM622 + 4% CA)//Li | 2.5–4.2 | 0.5 mV s−1 | 230 | 72.79% at 1C after 300 cycles | [62] |
| (96% NCM622 + 4% CA)//pre-lithiated HC | 2.5–4.2 | 0.5 mV s−1 | 130 | 71.6% at 0.1C after 500 cycles | [62] |
| (90% NCM111 + 6.7% graphene + 3.3% CNT)//Li | 2.5–4.6 | 0.1 mV s−1 | 440 | 93.8% at 1C after 50 cycles | [69] |
| Cathode | Anode | Voltage/V | Power Density/ (kW kg−1) | Energy Density/(Wh kg−1) | Ref. |
|---|---|---|---|---|---|
| 75% NCM523 + 25% AC | graphite | 2.5–4.0 | 2.38 | 36.2 | [35] |
| 75% NCM523 + 25% AC | HC | 2.5–4.2 | 6.5 | 66.6 | [45] |
| 25% NCM523 + 75% AC | pre-lithiated HC | 2.0–4.0 | 6.9 | 75.6 | [56] |
| 75% NCM523 + 25% AC | pre-lithiated HC | 2.2–3.8 | 4.2 | 20 | [94] |
| 60% NCM523 + 40% AC | pre-lithiated HC | 2.2–3.8 | 5 | 42 | [88] |
| 67% NCM111 + 33% AC | pre-lithiated SC | 2.5–4.0 | / | 173.3 | [61] |
| 90% NCM111 + 10% AC | AC | 0–2.8 | 7.989 | 42 | [46] |
| 12.5% NCM111 + 87.5% AC | LTO/MWCNTs | 1.5–2.8 | 0.993 | 52.2 | [51] |
| 96.25% NCM622 + 3.75% CA | pre-lithiated HC | 2.5–4.2 | / | 323.8 | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Liu, Z.; Chen, W.; Sun, X.; Zhang, X.; Wang, K.; Ma, Y. Battery-Type Lithium-Ion Hybrid Capacitors: Current Status and Future Perspectives. Batteries 2023, 9, 74. https://doi.org/10.3390/batteries9020074
Guo Z, Liu Z, Chen W, Sun X, Zhang X, Wang K, Ma Y. Battery-Type Lithium-Ion Hybrid Capacitors: Current Status and Future Perspectives. Batteries. 2023; 9(2):74. https://doi.org/10.3390/batteries9020074
Chicago/Turabian StyleGuo, Zhang, Zhien Liu, Wan Chen, Xianzhong Sun, Xiong Zhang, Kai Wang, and Yanwei Ma. 2023. "Battery-Type Lithium-Ion Hybrid Capacitors: Current Status and Future Perspectives" Batteries 9, no. 2: 74. https://doi.org/10.3390/batteries9020074






