Electrolytes, Additives and Binders for NMC Cathodes in Li-Ion Batteries—A Review
Abstract
:1. Introduction
2. Electrolytes
- (1)
- The viscosity of electrolytes, which influences ionic mobility, should be low (<2 cP);
- (2)
- The high dielectric constant (>20) of solvents helps to dissociate salt;
- (3)
- Ionic conductivity should be high enough with a value greater than 1 mS cm−1;
- (4)
- A wide electrochemical stability window (0.01–5 V vs. Li/Li+) of operation;
- (5)
- Chemical and thermal stability in a wide range of voltage and temperatures;
- (6)
- (7)
- Environment friendliness, cost-effectiveness, and safety in operating conditions.
2.1. Non-Aqueous Solvents
2.1.1. Carbonate Solvents
2.1.2. Ether Solvents
2.1.3. Other Solvents
2.2. Lithium Salts
2.3. Dual-Salt-Based Electrolytes
2.4. Additives
2.5. Ionic Liquid Electrolytes
2.6. Solid-State Electrolytes
2.6.1. Inorganic Solid-State Electrolytes
2.6.2. Gel Polymer Electrolyte
2.6.3. Solid Polymer Electrolyte
3. Binder
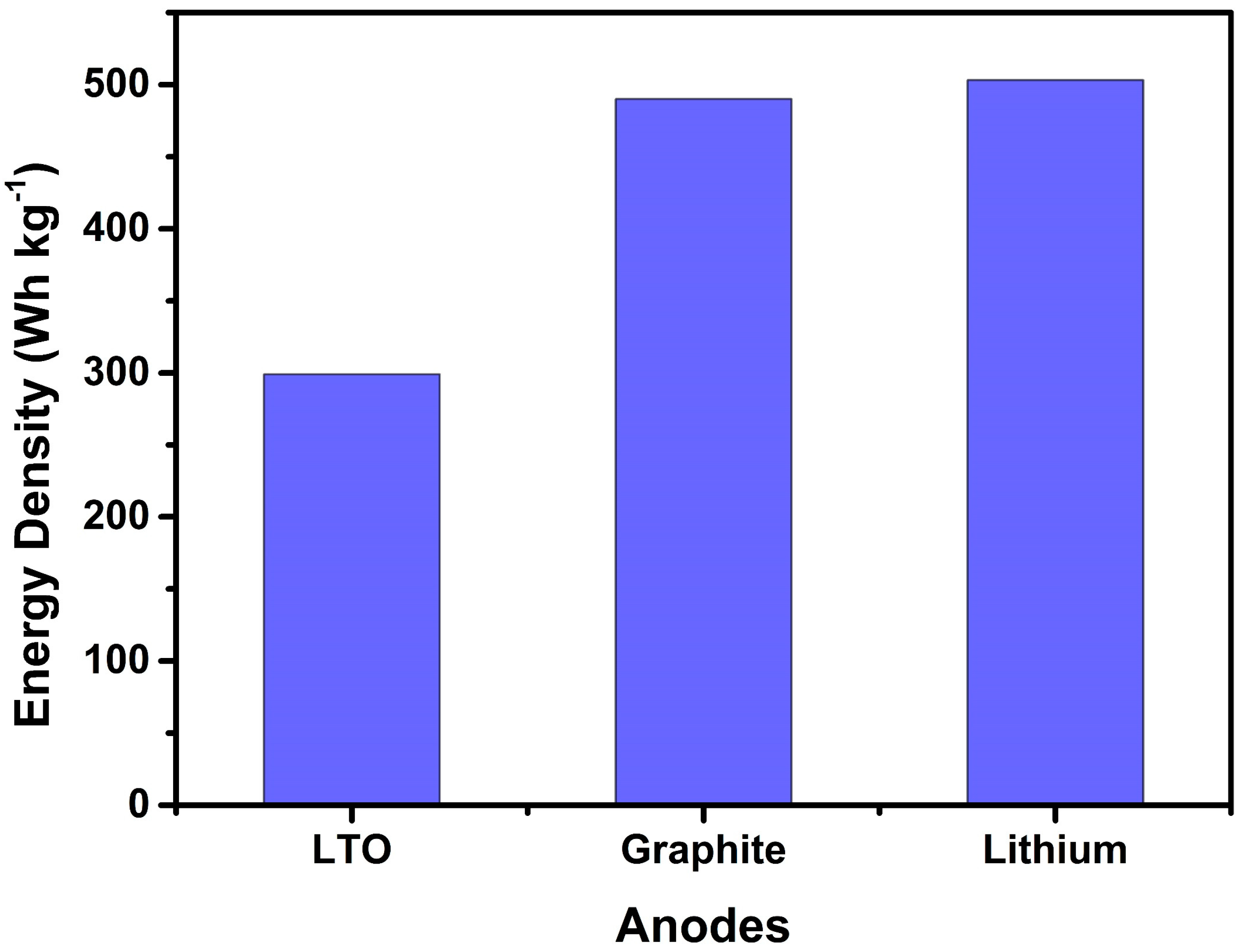
4. Anodes for NMC-Based Battery
5. NMC Compositions
5.1. LiNi1/3Mn1/3Co1/3O2 (NMC111)
5.2. LiNi0.4Mn0.4Co0.2O2 (NMC442)
5.3. LiNi0.5Mn0.3Co0.2O2 (NMC532)
5.4. LiNi0.6Mn0.2Co0.2O2 (NMC622)
5.5. LiNi0.8Mn0.1Co0.1O2 (NMC811)
6. Single-Crystal NMC
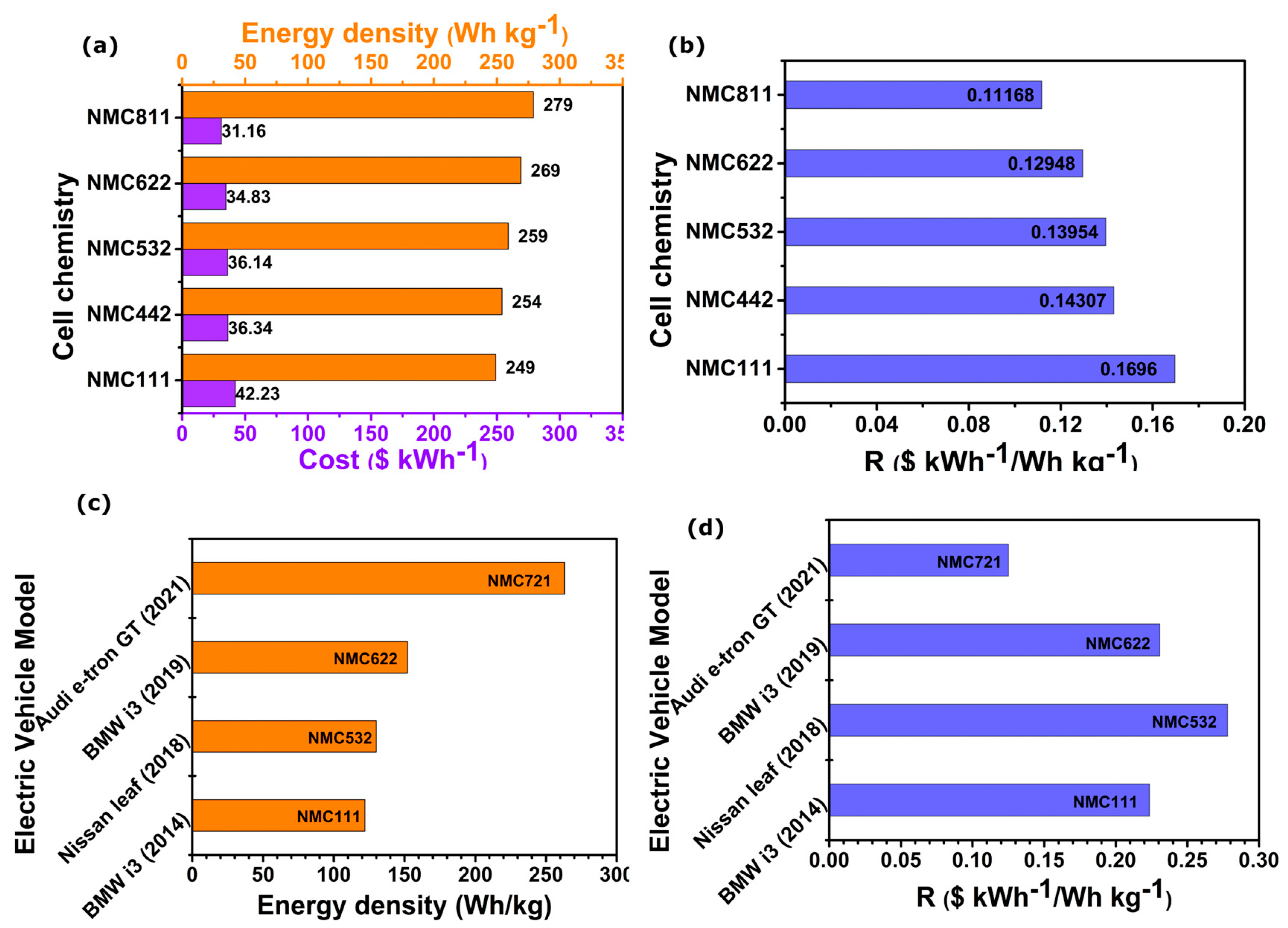
7. Commercial Aspects of NMC
8. Summary and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Z.; Yang, J.; Li, H.; Nuli, Y.; Wang, J. Electrolytes for Advanced Lithium Ion Batteries Using Silicon-Based Anodes. J. Mater. Chem. A 2019, 7, 9432–9446. [Google Scholar] [CrossRef]
- Morigaki, K.I. In Situ Analysis of the Interfacial Reactions between MCMB Electrode and Organic Electrolyte Solutions. J. Power Sources 2002, 103, 253–264. [Google Scholar] [CrossRef]
- Manthiram, A. A Reflection on Lithium-Ion Battery Cathode Chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, L.; Liu, Z.; Sun, Z.; Wu, T.; Gao, Y.; Li, H.; Li, F.; Niu, L. An Advanced Lithium Ion Battery Based on a High Quality Graphitic Graphene Anode and a Li[Ni0.6Co0.2Mn0.2]O2 Cathode. Electrochim. Acta 2018, 259, 48–55. [Google Scholar] [CrossRef]
- Gören, A.; Costa, C.M.; Silva, M.M.; Lanceros-Méndez, S. State of the Art and Open Questions on Cathode Preparation Based on Carbon Coated Lithium Iron Phosphate. Compos. Part B Eng. 2015, 83, 333–345. [Google Scholar] [CrossRef]
- Amine, K.; Liu, J.; Kang, S.; Belharouak, I.; Hyung, Y.; Vissers, D.; Henriksen, G. Improved Lithium Manganese Oxide Spinel/Graphite Li-Ion Cells for High-Power Applications. J. Power Sources 2004, 129, 14–19. [Google Scholar] [CrossRef]
- Xue, L.; Ueno, K.; Lee, S.Y.; Angell, C.A. Enhanced Performance of Sulfone-Based Electrolytes at Lithium Ion Battery Electrodes, Including the LiNi0.5Mn1.5O4 High Voltage Cathode. J. Power Sources 2014, 262, 123–128. [Google Scholar] [CrossRef]
- Malik, M.; Chan, K.H.; Azimi, G. Review on the Synthesis of LiNixMnyCo1-x-y O2 (NMC) Cathodes for Lithium-Ion Batteries. Mater. Today Energy 2022, 28, 101066. [Google Scholar] [CrossRef]
- Geldasa, F.T.; Kebede, M.A.; Shura, M.W.; Hone, F.G. Identifying Surface Degradation, Mechanical Failure, and Thermal Instability Phenomena of High Energy Density Ni-Rich NCM Cathode Materials for Lithiumion Batteries: A Review. RSC Adv. 2022, 12, 5891–5909. [Google Scholar] [CrossRef]
- Yan, W.; Yang, S.; Huang, Y.; Yang, Y.; Yuan, G. A Review on Doping/Coating of Nickel-Rich Cathode Materials for Lithium-Ion Batteries. J. Alloys Compd. 2020, 819, 153048. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation Mechanisms and Mitigation Strategies of Nickel-Rich NMC-Based Lithium-Ion Batteries; Springer: Singapore, 2020; Volume 3, ISBN 0123456789. [Google Scholar]
- Noh, H.J.; Youn, S.; Yoon, C.S.; Sun, Y.K. Comparison of the Structural and Electrochemical Properties of Layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) Cathode Material for Lithium-Ion Batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Tornheim, A.; Sharifi-Asl, S.; Garcia, J.C.; Bareño, J.; Iddir, H.; Shahbazian-Yassar, R.; Zhang, Z. Effect of Electrolyte Composition on Rock Salt Surface Degradation in NMC Cathodes during High-Voltage Potentiostatic Holds. Nano Energy 2019, 55, 216–225. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen Release and Its Effect on the Cycling Stability of LiNixMnyCozO2 (NMC) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Xie, Q.; You, Y.; Chi, M.; Manthiram, A. Long-Term Cyclability of NCM-811 at High Voltages in Lithium-Ion Batteries: An In-Depth Diagnostic Study. Chem. Mater. 2020, 32, 7796–7804. [Google Scholar] [CrossRef]
- Sun, H.H.; Manthiram, A. Impact of Microcrack Generation and Surface Degradation on a Nickel-Rich Layered Li[Ni0.9Co0.05Mn0.05]O2 Cathode for Lithium-Ion Batteries. Chem. Mater. 2017, 29, 8486–8493. [Google Scholar] [CrossRef]
- Jung, R.; Linsenmann, F.; Thomas, R.; Wandt, J.; Solchenbach, S.; Maglia, F.; Stinner, C.; Tromp, M.; Gasteiger, H.A. Nickel, Manganese, and Cobalt Dissolution from Ni-Rich NMC and Their Effects on NMC622-Graphite Cells. J. Electrochem. Soc. 2019, 166, A378–A389. [Google Scholar] [CrossRef] [Green Version]
- Tatara, R.; Yu, Y.; Karayaylali, P.; Chan, A.K.; Zhang, Y.; Jung, R.; Maglia, F.; Giordano, L.; Shao-Horn, Y. Enhanced Cycling Performance of Ni-Rich Positive Electrodes (NMC) in Li-Ion Batteries by Reducing Electrolyte Free-Solvent Activity. ACS Appl. Mater. Interfaces 2019, 11, 34973–34988. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Fukunaga, T.; Hazama, T.; Terasaki, M.; Mizutani, M.; Yamachi, M. Degradation Mechanism of Alkyl Carbonate Solvents Used in Lithium-Ion Cells during Initial Charging. J. Power Sources 1997, 68, 311–315. [Google Scholar] [CrossRef]
- Laszczynski, N.; Solchenbach, S.; Gasteiger, H.A.; Lucht, B.L. Understanding Electrolyte Decomposition of Graphite/NCM811 Cells at Elevated Operating Voltage. J. Electrochem. Soc. 2019, 166, A1853–A1859. [Google Scholar] [CrossRef]
- Strehle, B.; Solchenbach, S.; Metzger, M.; Schwenke, K.U.; Gasteiger, H.A. The Effect of CO2 on Alkyl Carbonate Trans-Esterification during Formation of Graphite Electrodes in Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A2513–A2526. [Google Scholar] [CrossRef]
- Xia, J.; Petibon, R.; Xiong, D.; Ma, L.; Dahn, J.R. Enabling Linear Alkyl Carbonate Electrolytes for High Voltage Li-Ion Cells. J. Power Sources 2016, 328, 124–135. [Google Scholar] [CrossRef]
- Xiong, D.J.; Bauer, M.; Ellis, L.D.; Hynes, T.; Hyatt, S.; Hall, D.S.; Dahn, J.R. Some Physical Properties of Ethylene Carbonate-Free Electrolytes. J. Electrochem. Soc. 2018, 165, A126–A131. [Google Scholar] [CrossRef]
- Logan, E.R.; Tonita, E.M.; Gering, K.L.; Ma, L.; Bauer, M.K.G.; Li, J.; Beaulieu, L.Y.; Dahn, J.R. A Study of the Transport Properties of Ethylene Carbonate-Free Li Electrolytes. J. Electrochem. Soc. 2018, 165, A705–A716. [Google Scholar] [CrossRef]
- Aurbach, D. Nonaqueous Electrochemistry; CRC Press: Boca Raton, FL, USA, 1999; ISBN 0824773349. [Google Scholar]
- Lee, J.; Kim, Y.J.; Jin, H.S.; Noh, H.; Kwack, H.; Chu, H.; Ye, F.; Lee, H.; Kim, H.T. Tuning Two Interfaces with Fluoroethylene Carbonate Electrolytes for High-Performance Li/LCO Batteries. ACS Omega 2019, 4, 3220–3227. [Google Scholar] [CrossRef] [PubMed]
- Flamme, B.; Rodriguez Garcia, G.; Weil, M.; Haddad, M.; Phansavath, P.; Ratovelomanana-Vidal, V.; Chagnes, A. Guidelines to Design Organic Electrolytes for Lithium-Ion Batteries: Environmental Impact, Physicochemical and Electrochemical Properties. Green Chem. 2017, 19, 1828–1849. [Google Scholar] [CrossRef]
- Koch, V.R.; Goldman, J.L.; Mattos, C.J.; Mulvaney, M. Specular Lithium Deposits from Lithium Hexafluoroarsenate/Diethyl Ether Electrolytes. J. Electrochem. Soc. 1982, 129, 1. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Jiao, S.; Mei, D.; Engelhard, M.H.; Li, Q.; Lee, H.; Niu, C.; Adams, B.D.; Wang, C.; et al. High-Concentration Ether Electrolytes for Stable High-Voltage Lithium Metal Batteries. ACS Energy Lett. 2019, 4, 896–902. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Cao, X.; Engelhard, M.H.; Liu, W.; Burton, S.D.; Lee, H.; Niu, C.; Matthews, B.E.; Zhu, Z.; et al. Enabling High-Voltage Lithium-Metal Batteries under Practical Conditions. Joule 2019, 3, 1662–1676. [Google Scholar] [CrossRef]
- Yamada, Y.; Yaegashi, M.; Abe, T.; Yamada, A. A Superconcentrated Ether Electrolyte for Fast-Charging Li-Ion Batteries. Chem. Commun. 2013, 49, 11194–11196. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Xu, Y.; Burton, S.D.; Gao, P.; Zhang, X.; Matthews, B.E.; Engelhard, M.H.; Zhong, L.; Bowden, M.E.; Xiao, B.; et al. Enabling Ether-Based Electrolytes for Long Cycle Life of Lithium-Ion Batteries at High Charge Voltage. ACS Appl. Mater. Interfaces 2020, 12, 54893–54903. [Google Scholar] [CrossRef]
- Amanchukwu, C.V.; Yu, Z.; Kong, X.; Qin, J.; Cui, Y.; Bao, Z. A New Class of Ionically Conducting Fluorinated Ether Electrolytes with High Electrochemical Stability. J. Am. Chem. Soc. 2020, 142, 7393–7403. [Google Scholar] [CrossRef] [PubMed]
- Dalavi, S.; Xu, M.; Ravdel, B.; Zhou, L.; Lucht, B.L. Nonflammable Electrolytes for Lithium-Ion Batteries Containing Dimethyl Methylphosphonate. J. Electrochem. Soc. 2010, 157, A1113. [Google Scholar] [CrossRef]
- Jin, Z.; Wu, L.; Song, Z.; Yan, K.; Zhan, H.; Li, Z. A New Class of Phosphates as Co-Solvents for Nonflammable Lithium Ion Batteries Electrolytes. ECS Electrochem. Lett. 2012, 1, 55–58. [Google Scholar] [CrossRef]
- Xu, M.; Lu, D.; Garsuch, A.; Lucht, B.L. Improved Performance of LiNi0.5Mn1.5O4 Cathodes with Electrolytes Containing Dimethylmethylphosphonate (DMMP). J. Electrochem. Soc. 2012, 159, A2130–A2134. [Google Scholar] [CrossRef]
- Von Aspern, N.; Leissing, M.; Christian Wölke, D.D.; Takeshi Kobayashi, M.B.; Stubbmann-Kazakova, O.; Kozel, V.; Röschenthaler, G.-V.; Smiatek, J.; Sascha Nowak, M.W.; Cekic-Laskovic, I. Non-Flammable Fluorinated Phosphorus III-Based Electrolytes for Advanced Lithium-Ion Battery Performance. ChemElectroChem 2020, 7, 1499–1508. [Google Scholar] [CrossRef]
- Gu, Y.; Fang, S.; Yang, L.; Hirano, S.I. Tris (2,2,2-Trifluoroethyl) Phosphate as a Cosolvent for a Nonflammable Electrolyte in Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 4919–4927. [Google Scholar] [CrossRef]
- Wu, W.; Bai, Y.; Wang, X.; Wu, C. Sulfone-Based High-Voltage Electrolytes for High Energy Density Rechargeable Lithium Batteries: Progress and Perspective. Chin. Chem. Lett. 2020, 32, 1309–1315. [Google Scholar] [CrossRef]
- Flamme, B.; Haddad, M.; Phansavath, P.; Ratovelomanana-Vidal, V.; Chagnes, A. Anodic Stability of New Sulfone-Based Electrolytes for Lithium-Ion Batteries. ChemElectroChem 2018, 5, 2279–2287. [Google Scholar] [CrossRef]
- Flamme, B.; Światowska, J.; Haddad, M.; Phansavath, P.; Ratovelomanana-Vidal, V.; Chagnes, A. Sulfone Based-Electrolytes for Lithium-Ion Batteries: Cycling Performances and Passivation Layer Quality of Graphite and LiNi1/3Mn1/3Co1/3O2 Electrodes. J. Electrochem. Soc. 2020, 167, 070508. [Google Scholar] [CrossRef]
- Su, C.C.; He, M.; Redfern, P.C.; Curtiss, L.A.; Shkrob, I.A.; Zhang, Z. Oxidatively Stable Fluorinated Sulfone Electrolytes for High Voltage High Energy Lithium-Ion Batteries. Energy Environ. Sci. 2017, 10, 900–904. [Google Scholar] [CrossRef]
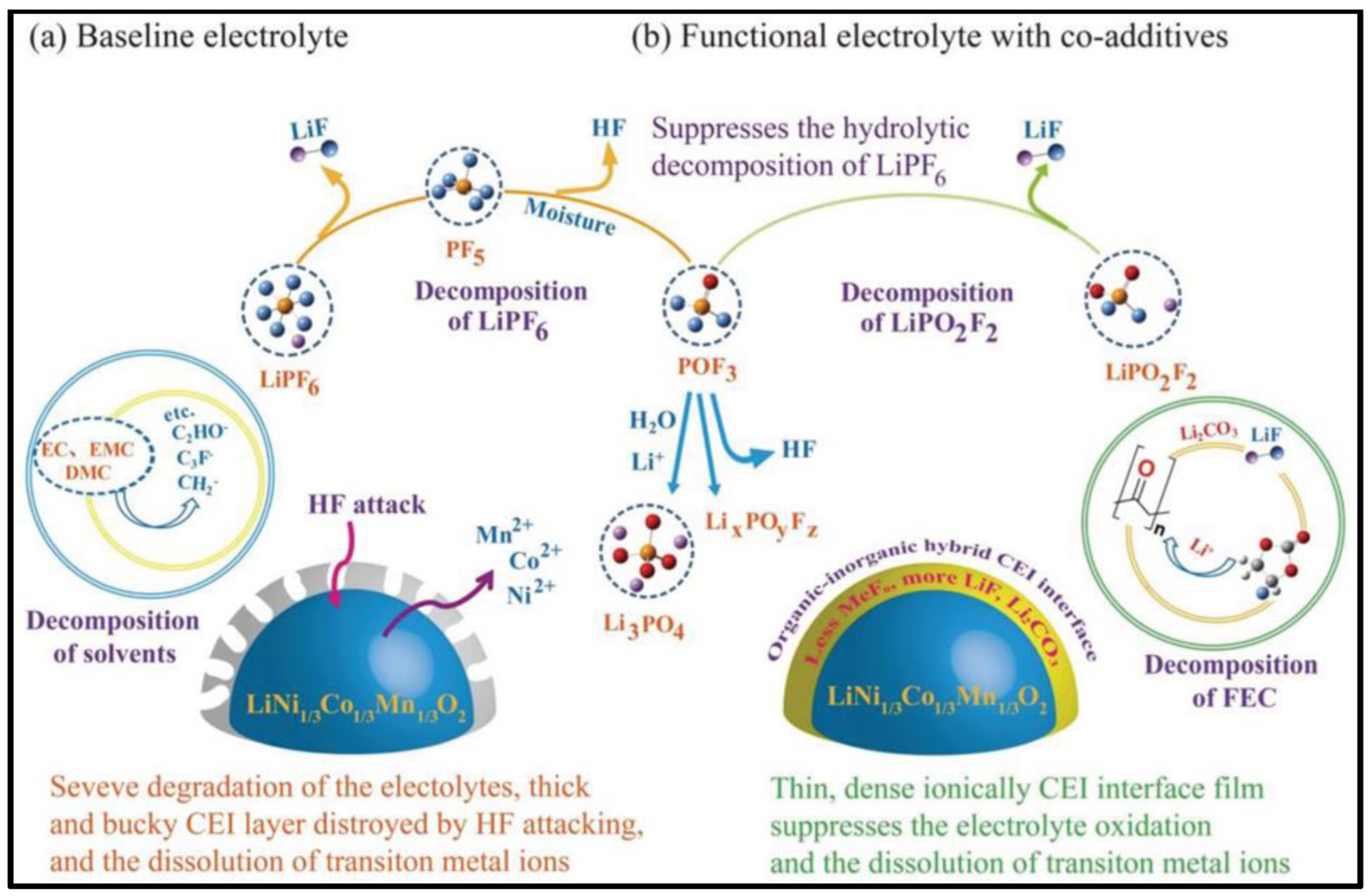
- Lux, S.F.; Chevalier, J.; Lucas, I.T.; Kostecki, R. HF Formation in LiPF6-Based Organic Carbonate Electrolytes. ECS Electrochem. Lett. 2013, 2, 2013–2016. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, W.; Zhang, Q.; Yang, G.; Zheng, J.; Tang, W.; Xu, Q.; Lai, C.; Yang, J.; Peng, C. Armoring LiNi1/3Co1/3Mn1/3O2 Cathode with Reliable Fluorinated Organic–Inorganic Hybrid Interphase Layer toward Durable High Rate Battery. Adv. Funct. Mater. 2020, 30, 2000396. [Google Scholar] [CrossRef]
- Lux, S.F.; Lucas, I.T.; Pollak, E.; Passerini, S.; Winter, M.; Kostecki, R. The Mechanism of HF Formation in LiPF6 Based Organic Carbonate Electrolytes. Electrochem. Commun. 2012, 14, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Dalavi, S.; Xu, M.; Knight, B.; Lucht, B.L. Effect of Added LiBOB on High Voltage (LiNi0.5Mn1.5O4) Spinel Cathodes. Electrochem. Solid-State Lett. 2012, 15, 28–31. [Google Scholar] [CrossRef]
- Reiter, J.; Nádherná, M.; Dominko, R. Graphite and LiCo1/3Mn1/3Ni1/3O2 Electrodes with Piperidinium Ionic Liquid and Lithium Bis(Fluorosulfonyl)Imide for Li-Ion Batteries. J. Power Sources 2012, 205, 402–407. [Google Scholar] [CrossRef]
- Xu, C.; Hernández, G.; Abbrent, S.; Kobera, L.; Konefal, R.; Brus, J.; Edström, K.; Brandell, D.; Mindemark, J. Unraveling and Mitigating the Storage Instability of Fluoroethylene Carbonate-Containing LiPF6 Electrolytes to Stabilize Lithium Metal Anodes for High-Temperature Rechargeable Batteries. ACS Appl. Energy Mater. 2019, 2, 4925–4935. [Google Scholar] [CrossRef]
- Shui Zhang, S. An Unique Lithium Salt for the Improved Electrolyte of Li-Ion Battery. Electrochem. Commun. 2006, 8, 1423–1428. [Google Scholar] [CrossRef]
- Gu, Y.; Fang, S.; Yang, L.; Hirano, S. ichi A Safe Electrolyte for High-Performance Lithium-Ion Batteries Containing Lithium Difluoro(Oxalato)Borate, Gamma-Butyrolactone and Non-Flammable Hydrofluoroether. Electrochim. Acta 2021, 394, 139120. [Google Scholar] [CrossRef]
- Xiang, H.; Shi, P.; Bhattacharya, P.; Chen, X.; Mei, D.; Bowden, M.E.; Zheng, J.; Zhang, J.G.; Xu, W. Enhanced Charging Capability of Lithium Metal Batteries Based on Lithium Bis(Trifluoromethanesulfonyl)Imide-Lithium Bis(Oxalato)Borate Dual-Salt Electrolytes. J. Power Sources 2016, 318, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zheng, J.; Engelhard, M.H.; Mei, D.; Li, Q.; Jiao, S.; Liu, N.; Zhao, W.; Zhang, J.G.; Xu, W. Effects of Imide-Orthoborate Dual-Salt Mixtures in Organic Carbonate Electrolytes on the Stability of Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2018, 10, 2469–2479. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Engelhard, M.H.; Mei, D.; Jiao, S.; Polzin, B.J.; Zhang, J.G.; Xu, W. Electrolyte Additive Enabled Fast Charging and Stable Cycling Lithium Metal Batteries. Nat. Energy 2017, 2, 17012. [Google Scholar] [CrossRef]
- Jiao, S.; Zheng, J.; Li, Q.; Li, X.; Engelhard, M.H.; Cao, R.; Zhang, J.G.; Xu, W. Behavior of Lithium Metal Anodes under Various Capacity Utilization and High Current Density in Lithium Metal Batteries. Joule 2018, 2, 110–124. [Google Scholar] [CrossRef] [Green Version]
- Jiao, S.; Ren, X.; Cao, R.; Engelhard, M.H.; Liu, Y.; Hu, D.; Mei, D.; Zheng, J.; Zhao, W.; Li, Q.; et al. Stable Cycling of High-Voltage Lithium Metal Batteries in Ether Electrolytes. Nat. Energy 2018, 3, 739–746. [Google Scholar] [CrossRef]
- Aurbach, D.; Gamolsky, K.; Markovsky, B.; Gofer, Y.; Schmidt, M.; Heider, U. On the Use of Vinylene Carbonate (VC) as an Additive to Electrolyte Solutions for Li-Ion Batteries. Electrochim. Acta 2002, 47, 1423–1439. [Google Scholar] [CrossRef]
- Burns, J.C.; Electrochem, J.; Soc, A.; Burns, J.C.; Petibon, R.; Nelson, K.J.; Sinha, N.N.; Kassam, A.; Way, B.M.; Dahn, J.R. Studies of the Effect of Varying Vinylene Carbonate (VC) Content in Lithium Ion Cells on Cycling Performance and Cell Impedance. J. Electrochem. Soc. 2013, 160, A1668. [Google Scholar] [CrossRef]
- Liu, Y.; Takeda, S.; Kaneko, I.; Yoshitake, H.; Mukai, T.; Yanagida, M.; Saito, Y.; Sakai, T. Understanding the Improved High-Temperature Cycling Stability of Comprehensive Analysis Approach Utilizing LC-MS and DART-MS. J. Phys. Chem. C 2018, 122, 5864–5870. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Cheng, X.B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene Carbonate Additives to Render Uniform Li Deposits in Lithium Metal Batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Salitra, G.; Markevich, E.; Afri, M.; Talyosef, Y.; Hartmann, P.; Kulisch, J.; Sun, Y.K.; Aurbach, D. High-Performance Cells Containing Lithium Metal Anodes, LiNi0.6Co0.2Mn0.2O2 (NCM 622) Cathodes, and Fluoroethylene Carbonate-Based Electrolyte Solution with Practical Loading. ACS Appl. Mater. Interfaces 2018, 10, 19773–19782. [Google Scholar] [CrossRef]
- Im, J.; Lee, J.; Ryou, M.-H.; Lee, Y.M.; Cho, K.Y. Fluorinated Carbonate-Based Electrolyte for High-Voltage Li(Ni0.5Mn0.3Co 0.2)O2/Graphite Lithium-Ion Battery. J. Electrochem. Soc. 2017, 164, A6381–A6385. [Google Scholar] [CrossRef]
- Mai, S.; Xu, M.; Liao, X.; Hu, J.; Lin, H.; Xing, L.; Liao, Y.; Li, X.; Li, W. Tris (Trimethylsilyl) Phosphite as Electrolyte Additive for High Voltage Layered Lithium Nickel Cobalt Manganese Oxide Cathode of Lithium Ion Battery. Electrochim. Acta 2014, 147, 565–571. [Google Scholar] [CrossRef]
- Wang, D.Y.; Xia, J.; Ma, L.; Nelson, K.J.; Harlow, J.E.; Xiong, D.; Downie, L.E.; Petibon, R.; Burns, J.C.; Xiao, A.; et al. A Systematic Study of Electrolyte Additives in Li[Ni1/3Mn1/3Co1/3]O2 (NMC)/Graphite Pouch Cells. J. Electrochem. Soc. 2014, 161, A1818–A1827. [Google Scholar] [CrossRef]
- Sinha, N.N.; Burns, J.C.; Dahn, J.R. Comparative Study of Tris (Trimethylsilyl) Phosphate and Tris (Trimethylsilyl) Phosphite as Electrolyte Additives for Li-Ion Cells. J. Electrochem. Soc. 2014, 161, A1084–A1089. [Google Scholar] [CrossRef]
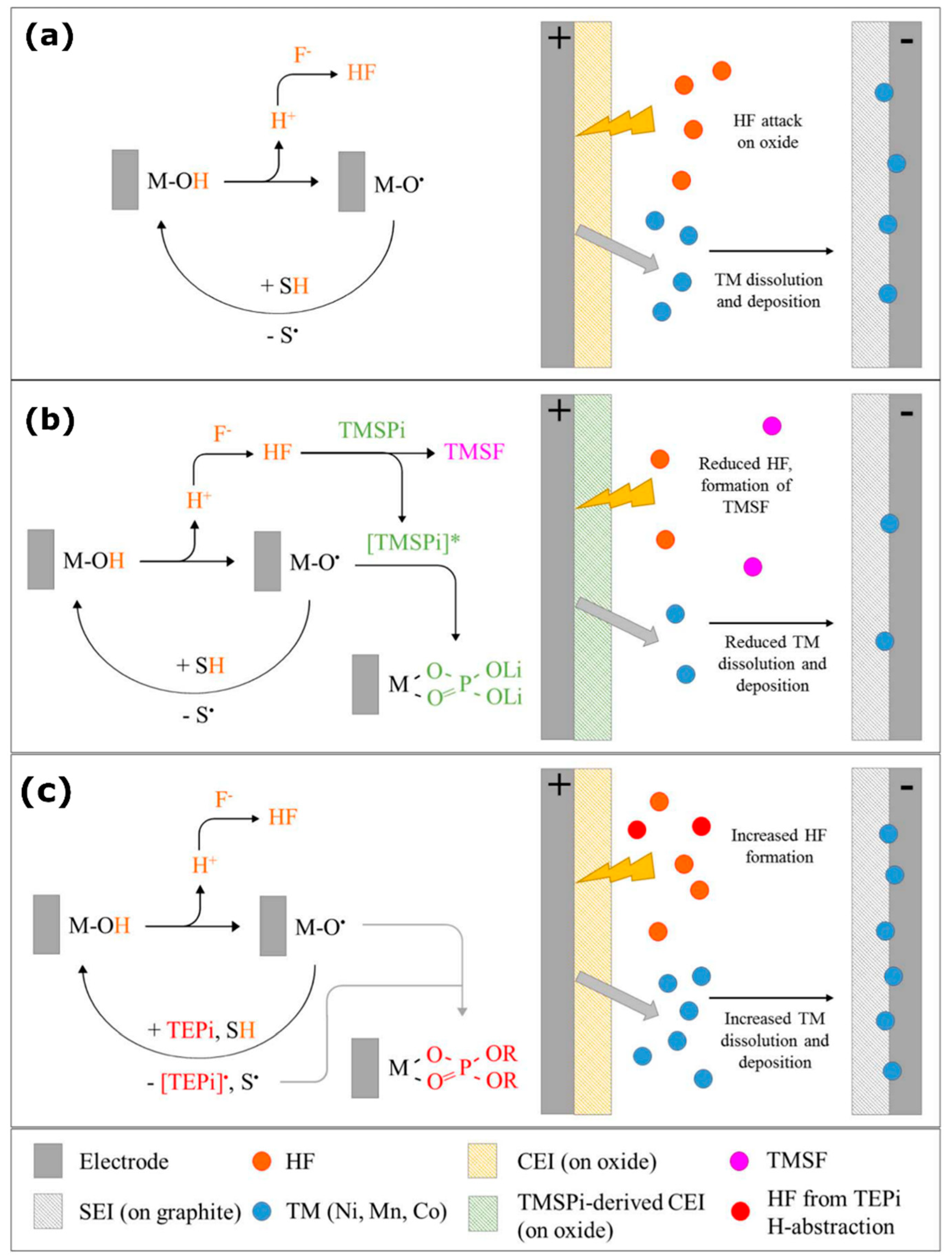
- Laveda, J.V.; Low, J.E.; Pagani, F.; Stilp, E.; Dilger, S.; Baran, V.; Heere, M.; Battaglia, C. Stabilizing Capacity Retention in NMC811/Graphite Full Cells via TMSPi Electrolyte Additives. ACS Appl. Energy Mater. 2019, 2, 7036–7044. [Google Scholar] [CrossRef]
- Peebles, C.; Sahore, R.; Gilbert, J.A.; Garcia, J.C.; Tornheim, A.; Bareño, J.; Iddir, H.; Liao, C.; Abraham, D.P. Tris (Trimethylsilyl) Phosphite (TMSPi) and Triethyl Phosphite (TEPi) as Electrolyte Additives for Lithium Ion Batteries: Mechanistic Insights into Differences during LiNi0.5Mn0.3Co0.2O2-Graphite Full Cell Cycling. J. Electrochem. Soc. 2017, 164, A1579–A1586. [Google Scholar] [CrossRef]
- Xia, J.; Sinha, N.N.; Chen, L.P.; Dahn, J.R. A Comparative Study of a Family of Sulfate Electrolyte Additives. J. Electrochem. Soc. 2014, 161, A264–A274. [Google Scholar] [CrossRef]
- Xia, J.; Ma, L.; Aiken, C.P.; Nelson, K.J.; Chen, L.P.; Dahn, J.R. Comparative Study on Prop-1-Ene-1,3-Sultone and Vinylene Carbonate as Electrolyte Additives for Li(Ni1/3Mn1/3Co1/3]O2/Graphite Pouch Cells. J. Electrochem. Soc. 2014, 161, A1634–A1641. [Google Scholar] [CrossRef]
- Madec, L.; Petibon, R.; Xia, J.; Sun, J.-P.; Hill, I.G.; Dahn, J.R. Understanding the Role of Prop-1-Ene-1,3-Sultone and Vinylene Carbonate in LiNi1/3Mn1/3Co1/3O2/Graphite Pouch Cells: Electrochemical, GC-MS and XPS Analysis. J. Electrochem. Soc. 2015, 162, A2635–A2645. [Google Scholar] [CrossRef]
- Ma, L.; Xia, J.; Dahn, J.R. Ternary Electrolyte Additive Mixtures for Li-Ion Cells That Promote Long Lifetime and Less Reactivity with Charged Electrodes at Elevated Temperatures. J. Electrochem. Soc. 2015, 162, A1170–A1174. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Lin, H.; Wang, X.; Xu, M.; Wang, Y.; Xing, L.; Li, W. Performance Improvement of Phenyl Acetate as Propylene Carbonate-Based Electrolyte Additive for Lithium Ion Battery by Fluorine-Substituting. J. Power Sources 2014, 267, 182–187. [Google Scholar] [CrossRef]
- An, Y.; Zuo, P.; Cheng, X.; Liao, L.; Yin, G. The Effects of LiBOB Additive for Stable SEI Formation of PP13TFSI-Organic Mixed Electrolyte in Lithium Ion Batteries. Electrochim. Acta 2011, 56, 4841–4848. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Bettge, M.; Abraham, D.P. Electrolyte Additive Combinations That Enhance Performance of High-Capacity Li1.2Ni0.15Mn0.55Co0.1O2-Graphite Cells. Electrochim. Acta 2013, 110, 191–199. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Bettge, M.; Abraham, D.P. Positive Electrode Passivation by LiDFOB Electrolyte Additive in High-Capacity Lithium-Ion Cells. J. Electrochem. Soc. 2012, 159, A2109–A2117. [Google Scholar] [CrossRef]
- Jiao, S.; Liu, J.; Xu, W.; Zhang, J. A Localized High-Concentration Electrolyte with Optimized Solvents and Lithium Di Fl Uoro(Oxalate)Borate Additive for Stable Lithium Metal Batteries. ACS Energy Lett. 2018, 3, 2059–2067. [Google Scholar] [CrossRef]
- Tan, S.; Shadike, Z.; Li, J.; Wang, X.; Yang, Y.; Lin, R.; Cresce, A.; Hu, J.; Hunt, A.; Waluyo, I.; et al. Additive Engineering for Robust Interphases to Stabilize High-Ni Layered Structures at Ultra-High Voltage of 4.8 V. Nat. Energy 2022, 7, 484–494. [Google Scholar] [CrossRef]
- Wang, C.; Yu, L.; Fan, W.; Liu, J.; Ouyang, L.; Yang, L.; Zhu, M. Lithium Difluorophosphate As a Promising Electrolyte Lithium Additive for High-Voltage Lithium-Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 2647–2656. [Google Scholar] [CrossRef]
- Wang, C.; Yu, L.; Fan, W.; Liu, R.; Liu, J.; Ouyang, L.; Yang, L.; Zhu, M. Enhanced High-Voltage Cyclability of LiNi0.5Co0.2Mn0.3O2-Based Pouch Cells via Lithium Difluorophosphate Introducing as Electrolyte Additive. J. Alloys Compd. 2018, 755, 1–9. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, H.; Yu, L.; Fan, W.Z.; Huang, D. Lithium Difluorophosphate as an Additive to Improve the Low Temperature Performance of LiNi0.5Co0.2Mn0.3O2/Graphite Cells. Electrochim. Acta 2016, 221, 107–114. [Google Scholar] [CrossRef]
- Qian, Y.; Kang, Y.; Hu, S.; Shi, Q.; Chen, Q.; Tang, X.; Xiao, Y.; Zhao, H.; Luo, G.; Xu, K.; et al. Mechanism Study of Unsaturated Tripropargyl Phosphate as An efficient electrolyte additive forming multifunctional interphases in lithium ion and lithium metal batteries. ACS Appl. Mater. Interfaces 2020, 12, 10443–10451. [Google Scholar] [CrossRef]
- Skyler, J.S. Challenges of the Future. Endocrinologist 1993, 3, 233–238. [Google Scholar] [CrossRef]
- Lewandowski, A.; Świderska-Mocek, A. Ionic Liquids as Electrolytes for Li-Ion Batteries-An Overview of Electrochemical Studies. J. Power Sources 2009, 194, 601–609. [Google Scholar] [CrossRef]
- Chaudoy, V.; Ghamouss, F.; Jacquemin, J.; Houdbert, J.C.; Tran-Van, F. On the Performances of Ionic Liquid-Based Electrolytes for Li-NMC Batteries. J. Solut. Chem. 2015, 44, 769–789. [Google Scholar] [CrossRef]
- Matsui, Y.; Kawaguchi, S.; Sugimoto, T.; Kikuta, M.; Higashizaki, T.; Kono, M.; Yamagata, M.; Ishikawa, M. Charge-Discharge Characteristics of a LiNi1/3Mn1/3Co1/3O2 Cathode in FSI-Based Ionic Liquids. Electrochemistry 2012, 80, 808–811. [Google Scholar] [CrossRef] [Green Version]
- Simonetti, E.; Maresca, G.; Appetecchi, G.B.; Kim, G.T.; Loeffler, N.; Passerini, S. Towards Li(Ni0.33Mn0.33Co0.33)O2/Graphite Batteries with Ionic Liquid-Based Electrolytes. I. Electrodes’ Behavior in Lithium Half-Cells. J. Power Sources 2016, 331, 426–434. [Google Scholar] [CrossRef]
- Evans, T.; Olson, J.; Bhat, V.; Lee, S.H. Effect of Organic Solvent Addition to PYR13FSI + LiFSI Electrolytes on Aluminum Oxidation and Rate Performance of Li(Ni1/3Mn1/3Co1/3)O2 Cathodes. J. Power Sources 2014, 265, 132–139. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on Solid Electrolytes for All-Solid-State Lithium-Ion Batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Kato, T.; Iwasaki, S.; Ishii, Y.; Motoyama, M.; West, W.C.; Yamamoto, Y.; Iriyama, Y. Preparation of Thick- Fi Lm Electrode-Solid Electrolyte Composites on Li7La3Zr2O12 and Their Electrochemical Properties. J. Power Sources 2016, 303, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, S.; Hamanaka, T.; Yamakawa, T.; West, W.C.; Yamamoto, K.; Motoyama, M.; Hirayama, T.; Iriyama, Y. Preparation of Thick-Film LiNi1/3Co1/3Mn1/3O2 Electrodes by Aerosol Deposition and Its Application to All-Solid-State Batteries. J. Power Sources 2014, 272, 1086–1090. [Google Scholar] [CrossRef] [Green Version]
- Yan, P.; Zheng, J.; Liu, J.; Wang, B.; Cheng, X.; Zhang, Y.; Sun, X.; Wang, C.; Zhang, J.G. Tailoring Grain Boundary Structures and Chemistry of Ni-Rich Layered Cathodes for Enhanced Cycle Stability of Lithium-Ion Batteries. Nat. Energy 2018, 3, 600–605. [Google Scholar] [CrossRef]
- Alexander, G.V.; Indu, M.S.; Kamakshy, S.; Murugan, R. Electrochimica Acta Development of Stable and Conductive Interface between Garnet Structured Solid Electrolyte and Lithium Metal Anode for High Performance Solid-State Battery. Electrochim. Acta 2020, 332, 135511. [Google Scholar] [CrossRef]
- Phillip, N.D.; Westover, A.S.; Daniel, C.; Veith, G.M. Structural Degradation of High Voltage Lithium Nickel Manganese Cobalt Oxide (NMC) Cathodes in Solid-State Batteries and Implications for Next Generation Energy Storage. ACS Appl. Energy Mater. 2020, 3, 1768–1774. [Google Scholar] [CrossRef]
- Yu, X.; Li, J.; Manthiram, A. Rational Design of a Laminated Dual-Polymer/Polymer–Ceramic Composite Electrolyte for High-Voltage All-Solid-State Lithium Batteries. ACS Mater. Lett. 2020, 2, 317–324. [Google Scholar] [CrossRef]
- Gupta, H.; Singh, S.K.; Singh, V.K.; Tripathi, A.K.; Srivastava, N.; Tiwari, R.K.; Mishra, R.; Meghnani, D.; Singh, R.K. Development of Polymer Electrolyte and Cathode Material for Li-Batteries. J. Electrochem. Soc. 2019, 166, A5187–A5192. [Google Scholar] [CrossRef]
- Kaboli, S.; Demers, H.; Paolella, A.; Darwiche, A.; Dontigny, M.; Clément, D.; Guerfi, A.; Trudeau, M.L.; Goodenough, J.B.; Zaghib, K. Behavior of Solid Electrolyte in Li-Polymer Battery with NMC Cathode via in-Situ Scanning Electron Microscopy. Nano Lett. 2020, 20, 1607–1613. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kobayashi, Y.; Tabuchi, M.; Shono, K.; Ohno, Y.; Mita, Y.; Miyashiro, H. Oxidation Reaction of Polyether-Based Material and Its Suppression in Lithium Rechargeable Battery Using 4 v Class Cathode, LiNi1/3Mn1/3Co1/3O2. ACS Appl. Mater. Interfaces 2013, 5, 12387–12393. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Chien, P.H.; Li, Y.; Dolocan, A.; Xu, H.; Xu, B.; Grundish, N.S.; Jin, H.; Hu, Y.Y.; Goodenough, J.B. Fast Li+ Conduction Mechanism and Interfacial Chemistry of a NASICON/Polymer Composite Electrolyte. J. Am. Chem. Soc. 2020, 142, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Biensan, P.; Simon, B.; Pérès, J.P.; De Guibert, A.; Broussely, M.; Bodet, J.M.; Perton, F. On Safety of Lithium-Ion Cells. J. Power Sources 1999, 81–82, 906–912. [Google Scholar] [CrossRef]
- Li, J.; Daniel, C.; Wood, D. Materials Processing for Lithium-Ion Batteries. J. Power Sources 2011, 196, 2452–2460. [Google Scholar] [CrossRef]
- Lux, S.F.; Schappacher, F.; Balducci, A.; Passerini, S.; Winter, M. Low Cost, Environmentally Benign Binders for Lithium-Ion Batteries. J. Electrochem. Soc. 2010, 157, A320. [Google Scholar] [CrossRef]
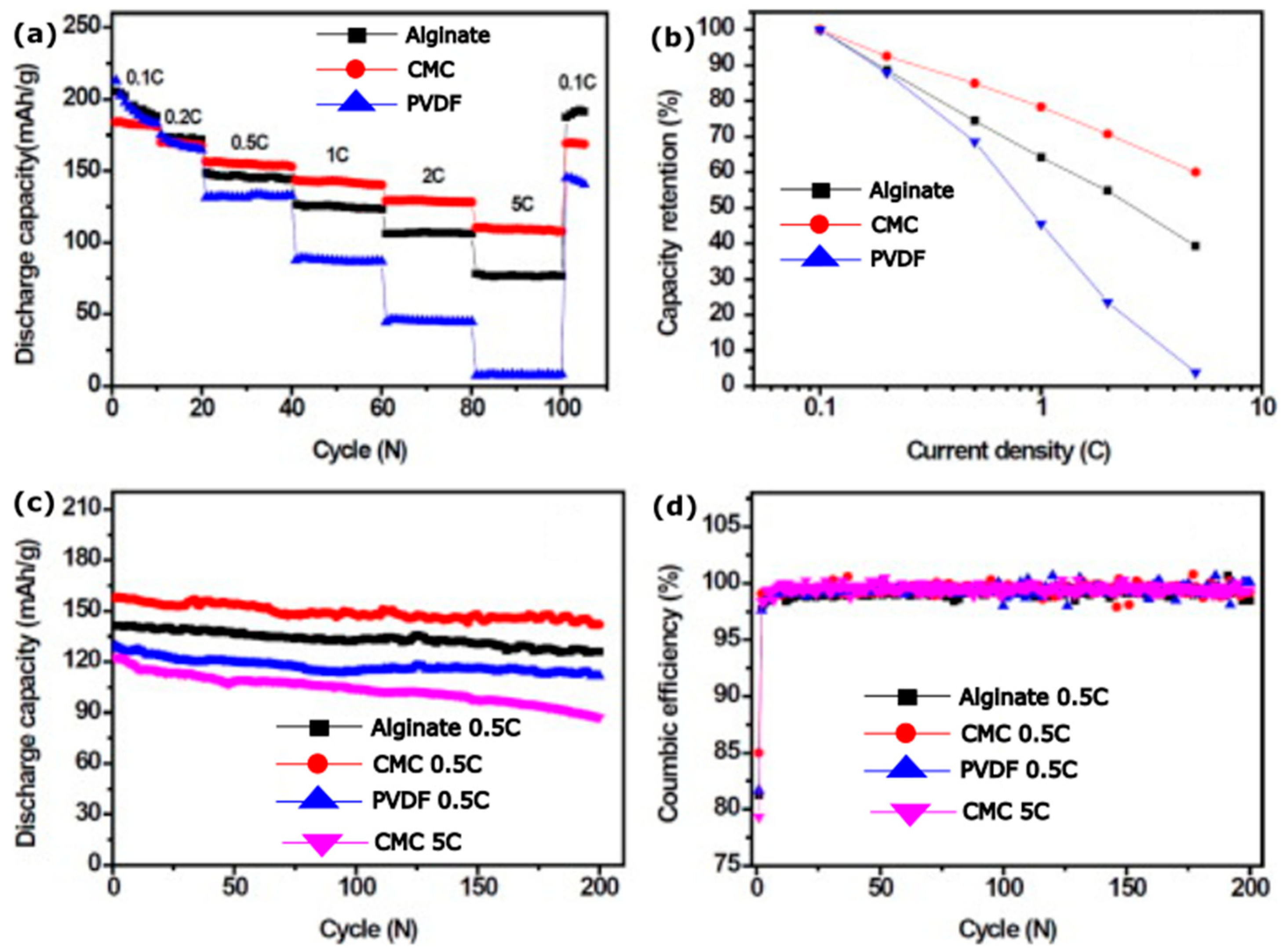
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A Major Constituent of Brown Algae for Use in High-Capacity Li-Ion Batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chou, S.L.; Gu, Q.F.; Liu, H.K.; Dou, S.X. The Effect of Different Binders on Electrochemical Properties of LiNi1/3Mn1/3Co1/3O2 Cathode Material in Lithium Ion Batteries. J. Power Sources 2013, 225, 172–178. [Google Scholar] [CrossRef]
- Loeffler, N.; Von Zamory, J.; Laszczynski, N.; Doberdo, I.; Kim, G.T.; Passerini, S. Performance of LiNi1/3Mn1/3Co1/3O2/Graphite Batteries Based on Aqueous Binder. J. Power Sources 2014, 248, 915–922. [Google Scholar] [CrossRef]
- Chen, Z.; Kim, G.; Chao, D.; Loe, N.; Copley, M.; Lin, J.; Shen, Z.; Passerini, S. Toward Greener Lithium-Ion Batteries: Aqueous Binder-Based Performance. J. Power Sources 2017, 372, 180–187. [Google Scholar] [CrossRef]
- Zhong, H.; Sun, M.; Li, Y.; He, J.; Yang, J.; Zhang, L. The Polyacrylic Latex: An Efficient Water-Soluble Binder for LiNi1/3Co1/3Mn1/3O2 Cathode in Li-Ion Batteries. J. Solid State Electrochem. 2016, 20, 1–8. [Google Scholar] [CrossRef]
- Memm, M.; Hoffmann, A.; Wohlfahrt-Mehrens, M. Water-Based LiNi1/3Mn1/3Co1/3O2-Cathodes with Good Electrochemical Performance by Use of Additives. Electrochim. Acta 2018, 260, 664–673. [Google Scholar] [CrossRef]
- Loeffler, N.; Kim, G.T.; Mueller, F.; Diemant, T.; Kim, J.K.; Behm, R.J.; Passerini, S. In Situ Coating of Li[Ni0.33Mn0.33Co0.33]O2 Particles to Enable Aqueous Electrode Processing. ChemSusChem 2016, 9, 1112–1117. [Google Scholar] [CrossRef]
- Bichon, M.; Sotta, D.; De Vito, E.; Porcher, W.; Lestriez, B. Performance and Ageing Behavior of Water-Processed LiNi0.5Mn0.3Co0.2O2/Graphite Lithium-Ion Cells. J. Power Sources 2021, 483, 229097. [Google Scholar] [CrossRef]
- Wood, M.; Li, J.; Ruther, R.E.; Du, Z.; Self, E.C.; Meyer, H.M.; Daniel, C.; Belharouak, I.; Wood, D.L. Chemical Stability and Long-Term Cell Performance of Low-Cobalt, Ni-Rich Cathodes Prepared by Aqueous Processing for High-Energy Li-Ion Batteries. Energy Storage Mater. 2020, 24, 188–197. [Google Scholar] [CrossRef]
- Kuo, J.-H.; Li, C.-C. Water-Based Process to the Preparation of Nickel-Rich Li(Ni0.8Co0.1Mn0.1)O2 Cathode. J. Electrochem. Soc. 2020, 167, 100504. [Google Scholar] [CrossRef]
- Brilloni, A.; Poli, F.; Spina, G.E.; Samorì, C.; Guidi, E.; Gualandi, C.; Maisuradze, M.; Giorgetti, M.; Soavi, F. Easy Recovery of Li-Ion Cathode Powders by the Use of Water-Processable Binders. Electrochim. Acta 2022, 418, 140376. [Google Scholar] [CrossRef]
- Vauthier, S.; Alvarez-Tirado, M.; Guzmán-González, G.; Tomé, L.; Cotte, S.; Castro, L.; Guéguen, A.; Mecerreyes, D.; Casado, N. High-Performance Pyrrolidinium-Based Poly (Ionic Liquid) Binders for Li-Ion and Li-Air Batteries. Mater. Today Chem. 2023, 27, 101293. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as Anode Materials: Fundamental Mechanism, Recent Progress and Advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.G. Lithium Metal Anodes for Rechargeable Batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Xu, H.; Wang, L.; Lu, X.; He, X. Li4Ti5O12 Spinel Anode: Fundamentals and Advances in Rechargeable Batteries. InfoMat 2022, 4, e12228. [Google Scholar] [CrossRef]
- Björklund, E.; Brandell, D.; Hahlin, M.; Edström, K.; Younesi, R. How the Negative Electrode Influences Interfacial and Electrochemical Properties of LiNi1/3Co1/3Mn1/3O2 Cathodes in Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A3054–A3059. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Jackson, D.; Dreibelbis, M.L.; Kuech, T.F.; Hamers, R.J. Anode-Originated SEI Migration Contributes to Formation of Cathode-Electrolyte Interphase Layer. J. Power Sources 2018, 373, 184–192. [Google Scholar] [CrossRef]
- Ohzuku, T.; Makimura, Y. Layered Lithium Insertion Material of LiCo1/3Ni1/3Mn1/3O2 for Lithium-Ion Batteries. Chem. Lett. 2001, 30, 642–643. [Google Scholar] [CrossRef]
- Wang, D.Y.; Dahn, J.R. A High Precision Study of Electrolyte Additive Combinations Containing Vinylene Carbonate, Ethylene Sulfate, Tris(Trimethylsilyl) Phosphate and Tris (Trimethylsilyl) Phosphite in Li[Ni1/3Mn1/3Co1/3]O2 /Graphite Pouch Cells. J. Electrochem. Soc. 2014, 161, A1890–A1897. [Google Scholar] [CrossRef]
- Wang, C.; Yu, L.; Fan, W.; Liu, J.; Ouyang, L.; Yang, L.; Zhu, M. 3,3′-(Ethylenedioxy)Dipropiononitrile as an Electrolyte Additive for 4.5 V LiNi1/3Co1/3Mn1/3O2/Graphite Cells. ACS Appl. Mater. Interfaces 2017, 9, 9630–9639. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, T.; Pan, Y.; Wang, W.; Fang, G.; Ding, K.; Wu, M. 3, 3′-Sulfonyldipropionitrile: A Novel Electrolyte Additive That Can Augment the High-Voltage Performance of LiNi1/3Co1/3Mn1/3O2/Graphite Batteries. J. Power Sources 2016, 319, 116–123. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, T.; Pan, Y.; Wang, W.; Fang, G.; Wu, M. High-Voltage Performance of LiNi1/3Co1/3Mn1/3O2/Graphite Batteries with Di(Methylsulfonyl) Methane as a New Sulfone-Based Electrolyte Additive. J. Power Sources 2015, 293, 196–202. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Z.; Xing, L.; Chen, D.; Rong, H.; Liu, Q.; Li, W. Enhanced High Voltage Performances of Layered Lithium Nickel Cobalt Manganese Oxide Cathode by Using Trimethylboroxine as Electrolyte Additive. Electrochim. Acta 2015, 176, 919–925. [Google Scholar] [CrossRef]
- Brox, S.; Röser, S.; Streipert, B.; Hildebrand, S.; Rodehorst, U.; Qi, X.; Wagner, R.; Winter, M.; Cekic-Laskovic, I. Innovative, Non-Corrosive LiTFSI Cyanoester-Based Electrolyte for Safer 4 V Lithium-Ion Batteries. ChemElectroChem 2017, 4, 304–309. [Google Scholar] [CrossRef]
- Schmiegel, J.-P.; Qi, X.; Klein, S.; Winkler, V.; Evertz, M.; Nölle, R.; Henschel, J.; Reiter, J.; Terborg, L.; Fan, Q.; et al. Improving the Cycling Performance of High-Voltage NMC111 || Graphite Lithium Ion Cells by an Effective Urea-Based Electrolyte Additive. J. Electrochem. Soc. 2019, 166, A2910–A2920. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, T.; Pan, Y.; Wang, W.; Fang, G.; Ding, K.; Wu, M. Enhancing the High-Voltage Cycling Performance of LiNi1/3Co1/3Mn1/3O2/Graphite Batteries Using Alkyl 3,3,3-Trifluoropropanoate as an Electrolyte Additive. ACS Appl. Mater. Interfaces 2017, 9, 18758–18765. [Google Scholar] [CrossRef]
- Belharouak, I.; Sun, Y.; Liu, J.; Amine, K. Li(Ni1/3Mn1/3Co1/3)O2 as a Suitable Cathode for High Power Applications. J. Power Sources 2003, 123, 247–252. [Google Scholar] [CrossRef]
- Qian, Y.; Niehoff, P.; Börner, M.; Grützke, M.; Mönnighoff, X.; Behrends, P.; Nowak, S.; Winter, M.; Schappacher, F.M. Influence of Electrolyte Additives on the Cathode Electrolyte Interphase (CEI) Formation on LiNi1/3Mn1/3Co1/3O2 in Half Cells with Li Metal Counter Electrode. J. Power Sources 2016, 329, 31–40. [Google Scholar] [CrossRef]
- Xu, C.; Renault, S.; Ebadi, M.; Wang, Z.; Björklund, E.; Guyomard, D.; Brandell, D.; Edström, K.; Gustafsson, T. LiTDI: A Highly Efficient Additive for Electrolyte Stabilization in Lithium-Ion Batteries. Chem. Mater. 2017, 29, 2254–2263. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Cheng, X.; Zuo, P.; Cui, Y.; Guan, T.; Du, C.; Gao, Y.; Yin, G. Enhancement of High Voltage Cycling Performance and Thermal Stability of LiNi1/3Co1/3Mn1/3O2 Cathode by Use of Boron-Based Additives. Solid State Ion. 2014, 263, 146–151. [Google Scholar] [CrossRef]
- Qian, Y.; Schultz, C.; Niehoff, P.; Schwieters, T.; Nowak, S.; Schappacher, F.M.; Winter, M. Investigations on the Electrochemical Decomposition of the Electrolyte Additive Vinylene Carbonate in Li Metal Half Cells and Lithium Ion Full Cells. J. Power Sources 2016, 332, 60–71. [Google Scholar] [CrossRef]
- Aiken, C.P.; Self, J.; Petibon, R.; Xia, X.; Paulsen, J.M.; Dahn, J.R. A Survey of In Situ Gas Evolution during High Voltage Formation in Li-Ion Pouch Cells. J. Electrochem. Soc. 2015, 162, A760–A767. [Google Scholar] [CrossRef] [Green Version]
- Self, J.; Aiken, C.P.; Petibon, R.; Dahn, J.R. Survey of Gas Expansion in Li-Ion NMC Pouch Cells. J. Electrochem. Soc. 2015, 162, A796–A802. [Google Scholar] [CrossRef]
- Nelson, K.J.; d’Eon, G.L.; Wright, A.T.B.; Ma, L.; Xia, J.; Dahn, J.R. Studies of the Effect of High Voltage on the Impedance and Cycling Performance of Li[Ni0.4Mn0.4Co0.2]O2/Graphite Lithium-Ion Pouch Cells. J. Electrochem. Soc. 2015, 162, A1046–A1054. [Google Scholar] [CrossRef]
- Ma, L.; Xia, J.; Dahn, J.R. Improving the High Voltage Cycling of Li[Ni0.42Mn0.42Co0.16]O2 (NMC442)/Graphite Pouch Cells Using Electrolyte Additives. J. Electrochem. Soc. 2014, 161, A2250–A2254. [Google Scholar] [CrossRef]
- Petibon, R.; Xia, J.; Ma, L.; Bauer, M.K.G.; Nelson, K.J.; Dahn, J.R. Electrolyte System for High Voltage Li-Ion Cells. J. Electrochem. Soc. 2016, 163, A2571–A2578. [Google Scholar] [CrossRef]
- Xia, J.; Nie, M.; Burns, J.C.; Xiao, A.; Lamanna, W.M.; Dahn, J.R. Fluorinated Electrolyte for 4.5 v Li(Ni0.4Mn0.4Co0.2)O2/Graphite Li-Ion Cells. J. Power Sources 2016, 307, 340–350. [Google Scholar] [CrossRef]
- Xia, J.; Dahn, J.R. Improving Sulfolane-Based Electrolyte for High Voltage Li-Ion Cells with Electrolyte Additives. J. Power Sources 2016, 324, 704–711. [Google Scholar] [CrossRef]
- Rong, H.; Xu, M.; Xie, B.; Huang, W.; Liao, X.; Xing, L.; Li, W. Performance Improvement of Graphite/LiNi0.4Co0.2Mn0.4O2 Battery at High Voltage with Added Tris (Trimethylsilyl) Phosphate. J. Power Sources 2015, 274, 1155–1161. [Google Scholar] [CrossRef]
- Lei, Q.; Yang, T.; Zhao, X.; Fan, W.; Wang, W.; Yu, L.; Guo, S.; Zuo, X.; Zeng, R.; Nan, J. Lithium Difluorophosphate as a Multi-Functional Electrolyte Additive for 4.4 V LiNi0.5Co0.2Mn0.3O2/Graphite Lithium Ion Batteries. J. Electroanal. Chem. 2019, 846, 113141. [Google Scholar] [CrossRef]
- Zuo, X.; Fan, C.; Liu, J.; Xiao, X.; Wu, J.; Nan, J. Lithium Tetrafluoroborate as an Electrolyte Additive to Improve the High Voltage Performance of Lithium-Ion Battery. J. Electrochem. Soc. 2013, 160, A1199–A1204. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, K.; Che, Y.; Liu, M.; Zhang, W.; Xing, L.; Wang, H.; Li, S.; Liu, X.; Li, W. A Novel Electrolyte Additive Enables High-Voltage Operation of Nickel-Rich Oxide/Graphite Cells. J. Phys. Chem. Lett. 2021, 12, 4327–4338. [Google Scholar] [CrossRef]
- Guo, R.; Che, Y.; Lan, G.; Lan, J.; Li, J.; Xing, L.; Xu, K.; Fan, W.; Yu, L.; Li, W. Tailoring Low-Temperature Performance of a Lithium-Ion Battery via Rational Designing Interphase on an Anode. ACS Appl. Mater. Interfaces 2019, 11, 38285–38293. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Fan, C.; Liu, J.; Xiao, X.; Wu, J.; Nan, J. Effect of Tris(Trimethylsilyl)Borate on the High Voltage Capacity Retention of LiNi0.5Co0.2Mn0.3O2/Graphite Cells. J. Power Sources 2013, 229, 308–312. [Google Scholar] [CrossRef]
- Rong, H.; Xu, M.; Zhu, Y.; Xie, B.; Lin, H.; Liao, Y.; Xing, L.; Li, W. A Novel Imidazole-Based Electrolyte Additive for Improved Electrochemical Performance of High Voltage Nickel-Rich Cathode Coupled with Graphite Anode Lithium Ion Battery. J. Power Sources 2016, 332, 312–321. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoon, S.; Hwang, E.H.; Kwon, Y.G.; Lee, Y.G.; Cho, K.Y. [4,4″-Bi(1,3,2-Dioxathiolane)] 2,2″-Dioxide: A Novel Cathode Additive for High-Voltage Performance in Lithium Ion Batteries. J. Power Sources 2018, 378, 112–118. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, T.; Xiong, J.; Zhu, B.; Cheng, Y.J.; Xia, Y. Stable Electrode/Electrolyte Interface for High-Voltage NCM 523 Cathode Constructed by Synergistic Positive and Passive Approaches. ACS Appl. Mater. Interfaces 2021, 13, 57107–57117. [Google Scholar] [CrossRef]
- Jung, S.K.; Gwon, H.; Hong, J.; Park, K.Y.; Seo, D.H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the Degradation Mechanisms of LiNi0.5Co0.2Mn0.3O2 Cathode Material in Lithium Ion Batteries. Adv. Energy Mater. 2014, 4, 1300787. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Li, N.; Tong, W. Influence of Charge Cutoff Voltage on the Cycling Behavior of LiNi0.5Mn0.3Co0.2O2 Cathode. J. Electrochem. Soc. 2020, 167, 120509. [Google Scholar] [CrossRef]
- Hong, P.; Xu, M.; Liao, B.; Wu, Y.; Lin, N.; Huang, Q.; Li, W. Enhancing the Cycling Performance of High Voltage (4.5 V) Li/LiNi0.5Mn0.3Co0.2O2 Cell by Tailoring Sulfur-Derivative Cathode Passivation Film. J. Electrochem. Soc. 2017, 164, A2914–A2921. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Li, W.; Liu, X.; Zhang, J. Enhancing the High Voltage Interface Compatibility of LiNi0.5Co0.2Mn0.3O2 in the Succinonitrile-Based Electrolyte. Electrochim. Acta 2019, 298, 818–826. [Google Scholar] [CrossRef]
- Jung, R.; Strobl, P.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Temperature Dependence of Oxygen Release from LiNi0.6Mn0.2Co0.2O2 (NMC622) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A2869–A2879. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yang, T.; Li, S.; Lu, J.; Zhao, X.; Fan, W.; Fan, C.; Zuo, X.; Tie, S.; Nan, J. 1,4-Phenylene Diisocyanate (PPDI)-Containing Low H2O/HF and Multi-Functional Electrolyte for LiNi0.6Co0.2Mn0.2O2 /Graphite Batteries with Enhanced Performances. J. Power Sources 2021, 483, 229172. [Google Scholar] [CrossRef]
- Liao, B.; Hu, X.; Xu, M.; Li, H.; Yu, L.; Fan, W.; Xing, L.; Liao, Y.; Li, W. Constructing Unique Cathode Interface by Manipulating Functional Groups of Electrolyte Additive for Graphite/LiNi0.6Co0.2Mn0.2O2 Cells at High Voltage. J. Phys. Chem. Lett. 2018, 9, 3434–3445. [Google Scholar] [CrossRef]
- Lu, J.; Wang, W.; Yang, T.; Li, S.; Zhao, X.; Fan, W.; Fan, C.; Zuo, X.; Nan, J. Hexamethylene Diisocyanate (HDI)-Functionalized Electrolyte Matching LiNi0.6Co0.2Mn0.6O2/Graphite Batteries with Enhanced Performances. Electrochim. Acta 2020, 352, 136456. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, M.; Wu, S.; Tian, Y.; Cao, Z.; Xing, L.; Li, W. Insight into the Mechanism of Improved Interfacial Properties between Electrodes and Electrolyte in the Graphite/LiNi0.6Mn0.2Co0.2O2 Cell via Incorporation of 4-Propyl-[1,3,2]Dioxathiolane-2,2-Dioxide (PDTD). ACS Appl. Mater. Interfaces 2018, 10, 16400–16409. [Google Scholar] [CrossRef]
- Yan, X.; Chen, C.; Zhu, X.; Pan, L.; Zhao, X.; Zhang, L. Aminoalkyldisiloxane as Effective Electrolyte Additive for Improving High Temperature Cycle Life of Nickel-Rich LiNi0.6Co0.2Mn0.2O2/Graphite Batteries. J. Power Sources 2020, 461, 228099. [Google Scholar] [CrossRef]
- Che, Y.; Lin, X.; Xing, L.; Guan, X.; Guo, R.; Lan, G.; Zheng, Q.; Zhang, W.; Li, W. Protective Electrode/Electrolyte Interphases for High Energy Lithium-Ion Batteries with p-Toluenesulfonyl Fluoride Electrolyte Additive. J. Energy Chem. 2020, 52, 361–371. [Google Scholar] [CrossRef]
- Qin, Z.; Hong, S.; Hong, B.; Duan, B.; Lai, Y.; Feng, J. Triisopropyl Borate as an Electrolyte Additive for Improving the High Voltage. J. Electroanal. Chem. 2019, 854, 113506. [Google Scholar] [CrossRef]
- Deng, B.; Wang, H.; Ge, W.; Li, X.; Yan, X.; Chen, T.; Qu, M.; Peng, G. Investigating the Influence of High Temperatures on the Cycling Stability of a LiNi0.6Co0.2Mn0.2O2 Cathode Using an Innovative Electrolyte Additive. Electrochim. Acta 2017, 236, 61–71. [Google Scholar] [CrossRef]
- Wang, H.; Sun, D.; Li, X.; Ge, W.; Deng, B.; Qu, M.; Peng, G. Alternative Multifunctional Cyclic Organosilicon as an Efficient Electrolyte Additive for High Performance Lithium-Ion Batteries. Electrochim. Acta 2017, 254, 112–122. [Google Scholar] [CrossRef]
- Gao, H.; Maglia, F.; Lamp, P.; Amine, K.; Chen, Z. Mechanistic Study of Electrolyte Additives to Stabilize High-Voltage Cathode-Electrolyte Interface in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 44542–44549. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, J.; He, J.; Wang, H.; Jiang, G.; Qi, S.; Ma, J. Stabilizing the Cycling Stability of Rechargeable Lithium Metal Batteries with Tris(Hexafluoroisopropyl)Phosphate Additive. Sci. Bull. 2022, 67, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zuo, X.; Zhang, L.; Huang, W.; Chen, Q.; Zhu, T.; Liu, J.; Nan, J. Nonflammable LiTFSI-Ethylene Carbonate/1, 2-Dimethoxyethane Electrolyte for High-Safety Li-Ion Batteries Electrolyte for High-Safety Li-Ion Batteries. J. Electrochem. Soc. 2020, 167, 090520. [Google Scholar] [CrossRef]
- Su, C.; He, M.; Amine, R.; Chen, Z.; Sahore, R.; Dietz, N.; Amine, K. Cyclic Carbonate for Highly Stable Cycling of High Voltage Lithium Metal Batteries. Energy Storage Mater. 2019, 17, 284–292. [Google Scholar] [CrossRef]
- Lee, W.J.; Prasanna, K.; Jo, Y.N.; Kim, K.J.; Kim, H.S.; Lee, C.W. Depth Profile Studies on Nickel Rich Cathode Material Surfaces after Cycling with an Electrolyte Containing Vinylene Carbonate at Elevated Temperature. Phys. Chem. Chem. Phys. 2014, 16, 17062–17071. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Zhang, Z.; Fan, J.; Qi, W.; Chen, S. Fluoroethylene Carbonate as an Electrolyte Additive for Improving Interfacial Stability of High-Voltage LiNi0.6Co0.2Mn0.2O2 Cathode. Ionics 2019, 25, 1035–1043. [Google Scholar] [CrossRef]
- Beltrop, K.; Klein, S.; No, R.; Wilken, A.; Lee, J.J.; Ko, T.K.; Reiter, J.; Tao, L.; Liang, C.; Winter, M.; et al. Triphenylphosphine Oxide as Highly E Ff Ective Electrolyte Additive for Graphite/NMC811 Lithium Ion Cells. Chem. Mater. 2018, 30, 2726–2741. [Google Scholar] [CrossRef]
- Lan, G.; Xing, L.; Bedrov, D.; Chen, J.; Guo, R.; Che, Y.; Li, Z.; Zhou, H.; Li, W. Enhanced Cyclic Stability of Ni-Rich Lithium Ion Battery with Electrolyte Film-Forming Additive. J. Alloys Compd. 2020, 821, 153236. [Google Scholar] [CrossRef]
- Lu, J.; Xu, X.; Fan, W.; Xin, Y.; Wang, W.; Fan, C.; Cheng, P.; Zhao, J.; Liu, J.; Huo, Y. Phenyl 4-Fluorobenzene Sulfonate as a Versatile Film-Forming Electrolyte Additive for Wide-Temperature-Range NCM811//Graphite Batteries. ACS Appl. Energy Mater. 2022, 5, 6324–6334. [Google Scholar] [CrossRef]
- Qiu, Y.; Lu, D.; Gai, Y.; Cai, Y. Adiponitrile (ADN): A Stabilizer for the LiNi0.8Co0.1Mn0.1O2 (NCM811) Electrode/Electrolyte Interface of a Graphite/NCM811 Li-Ion Cell. ACS Appl. Mater. Interfaces 2022, 14, 11398–11407. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Yang, T.; Wang, W.; Lu, J.; Fan, W.; Zhao, X.; Zuo, X.; Tie, S.; Nan, J. 3,3-Diethylene Di-Sulfite (DES) as a High-Voltage Electrolyte Additive for 4.5 V LiNi0.8Co0.1Mn0.1O2/Graphite Batteries with Enhanced Performances. ChemElectroChem 2021, 8, 745–754. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, X.; Wei, P.; Sun, S.; Xu, Y.; Li, Q.; Fang, C.; Han, J. Tailoring Electrolyte Enables High-Voltage Ni-Rich NCM Cathode against Aggressive Cathode Chemistries for Li-Ion Batteries. Sci. Bull. 2022, 67, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, H.; Wang, P.; Fu, X.L.; Zhang, N.S.; Zhao, D.N.; Li, S.Y.; Cui, X.L. Adjusting the Solvation Structure with Tris(Trimethylsilyl)Borate Additive to Improve the Performance of LNCM Half Cells. J. Energy Chem. 2022, 67, 55–64. [Google Scholar] [CrossRef]
- Jung, K.; Oh, S.H.; Yim, T. Triphenyl Phosphate as an Efficient Electrolyte Additive for Ni-Rich Ncm Cathode Materials. J. Electrochem. Sci. Technol. 2021, 12, 67–73. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Jia, M.; Peng, L.; Zhang, N.; Qi, S.; Zhang, L. Bifunctional Additive Phenyl Vinyl Sulfone for Boosting Cyclability of Lithium Metal Batteries. Green Chem. Eng. 2022, 4, 49–56. [Google Scholar] [CrossRef]
- Li, G.; Liao, Y.; Li, Z.; Xu, N.; Lu, Y.; Lan, G.; Sun, G.; Li, W. Constructing a Low-Impedance Interface on a High-Voltage LiNi0.8Co0.1Mn0.1O2 Cathode with 2,4,6-Triphenyl Boroxine as a Film-Forming Electrolyte Additive for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 37013–37026. [Google Scholar] [CrossRef]
- Zhang, L.; Min, F.; Luo, Y.; Dang, G.; Gu, H.; Dong, Q.; Zhang, M.; Sheng, L.; Shen, Y.; Chen, L.; et al. Practical 4.4 V Li||NCM811 Batteries Enabled by a Thermal Stable and HF Free Carbonate-Based Electrolyte. Nano Energy 2022, 96, 107122. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, X.; Yin, J.; Wu, H.; Zhu, X.; Gao, Y. Multifunctional Electrolyte Additive for Bi-Electrode Interphase Regulation and Electrolyte Stabilization in Li/LiNi0.8Co0.1Mn0.1O2 Batteries. ACS Appl. Mater. Interfaces 2022, 14, 38758–38768. [Google Scholar] [CrossRef]
- Hu, H.; Wang, W.; Zeng, X.; Fan, W.; Fan, C.; Nan, J. Interfacial Film Regulation and Electrochemical Performance Using Cyclopropane Sulphonic Amide Functionalized Electrolyte to Stabilize Lithium Metal Batteries with a LiNi0.8Mn0.1Co0.1O2 Cathode. ACS Appl. Energy Mater. 2022, 5, 5053–5063. [Google Scholar] [CrossRef]
- You, B.; Wang, Z.; Shen, F.; Chang, Y.; Peng, W.; Li, X.; Guo, H.; Hu, Q.; Deng, C.; Yang, S.; et al. Research Progress of Single-Crystal Nickel-Rich Cathode Materials for Lithium Ion Batteries. Small Methods 2021, 5, 2100234. [Google Scholar] [CrossRef] [PubMed]
- Langdon, J.; Manthiram, A. A Perspective on Single-Crystal Layered Oxide Cathodes for Lithium-Ion Batteries. Energy Storage Mater. 2021, 37, 143–160. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liu, J.; Yan, Z.; Chen, J. Syntheses, Challenges and Modifications of Single-Crystal Cathodes for Lithium-Ion Battery. J. Energy Chem. 2021, 63, 217–229. [Google Scholar] [CrossRef]
- Klein, S.; Bärmann, P.; Fromm, O.; Borzutzki, K.; Reiter, J.; Fan, Q.; Winter, M.; Placke, T.; Kasnatscheew, J. Prospects and Limitations of Single-Crystal Cathode Materials to Overcome Cross-Talk Phenomena in High-Voltage Lithium Ion Cells. J. Mater. Chem. A 2021, 9, 7546–7555. [Google Scholar] [CrossRef]
- Hall, D.S.; Soc, J.E.; Cells, L.; Hall, D.S. Dioxazolone and Nitrile Sulfite Electrolyte Additives for Lithium-Ion Cells Dioxazolone and Nitrile Sulfite Electrolyte Additives For. J. Electrochem. Soc. 2018, 165, A2961–A2967. [Google Scholar] [CrossRef]
- Harlow, J.E.; Ma, X.; Li, J.; Logan, E.; Liu, Y.; Zhang, N.; Ma, L.; Glazier, S.L.; Cormier, M.M.E.; Genovese, M.; et al. A Wide Range of Testing Results on an Excellent Lithium-Ion Cell Chemistry to Be Used as Benchmarks for New Battery Technologies. J. Electrochem. Soc. 2019, 166, A3031–A3044. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ma, X.; Dahn, J.R. Synthesis of Single Crystal LiNi0.6Mn0.2Co0.2O2 with Enhanced Electrochemical Performance for Lithium Ion Batteries. J. Electrochem. Soc. 2018, 165, A1038–A1045. [Google Scholar] [CrossRef]
- Logan, E.R.; Hebecker, H.; Ma, X.; Quinn, J.; HyeJeong, Y.; Kumakura, S.; Paulsen, J.; Dahn, J.R. A Comparison of the Performance of Different Morphologies of LiNi0.8Mn0.1Co0.1O2 Using Isothermal Microcalorimetry, Ultra-High Precision Coulometry, and Long-Term Cycling. J. Electrochem. Soc. 2020, 167, 060530. [Google Scholar] [CrossRef]
- Song, W.; Harlow, J.; Logan, E.; Hebecker, H.; Coon, M.; Molino, L.; Johnson, M.; Dahn, J.; Metzger, M. A Systematic Study of Electrolyte Additives in Single Crystal and Bimodal LiNi0.8Mn0.1Co0.1O2/Graphite Pouch Cells. J. Electrochem. Soc. 2021, 168, 090503. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Li, H.; Liu, A.; Huang, Q.; Ma, L.; Li, Y.; Dahn, J.R. Structural, Electrochemical, and Thermal Properties of Nickel-Rich LiNixMnyCozO2 Materials. Chem. Mater. 2018, 30, 8852–8860. [Google Scholar] [CrossRef]
- Ge, M.; Wi, S.; Liu, X.; Bai, J.; Ehrlich, S.; Lu, D.; Lee, W.K.; Chen, Z.; Wang, F. Kinetic Limitations in Single-Crystal High-Nickel Cathodes. Angew. Chem. Int. Ed. 2021, 60, 17350–17355. [Google Scholar] [CrossRef]
- Lee, S.; Jin, W.; Kim, S.H.; Joo, S.H.; Nam, G.; Oh, P.; Kim, Y.; Kwak, S.K.; Cho, J. Oxygen Vacancy Diffusion and Condensation in Lithium-Ion Battery Cathode Materials. Angew. Chem. 2019, 131, 10588–10595. [Google Scholar] [CrossRef]
- Han, Y.; Heng, S.; Wang, Y.; Qu, Q.; Zheng, H. Anchoring Interfacial Nickel Cations on Single-Crystal LiNi0.8Co0.1Mn0.1O2 Cathode Surface via Controllable Electron Transfer. ACS Energy Lett. 2020, 5, 2421–2433. [Google Scholar] [CrossRef]
- Ryu, H.H.; Namkoong, B.; Kim, J.H.; Belharouak, I.; Yoon, C.S.; Sun, Y.K. Capacity Fading Mechanisms in Ni-Rich Single-Crystal NCM Cathodes. ACS Energy Lett. 2021, 6, 2726–2734. [Google Scholar] [CrossRef]
- Han, G.M.; Kim, Y.S.; Ryu, H.H.; Sun, Y.K.; Yoon, C.S. Structural Stability of Single-Crystalline Ni-Rich Layered Cathode upon Delithiation. ACS Energy Lett. 2022, 7, 2919–2926. [Google Scholar] [CrossRef]
- Li, X.; Peng, W.; Tian, R.; Song, D.; Wang, Z.; Zhang, H.; Zhu, L.; Zhang, L. Excellent Performance Single-Crystal NCM Cathode under High Mass Loading for All-Solid-State Lithium Batteries. Electrochim. Acta 2020, 363, 137185. [Google Scholar] [CrossRef]
- Wang, C.; Hwang, S.; Jiang, M.; Liang, J.; Sun, Y.; Adair, K.; Zheng, M.; Mukherjee, S.; Li, X.; Li, R.; et al. Deciphering Interfacial Chemical and Electrochemical Reactions of Sulfide-Based All-Solid-State Batteries. Adv. Energy Mater. 2021, 11, 2100210. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, B.; Zhao, J.; Zhao, W.; Liang, Z.; Su, Y.; Xie, C.; Zhou, K.; Xiang, Y.; Zhu, J.; et al. Electrochemo-Mechanical Effects on Structural Integrity of Ni-Rich Cathodes with Different Microstructures in All Solid-State Batteries. Adv. Energy Mater. 2021, 11, 2003583. [Google Scholar] [CrossRef]
- Doerrer, C.; Capone, I.; Narayanan, S.; Liu, J.; Grovenor, C.R.M.; Pasta, M.; Grant, P.S. High Energy Density Single-Crystal NMC/Li6PS5Cl Cathodes for All-Solid-State Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 37809–37815. [Google Scholar] [CrossRef]
- Conforto, G.; Ruess, R.; Schröder, D.; Trevisanello, E.; Fantin, R.; Richter, F.H.; Janek, J. Editors’ Choice—Quantification of the Impact of Chemo-Mechanical Degradation on the Performance and Cycling Stability of NCM-Based Cathodes in Solid-State Li-Ion Batteries. J. Electrochem. Soc. 2021, 168, 070546. [Google Scholar] [CrossRef]
- Guo, X.; Hao, L.; Yang, Y.; Wang, Y.; Lu, Y.; Yu, H. High Cathode Utilization Efficiency through Interface Engineering in All-Solid-State Lithium-Metal Batteries. J. Mater. Chem. A 2019, 7, 25915–25924. [Google Scholar] [CrossRef]
- Wang, C.; Yu, R.; Hwang, S.; Liang, J.; Li, X.; Zhao, C.; Sun, Y.; Wang, J.; Holmes, N.; Li, R.; et al. Single Crystal Cathodes Enabling High-Performance All-Solid-State Lithium-Ion Batteries. Energy Storage Mater. 2020, 30, 98–103. [Google Scholar] [CrossRef]
- Jeon, H.; Kwon, D.H.; Kim, H.; Lee, J.H.; Jun, Y.; Son, J.W.; Park, S. Tailoring Shape and Exposed Crystal Facet of Single-Crystal Layered-Oxide Cathode Particles for All-Solid-State Batteries. Chem. Eng. J. 2022, 445, 136828. [Google Scholar] [CrossRef]
- Yi, M.; Li, J.; Fan, X.; Bai, M.; Zhang, Z.; Hong, B.; Zhang, Z.; Hu, G.; Jiang, H.; Lai, Y. Single Crystal Ni-Rich Layered Cathodes Enabling Superior Performance in All-Solid-State Batteries with PEO-Based Solid Electrolytes. J. Mater. Chem. A 2021, 9, 16787–16797. [Google Scholar] [CrossRef]
- Yi, M.; Li, J.; Wang, M.; Fan, X.; Hong, B.; Zhang, Z.; Zhang, Z.; Jiang, H.; Wang, A.; Lai, Y. Suppressing Structural Degradation of Single Crystal Nickel-Rich Cathodes in PEO-Based All-Solid-State Batteries: Mechanistic Insight and Performance. Energy Storage Mater. 2023, 54, 579–588. [Google Scholar] [CrossRef]
- Ma, X.; Vanaphuti, P.; Fu, J.; Hou, J.; Liu, Y.; Zhang, R.; Bong, S.; Yao, Z.; Yang, Z.; Wang, Y. A Universal Etching Method for Synthesizing High-Performance Single Crystal Cathode Materials. Nano Energy 2021, 87, 106194. [Google Scholar] [CrossRef]
- Kimijima, T.; Zettsu, N.; Teshima, K. Growth Manner of Octahedral-Shaped Li(Ni1/3Co1/3Mn1/3)O2 Single Crystals in Molten Na2SO4. Cryst. Growth Des. 2016, 16, 2618–2623. [Google Scholar] [CrossRef]
- Li, F.; Kong, L.; Sun, Y.; Jin, Y.; Hou, P. Micron-Sized Monocrystalline LiNi1/3Co1/3Mn1/3O2 as High-Volumetric-Energy-Density Cathode for Lithium-Ion Batteries. J. Mater. Chem. A 2018, 6, 12344–12352. [Google Scholar] [CrossRef]
- Huang, Z.D.; Liu, X.M.; Oh, S.W.; Zhang, B.; Ma, P.C.; Kim, J.K. Microscopically Porous, Interconnected Single Crystal LiNi1/3Co1/3Mn1/3O2 Cathode Material for Lithium Ion Batteries. J. Mater. Chem. 2011, 21, 10777–10784. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, G.; Lin, M.; Zhao, W.; Li, D.; Guan, X.; Ji, Y.; Ortiz, G.F.; Yang, Y. Toward a Stable Solid-Electrolyte-Interfaces on Nickel-Rich Cathodes: LiPO2F2 Salt-Type Additive and Its Working Mechanism for LiNi0.5Mn0.25Co0.25O2 Cathodes. J. Power Sources 2018, 380, 149–157. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Ou, X.; Zhang, J.; Zhang, B.; Wang, D.; Hu, G. Unravelling the Influence of Quasi Single-Crystalline Architecture on High-Voltage and Thermal Stability of LiNi0.5Co0.2Mn0.3O2 Cathode for Lithium-Ion Batteries. Chem. Eng. J. 2020, 393, 124709. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, L.; Huang, S.; Shang, W.; Kong, L.; Sun, M.; Chen, L.; Ren, W. Single-Crystal LiNi0.5Co0.2Mn0.3O2: A High Thermal and Cycling Stable Cathodes for Lithium-Ion Batteries. J. Mater. Sci. 2020, 55, 2913–2922. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, L.; Yu, H.; Xu, F.; Wei, W.; Fan, L. Critical Rate Capability Barrier by the (001) Microtexture of a Single-Crystal Cathode for Long Lifetime Lithium-Ion Batteries. J. Mater. 2022, 8, 649–655. [Google Scholar] [CrossRef]
- Li, J.; Cameron, A.R.; Li, H.; Glazier, S.; Xiong, D.; Chatzidakis, M.; Allen, J.; Botton, G.A.; Dahn, J.R. Comparison of Single Crystal and Polycrystalline LiNi0.5Mn0.3Co0.2O2 Positive Electrode Materials for High Voltage Li-Ion Cells. J. Electrochem. Soc. 2017, 164, A1534–A1544. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Stone, W.; Glazier, S.; Dahn, J.R. Development of Electrolytes for Single Crystal NMC532/Artificial Graphite Cells with Long Lifetime. J. Electrochem. Soc. 2018, 165, A626–A635. [Google Scholar] [CrossRef]
- Wang, L.; Wu, B.; Mu, D.; Liu, X.; Peng, Y.; Xu, H.; Liu, Q.; Gai, L.; Wu, F. Single-Crystal LiNi0.6Co0.2Mn0.2O2 as High Performance Cathode Materials for Li-Ion Batteries. J. Alloys Compd. 2016, 674, 360–367. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Zhao, Y.; Jiang, G.; Gong, R.; Li, Y.; Meng, Q.; Dong, P. Surface Growth and Intergranular Separation of Polycrystalline Particles for Regeneration of Stable Single-Crystal Cathode Materials. ACS Appl. Mater. Interfaces 2022, 14, 29886–29895. [Google Scholar] [CrossRef]
- Huang, B.; Wang, M.; Zuo, Y.; Zhao, Z.; Zhang, X.; Gu, Y. The Effects of Reheating Process on the Electrochemical Properties of Single Crystal LiNi0.6Mn0.2Co0.2. Solid State Ion. 2020, 345, 115200. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, F.; Zhang, Y. Micron-Sized Monodisperse Particle LiNi0.6Co0.2Mn0.2O2 Derived by Oxalate Solvothermal Process Combined with Calcination as Cathode Material for Lithium-Ion Batteries. Materials 2021, 14, 2576. [Google Scholar] [CrossRef]
- Huang, B.; Wang, M.; Zhang, X.; Xu, G.; Gu, Y. Optimized Preparation of LiNi0.6Mn0.2Co0.2O2 with Single Crystal Morphology Cathode Material for Lithium-Ion Batteries. Ionics 2020, 26, 2689–2698. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, Y.; Li, L.; Zhang, R.; Xu, J.; Cheng, Z.; Xie, S.; Wang, H.; Rao, Q.; He, Y.; et al. Single-Crystal Nickel-Rich Layered-Oxide Battery Cathode Materials: Synthesis, Electrochemistry, and Intra-Granular Fracture. Energy Storage Mater. 2020, 27, 140–149. [Google Scholar] [CrossRef]
- Zhang, H.; Cen, T.; Tian, Y.; Zhang, X. Synthesis of High-Performance Single-Crystal LiNi0.8Co0.1Mn0.1O2 Cathode Materials by Controlling Solution Super-Saturation. J. Power Sources 2022, 532, 231037. [Google Scholar] [CrossRef]
- Xu, X.; Huo, H.; Jian, J.; Wang, L.; Zhu, H.; Xu, S.; He, X.; Yin, G.; Du, C.; Sun, X. Radially Oriented Single-Crystal Primary Nanosheets Enable Ultrahigh Rate and Cycling Properties of LiNi0.8Co0.1Mn0.1O2 Cathode Material for Lithium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1803963. [Google Scholar] [CrossRef]
- Guo, F.; Xie, Y.; Zhang, Y. Low-Temperature Strategy to Synthesize Single-Crystal LiNi0.8 Co0.1Mn0.1O2 with Enhanced Cycling Performances as Cathode Material for Lithium-Ion Batteries. Nano Res. 2022, 15, 2052–2059. [Google Scholar] [CrossRef]
- Azhari, L.; Meng, Z.; Yang, Z.; Gao, G.; Han, Y.; Wang, Y. Underlying Limitations behind Impedance Rise and Capacity Fade of Single Crystalline Ni-Rich Cathodes Synthesized via a Molten-Salt Route. J. Power Sources 2022, 545, 231963. [Google Scholar] [CrossRef]
- Zheng, L.; Bennett, J.C.; Obrovac, M.N. All-Dry Synthesis of Single Crystal NMC Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2020, 167, 130536. [Google Scholar] [CrossRef]
- Huang, B.; Wang, M.; Zhang, X.; Zhao, Z.; Chen, L.; Gu, Y. Synergistic Coupling Effect of Single Crystal Morphology and Precursor Treatment of Ni-Rich Cathode Materials. J. Alloys Compd. 2020, 830, 154619. [Google Scholar] [CrossRef]
- Zou, Y.G.; Meng, F.; Xiao, D.; Sheng, H.; Chen, W.P.; Meng, X.H.; Du, Y.H.; Gu, L.; Shi, J.L.; Guo, Y.G. Constructing a Stable Interfacial Phase on Single-Crystalline Ni-Rich Cathode via Chemical Reaction with Phosphomolybdic Acid. Nano Energy 2021, 87, 106172. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, G.; Peng, Z.; Cao, Y.; Li, L.; Tan, C.; Wang, Y.; Wang, W.; Du, K. Synthesis and Characterization of Mono-Dispersion LiNi0.8Co0.1Mn0.1O2 Micrometer Particles for Lithium-Ion Batteries. Ceram. Int. 2021, 47, 25680–25688. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, J.; Cao, G.; Li, Y.; Zhou, Y.; Deng, S.; Hai, C. Flux-Free Synthesis of Single-Crystal LiNi0.8Co0.1Mn0.1O2 Boosts Its Electrochemical Performance in Lithium Batteries. J. Power Sources 2020, 464, 228207. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, Z.; Wei, W.; Zhou, L. Superior Cycle Stability of Single Crystal Nickel-Rich Layered Oxides with Micron-Scale Grain Size as Cathode Material for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 5031–5041. [Google Scholar] [CrossRef]
- Kim, Y. Lithium Nickel Cobalt Manganese Oxide Synthesized Using Alkali Chloride Flux: Morphology and Performance as a Cathode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2012, 4, 2329–2333. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.; Tan, X.; Wang, Z.; Cai, Z.; Nan, J.; Xing, Z.; Li, H. Crack-Free Single-Crystal LiNi0.83Co0.10Mn0.07O2 as Cycling/Thermal Stable Cathode Materials for High-Voltage Lithium-Ion Batteries. Electrochim. Acta 2021, 365, 137380. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, J.; Liang, Z.; Potapenko, H.; Zhou, M.; Tang, X.; Zhong, S. The Use of a Single-Crystal Nickel-Rich Layered NCM Cathode for Excellent Cycle Performance of Lithium-Ion Batteries. New J. Chem. 2021, 45, 3652–3659. [Google Scholar] [CrossRef]
- Zhu, H.; Tang, Y.; Wiaderek, K.M.; Borkiewicz, O.J.; Ren, Y.; Zhang, J.; Ren, J.; Fan, L.; Li, C.C.; Li, D.; et al. Spontaneous Strain Buffer Enables Superior Cycling Stability in Single-Crystal Nickel-Rich NCM Cathode. Nano Lett. 2021, 21, 9997–10005. [Google Scholar] [CrossRef]
- Wang, J.; Lu, X.; Zhang, Y.; Zhou, J.; Wang, J.; Xu, S. Grain Size Regulation for Balancing Cycle Performance and Rate Capability of LiNi0.9Co0.055Mn0.045O2 Single Crystal Nickel-Rich Cathode Materials. J. Energy Chem. 2022, 65, 681–687. [Google Scholar] [CrossRef]
- Lee, S.H.; Sim, S.J.; Jin, B.S.; Kim, H.S. High Performance Well-Developed Single Crystal LiNi0.91Co0.06Mn0.03O2 Cathode via LiCl-NaCl Flux Method. Mater. Lett. 2020, 270, 127615. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium Ion, Lithium Metal, and Alternative Rechargeable Battery Technologies: The Odyssey for High Energy Density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Wentker, M.; Greenwood, M.; Leker, J. A Bottom-up Approach to Lithium-Ion Battery Cost Modeling with a Focus on Cathode Active Materials. Energies 2019, 12, 504. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, A.; Nagmani; Puravankara, S. Opportunities in Na/K [Hexacyanoferrate] Frameworks for Sustainable Non-Aqueous Na+/K+batteries. Sustain. Energy Fuels 2022, 6, 550–595. [Google Scholar] [CrossRef]
- Nagmani, D.P.; Tyagi, A.; Puravankara, S. Lithium-Ion Battery Technologies for Electric Mobility–State-of-the-Art Scenario. ARAI J. Mobil. Technol. 2022, 2, 233–248. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and Cost of Materials for Lithium-Based Rechargeable Automotive Batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Yim, C.H.; Karkar, Z.; Abu-Lebdeh, Y. On the Current and Future Outlook of Battery Chemistries for Electric Vehicles—Mini Review. Batteries 2022, 8, 70. [Google Scholar] [CrossRef]





| Solvent | Dielectric Constant | Viscosity (cP) (25 °C) | Ionic Conductivity (mS cm−1) (1 M LiPF6) | Melting Point (°C) | Oxidation Potential (V vs. Li/Li+) |
|---|---|---|---|---|---|
| EC | 89.78@40 °C | 1.93@40 °C | 8.3 | 36.4 | 5.5 |
| PC | 64.95 | 2.51 | 5.6 | −54.5 | 5.2 |
| DMC | 3.107 | 0.59 | 6.0 | 4.6 | 5.5 |
| DEC | 2.820@20 °C | 0.748 | 4.2 | −43 | 5.2 |
| EMC | 2.958 | 0.65 | 3.5 | −53 | 6.1 # |
| FEC | 110 | 4.1 | 5.0 | 18 | 6.6 |
| Salt | Mol. Wt. | Melting Point (Tm)(°C) | Tdecomposition (°C) in Solution | Al Collector Corrosion |
|---|---|---|---|---|
| LiPF6 | 151.9 | 200 | ~80 (EC/DMC) | N |
| LiBF4 | 93.9 | 293 | >100 | N |
| LiAsF6 | 195.9 | 340 | >100 | N |
| LiClO4 | 106.4 | 236 | >100 | N |
| LiTFSI | 286.9 | 234 | >100 | Y |
| Binders | Structure | Solvent | Properties |
|---|---|---|---|
| PVDF | 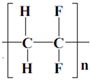 | NMP | Linear crystalline thermoplastic fluoropolymer |
| CMC | 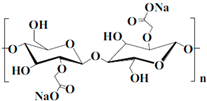 | Water | Linear polymer; environmentally friendly; high viscous; and low ionic impedance |
| Na-alginate | 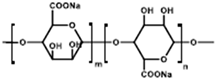 | Water | Self-healing effect; uniform distribution of carboxyl group; low ionic impedance |
| LA132 | 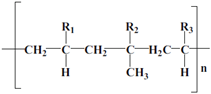 (R1-Acrylamide, R2-Carboxylic acid lithium, R3 Cyano-) | Water | Co-polymer of acrylamide, lithium methacrylate, and acrylonitrile; high adhesion property |
| PAA | 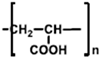 | Water | Linear polymer with uniform distribution of functional group; tuneable mechanical properties |
| PDADMA | 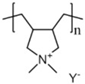 Y = FSI, TFSI, CFSO, BETI | Water | Ionic conductive polymers; wide electrochemical stability window |
| Electrodes | Electrolytes | Additives | Voltage Range | Sp. Capacity (mAh g−1) (C-Rate) | Capacity Retention, % (Cycles, C-Rate) |
|---|---|---|---|---|---|
| NMC111/graphite [14] | 1 M LiPF6 in EC: EMC (3:7, w/w) | 3–4.2 V 3–4.4 V 3–4.6 V | 140.2 (1 C) 162.8(1 C) 183.4(1 C) | 93% (295, 1 C) 94% (295, 1 C) 42% (295, 1 C) | |
| NMC111/graphite [79] | 1 M LiPF6 in EC: DEC (1:3, w/w) | 1 wt% LiDFP | 3–4.5 V | 92.6% (100, 1 C) | |
| NMC111/graphite [127] | 1 M LiPF6 in EC: EMC (3:7, w/w) | 0.1 wt% MUI | 2.8–4.6 V | 197.3 (0.1 C) | 63% (200, 0.3 C) |
| NMC111/graphite [65] | 1 M LiPF6 in EC: EMC (3:7, w/w) | 2 wt% VC 2 wt% VC + 1 wt% MMDS 2 wt% VC + 1 wt% DTD 2 wt% VC + 1 wt% MMDS + 1 wt% TTSPi 2 wt% VC + 1 wt% DTD + 1 wt% TTSPi 3 wt% PES 2 wt% PES + 1 wt% MMDS 2 wt% PES + 1 wt% DTD 2 wt% PES + 1 wt% TTSPi 2 wt% PES + 1 wt% MMDS + 1 wt% TTSPi 2 wt%PES + 1 wt%DTD + 1 wt%TTSPi | 2.8–4.2 V | 84% (500, 0.4 C) 87% (500, 0.4 C) 86% (500, 0.4 C) 88% (500, 0.4 C) 90% (500, 0.4 C) 87% (500, 0.4 C) 80% (500, 0.4 C) 89% (500, 0.4 C) 84% (500, 0.4 C) 89% (500, 0.4 C) 91% (500, 0.4 C) | |
| NMC111/graphite [72] | 1 M LiPF6 in EC: EMC (3:7, w/w) | 2 wt% PES + 1 wt% MMDS + 1 wt% TTSPi | 2.8–4.2 V (55 °C) | >80% (900, 0.4 C) | |
| NMC111/graphite [121] | 1 M LiPF6 in EC: EMC (3:7, w/w) | 2 wt% VC + 1 wt% DTD + 0.5 wt% TTSP + 0.5 wt% TTSPi | 2.8–4.2 V (40 °C) | 97% (500, C/2.2) | |
| NMC111/graphite [126] | 1 M LiTFSI in MCP 1 M LiPF6 in MCP 1 M LiPF6 in PC | 3 wt% FEC | 2.8–4.2 V | 94.4% (195, 1 C) 87.6% (195, 1 C) 92.3% (195, 1 C) | |
| NMC111/graphite [122] | 1 M LiPF6 in EC: DEC (1:3, w/w) | 0.5 wt% EDPN | 3–4.5 V 3–4.2 V | 156.2 (1 C) | 83.9% (100, 1 C) 91% (100, 1 C) |
| NMC111/graphite [123] | 1 M LiPF6 in EC: DMC: EMC (1:1:1, w/w) | 0.2 wt% SDPN | 3–4.6 V | 77.3% (100, 0.2 C) | |
| NMC111/graphite [124] | 1 M LiPF6 in EC: DMC: EMC (1:1:1, w/w) | 0.1 wt% DMSM | 3–4.6 V | 175.1 (0.2 C) | 80.1% (100, 0.2 C) |
| NMC111/graphite [128] | 1 M LiPF6 in EC: DMC: EMC (1:1:1, w/w) | 0.2 wt% TFPM 0.5 wt% TFPE | 3–4.6 V | 75.4% (100, 0.2 C) 76.1% (100, 0.2 C) | |
| NMC111/Li [12] | 1 M LiPF6 in EC: DEC (1:1, v/v) | 3–4.3 V (55 °C) | 163 (0.1 C) | 92.4% (100, 0.5 C) | |
| NMC111/Li [120] | 1 M LiPF6 in EC: DMC (3:7, v/v) | 3.5–4.2 V | 150 (0.17 mA/cm2) | ||
| NMC111/Li [129] | 1.2 M LiPF6 in EC: PC: DMC (1:1:3, w/w) | 2.9–4.6 V | 200 (0.1 mA/cm2) | ||
| NMC111/Li [130] | 1 M LiPF6 in EC: EMC (3:7, w/w) | 2 vol% VC 2 vol% FEC 2 vol% ES | 2.5–4.2 V | 121 (0.1 C) 121 (0.1 C) 108 (0.1 C) | 65% (150, 1 C) 95% (150, 1 C) 56% (150, 1 C) |
| NMC111/Li [64] | 1 M LiPF6 in EC: DMC (1:2, v/v) | 0.5 wt% TMSPI | 3–4.5 V | 91.2% (100, 0.5 C) | |
| NMC111/Li [46] | 1 M LiPF6 in EC: DMC: EMC (1:1:1 w/w) | 1 wt% LiDFP 1 wt% LiDFP + 10 wt% FEC | 2.8–4.3 V | 67% (400, 1 C) 86.2% (400, 1 C) | |
| NMC111/Li [131] | 1 M LiPF6 in EC: DEC (1:1 v/v) | 2 wt% LiTDI | 3–4.2 V (55 °C) | 80% (830, 1 C) | |
| NMC111/Li [132] | 1 M LiPF6 in EC: EMC: DEC (1:1:1, v/v/v) | 0.5 wt% LiBOB 0.2 wt% LiDFOB | 3–4.6 V | 191 (0.6 C) 187.2 (0.6 C) | 91.8% (60, 0.6 C) 88.2% (60, 0.6 C) |
| NMC111/Li [125] | 1 M LiPF6 in EC: DEC: DMC (3:5:2, w/w) | 3 wt% TMB | 3–4.5 V | 154 (0.5 C) | 99% (300, 1 C) |
| NMC111/Li [133] | 1 M LiPF6 in EC: EMC (3:7, w/w) | 1 vol% VC | 3–4.2 V | 137 (0.1 C) | 55% (200, 1 C) |
| NMC111/MCMB [133] | 1 M LiPF6 in EC: EMC (3:7, w/w) | 1 vol% VC | 3–4.2 V | 132.5 (0.1 C) | 97% (200, 1 C) |
| Electrodes | Electrolytes | Additives | Voltage Range | Sp. Capacity (mAh g−1) (C-Rate) | Capacity Retention, % (Cycles, C-Rate) |
|---|---|---|---|---|---|
| NMC442/graphite [72,137] | 1 M LiPF6 in EC: EMC (3:7, w/w) | 2 wt%PES + 1 wt%MMDS + 1 wt%TTSPi | 3–4.4 V (45 °C) | 85% (500, C/2.5) | |
| NMC442/graphite [138] | 1 M LiPF6 in EMC:VC (98:2, w/w) | 1% TAP or 1% PPF | 2.8–4.4 (55 °C) | 80% (350, C/2.5) | |
| NMC442/graphite [139] | 1 M LiPF6 in FEC: TFEC (1:1, w/w) | 1 wt% PES | 2.8–4.5 (40 °C) | ~80% (800, C/2.4) | |
| NMC442/graphite [140] | 1 M LiPF6 in SL: EMC (3:7, w/w) | 2 wt% VC + 2 wt% TAP | 2.8–4.4 (40 °C) | 80% (500, C/2.4) | |
| NMC442/graphite [141] | 1 M LiPF6 in EC: DMC: EMC (1:1:1, v/v) | 1 wt% TMSP | 2.75–4.35 V | 164.6(1 C) | 90.8%(70, 1 C) |
| Electrodes | Electrolytes | Additives | Voltage Range | Sp. Capacity (mAh g−1) (C-Rate) | Capacity Retention, % (Cycles, C-Rate) |
|---|---|---|---|---|---|
| NMC532/graphite [60] | 1 M LiPF6 in EC: DEC (1:1 v/v) | 1 wt% VC | 2.5–4.2 V (60 °C) | 159 (75 mA gm−1) | 79% (100, 75 mA gm−1) |
| NMC532/graphite [68] | 1.2 M LiPF6 in EC: EMC (3:7 w/w) | 1 wt% TMSPi 1 wt% TEPi | 3–4.4 V | 190 (0.1 C) 194.5 (0.1 C) | 88.8% (119, 0.3 C) 81.7% (119, 0.3 C) |
| NMC532/graphite [80] | 1 M LiPF6 in EC: DEC (1:3 w/w) | 1 wt% LiDFP | 3–4.5 V | 152.8 (0.1 C) | 93.8% (100, 1 C) |
| NMC532/graphite [142] | 1 M LiPF6 in EC: EMC (1:2 w/w) | 2 wt% LiDFP | 2.75–4.4 V (25 °C) 2.75–4.4 V (45 °C) 2.75–4.2 V (−10 °C) | 93% (150, 1 C) 86% (150, 1 C) 92.7% (100, 0.3 C) | |
| NMC532/graphite [143] | 1 M LiPF6 in EC: EMC (1:2 w/w) | 1 wt% LiBF4 | 3–4.5 V | 178.1 (1 C) | 90.1% (100, 1 C) |
| NMC532/graphite [144] | 1 M LiPF6 in EC: EMC: DEC (3:5:2 w/w) | 1 wt% NOB | 2.75–4.5 V (25 °C) | 73% (100, 1 C) | |
| NMC532/graphite [145] | 1 M LiPF6 in EC: EMC (1:2 w/w) | 0.5 wt% DMS 0.5 wt% DTD | 2.75–4.2 V (−10 °C) 2.75–4.2 V (25 °C) 2.75–4.2 V (45 °C) 2.75–4.2 V (−10 °C) | 98.84% (50, 0.2 C) 89.04% (350, 1 C) 95.3% (100, 1 C) 81.14% (50, 0.2 C) | |
| NMC532/graphite [146] | 1 M LiPF6 in EC: EMC (1:2 w/w) | 0.5 wt% TMSB | 2.5–4.4 V 3–4.4 V | 181 (0.2 C) 167.9 (1 C) | 92.3% (150, 1 C) |
| NMC532/graphite [147] | 1 M LiPF6 in EC: DEC: EMC (3:2:5, w/w) | 0.25 wt% SDM | 2.75–4.5 V | 96.9% (50, 0.2 C) | |
| NMC532/Li [150] | 1 M LiPF6 in EC: DMC (1:1 v/v) | 3–4.3 V | 150 (0.4 C) | 95% (50, 0.4 C) | |
| NMC532/Li [151] | 1 M LiPF6 in EC: DEC (1:1 v/v) | 3–4.4 V | 179.6 (0.2 C) | 94.8% (80, 0.2 C) | |
| NMC532/Li [61] | 1 M LiPF6 in EC: DEC (1:1 v/v) | 5 vol% FEC | 3–4.3 V | 154 (1 C) | 65% (100, 1 C) |
| NMC532/Li [152] | 1 M LiPF6 in EC: EMC (3:7 v/v) | 2 wt% DTD | 2.75–4.5 V | 84% (100, 0.5 C) | |
| NMC532/Li [148] | 1 M LiPF6 in EC: EMC (3:7 v/v) | 2 wt% BDTD | 3–4.6 V | 189 (0.5 C) | 91.6% (100, 0.5 C) |
| NMC532/Li [153] | 1 M LiPF6 in EC: DMC: DEC (1:1:1 v/v) 1 M LiBF4 in FEC:SN (1:4 w/w) | 3–4.5 V 3–4.7 V 3–4.5 V 3–4.7 V | 56.3% (100, 0.5 C) 35.5% (100, 0.5 C) 81.6% (100, 0.5 C) 73.6% (100, 0.5 C) | ||
| NMC532/Li [149] NMC532/graphite | 1 M LiPF6 in EC: DMC (3:7 v/v) | 0.01 mg ml−1 Li2S + 0.5 vol% AN | 3–4.5 V 2.8–4.5 V | 80.74% (200, 1 C) 81% (180, 0.5 C) |
| Electrodes | Electrolytes | Additives | Voltage Range | Sp. Capacity (mAh g−1) (C-Rate) | Capacity Retention, % (Cycles, C-Rate) |
|---|---|---|---|---|---|
| NMC622/graphite [154] | 1 M LiPF6 in EC: EMC (3:7 w/w) | 3–4.4 V (25 °C) 3–4.4 V (40 °C) 3–4.4 V (50 °C) | 174 (1 C) 184 (1 C) 190 (1 C) | 84% (308, 1 C) 85% (308, 1 C) 73% (308, 1 C) | |
| NMC622/graphite [166] | 2.3 mol kg−1 LiTFSI in EC: DME (1:2 v/v) | 2.75–4.2 V | 169.3 (0.5 C) | 98.7% (50, 0.5 C) | |
| NMC622/graphite [155] | 1 M LiPF6 in EC: EMC (1:2, w/w) | 0.5 wt% PPDI | 3–4.2 V (rt) 3–4.2 V (45 °C) | 84.9% (600, 1 C) 81.3% (300,1 C) | |
| NMC622/graphite [156] | 1 M LiPF6 in EC: EMC (1:2, w/w) | 1 wt% CEP | 3–4.5 V | 81.5% (50, 1 C) | |
| NMC622/graphite [157] | 1 M LiPF6 in EC: EMC (1:2, w/w) | 0.1 wt% HDI | 3–4.2 V | 82.9% (600, 1 C) | |
| NMC622/graphite [158] | 1 M LiPF6 in EC: EMC (1:2, w/w) | 1 wt% PDTD | 3–4.2 V | 83.7% (500, 1 C) | |
| NMC622/Li [159] NMC622/graphite | 1 M LiPF6 in EC: EMC: DMC (1:1:1, v/v) | 0.25 wt% DSON | 3–4.3 V 3–4.2 V (55 °C) | 84.7% (100, 1 C) 66.8% (50, 0.5 C) | |
| NMC622/graphite [160] NMC622/Li | 1 M LiPF6 in EC: DEC: EMC (3:2:5, w/w) | 1 wt% pTSF | 3–4.35 V (25 °C) 3–4.35 V (55 °C) | 88% (600, 1 C) 75% (300, 1 C) 89% (100, 1 C) | |
| NMC622/Li [167] | 1.2 M LiPF6 in DFEC: EMC (3:7 v/v) | 3–4.4 V | 189.9 (0.1 C) | 83% (400, C/3) | |
| NMC622/Li [168] | 1.15 M LiPF6 in EC: EMC (3:7, v/v) | 2 wt% VC | 3–4.3 V (60 °C) | 180.9 (C/5) | 91.2% (60, 1 C) |
| NMC622/Li [169] | 1 M LiPF6 in EC: EMC: DEC (1:1:1, v/v/v) | 10 wt% FEC | 2.8–4.6 V | 196.3 (1 C) | 87.3% (100, 1 C) |
| NMC622/Li [161] | 1 M LiPF6 in EC: EMC: DEC (1:1:1, w/w/w) | 1 wt% TIB | 3–4.5 V | 183.4 (1 C) | 82.7% (300, 1 C) |
| NMC622/Li [162] | 1 M LiPF6 in EC: EMC: DMC (1:1:1, v/v/v) | 1 wt% DPDMS | 2.8–4.3 V | 168.2 (1 C, 25 °C) | 93.3% (200, 2 C, 55 °C) |
| NMC622/Li [163] | 1 M LiPF6 in EC: EMC: DMC (1:1:1, v/v/v) | 0.5 wt% ViD4 0.5 wt% D4 0.5 wt% OMCTS | 3–4.5 V | 187.2 (0.2 C) 187.7 (0.2 C) 187 (0.2 C) | 83.6% (150, 1 C) 81.3% (150, 1 C) 81.9% (150, 1 C) |
| NMC622/Li [164] | 1 M LiPF6 in EC: EMC (3:7, w/w) | 0.5 wt% LiDFOB 0.25 wt% 3HT | 2.8–4.5 V | 91.6% (50, 0.1 C) 93.5% (50, 0.1 C) | |
| NMC622/Li [165] | 1 M LiPF6 in PC: EMC: TEP (42.5:42.5:15, v/v) | 2 wt% THFP | 2.8–4.3 V | 160 (100 mA g−1) | 82% (200, 100 mA g−1) |
| Electrodes | Electrolytes | Additives | Voltage Range | Sp. Capacity (mAh g−1) (C-Rate) | Capacity Retention, % (Cycles, C-Rate) |
|---|---|---|---|---|---|
| NMC811/graphite [14] | 1 M LiPF6 in EC: EMC (3:7 w/w) | 3–4.0 V 3–4.1 V 3–4.2 V | 131.9 (1 C) 149.3 (1 C) 172.5 (1 C) | 90% (296, 1 C) 77% (296, 1 C) 66% (296, 1 C) | |
| NMC811/graphite [67] | 1 M LiPF6 in EC: DMC (1:1, v/v) | 1 vol% VC+ 1 vol% TMSPi | 2.75–4.2 V | 91% (200, C/3) | |
| NMC811/graphite [170] | 1 M LiPF6 in EC: EMC (3:7, w/w) | 0.5 wt% TPPO | 2.8–4.3 V | 198 (0.1 C) | 92% (97, 0.5 C) |
| NMC811/graphite [171] | 1 M LiPF6 in EC: DMC (3:7, w/w) | 1 wt% PTSS | 3–4.35 V | 117 (0.2 C) | 62.7% (100, 1 C) |
| NMC811/graphite [172] | 1 M LiPF6 in EMC:EC: DMC (5:3:2, w/w) | 1 w% PFBS | 3–4.2 V (25 °C) 3–4.2 V (45 °C) | 89.9% (400, 1 C) 89.01% (400, 1 C) | |
| NMC811/graphite [173] | 1 M LiPF6 in EC: EMC (3:7, v/v) | 0.5 wt%ADN | 2.7–4.3 V | 85.60% (200, 0.3 C) | |
| NMC811/graphite [174] | 1 M LiPF6 in EC: EMC (1:2, w/w) | 0.25 wt% DES 1 wt% DES | 2.75–4.3 V 2.75–4.5 V | 77.25% (300, 1 C) 82.53% (150, 1 C) | |
| NMC811/Li [175] NMC811/graphite NMC811/Li Pouch | 1.1 M LiPF6 in EC: DEC (1:1, v/v) | 0.1 M LiDFOB + 2 wt% TMSP | 2.7–4.5 V 2.7–4.7 V 2.7–4.3 V (45 °C) 2.7–4.5 V (45 °C) 2.7–4.5 V 2.7–4.5 V | 74.5% (800, 1 C) 76.3% (500, 1 C) 85% (500, 1 C) 90% (400, 1 C) 82.8% (500, 1 C) 94% (200, 1 C) | |
| NMC811/Li [176] | 1 M LiPF6 in EC: DEC (1:1, v/v) | 0.5 wt% TMSB | 2.7–4.3 V | 165.8 (0.2 C) | |
| NMC811/Li [177] | 1 M LiPF6 in EC: EMC (1:2, v/v) | 2 wt% TPPa | 3–4.3 V (55 °C) | 210.5 (0.1 C) | 63.5% (100, 1 C) |
| NMC811/Li [178] | 1 M LiPF6 and 0.1 M LiDFOB in EC: FEC: EMC: DEC (2:1:5:2 w/w) | 1 wt% PVS | 3–4.3 V | 80.8% (400, 0.5 C) 80.0% (400, 1 C) | |
| NMC811/Li [179] | 1 M LiPF6 in EC: EMC: DEC (5:3:2, w/w/w) | 5 wt% TPBX | 3–4.35 V | 192.1 (1 C) | 78% (100, 1 C) |
| NMC811/Li [180] | 1 M LiPF6 in FEC: DMC (1:1, v/v) | 3 wt% PFN 3 wt%PFN + 2 wt% LiDFOB | 2.7–4.4 V | 92.5% (50, 0.5 C) 84.2% (100, 0.5 C) | |
| NMC811/Li [181] | 1 M LiPF6 in EC: DMC: EMC (1:1:1, v/v) | 0.2 wt% TMSTFA | 3–4.3 V | 149 (1 C) | 80% (200, 1 C) |
| NMC811/Li [182] | 1 M LiFSI in DOL: DME (1:1, w/w) | 1 wt% CPSA | 3–4.3 V | 157.08 (0.5 C) | 82.39% (180, 0.5 C) |
| Electrodes | Electrolytes | Additives | Voltage Range (V) | Temperature (°C) | Sp. Capacity (mAh g−1) (C-Rate) | Capacity Retention, % (Cycles, C-Rate) |
|---|---|---|---|---|---|---|
| NMC111/Li [208] | 1 M LiPF6 in EC: EMC (3:7 w/w) | 2.8–4.3 V | 163.9 (0.1 C) | ~85% (300, 0.5 C) | ||
| NMC111/Li [209] | 1 M LiPF6 in EC: DMC (3:7 v/v) | 2.8–4.4 V | 25 | 160 (0.1 C) | ||
| NMC111/Li [210] | 1 M LiPF6 in EC: DMC (1:1 v/v) | 2.5–4.4 V | 25 55 | 171 (0.1 C) | 97.2% (100, 0.1 C) 96.2% (80, 0.1 C) | |
| NMC111/Li [211] | 1 M LiPF6 in EC: DEC: EMC (1:1:1 v/v) | 2.8–4.4 V | 173.1 (0.1 C) | 88.7% (50, 0.1 C) | ||
| LiNi0.5Co0.25Mn0.25O2/Li [212] | 1 M LiClO4 in EC: EMC (3:7 w/w) | 1 wt% LiPO2F2 | 3–4.3 V | 25 55 | 154 (1 C) 156 (1 C) | 95.3% (200, 1 C) 91.6% (100, 1 C) |
| NMC532/Li [213] NMC532/graphite | 1 M LiPF6 in EC: DEC (1:1 v/v) | 2.75–4.4 V 2.75–4.6 V 2.75–4.4 V 2.75–4.4 V | 25 25 55 45 | 177.5 (1 C) 187.4 (1 C) 175.9 (1 C) 172.6 (1 C) | 90.6% (100, 1 C) 81% (100, 1 C) 92.6% (100, 1 C) 98.7% (500, 1 C) | |
| NMC532/Li [214] | 1 M LiPF6 in EC: EMC: DMC (3:2:5 v/v) | 3–4.5 V | 167.3 (1 C) | 90.3% (100, 1 C) | ||
| NMC532/graphite [215] | 1 M LiPF6 in EMC: DEC: DMC (1:1:1 v/v) | 3–4.2 V | 25 | 141.2 (1 C) | 99% (1000, 1 C) | |
| NMC532/graphite [216] | 1 M LiPF6 in EC: EMC (3:7 w/w) 1 M LiPF6 in EMC | 2 wt% PES + 1 wt% TTSPi + 1 wt% DTD 5 wt% FEC | 3–4.4 V | 40 | ~97% (800, C/2) ~96% (800, C/2) | |
| NMC532/graphite [217] | 1 M LiPF6 in EC: EMC (3:7 w/w) | 1 wt% DTD 2 wt% FEC 2 wt% VC 2 wt% FEC + 1 wt% DTD 2 wt% VC + 1 wt% DTD | 3–4.2 V | 40 | 96.8% (400, C/3) 96.4% (400, C/3) 95.8% (400, C/3) 97.6% (400, C/3) 97.3% (400, C/3) | |
| NMC622/Li [218] | 1 M LiPF6 in EC: DEC: DMC (1:1:1 v/v) | 2.8–4.3 V | 183.7 (0.2 C) | 89.93% (100,0.2 C) | ||
| NMC622/Li [219] NMC622/graphite | 1 M LiPF6 in EC: DEC: DMC (1:1:1 v/v) | 2.8–4.3 V 2.8–4.35 V | 25 25 | 171.3 (0.1 C) 179.3 (0.1 C) | 83.3% (200, 1 C) 85.24% (800, 1 C) | |
| NMC622/Li [220] | 1 M LiPF6 in EC: DMC: EMC (1:1:1 v/v) | 2.8–4.5 V | 190.4 (0.1 C) | 96.5% (50, 1 C) | ||
| NMC622/Li [221] | 1 M LiPF6 in EC: DMC (1:1 v/v) | 2.8–4.3 V | 175.5 (0.1 C) | 84.4% (100, 1 C) | ||
| NMC622/Li [222] | 1 M LiPF6 in EC: DMC (1:1 v/v) | 2.8–4.5 V | 190.1 (1 C) | 96% (50, 1 C) | ||
| NMC622/Li [208] | 1 M LiPF6 in EC: EMC (3:7 w/w) | 2.8–4.3 V | 176 (0.1 C) | 87.1% (200, 0.5 C) | ||
| NMC622/Li [223] NMC622/graphite | 1 M LiPF6 in EC: EMC (3:7 w/w) | 2 wt% VC | 2.8–4.3 V | 30 55 30 | 183 (0.1 C) | ~94% (300, 1 C) ~85% (300, 1 C) 80% (800, 1 C) |
| NMC622/Li [189] | 1 M LiPF6 in EC: DEC (1:2 v/v) | 2 wt% FEC + 1 wt% DTD | 3–4.4 V | 30 | ~96% (50, C/5) | |
| NMC811/Li [224] | 1 M LiPF6 in EC: DMC (1:1 v/v) | 2.7–4.3 V | 25 | 206.3 (0.1 C) | 93% (100, 0.1 C) | |
| NMC811/Li [225] | 1 M LiPF6 in EC: DMC (1:1 v/v) | 3–4.3 V | 203.4 (0.1 C) | 95.5% (300, 1 C) | ||
| NMC811/Li [226] | 1 M LiPF6 in EC: DMC (1:1 v/v) | 2.8–4.3 V | 226.9 (0.1 C) | 95.1% (100, 1 C) | ||
| NMC811/Li [227] | 1 M LiPF6 in EC: EMC (3:7 w/w) | 2.8–4.5 V | 199 (C/2) | |||
| NMC811/Li [228] | 1 M LiPF6 in EC: DEC: FEC (3:6:1 v/v) | 2.5–4.3 V | 30 | 162 (C/20) | 90% (90, C/5) | |
| NMC811/Li [229] | 1 M LiPF6 in EC: DMC: EMC (1:1:1 v/v) | 2.8–4.5 V | 221.2 (0.1 C) | 91.6% (50, 1 C) | ||
| NMC811/Li [230] | 1 M LiPF6 in EC: DMC: DEC (1:1:1 v/v) | 3–4.3 V | 206 (0.1 C) | 92% (200, 0.5 C) | ||
| NMC811/Li [231] | 1 M LiPF6 in EC: DMC: EMC (1:1:1 v/v) | 2.8–4.3 V | 196.7 (0.2 C) | 95.2% (100, 1 C) | ||
| NMC811/Li [232] | 1 M LiPF6 in EC: DMC: EMC (1:1:1 v/v) | 3–4.4 V | 25 55 25 | 216 (0.1 C) 209 (1 C) | 81.8% (200, 1 C) 85.3% (100, 1 C) 85% (100, 4 C) | |
| NMC811/Li [233] | 1 M LiPF6 in EC: DMC: EMC (1:1:1 w/w) | 2.8–4.3 V | 198.9 (0.1 C) | 96.2% (150, 1 C) | ||
| NMC811/Li [234] | 1.15 M LiPF6 in EC: DMC: EMC (3:3:4 v/v) | 3–4.45 V | 176 (0.1 C) | |||
| NMC811/Li [190] | 1.5 M LiPF6 in EC: EMC: DMC (25:5:70 v/v) | 2 wt% FEC + 1 wt% LFO | 3–4.2 V | 55 | ~85% (1000, C/3) | |
| LiNi0.83Mn0.07Co0.10O2/Li [235] LiNi0.83Mn0.07Co0.10O2/graphite | 1 M LiPF6 in EC: DEC (3:7 w/w) | 3–4.5 V 3–4.9 V 3–4.35 V | 94.5% (30, 0.5 C) 93.3% (15, 0.1 C) 84.8% (400, 0.5 C) | |||
| LiNi0.83Mn0.05Co0.12O2/Li [236] | 1 M LiPF6 in EC: DEC (1:1 v/v) | 2.75–4.3 V | 25 | 209.7 (0.1 C) | 99.56% (100, 0.2 C) | |
| LiNi0.83Mn0.05Co0.12O2/Li [237] | 1.2 M LiPF6 in EC: EMC (3:7 v/v) | 2 wt% VC | 2.8–4.3 V | 209 (0.1 C) | 96.6% (100, 1 C) 93.1% (200, 1 C) | |
| LiNi0.9Co0.055Mn0.045O2/Li [238] | 1 M LiPF6 in EC: DMC: EMC (1:1:1 v/v) | 2.8–4.3 V | 27 | 220.6 (0.1 C) | 93% (50, 1 C) | |
| LiNi0.91Mn0.03Co0.06O2/Li [239] | 1 M LiPF6 in EC: DMC: EMC (1:1:1 v/v) | 3–4.3 V | 203.8 (0.1) | 80.8% (70, 0.5 C) |
| Cell Chemistry (Cathode/ Anode) | Cell Specification | Battery Packs | Electric Vehicle Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Producer | Type | Capacity (Ah) | Voltage(V) | Specific Energy (Wh kg−1) | Energy Density (Wh L−1) | Energy (kWh) | Range (km) | ||
| NMC/LTO | Toshiba | Prismatic | 20 | 2.30 | 89 | 200 | 20 | 130 | Honda Fit EV (2013) |
| NMC111/C | Li-Tec | Pouch | 52 | 3.65 | 152 | 316 | 17 | 145 | Smart Fortwo EV (2013) |
| NMC111/C | Samsung SDI | Prismatic | 60 | 3.7 | 122 | 228 | 22 | 130 | BMW i3 (2014) |
| NMC622/C | SK Innovation | Pouch | 38 | 3.70 | 27 | 145 | Kia Soul EV (2014) | ||
| NMC/C | Panasonic | Prismatic | 25 | 3.70 | 130 | 215 | 24 | 190 | VW e-Golf (2015) |
| NMC/C | LG Chem | Pouch | 56 | 3.65 | 186 | 393 | 60 | 383 | Chevrolet Bolt (2016) |
| NMC111/C | Samsung SDI | Prismatic | 94 | 3.7 | 189 | 357 | 33 | 183 | BMW i3 (2017) |
| NMC721/C | LG Chem | Pouch | 59 | 3.70 | 241 | 466 | 41 | 400 | Renault Zoe (2017) |
| NMC532/C | AESC | Pouch | 56.3 | 3.65 | 130 | 205 | 39 | 240 | Nissan Leaf (2018) |
| NMC622/C | Samsung SDI | Prismatic | 120 | 3.7 | 42 | 246 | BMW i3 (2019) | ||
| NMC721/C | LG Chem | Pouch | 64.6 | 3.7 | 259 | 648 | 93.4 | 100 | Porsche Taycan (2019) |
| NMC532/C | LG Chem | Pouch | 78 | 292 | Volvo XC40 (2019) | ||||
| NMC721/C | LG Chem | Pouch | 145 | 1.85 | 164 | 267 | 58 | 350–544 | Volkswagen ID.3 |
| NMC622/C | LG Chem | Pouch | 55 | 3.75 | 151 | 228 | 65 | 417 | Chevrolet Bolt (2020) |
| NMC622/C (Li-ion Polymer) | SK Innovation | Pouch | 180 | 3.56 | 250 | 64 | 391 | Kia Soul EV (2020) | |
| NMC622/C | LG Chem | Laminated | 56.3 | 151 | 62 | 364 | Nissan Leaf E plus (2020) | ||
| NMC721/C | LG Chem | Pouch | 64.6 | 3.65 | 263 | 648 | 85 | 392 | Audi e-tron GT (2021) |
| NMC721/C | LG Chem | Pouch | 78 | 3.65 | 156 | 77 | 305 | Audi Q4 e-tron-SUV (2021) | |
| NMC622/C | LG Chem (Umicore) | Pouch | 60 | 142 | 164 | 64 | 484 | Hyundai KONA Electric (2021) | |
| NMC/C (Li-ion Polymer) | LG Chem | Pouch | 77 | 488 | Hyundai ioniq 5-LR AWD (2021) | ||||
| NMC811/C (Li-ion Polymer) | SK Innovation | Pouch | 180 | 3.56 | 250 | 64 | 370 | Kia Niro (2021) | |
| NMC811/C (Li-ion Polymer) | LG Chem | Pouch | 11.2 | 163 | 230 | 78 | 303 | Kia EV6-LR AWD (2021) | |
| Electrodes | Electrolytes | Additives | Voltage Range | Capacity Reported (mAh g−1) | Cycle Stability |
|---|---|---|---|---|---|
| NMC111/graphite | 1 M LiPF6 in EC: EMC (3:7, w/w) | 0.1 wt% MUI without | 2.8–4.6 V | 197.3 (0.1 C) 197.5 (0.1 C) | 63% (200, 0.3 C) 47% (200, 0.3 C) |
| NMC442/graphite | 1 M LiPF6 in EC: DMC: EMC (1:1:1, v/v) | 1 wt% TMSP without | 2.75–4.35 V | 164.6 (1 C) | 90.8% (70, 1 C) |
| NMC532/graphite | 1.2 M LiPF6 in EC: EMC (3:7 w/w) | 1 wt% TMSPi without | 3–4.4 V | 190 (0.1 C) 191 (0.1 C) | 88.8% (119, 0.3 C) 85.6% (119, 0.3 C) |
| NMC622/graphite | 1 M LiPF6 in EC: EMC (1:2, w/w) | 1 wt% CEP without | 3–4.5 V | 192 187 | 81.5% (50, 1 C) 27.4% (50, 1 C) |
| NMC811/graphite | 1 M LiPF6 in EC: EMC (3:7, w/w) | 0.5 wt% TPPO without | 2.8–4.3 V | 198 (0.1 C) 163 (0.1 C) | 92% (97, 0.5 C) 86% (97, 0.5 C) |
| NMC111/Li | 1 M LiPF6 in EC: EMC: DEC (1:1:1, v/v/v) | 0.5 wt% LiBOB without | 3–4.6 V | 191 (0.6 C) 183.7 (0.6 C) | 91.8% (60, 0.6 C) 78.8% (60, 0.6 C) |
| NMC532/Li | 1 M LiPF6 in EC: EMC (3:7 v/v) | 2 wt% BDTD without | 3–4.6 V | 189 (0.5 C) 192.9 (0.5 C) | 91.6% (100, 0.5 C) 87.2% (100, 0.5 C) |
| NMC622/Li | 1 M LiPF6 in EC: EMC: DEC (1:1:1, v/v/v) | 10 wt% FEC without | 2.8–4.6 V | 196.3 (1 C) 187.8 (1 C) | 87.3% (100, 1 C) 60.8% (100, 1 C) |
| NMC811/Li | 1 M LiPF6 in EC: EMC: DEC (5:3:2, w/w/w) | 5 wt% TPBX without | 3–4.35 V | 192.1 (1 C) 189.7 (1 C) | 78% (100, 1 C) 57% (100, 1 C) |
| Electrodes | Electrolytes | Voltage Range (V) | Capacity Reported (mAh g−1) | Cycle Stability |
|---|---|---|---|---|
| NMC111/Li | 1 M LiPF6 in EC: DMC (1:1 v/v) | 2.5–4.4 V | 171 (0.1 C) | 97.2% (100, 0.1 C) |
| NMC532/Li | 1 M LiPF6 in EC: DEC (1:1 v/v) | 2.75–4.4 V | 177.5 (1 C) | 90.6% (100, 1 C) |
| NMC622/Li | 1 M LiPF6 in EC: DMC: EMC (1:1:1 v/v) | 2.8–4.5 V | 190.4 (0.1 C) | 96.5% (50, 1 C) |
| NMC811/Li | 1 M LiPF6 in EC: DMC (1:1 v/v) | 2.8–4.3 V | 226.9 (0.1 C) | 95.1% (100, 1 C) |
| LiNi0.83Mn0.05Co0.12O2/Li | 1 M LiPF6 in EC: DEC (1:1 v/v) | 2.75–4.3 V | 209.7 (0.1 C) | 99.56% (100, 0.2 C) |
| LiNi0.9Co0.055Mn0.045O2/Li | 1M LiPF6 in EC: DMC: EMC (1:1:1 v/v) | 2.8–4.3 V | 220.6 (0.1 C) | 93% (50, 1 C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, D.; Manna, S.; Puravankara, S. Electrolytes, Additives and Binders for NMC Cathodes in Li-Ion Batteries—A Review. Batteries 2023, 9, 193. https://doi.org/10.3390/batteries9040193
Das D, Manna S, Puravankara S. Electrolytes, Additives and Binders for NMC Cathodes in Li-Ion Batteries—A Review. Batteries. 2023; 9(4):193. https://doi.org/10.3390/batteries9040193
Chicago/Turabian StyleDas, Dhrubajyoti, Sanchita Manna, and Sreeraj Puravankara. 2023. "Electrolytes, Additives and Binders for NMC Cathodes in Li-Ion Batteries—A Review" Batteries 9, no. 4: 193. https://doi.org/10.3390/batteries9040193
APA StyleDas, D., Manna, S., & Puravankara, S. (2023). Electrolytes, Additives and Binders for NMC Cathodes in Li-Ion Batteries—A Review. Batteries, 9(4), 193. https://doi.org/10.3390/batteries9040193






