Influence of the Thermal Treatment on the Structure and Cycle Life of Copper Hexacyanoferrate for Aqueous Zinc-Ion Batteries
Abstract
:1. Introduction
2. Experimental Methods
2.1. Material Synthesis
2.2. Electrochemical Characterization
2.3. Material Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Delucchi, M.A.; Jacobson, M.Z. Providing All Global Energy with Wind, Water, and Solar Power, Part II: Reliability, System and Transmission Costs, and Policies. Energy Policy 2011, 39, 1170–1190. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Delucchi, M.A. Providing All Global Energy with Wind, Water, and Solar Power, Part I: Technologies, Energy Resources, Quantities and Areas of Infrastructure, and Materials. Energy Policy 2011, 39, 1154–1169. [Google Scholar] [CrossRef]
- Barton, J.P.; Infield, D.G. Energy Storage and Its Use with Intermittent Renewable Energy. IEEE Trans. Energy Convers. 2004, 19, 441–448. [Google Scholar] [CrossRef]
- Zampardi, G.; la Mantia, F. Prussian Blue Analogues as Aqueous Zn-Ion Batteries Electrodes: Current Challenges and Future Perspectives. Curr. Opin. Electrochem. 2020, 21, 84–92. [Google Scholar] [CrossRef]
- Zampardi, G.; la Mantia, F. Open Challenges and Good Experimental Practices in the Research Field of Aqueous Zn-Ion Batteries. Nat. Commun. 2022, 13, 687. [Google Scholar] [CrossRef] [PubMed]
- Zampardi, G.; Warnecke, M.; Tribbia, M.; Glenneberg, J.; Santos, C.; la Mantia, F. Effect of the Reactants Concentration on the Synthesis and Cycle Life of Copper Hexacyanoferrate for Aqueous Zn-Ion Batteries. Electrochem. Commun. 2021, 126, 107030. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Towards High-Voltage Aqueous Metal-Ion Batteries beyond 1.5 V: The Zinc/Zinc Hexacyanoferrate System. Adv. Energy Mater. 2015, 5, 1400930. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Blay, V.; Galian, R.E.; Muresan, L.M.; Pankratov, D.; Pinyou, P.; Zampardi, G.; Blay, V.; Galian, R.E.; Muresan, L.M.; Pankratov, D.; et al. Research Frontiers in Energy-Related Materials and Applications for 2020–2030. Adv. Sustain. Syst. 2020, 4, 1900145. [Google Scholar] [CrossRef]
- Kim, D.S.; Yoo, H.; Park, M.S.; Kim, H. Boosting the Sodium Storage Capability of Prussian Blue Nanocubes by Overlaying PEDOT:PSS Layer. J. Alloys Compd. 2019, 791, 385–390. [Google Scholar] [CrossRef]
- Xu, L.; Ma, J.; Guo, P.; Su, C. PEDOT-PSS-Coated FeFe(CN)6 Composite Cathode for Lithium-Ion Batteries with the Improved Electrochemical Performances. Nano 2019, 14, 93–101. [Google Scholar] [CrossRef]
- Eckert, M.; Peters, W.; Drillet, J.F. Fast Microwave-Assisted Hydrothermal Synthesis of Pure Layered δ-MnO2 for Multivalent Ion Intercalation. Materials 2018, 11, 2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Xie, L.; Zhang, W.; Dai, Z.; Wei, W.; Luo, S.; Chen, X.; Chen, W.; Rao, F.; Wang, L.; et al. Conjugated System of PEDOT:PSS-Induced Self-Doped PANI for Flexible Zinc-Ion Batteries with Enhanced Capacity and Cyclability. ACS Appl. Mater. Interfaces 2019, 11, 30943–30952. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Guo, J.; Xia, C.; Wang, W.; Alshareef, H.N. Zinc-Ion Batteries: Materials, Mechanisms, and Applications. Mater. Sci. Eng. R Rep. 2019, 135, 58–84. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Ding, J.; Han, X.; Deng, Y.; Wu, T.; Amine, K.; Hu, W.; Zhong, C.; Lu, J. Understanding the Gap between Academic Research and Industrial Requirements in Rechargeable Zinc-Ion Batteries. Batter. Supercaps 2021, 4, 60–71. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.; Park, Y.; Choi, J.W. Aqueous Zinc Ion Batteries: Focus on Zinc Metal Anodes. Chem. Sci. 2020, 11, 2028–2044. [Google Scholar] [CrossRef] [Green Version]
- Oberholzer, P.; Tervoort, E.; Bouzid, A.; Pasquarello, A.; Kundu, D. Oxide versus Nonoxide Cathode Materials for Aqueous Zn Batteries: An Insight into the Charge Storage Mechanism and Consequences Thereof. ACS Appl. Mater. Interfaces 2019, 11, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Tribbia, M.; Glenneberg, J.; Zampardi, G.; la Mantia, F. Highly Efficient, Dendrite-Free Zinc Electrodeposition in Mild Aqueous Zinc-Ion Batteries through Indium-Based Substrates. Batter. Supercaps 2022, 5, e202100381. [Google Scholar] [CrossRef]
- Kasiri, G.; Glenneberg, J.; Kun, R.; Zampardi, G.; la Mantia, F. Microstructural Changes of Prussian Blue Derivatives during Cycling in Zinc-Containing Electrolytes. ChemElectroChem 2020, 7, 3301–3310. [Google Scholar] [CrossRef]
- Gupta, T.; Kim, A.; Phadke, S.; Biswas, S.; Luong, T.; Hertzberg, B.J.; Chamoun, M.; Evans-Lutterodt, K.; Steingart, D.A. Improving the Cycle Life of a High-Rate, High-Potential Aqueous Dual-Ion Battery Using Hyper-Dendritic Zinc and Copper Hexacyanoferrate. J. Power Sources 2016, 305, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Jayalakshmi, M.; Scholz, F. Charge-Discharge Characteristics of a Solid-State Prussian Blue Secondary Cell. J. Power Sources 2000, 87, 212–217. [Google Scholar] [CrossRef]
- Chen, R.; Huang, Y.; Xie, M.; Zhang, Q.; Zhang, X.; Li, L.; Wu, F. Preparation of Prussian Blue Submicron Particles with a Pore Structure by Two-Step Optimization for Na-Ion Battery Cathodes. ACS Appl. Mater. Interfaces 2016, 8, 16078–16086. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, M.; Scholz, F. Performance Characteristics of Zinc HexacyanoferraterPrussian Blue and Copper HexacyanoferraterPrussian Blue Solid State Secondary Cells. J. Power Sources 2000, 91, 217–223. [Google Scholar] [CrossRef]
- Widmann, A.; Kahlert, H.; Wulff, H.; Scholz, F. Electrochemical and Mechanochemical Formation of Solid Solutions of Potassium Copper(II)/Zinc(II) Hexacyanocobaltate(III)/Hexacyanoferrate(III) KCuxZn1−x[Hcc]x [Hcf]1−x. J. Solid State Electrochem. 2005, 9, 380–389. [Google Scholar] [CrossRef]
- Itaya, K.; Uchida, I.; Neff, V.D. Mixed-Valence Compounds; Theory and Applica-Tions in Chemistry; Nato Advanced Study Institutes Series D; Reidel: Doredrecht, The Netherlands, 1986; Volume 19, p. 4575. [Google Scholar]
- Soto, M.B.; Scholz, F. The Thermodynamics of the Insertion Electrochemistry of Solid Metal Hexacyanometallates. J. Electroanal. Chem. 2002, 521, 183–189. [Google Scholar] [CrossRef]
- Doménech, A.; Montoya, N.; Scholz, F. Estimation of Individual Gibbs Energies of Cation Transfer Employing the Insertion Electrochemistry of Solid Prussian Blue. J. Electroanal. Chem. 2011, 657, 117–122. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Tang, Y.; Bin Xu, B.; Liang, C.; Yan, M.; Jiang, Y. Manganese Hexacyanoferrate Reinforced by PEDOT Coating towards High-Rate and Long-Life Sodium-Ion Battery Cathode. J. Mater. Chem. A 2020, 8, 3222–3227. [Google Scholar] [CrossRef]
- Shkreba, E.V.; Apraksin, R.V.; Tolstopjatova, E.G.; Kondratiev, V.V. Cathode Material for Sodium-Ion Batteries Based on Manganese Hexacyanoferrate: The Role of the Binder Component. J. Solid State Electrochem. 2020, 24, 3049–3057. [Google Scholar] [CrossRef]
- Liu, S.; Pan, G.L.; Li, G.R.; Gao, X.P. Copper Hexacyanoferrate Nanoparticles as Cathode Material for Aqueous Al-Ion Batteries. J. Mater. Chem. A 2014, 3, 959–962. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, B.; Wang, Y. Copper Hexacyanoferrate with a Well-Defined Open Framework as a Positive Electrode for Aqueous Zinc Ion Batteries. Mater. Chem. Phys. 2015, 149–150, 601–606. [Google Scholar] [CrossRef]
- Xue, Q.; Li, L.; Huang, Y.; Huang, R.; Wu, F.; Chen, R. Polypyrrole-Modified Prussian Blue Cathode Material for Potassium Ion Batteries via in Situ Polymerization Coating. ACS Appl. Mater. Interfaces 2019, 11, 22339–22345. [Google Scholar] [CrossRef]
- Hong, S.F.; Chen, L.C. Nano-Prussian Blue Analogue/PEDOT:PSS Composites for Electrochromic Windows. Sol. Energy Mater. Sol. Cells 2012, 104, 64–74. [Google Scholar] [CrossRef]
- Kjeldgaard, S.; Wagemaker, M.; Iversen, B.B.; Bentien, A. Sample Dependent Performance of Aqueous Copper Hexacyanoferrate/Zinc Batteries. Mater. Adv. 2021, 2, 2036–2044. [Google Scholar] [CrossRef]
- Renman, V.; Ojwang, D.O.; Valvo, M.; Gómez, C.P.; Gustafsson, T.; Svensson, G. Structural-Electrochemical Relations in the Aqueous Copper Hexacyanoferrate-Zinc System Examined by Synchrotron X-Ray Diffraction. J. Power Sources 2017, 369, 146–153. [Google Scholar] [CrossRef]
- Zampardi, G.; Sokolov, S.V.; Batchelor-McAuley, C.; Compton, R.G. Potassium (De-)Insertion Processes in Prussian Blue Particles: Ensemble versus Single Nanoparticle Behaviour. Chem. A Eur. J. 2017, 23, 14338–14344. [Google Scholar] [CrossRef] [PubMed]
- Zampardi, G.; Compton, R.G. Fast Electrodeposition of Zinc onto Single Zinc Nanoparticles. J. Solid State Electrochem. 2020, 24, 2695–2702. [Google Scholar] [CrossRef] [Green Version]
- Ojwang, D.O.; Grins, J.; Wardecki, D.; Valvo, M.; Renman, V.; Häggström, L.; Ericsson, T.; Gustafsson, T.; Mahmoud, A.; Hermann, R.P.; et al. Structure Characterization and Properties of K-Containing Copper Hexacyanoferrate. Inorg. Chem. 2016, 55, 5924–5934. [Google Scholar] [CrossRef]
- Kuperman, N.; Cairns, A.; Goncher, G.; Solanki, R. Structural Water Enhanced Intercalation of Magnesium Ions in Copper Hexacyanoferrate Nonaqueous Batteries. Electrochim. Acta 2020, 362, 137077. [Google Scholar] [CrossRef]
- Görlin, M.; Ojwang, D.O.; Lee, M.T.; Renman, V.; Tai, C.W.; Valvo, M. Aging and Charge Compensation Effects of the Rechargeable Aqueous Zinc/Copper Hexacyanoferrate Battery Elucidated Using in Situ X-Ray Techniques. ACS Appl. Mater. Interfaces 2021, 13, 59962–59974. [Google Scholar] [CrossRef]
- Gerber, S.J.; Erasmus, E. Electronic Effects of Metal Hexacyanoferrates: An XPS and FTIR Study. Mater. Chem. Phys. 2018, 203, 73–81. [Google Scholar] [CrossRef]
- Ng, C.W.; Ding, J.; Shi, Y.; Gan, L.M. Structure and Magnetic Properties of Copper(II) Hexacyanoferrate(III) Compound. J. Phys. Chem. Solids 2001, 62, 767–775. [Google Scholar] [CrossRef]
- Trócoli, R.; la Mantia, F. An Aqueous Zinc-Ion Battery Based on Copper Hexacyanoferrate. ChemSusChem 2015, 8, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.P.; Massoni, N.; Grandjean, A. Tuning the Thermal Stability of Copper(II) Hexacyanoferrate(II) Nanoparticles. J. Therm. Anal. Calorim. 2020, 145, 2353–2362. [Google Scholar] [CrossRef]
- Shi, N.; Gao, Q.; Sanson, A.; Li, Q.; Fan, L.; Ren, Y.; Olivi, L.; Chen, J.; Xing, X. Negative Thermal Expansion in Cubic FeFe(CN)6 Prussian Blue Analogues. Dalton Trans. 2019, 48, 3658–3663. [Google Scholar] [CrossRef]
- Hu, J.; Tao, H.; Chen, M.; Zhang, Z.; Cao, S.; Shen, Y.; Jiang, K.; Zhou, M. Interstitial Water Improves Structural Stability of Iron Hexacyanoferrate for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 12234–12242. [Google Scholar] [CrossRef]
- Hesse, H.C.; Schimpe, M.; Kucevic, D.; Jossen, A. Lithium-Ion Battery Storage for the Grid—A Review of Stationary Battery Storage System Design Tailored for Applications in Modern Power Grids. Energies 2017, 10, 2107. [Google Scholar] [CrossRef] [Green Version]

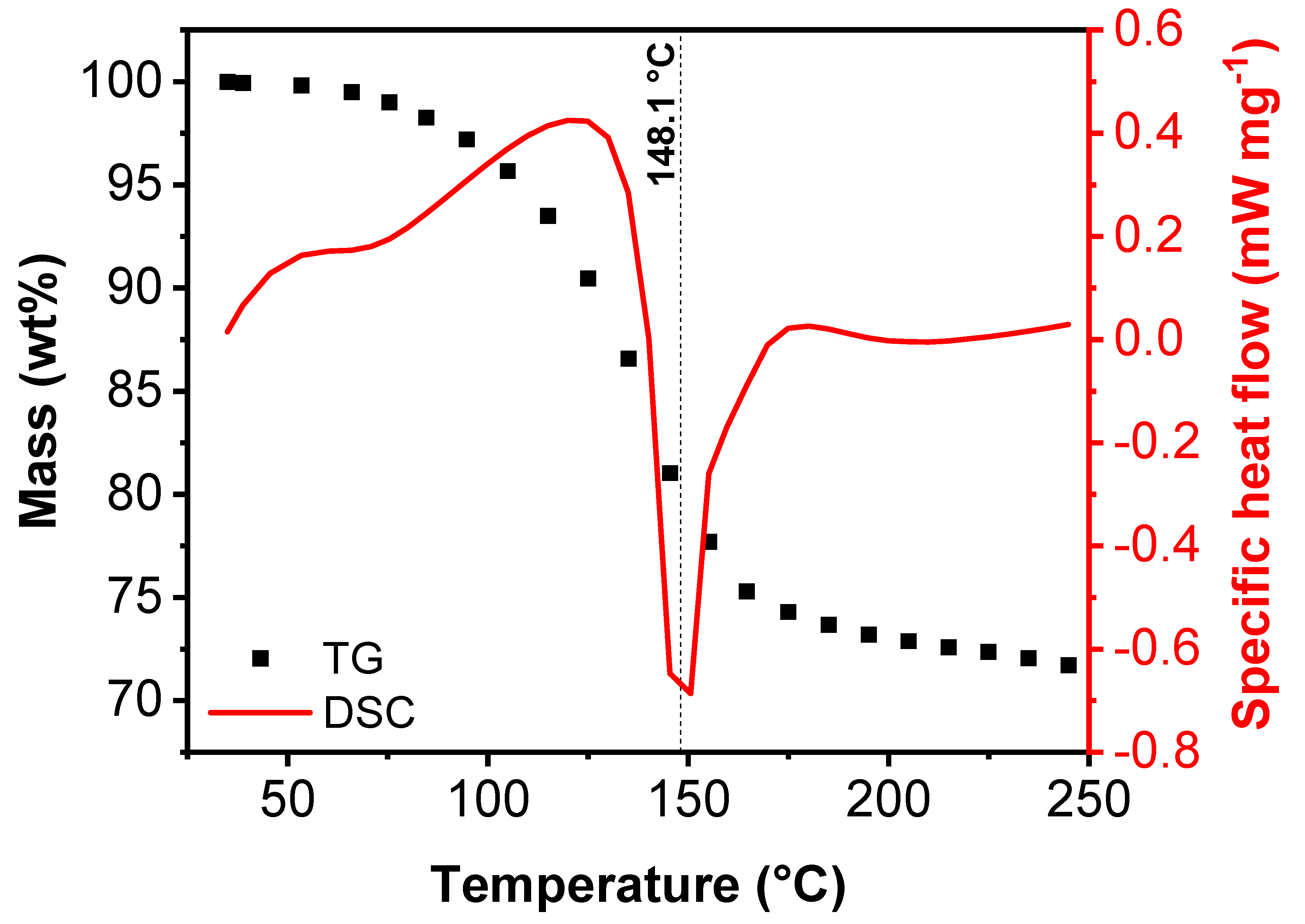
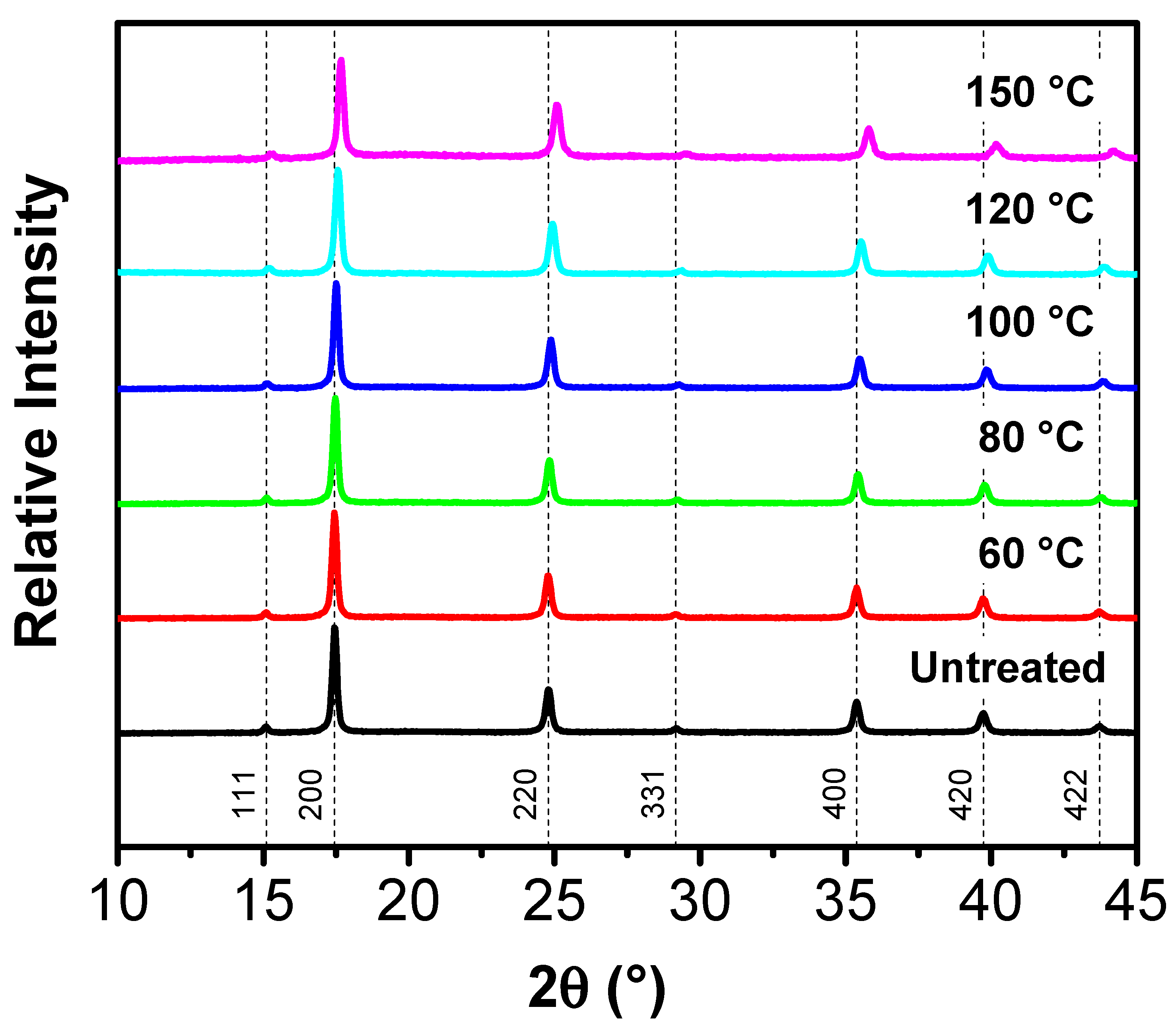



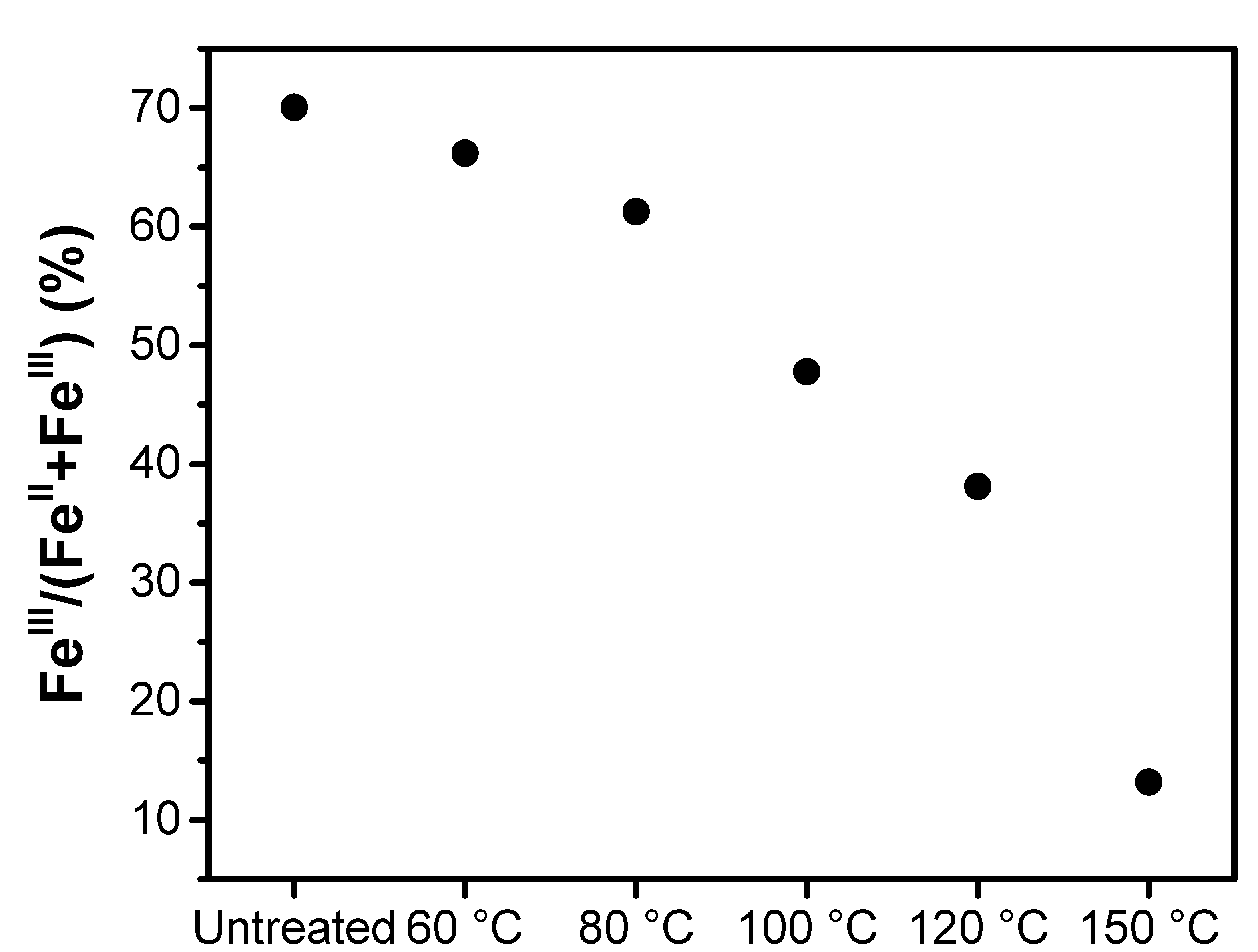
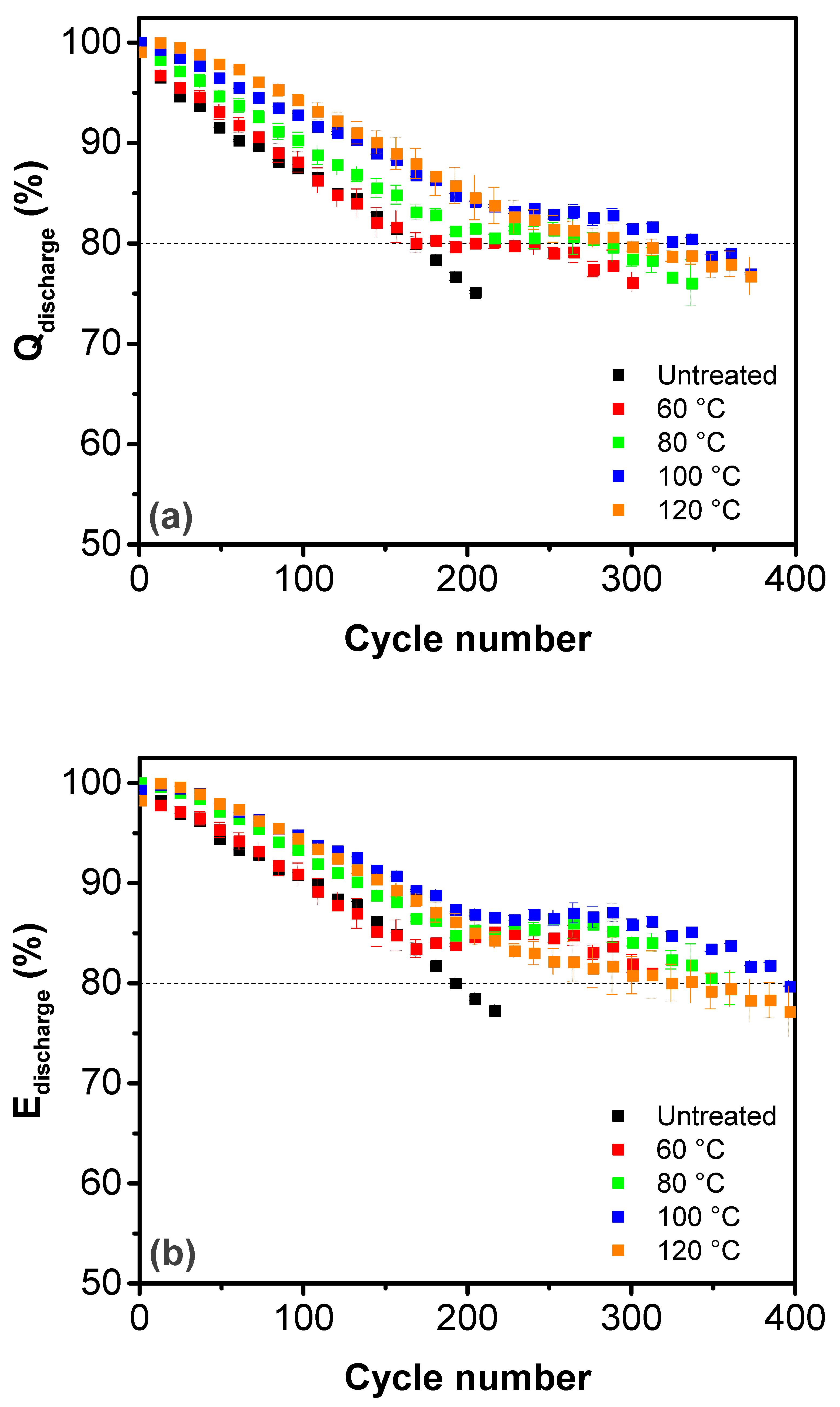
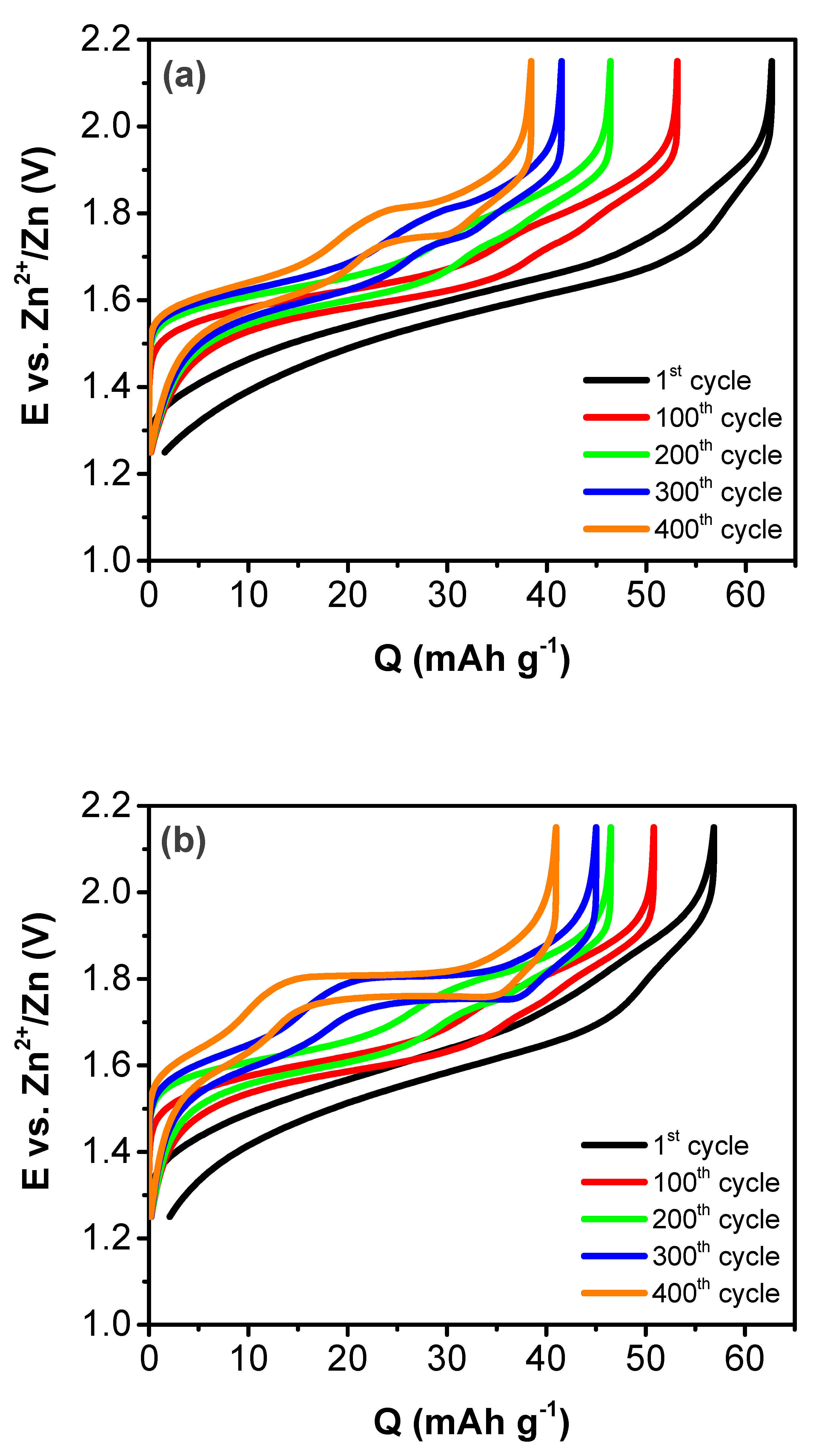
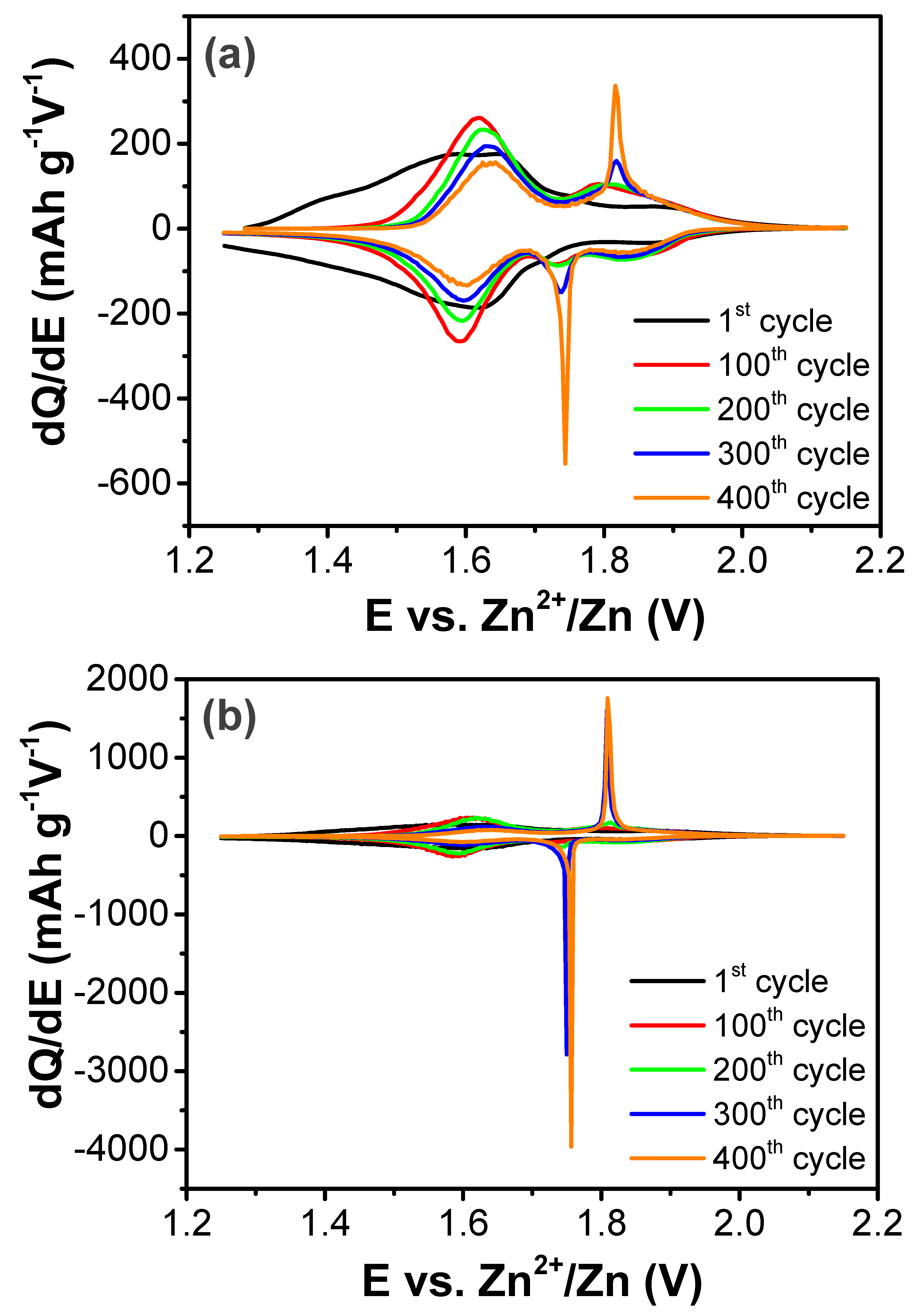
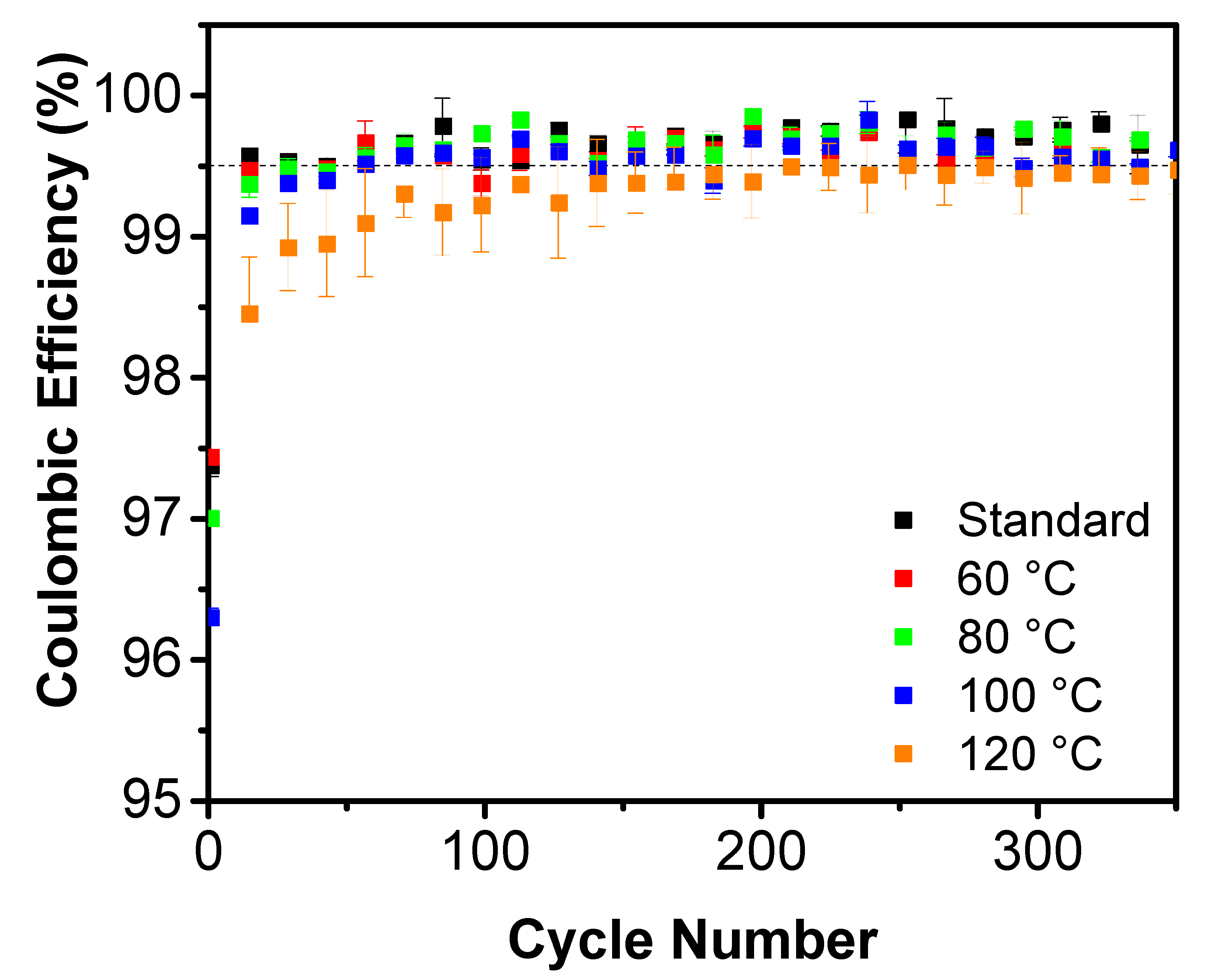
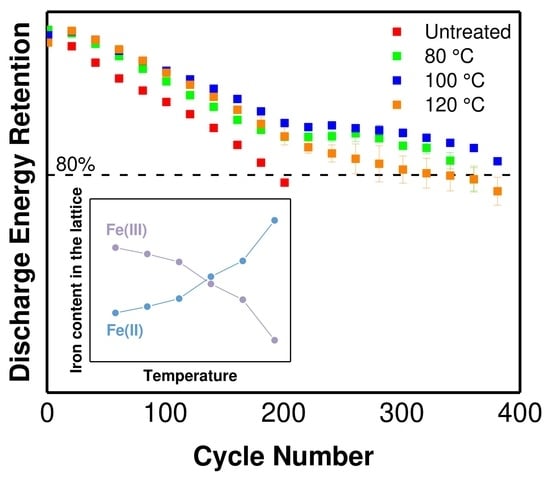
| Treatment Temperature (°C) | OCP (V) | Q0,charge (mAh g−1) | Q0,discharge (mAh g−1) | E0,discharge (mWh g−1) | Cycle Life Based on Qdischarge | Cycle Life Based on Edischarge |
|---|---|---|---|---|---|---|
| Untreated | 1.69 | 13.6 ± 1.3 | 58.1 ± 1.3 | 92.5 ± 2.1 | 170 | 190 |
| 60 | 1.67 | 19.6 ± 0.2 | 56.4 ± 0.2 | 89.6 ± 0.2 | 210 | 320 |
| 80 | 1.65 | 21.8 ± 0.2 | 54.6 ± 0.2 | 87.0 ± 0.4 | 280 | 350 |
| 100 | 1.62 | 29.4 ± 0.2 | 54.5 ± 0.2 | 87.4 ± 0.4 | 340 | 400 |
| 120 | 1.48 | 46.6 ± 1.9 | 49.8 ± 1.9 | 83.3 ± 0.8 | 290 | 340 |
| 150 | 1.32 | - | 15.2 ± 1.2 | 26.3 ± 2.1 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baghodrat, M.; Zampardi, G.; Glenneberg, J.; La Mantia, F. Influence of the Thermal Treatment on the Structure and Cycle Life of Copper Hexacyanoferrate for Aqueous Zinc-Ion Batteries. Batteries 2023, 9, 170. https://doi.org/10.3390/batteries9030170
Baghodrat M, Zampardi G, Glenneberg J, La Mantia F. Influence of the Thermal Treatment on the Structure and Cycle Life of Copper Hexacyanoferrate for Aqueous Zinc-Ion Batteries. Batteries. 2023; 9(3):170. https://doi.org/10.3390/batteries9030170
Chicago/Turabian StyleBaghodrat, Mohsen, Giorgia Zampardi, Jens Glenneberg, and Fabio La Mantia. 2023. "Influence of the Thermal Treatment on the Structure and Cycle Life of Copper Hexacyanoferrate for Aqueous Zinc-Ion Batteries" Batteries 9, no. 3: 170. https://doi.org/10.3390/batteries9030170
APA StyleBaghodrat, M., Zampardi, G., Glenneberg, J., & La Mantia, F. (2023). Influence of the Thermal Treatment on the Structure and Cycle Life of Copper Hexacyanoferrate for Aqueous Zinc-Ion Batteries. Batteries, 9(3), 170. https://doi.org/10.3390/batteries9030170