High-Energy and High-Power Primary Li-CFx Batteries Enabled by the Combined Effects of the Binder and the Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trahey, L.; Brushett, F.R.; Balsara, N.P.; Ceder, G.; Cheng, L.; Chiang, Y.M.; Hahn, N.T.; Ingram, B.J.; Minteer, S.D.; Moore, J.S.; et al. Energy storage emerging: A perspective from the Joint Center for Energy Storage Research. Proc. Natl. Acad. Sci. USA 2020, 117, 12550–12557. [Google Scholar] [CrossRef]
- Yang, X.G.; Liu, T.; Ge, S.; Rountree, E.; Wang, C.Y. Challenges and key requirements of batteries for electric vertical takeoff and landing aircraft. Joule 2021, 5, 1644–1659. [Google Scholar] [CrossRef]
- Bills, A.; Sripad, S.; Fredericks, W.L.; Singh, M.; Viswanathan, V. Performance metrics required of next-generation batteries to electrify commercial aircraft. ACS Energy Lett. 2020, 5, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Krause, F.; Ruiz, J.; Jones, S.; Brandon, E.; Darcy, E.; Iannello, C.; Bugga, R. Performance of commercial Li-ion cells for future NASA missions and aerospace applications. J. Electrochem. Soc. 2021, 168, 040504. [Google Scholar] [CrossRef]
- Krause, F.C.; Jones, J.P.; Jones, S.C.; Pasalic, J.; Billings, K.J.; West, W.C.; Smart, M.C.; Bugga, R.V.; Brandon, E.J.; Destephen, M. High specific energy lithium primary batteries as power sources for deep space exploration. J. Electrochem. Soc. 2018, 165, A2312. [Google Scholar] [CrossRef]
- Ndzebet, E.; Destephen, M.; Zhang, D.; Darch, D. High Power and High Rate Li/CFx-MnO2 Pouch Cell Hybrid Technology. In Proceedings of the 48th Power Sources Conference, Denver, CO, USA, 11–14 June 2018; pp. 558–561. [Google Scholar]
- Greatbatch, W.; Holmes, C.; Takeuchi, E.; Ebel, S. Lithium/carbon monofluoride (Li/CFx): A new pacemaker battery. Pacing Clin. Electrophysiol. 1996, 19, 1836–1840. [Google Scholar] [CrossRef]
- Ruff, O.; Bretschneider, O. Die Reaktionsprodukte der verschiedenen Kohlenstoffformen mit Fluor II (Kohlenstoff-monofluorid). Z. Anorg. Allg. Chem. 1934, 217, 1–18. [Google Scholar] [CrossRef]
- Rüdorff, W.; Rüdorff, G. Zur Konstitution des Kohlenstoff-Monofluorids. Z. Anorg. Chem. 1947, 253, 281–296. [Google Scholar] [CrossRef]
- Watanabe, N.; Fukuda, M. Primary Cell for Electric Batteries. U.S. Patent 3,536,532, 27 October 1970. [Google Scholar]
- Watanabe, K.; Fukuda, M. High Energy Density Battery. U.S. Patent 3,700,502, 24 October 1972. [Google Scholar]
- Fukuda, M.; Iijima, T.; Toyoguchi, Y. Active Material for Positive Electrode of Battery. U.S. Patent 4,271,242, 2 June 1981. [Google Scholar]
- Sharma, N.; Dubois, M.; Guérin, K.; Pischedda, V.; Radescu, S. Fluorinated (Nano) Carbons: CFx Electrodes and CFx-Based Batteries. Energy Technol. 2021, 9, 2000605. [Google Scholar] [CrossRef]
- Ahmad, Y.; Batisse, N.; Chen, X.; Dubois, M. Preparation and Applications of Fluorinated Graphenes. C 2021, 7, 20. [Google Scholar] [CrossRef]
- Wang, D.; Wang, G.; Zhang, M.; Cui, Y.; Yu, J.; Shi, S. Composite cathode materials for next-generation lithium fluorinated carbon primary batteries. J. Power Sources 2022, 541, 231716. [Google Scholar] [CrossRef]
- Groult, H.; Tressaud, A. Use of inorganic fluorinated materials in lithium batteries and in energy conversion systems. Chem. Commun. 2018, 54, 11375–11382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Takeuchi, K.J.; Takeuchi, E.S.; Marschilok, A.C. Progress towards high-power Li/CF x batteries: Electrode architectures using carbon nanotubes with CF x. Phys. Chem. Chem. Phys. 2015, 17, 22504–22518. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Jiang, C.; Jiang, J.; Zou, J.; Ran, Q.; Wang, X.; Niu, X.; Wang, L. Fluorinated Carbons as Rechargeable Li-Ion Battery Cathodes in the Voltage Window of 0.5–4.8 V. ACS Appl. Mater. Interfaces 2021, 13, 30576–30582. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Xie, J.Y.; Fu, Z.W. Rechargeable room-temperature CF x-sodium battery. ACS Appl. Mater. Interfaces 2014, 6, 2209–2212. [Google Scholar] [CrossRef]
- Whittingham, M.S. Mechanism of reduction of the fluorographite cathode. J. Electrochem. Soc. 1975, 122, 526. [Google Scholar] [CrossRef]
- Watanabe, N.; Nakajima, T.; Hagiwara, R. Discharge reaction and overpotential of the graphite fluoride cathode in a nonaqueous lithium cell. J. Power Sources 1987, 20, 87–92. [Google Scholar] [CrossRef]
- Watanabe, N.; Hagiwara, R.; Nakajima, T.; Touhara, H.; Ueno, K. Solvents effects on electrochemical characteristics of graphite fluoride—lithium batteries. Electrochim. Acta 1982, 27, 1615–1619. [Google Scholar] [CrossRef]
- Jang, B.Z.; Liu, C.; Neff, D.; Yu, Z.; Wang, M.C.; Xiong, W.; Zhamu, A. Graphene surface-enabled lithium ion-exchanging cells: Next-generation high-power energy storage devices. Nano Lett. 2011, 11, 3785–3791. [Google Scholar] [CrossRef]
- Liu, C.; Zhamu, A.; Neff, D.; Jang, B.Z. Lithium Super-Battery with a Functionalized Nano Graphene Cathode. U.S. Patent 8,795,899, 5 August 2014. [Google Scholar]
- Kim, H.; Park, K.Y.; Hong, J.; Kang, K. All-graphene-battery: Bridging the gap between supercapacitors and lithium ion batteries. Sci. Rep. 2014, 4, 5278. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Park, Y.U.; Park, K.Y.; Lim, H.D.; Hong, J.; Kang, K. Novel transition-metal-free cathode for high energy and power sodium rechargeable batteries. Nano Energy 2014, 4, 97–104. [Google Scholar] [CrossRef]
- Kornilov, D.; Penki, T.R.; Cheglakov, A.; Aurbach, D. Li/graphene oxide primary battery system and mechanism. Battery Energy 2022, 1, 20210002. [Google Scholar] [CrossRef]
- Zhang, S.S.; Foster, D.; Wolfenstine, J.; Read, J. Electrochemical characteristic and discharge mechanism of a primary Li/CFx cell. J. Power Sources 2009, 187, 233–237. [Google Scholar] [CrossRef]
- Sayahpour, B.; Hirsh, H.; Bai, S.; Schorr, N.B.; Lambert, T.N.; Mayer, M.; Bao, W.; Cheng, D.; Zhang, M.; Leung, K.; et al. Revisiting discharge mechanism of CFx as a high energy density cathode material for lithium primary battery. Adv. Energy Mater. 2022, 12, 2103196. [Google Scholar] [CrossRef]
- Yazami, R.; Hamwi, A.; Guérin, K.; Ozawa, Y.; Dubois, M.; Giraudet, J.; Masin, F. Fluorinated carbon nanofibres for high energy and high power densities primary lithium batteries. Electrochem. Commun. 2007, 9, 1850–1855. [Google Scholar] [CrossRef]
- Lam, P.; Yazami, R. Physical characteristics and rate performance of (CFx) n (0.33 < × < 0.66) in lithium batteries. J. Power Sources 2006, 153, 354–359. [Google Scholar]
- Luo, Z.; Wang, X.; Chen, D.; Chang, Q.; Xie, S.; Ma, Z.; Lei, W.; Pan, J.; Pan, Y.; Huang, J. Ultrafast Li/fluorinated graphene primary batteries with high energy density and power density. ACS Appl. Mater. Interfaces 2021, 13, 18809–18820. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cai, S.; Wu, L.; Yang, W.; Xie, J.; Wen, W.; Zheng, J.C.; Zhu, Y. Surface modified CFx cathode material for ultrafast discharge and high energy density. J. Mater. Chem. A 2014, 2, 20896–20901. [Google Scholar] [CrossRef]
- Peng, C.; Kong, L.; Li, Y.; Fu, H.; Sun, L.; Feng, Y.; Feng, W. Fluorinated graphene nanoribbons from unzipped single-walled carbon nanotubes for ultrahigh energy density lithium-fluorinated carbon batteries. Sci. China Mater. 2021, 64, 1367–1377. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, P.; Lu, J.; Liu, Z. The electrochemical performance of fluorinated ketjenblack as a cathode for lithium/fluorinated carbon batteries. RSC Adv. 2021, 11, 25461–25470. [Google Scholar] [CrossRef]
- Wang, K.; Feng, Y.; Kong, L.; Peng, C.; Hu, Y.; Li, W.; Li, Y.; Feng, W. The fluorination of boron-doped graphene for CFx cathode with ultrahigh energy density. Energy Environ. Mater. 2022, e12437. [Google Scholar] [CrossRef]
- Li, Q.; Xue, W.; Sun, X.; Yu, X.; Li, H.; Chen, L. Gaseous electrolyte additive BF3 for high-power Li/CFx primary batteries. Energy Storage Mater. 2021, 38, 482–488. [Google Scholar] [CrossRef]
- Rangasamy, E.; Li, J.; Sahu, G.; Dudney, N.; Liang, C. Pushing the theoretical limit of Li-CF x batteries: A tale of bifunctional electrolyte. J. Am. Chem. Soc. 2014, 136, 6874–6877. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.C.; Hossain, S. Polymer Materials as Binder for a CFx Cathode. U.S. Patent Application 13/010,431, 28 July 2011. [Google Scholar]
- Németh, K. Materials design by quantum-chemical and other theoretical/computational means: Applications to energy storage and photoemissive materials. Int. J. Quantum Chem. 2014, 114, 1031–1035. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Németh, K.; Bareño, J.; Dogan, F.; Bloom, I.D.; Shaw, L.L. Experimental and theoretical investigations of functionalized boron nitride as electrode materials for Li-ion batteries. RSC Adv. 2016, 6, 27901–27914. [Google Scholar] [CrossRef]
- Németh, K. Simultaneous oxygen and boron trifluoride functionalization of hexagonal boron nitride: A designer cathode material for energy storage. Theor. Chem. Accounts 2018, 137, 157. [Google Scholar] [CrossRef] [Green Version]
- Németh, K. Radical anion functionalization of two-dimensional materials as a means of engineering simultaneously high electronic and ionic conductivity solids. Nanotechnology 2021, 32, 245709. [Google Scholar] [CrossRef]
- Nemeth, K. Functionalized Boron Nitride Materials as Electroactive Species in Electrochemical Energy Storage Devices. U.S. Patent 10,693,137, 23 June 2020. [Google Scholar]
- Nemeth, K. Radical Anion Functionalization of Two-Dimensional Materials. U.S. Patent 11,453,596 B2, 7 September 2022. [Google Scholar]
- Marshall, J.E.; Zhenova, A.; Roberts, S.; Petchey, T.; Zhu, P.; Dancer, C.E.; McElroy, C.R.; Kendrick, E.; Goodship, V. On the solubility and stability of polyvinylidene fluoride. Polymers 2021, 13, 1354. [Google Scholar] [CrossRef]
- Zor, C.; Subaşı, Y.; Haciu, D.; Somer, M.; Afyon, S. Guide to water free lithium bis (oxalate) borate (LiBOB). J. Phys. Chem. C 2021, 125, 11310–11317. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Zhu, Y.; Che, J.; Xiao, Y. Two-dimensional transparent hydrophobic coating based on liquid-phase exfoliated graphene fluoride. Carbon 2013, 63, 149–156. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, Y.; Yu, M.; Lang, H.; Cao, X.; Zou, K. Dynamic sliding enhancement on the friction and adhesion of graphene, graphene oxide, and fluorinated graphene. ACS Appl. Mater. Interfaces 2018, 10, 8214–8224. [Google Scholar] [CrossRef] [PubMed]
- Huo, H. High Energy High Power Primary Lithium Batteries with Graphite Fluoride and Functionalized Boron Nitride Cathodes. Master’s Thesis, Illinois Institute of Technology, Chicago, IL, USA, 2022. [Google Scholar]
- Tatagari, V.R. A Functionalized 2D Boron Nitride Electrode for Rechargeable Batteries. Master’s Thesis, Illinois Institute of Technology, Chicago, IL, USA, 2021. [Google Scholar]
- Li, Y.Y.; Liu, C.; Chen, L.; Wu, X.Z.; Zhou, P.F.; Shen, X.Y.; Zhou, J. Multi-layered fluorinated graphene cathode materials for lithium and sodium primary batteries. Rare Metals 2022, 42, 940–953. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.; Zheng, J.; Deng, Y.; Warren, A.; Zhang, Q.; Archer, L. Designing electrolytes with polymerlike glass-forming properties and fast ion transport at low temperatures. Proc. Natl. Acad. Sci. USA 2020, 117, 26053–26060. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Kim, M.S.; Archer, L.A. Stable artificial solid electrolyte interphases for lithium batteries. Chem. Mater. 2017, 29, 4181–4189. [Google Scholar] [CrossRef]
- Bai, L.; Xu, Y.; Liu, A.; Dong, L.; Zhang, K.; Li, W.S.; Zhao, F.G. Unusual graphite fluoride hydrolysis toward unconventional graphene oxide for high-performance supercapacitors and Li-ion batteries. Chem. Eng. J. 2022, 434, 134639. [Google Scholar] [CrossRef]
- Bakandritsos, A.; Pykal, M.; Błoński, P.; Jakubec, P.; Chronopoulos, D.D.; Poláková, K.; Georgakilas, V.; Čépe, K.; Tomanec, O.; Ranc, V.; et al. Cyanographene and graphene acid: Emerging derivatives enabling high-yield and selective functionalization of graphene. ACS Nano 2017, 11, 2982–2991. [Google Scholar] [CrossRef] [Green Version]
- Siedle, A.; Losovyj, Y.; Stein, B.D.; Pink, M.; Werner-Zwanziger, U. Cyanographite. J. Phys. Chem. C 2022, 126, 3001–3008. [Google Scholar] [CrossRef]
- Pang, C.; Ding, F.; Sun, W.; Liu, J.; Hao, M.; Wang, Y.; Liu, X.; Xu, Q. A novel dimethyl sulfoxide/1,3-dioxolane based electrolyte for lithium/carbon fluorides batteries with a high discharge voltage plateau. Electrochim. Acta 2015, 174, 230–237. [Google Scholar] [CrossRef]
- Watanabe, M.; Kanba, M.; Nagaoka, K.; Shinohara, I. Ionic conductivity of hybrid films composed of polyacrylonitrile, ethylene carbonate, and LiClO4. J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 939–948. [Google Scholar] [CrossRef]
- Chen, H.; Lin, F.; Chen, C. Polyacrylonitrile electrolytes 1. A novel high-conductivity composite polymer electrolyte based on PAN, LiClO4 and a-Al2O3. Solid State Ionics 2002, 150, 327–335. [Google Scholar] [CrossRef]
- Wong, C.Y.; Kennepohl, D.K.; Cavell, R.G. Neutral six-coordinate phosphorus. Chem. Rev. 1996, 96, 1917–1952. [Google Scholar] [CrossRef]
- Amereller, M.; Multerer, M.; Schreiner, C.; Lodermeyer, J.; Schmid, A.; Barthel, J.; Gores, H.J. Investigation of the hydrolysis of lithium bis [1,2-oxalato (2-)-O, O’] borate (LiBOB) in water and acetonitrile by conductivity and NMR measurements in comparison to some other borates. J. Chem. Eng. Data 2009, 54, 468–471. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, M. Recent Advances in Organocatalytic Asymmetric Morita–Baylis–Hillman/aza-Morita–Baylis–Hillman Reactions. Chem. Rev. 2013, 113, 6659–6690. [Google Scholar] [CrossRef] [PubMed]
- De Yoreo, J.J.; Vekilov, P.G. Principles of crystal nucleation and growth. Rev. Mineral. Geochem. 2003, 54, 57–93. [Google Scholar] [CrossRef] [Green Version]
- Vekilov, P.G. The two-step mechanism of nucleation of crystals in solution. Nanoscale 2010, 2, 2346–2357. [Google Scholar] [CrossRef]
- Chung, T.C.; Schlesinger, Y.; Etemad, S.; Macdiarmid, A.; Heeger, A. Optical studies of pyrolyzed polyacrylonitrile. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 1239–1246. [Google Scholar] [CrossRef]
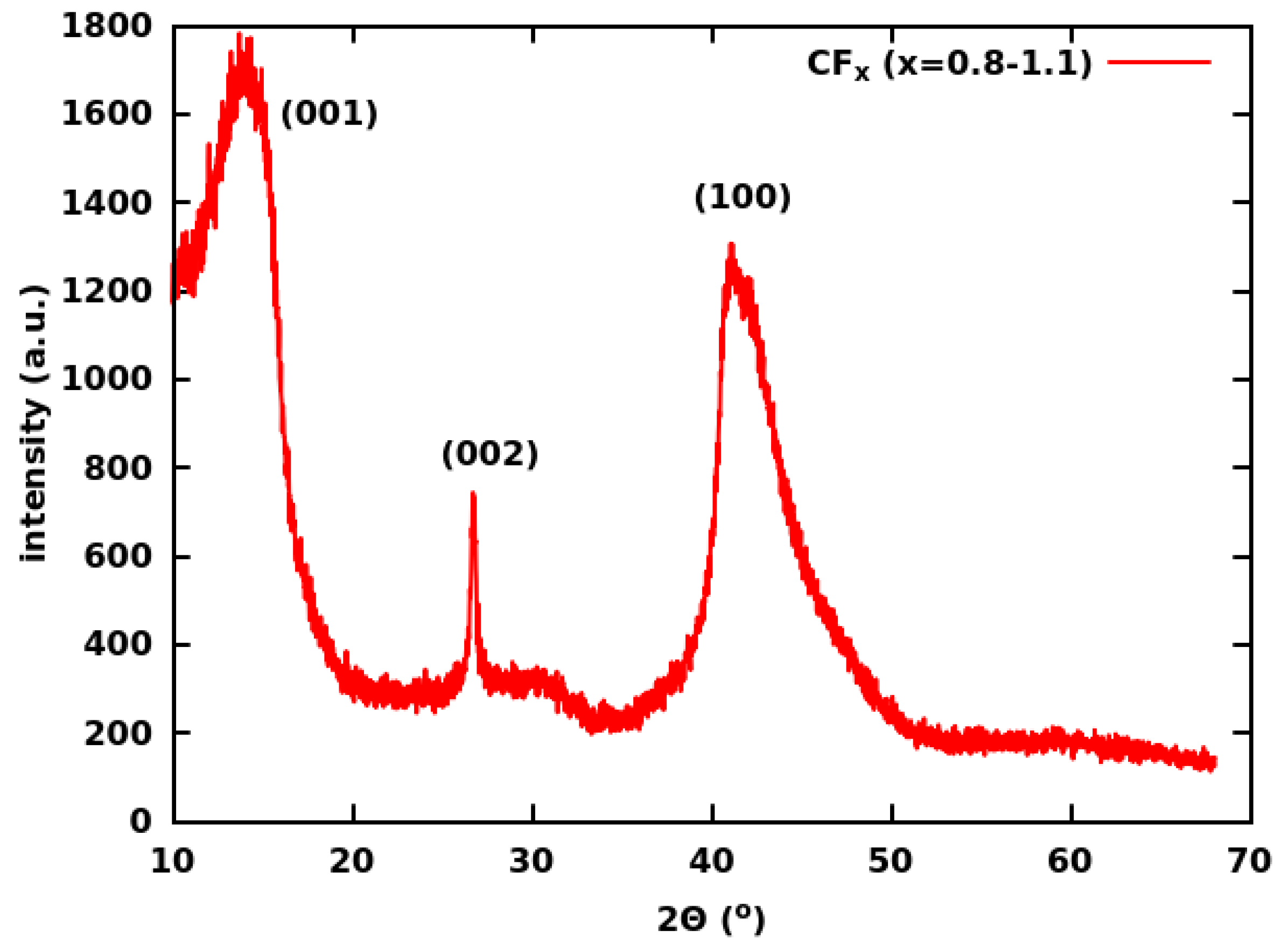



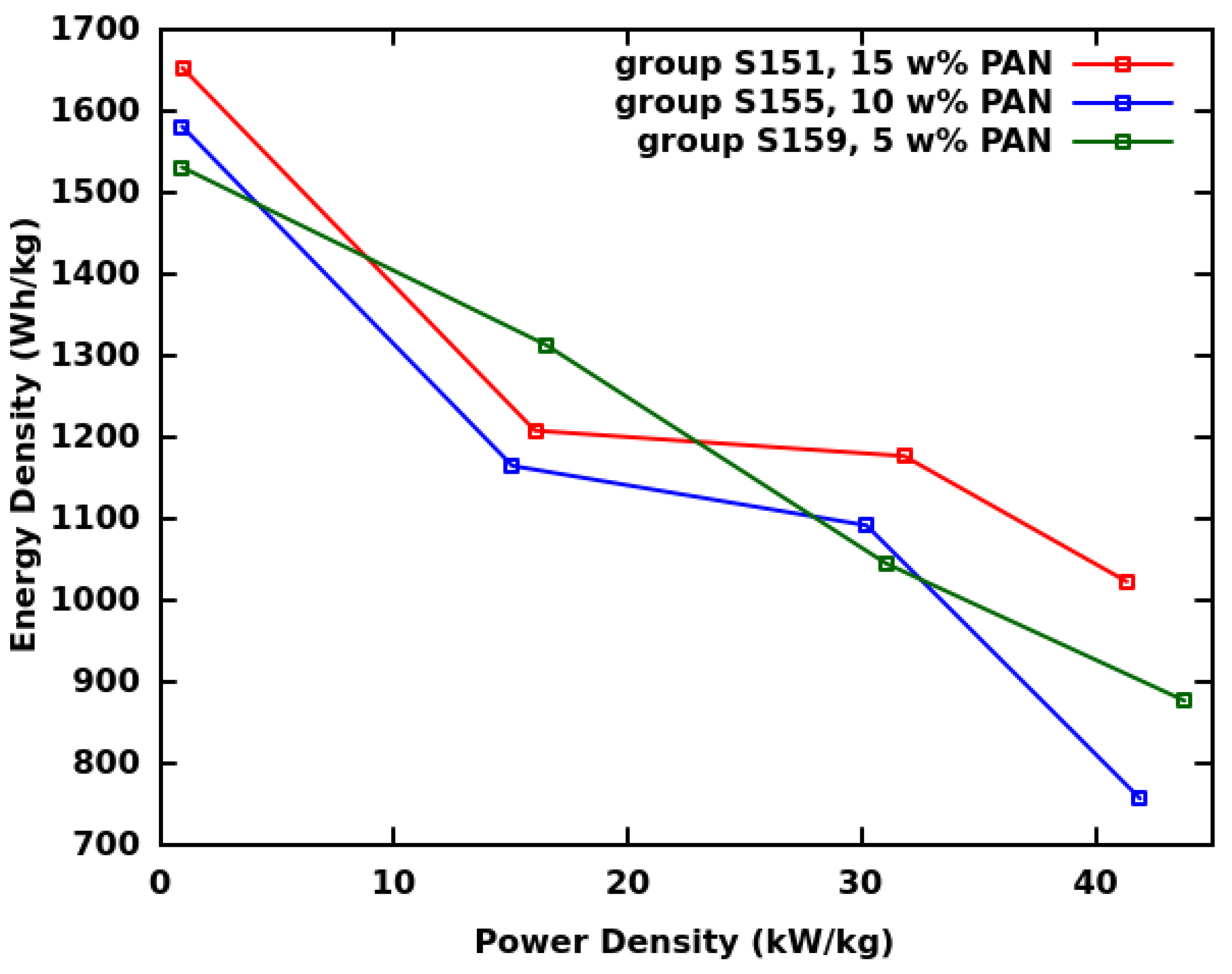
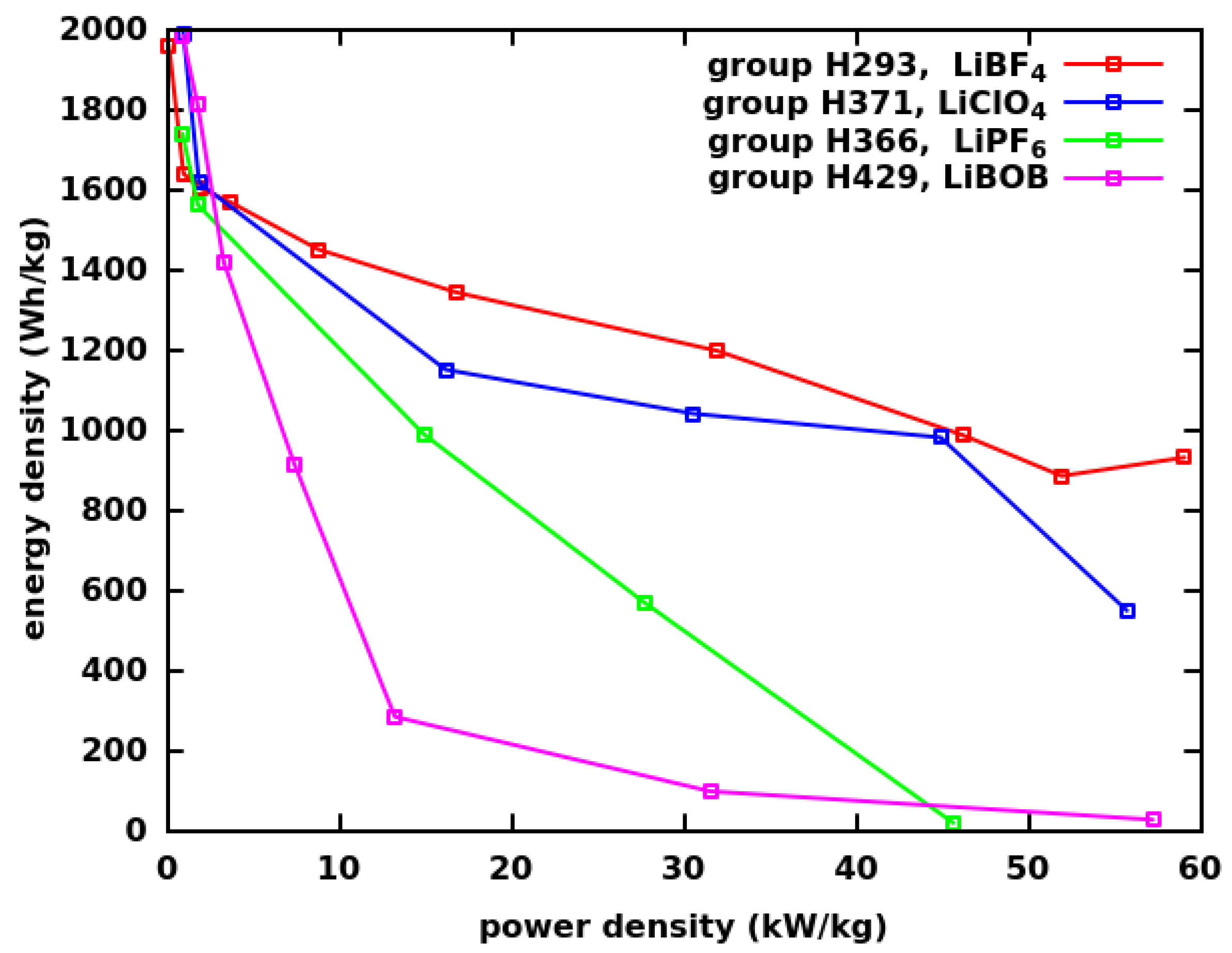
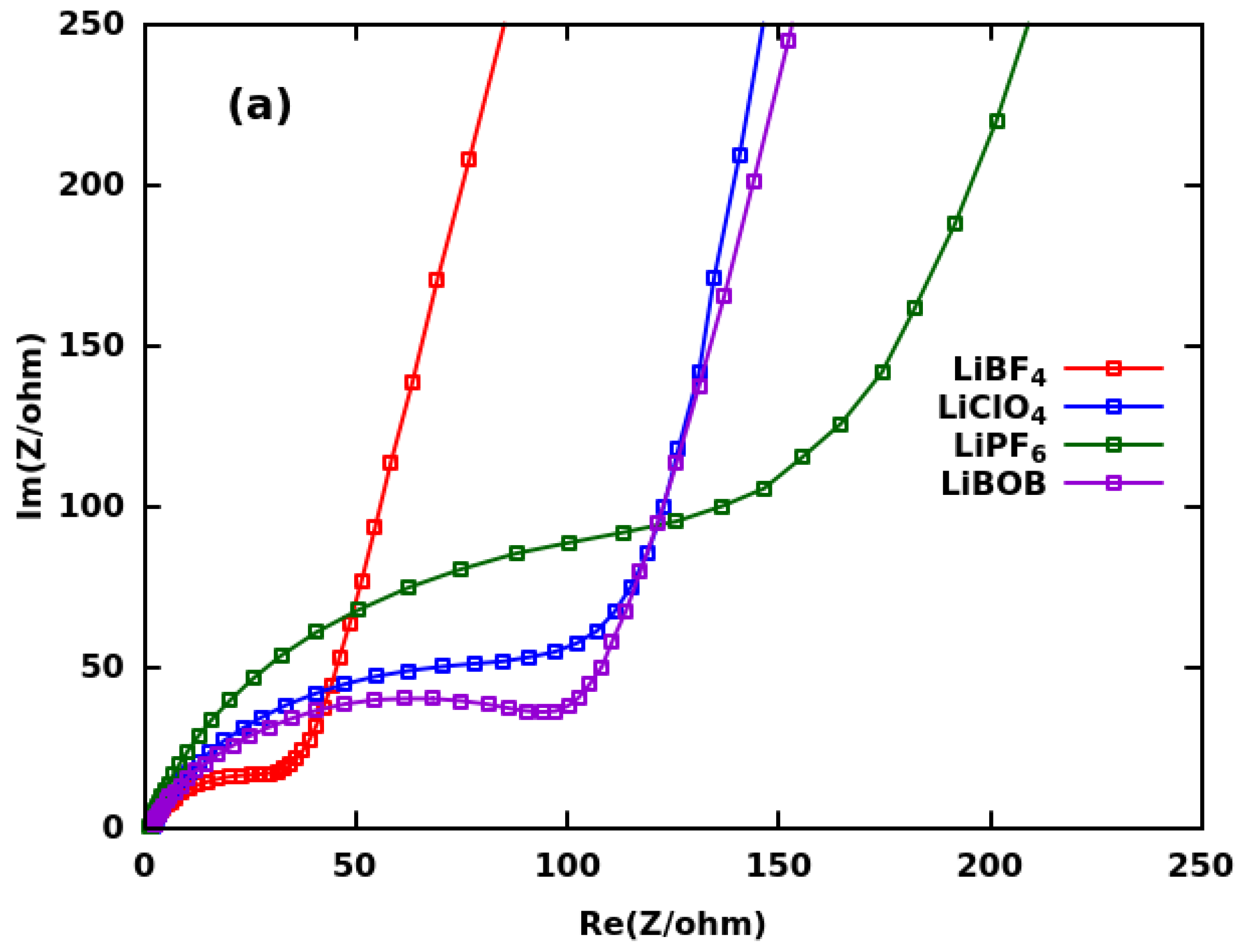
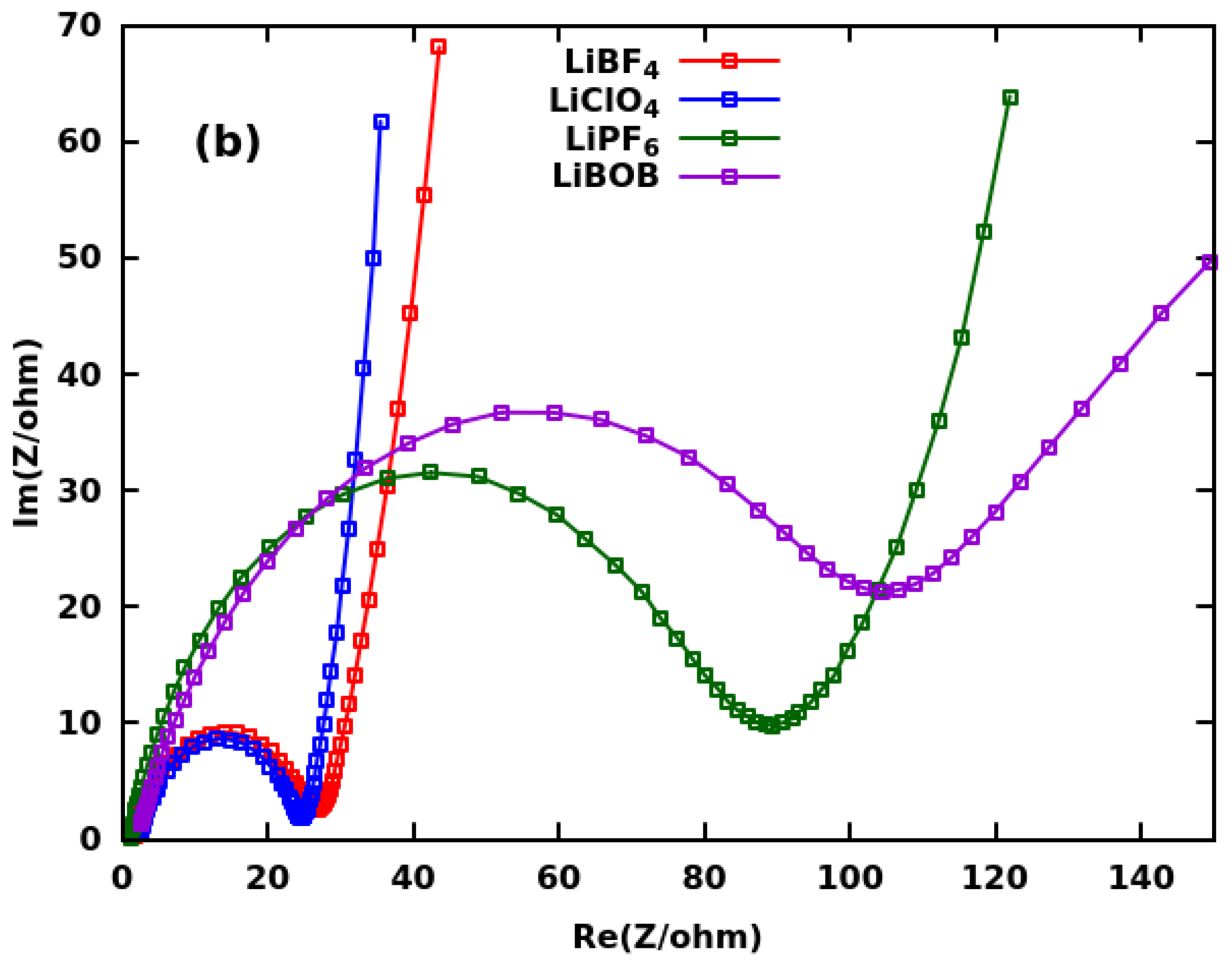



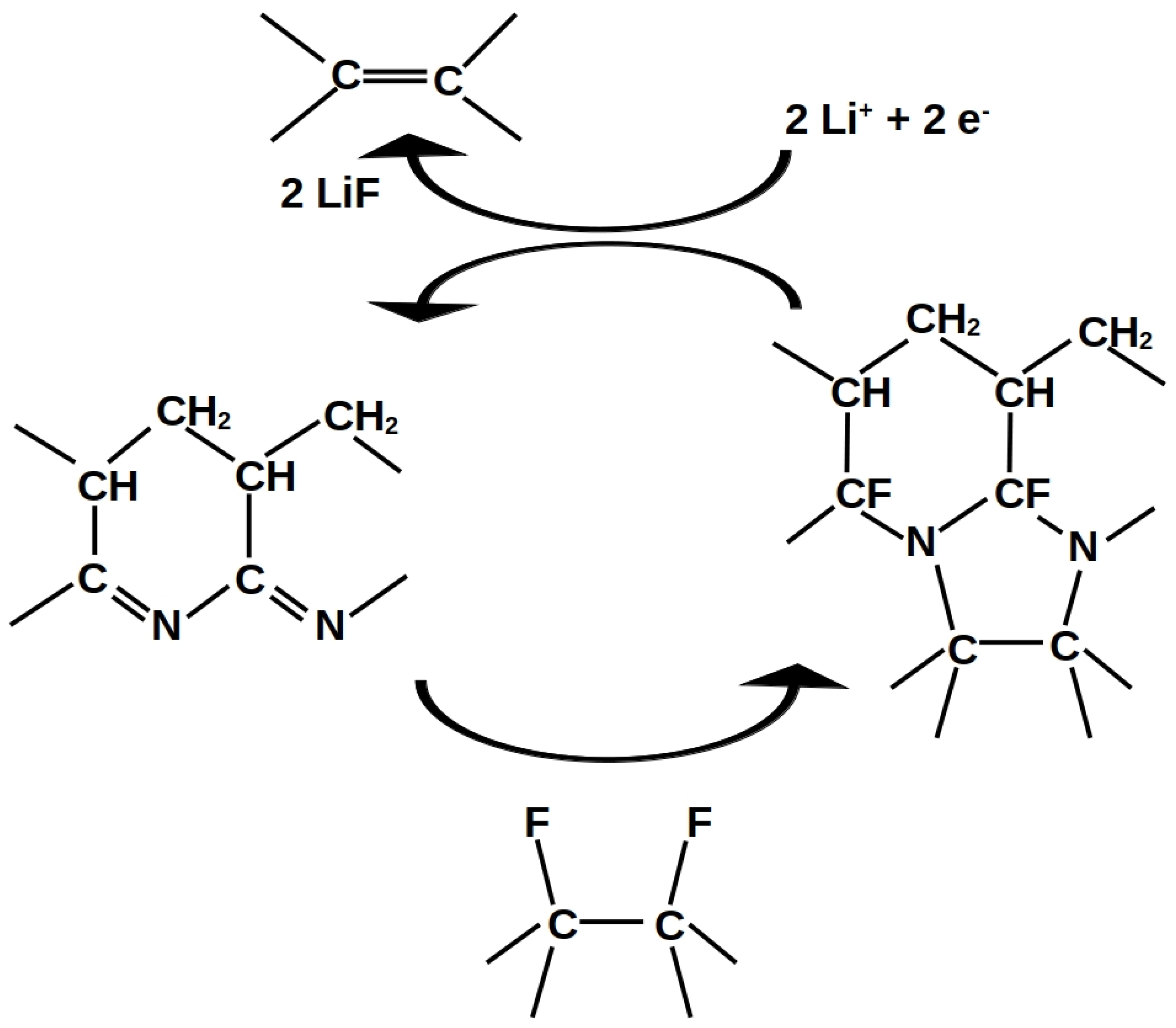
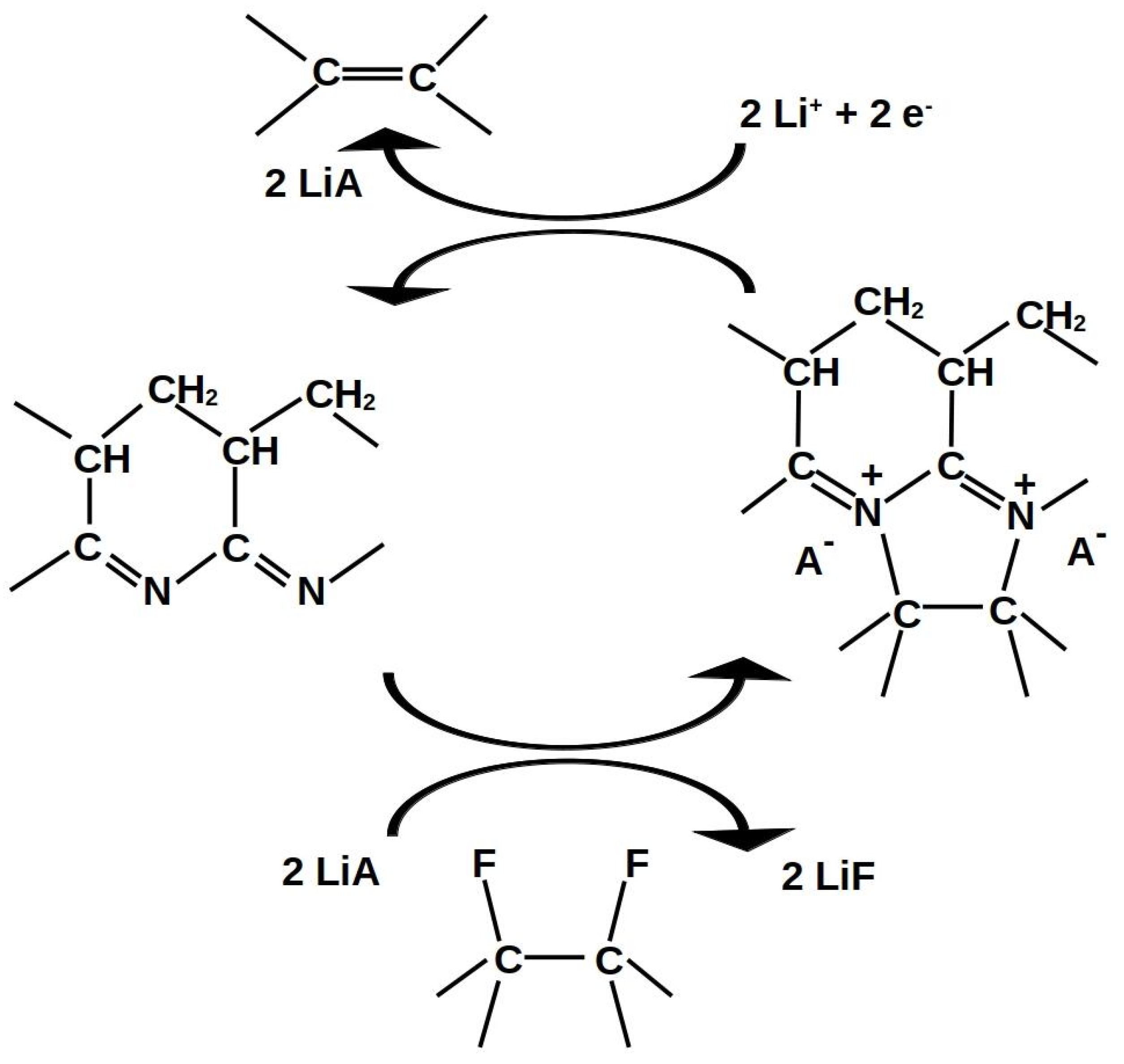
| Group | Ultrasonication of CF | Thickness | Binder | Electrolyte | ||
|---|---|---|---|---|---|---|
| ID | Solvent | Additive | (m) | Salt | Solvent | |
| H293 | EtOH | - | 100 | PAN | LiBF | PC:DME:DOL |
| H314 | EtOH | - | 250 | PAN | LiBF | PC:DME:DOL |
| H214 | EtOH | - | 100 | PVDF | LiBF | PC:DME:DOL |
| H321 | EtOH | - | 250 | PVDF | LiBF | PC:DME:DOL |
| H366 | EtOH | - | 100 | PAN | LiPF | PC:DME:DOL |
| H371 | EtOH | - | 100 | PAN | LiClO | PC:DME:DOL |
| H429 | EtOH | - | 100 | PAN | LiBOB | PC:DME:DOL |
| H101 | FB·OEt | - | 100 | PVDF | LiBF | PC:DME:DOL |
| H274 | FB·OEt | LiOX·BF | 100 | PAN | LiBF | PC:DME:DOL |
| H304 | no ultrasonication | 100 | PAN | LiBF | PC:DME:DOL | |
| H179 | FB·OEt | LiOX·BF | 100 | PAN | LiBF | DMSO:DOL |
| H387 | EtOH | - | 100 | PVDF | LiBF | EC:DMC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, H.; Radhakrishnan, S.; Shaw, L.L.; Németh, K. High-Energy and High-Power Primary Li-CFx Batteries Enabled by the Combined Effects of the Binder and the Electrolyte. Batteries 2023, 9, 268. https://doi.org/10.3390/batteries9050268
Huo H, Radhakrishnan S, Shaw LL, Németh K. High-Energy and High-Power Primary Li-CFx Batteries Enabled by the Combined Effects of the Binder and the Electrolyte. Batteries. 2023; 9(5):268. https://doi.org/10.3390/batteries9050268
Chicago/Turabian StyleHuo, Haobin, Sivaviswa Radhakrishnan, Leon L. Shaw, and Károly Németh. 2023. "High-Energy and High-Power Primary Li-CFx Batteries Enabled by the Combined Effects of the Binder and the Electrolyte" Batteries 9, no. 5: 268. https://doi.org/10.3390/batteries9050268
APA StyleHuo, H., Radhakrishnan, S., Shaw, L. L., & Németh, K. (2023). High-Energy and High-Power Primary Li-CFx Batteries Enabled by the Combined Effects of the Binder and the Electrolyte. Batteries, 9(5), 268. https://doi.org/10.3390/batteries9050268










