Effectively Elevating Ceramic Fillers’ Dispersity in Gel Hybrid Electrolyte through Bridge–Linked Construction for High–Performance Lithium Metal Batteries
Abstract
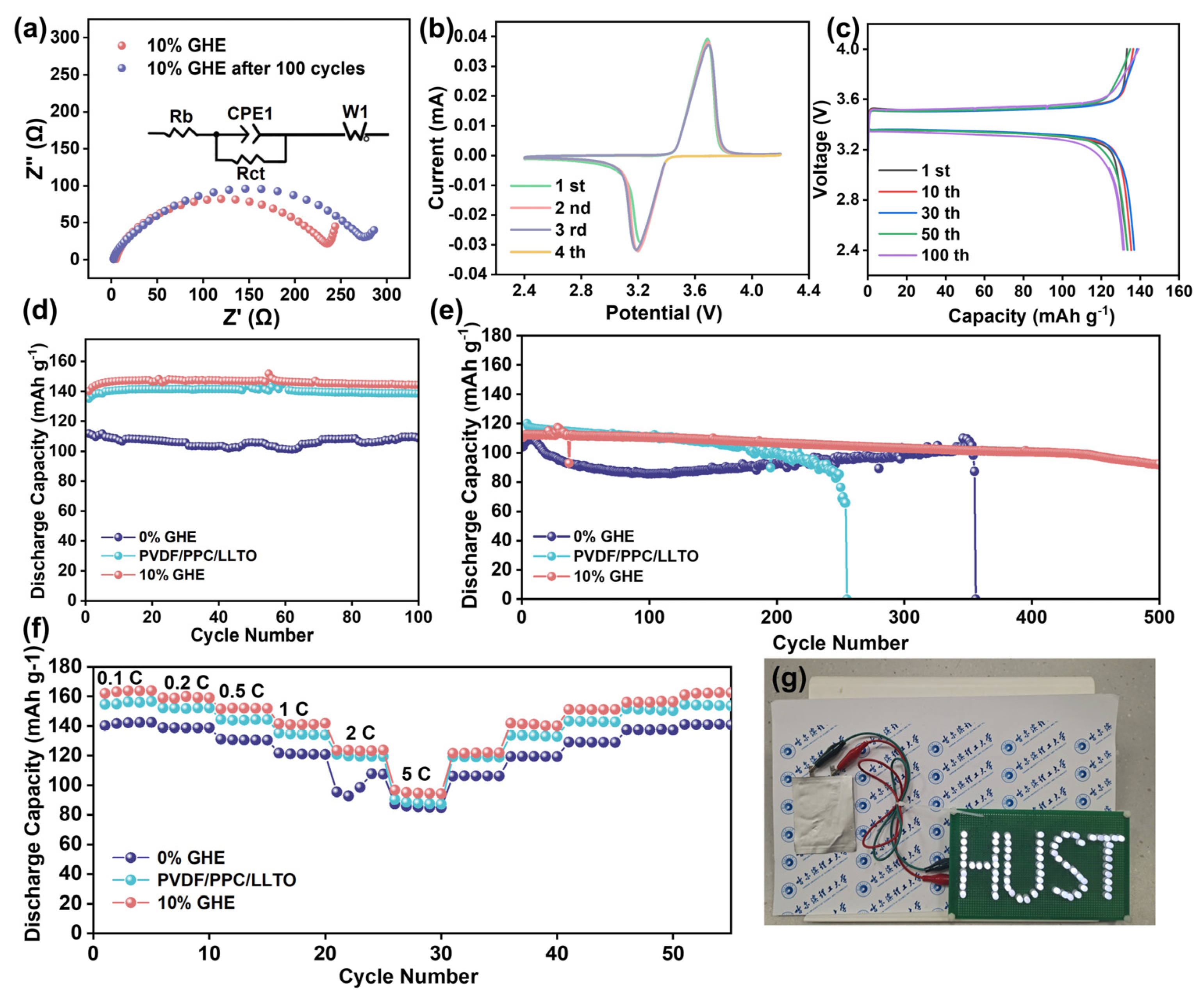
:1. Introduction
2. Materials and Methods
2.1. Chemicals
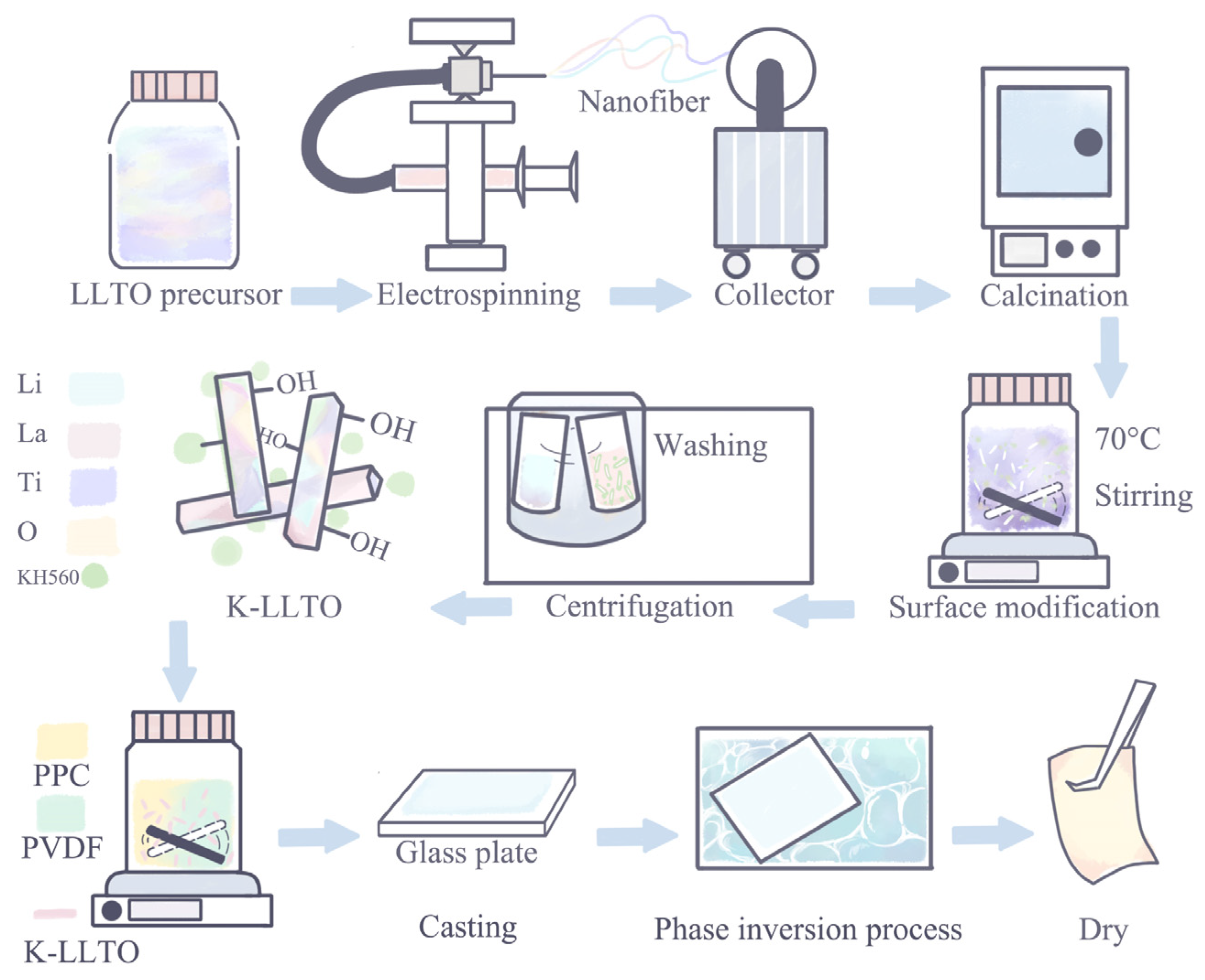
2.2. Synthesis of LLTO Nanorods
2.3. Synthesis of K–LLTO Nanorods
2.4. Fabrication of K–LLTO Doped Dry GHEs (dGHEs) and GHEs
2.5. Characterization
2.6. Electrochemical Measurements and Analysis
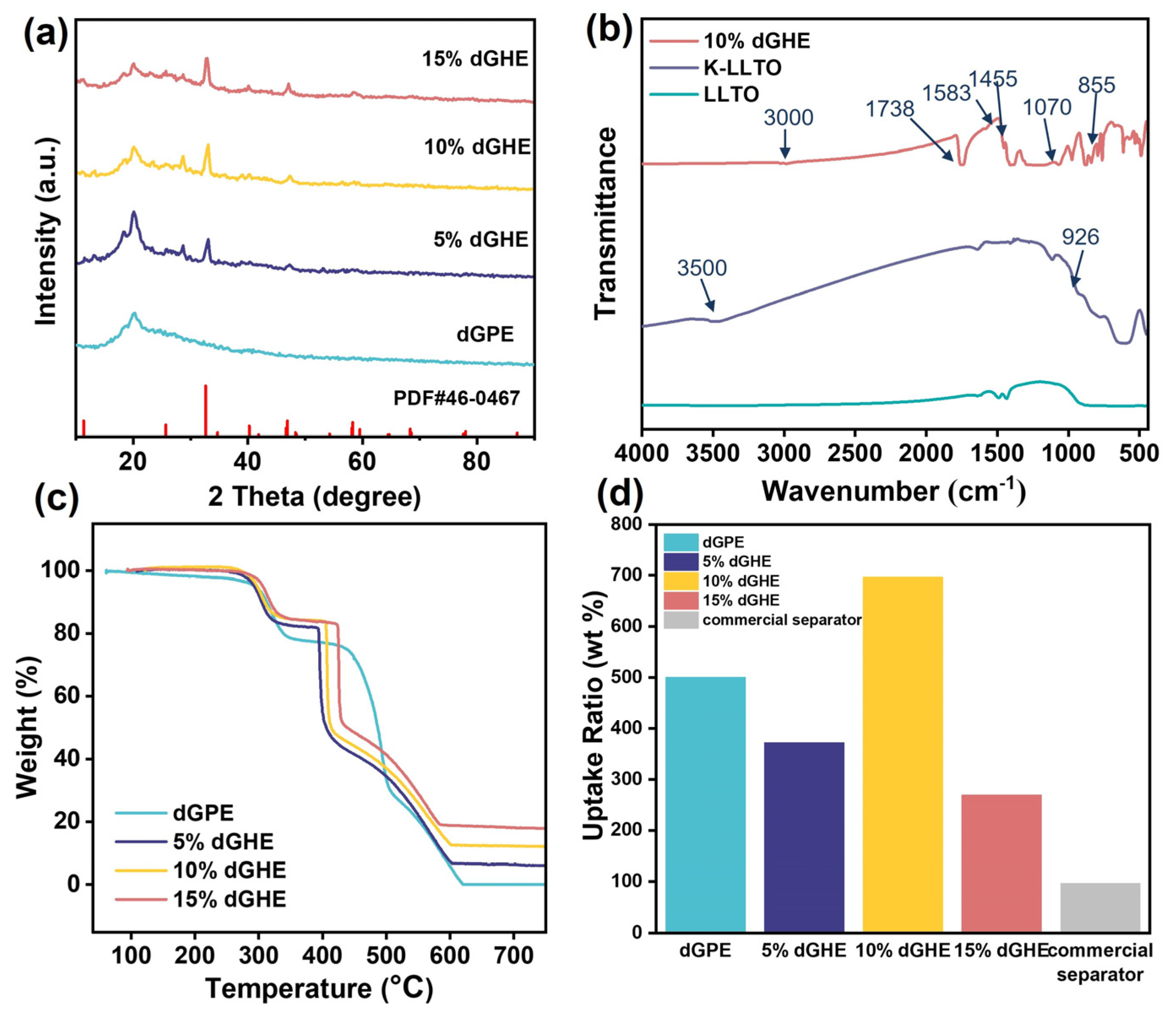
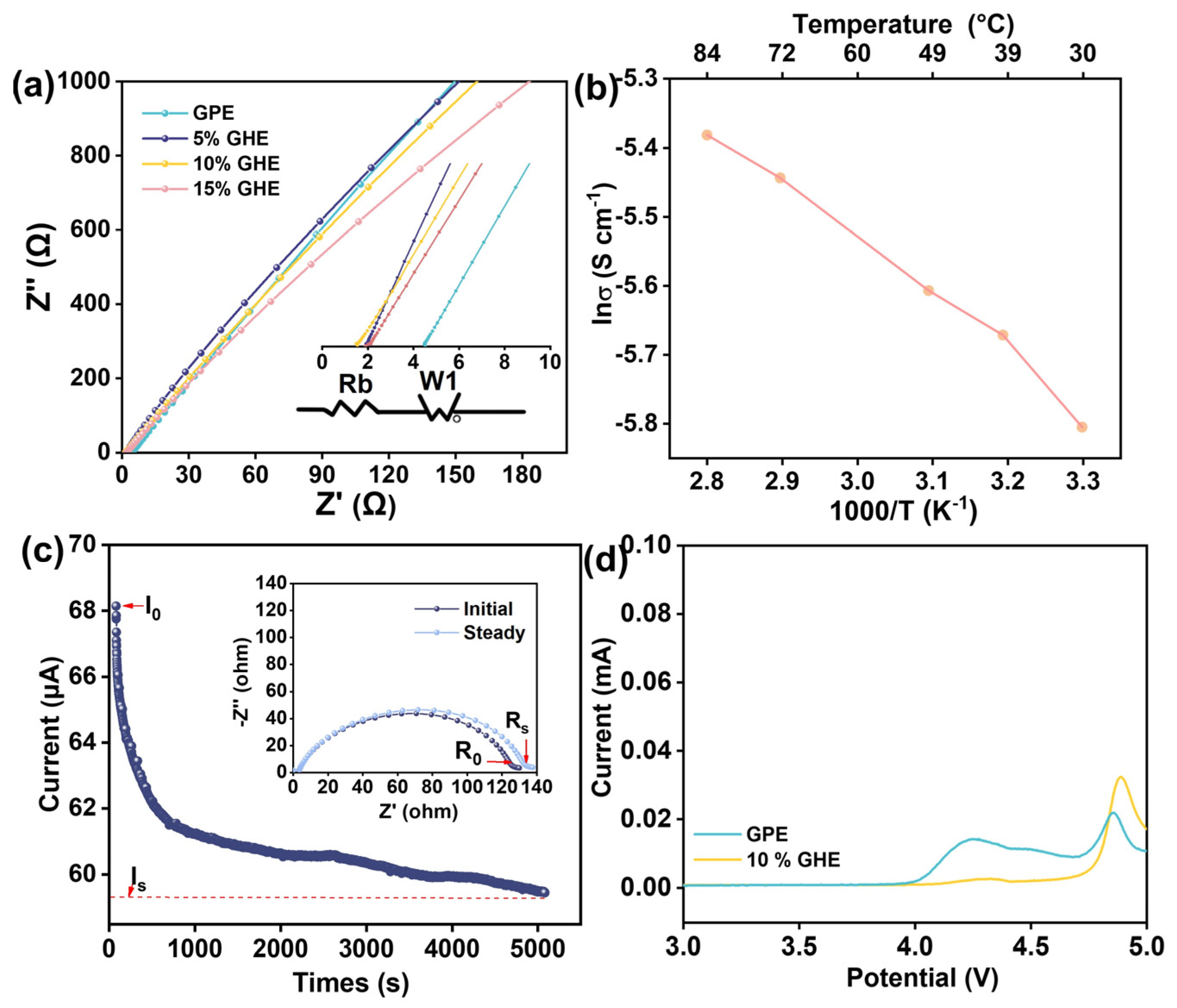
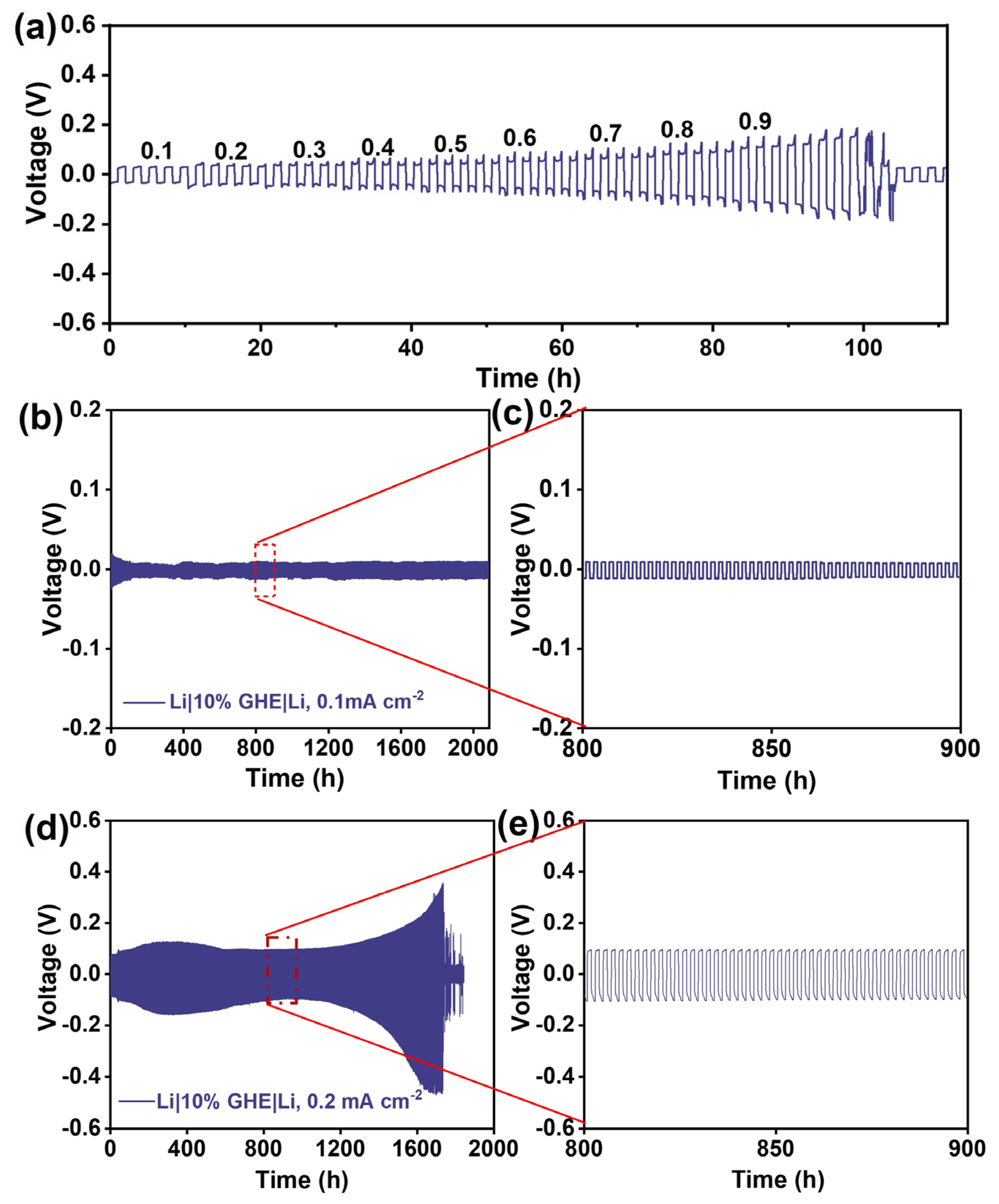
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, H.P.; Zarrabeitia, M.; Chen, Z.; Jovanovic, S.; Merz, S.; Granwehr, J.; Passerini, S.; Bresser, D. Polysiloxane-based single-ion conducting polymer blend electrolyte comprising small-molecule organic carbonates for high-energy and high-power lithium-metal batteries. Adv. Energy Mater. 2022, 12, 2200013. [Google Scholar] [CrossRef]
- Xu, L.; Lu, Y.; Zhao, C.Z.; Yuan, H.; Zhu, G.L.; Hou, L.P.; Zhang, Q.; Huang, J.Q. Toward the scale-up of solid-state lithium metal batteries: The gaps between lab-level cells and practical large-format batteries. Adv. Energy Mater. 2020, 11, 2002360. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Chen, Z.; Kim, G.-T.; Wang, Z.; Bresser, D.; Qin, B.; Geiger, D.; Kaiser, U.; Wang, X.; Shen, Z.X.; Passerini, S. 4-V flexible all-solid-state lithium polymer batteries. Nano Energy 2019, 64, 103986. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer electrolytes for lithium-ion batteries: State of the art and perspectives. ChemSusChem 2015, 8, 2154–2175. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, J.; Wang, Y.; Song, M.; Long, L.; Siyal, S.H.; Yang, X.; Sui, G. Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 2018, 37, 126–142. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Wang, S.-H.; Lin, Y.-Y.; Teng, C.-Y.; Chen, Y.-M.; Kuo, P.-L.; Lee, Y.-L.; Hsieh, C.-T.; Teng, H. Immobilization of Anions on Polymer Matrices for Gel Electrolytes with High Conductivity and Stability in Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 14776–14787. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qiu, X.; Bai, Z.; Fan, L.-Z. Enhancing interfacial stability in solid-state lithium batteries with polymer/garnet solid electrolyte and composite cathode framework. J. Energy Chem. 2021, 52, 210–217. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Dong, T.; Zhang, M.; Chai, J.; Dong, S.; Wu, T.; Zhou, X.; Cui, G. Aliphatic Polycarbonate-Based Solid-State Polymer Electrolytes for Advanced Lithium Batteries: Advances and Perspective. Small 2018, 14, e1800821. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Deng, Y.; Chen, G. Status and prospect of garnet/polymer solid composite electrolytes for all-solid-state lithium batteries. J. Energy Chem. 2020, 50, 154–177. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, S.; Liu, J.; He, T.; Sun, L.; Xu, D.; Yu, X.; Luo, Y.; Zhou, W.; Wu, J. High-performance electrospun poly(vinylidene fluoride)/poly(propylene carbonate) gel polymer electrolyte for lithium-ion batteries. J. Phys. Chem. C 2015, 119, 27882–27891. [Google Scholar] [CrossRef]
- Ye, F.; Liao, K.; Ran, R.; Shao, Z. Recent Advances in Filler Engineering of Polymer Electrolytes for Solid-State Li-Ion Batteries: A Review. Energy Fuels 2020, 34, 9189–9207. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Zhang, J.; Jing, M.; Yao, S.; Shen, X.; Li, S.; Tu, F. Approaching high performance PVDF-HFP based solid composite electrolytes with LLTO nanorods for solid-state lithium-ion batteries. Int. J. Energy Res. 2021, 45, 7663–7674. [Google Scholar] [CrossRef]
- Inaguma, Y.; Liquan, C.; Itoh, M.; Nakamura, T.; Uchida, T.; Ikuta, H.; Wakihara, M. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 1993, 86, 689–693. [Google Scholar] [CrossRef]
- Fan, L.-Z.; He, H.; Nan, C.-W. Tailoring inorganic–polymer composites for the mass production of solid-state batteries. Nat. Rev. Mater. 2021, 6, 1003–1019. [Google Scholar] [CrossRef]
- Bi, J.; Mu, D.; Wu, B.; Fu, J.; Yang, H.; Mu, G.; Zhang, L.; Wu, F. A hybrid solid electrolyte Li0.33La0.557TiO3/poly(acylonitrile) membrane infiltrated with a succinonitrile-based electrolyte for solid state lithium-ion batteries. J. Mater. Chem. A 2020, 8, 706–713. [Google Scholar] [CrossRef]
- Li, B.; Su, Q.; Yu, L.; Wang, D.; Ding, S.; Zhang, M.; Du, G.; Xu, B. Li0.35La0.55TiO3 nanofibers enhanced poly(vinylidene fluoride)-based composite polymer electrolytes for all-solid-state batteries. ACS Appl. Mater. Interfaces 2019, 11, 42206–42213. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zhang, X.; Liu, T.; Lin, Y.H.; Li, L.; Shen, Y.; Nan, C.W. Lithium-salt-rich PEO/Li0.3La0.557TiO3 interpenetrating composite electrolyte with three-dimensional ceramic nano-backbone for all-solid-state lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 24791–24798. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, S.; Shen, L.; Liu, Q.; Ma, J.; Lv, W.; He, Y.; Yang, Q. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Cheng, Y.; Sun, S.; Ke, X.; Liu, L.; Shi, Z. The critical role of inorganic nanofillers in solid polymer composite electrolyte for Li+ transportation. Carbon Energy 2021, 3, 482–508. [Google Scholar] [CrossRef]
- Yang, H.; Bright, J.; Chen, B.; Zheng, P.; Gao, X.; Liu, B.; Kasani, S.; Zhang, X.; Wu, N. Chemical interaction and enhanced interfacial ion transport in a ceramic nanofiber–polymer composite electrolyte for all-solid-state lithium metal batteries. J. Mater. Chem. A 2020, 8, 7261–7272. [Google Scholar] [CrossRef]
- Liang, J.; Li, A. Inorganic Particle Size and Content Effects on Tensile Strength of Polymer Composites. J. Reinf. Plast. Compos. 2010, 29, 2744–2752. [Google Scholar] [CrossRef]
- Zhang, H.; An, X.; Long, Y.; Cao, H.; Cheng, Z.; Liu, H.; Ni, Y. A thin and flexible solid electrolyte templated by controllable porous nanocomposites toward extremely high performance all-solid-state lithium-ion batteries. Chem. Eng. J. 2021, 425, 130632. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Kou, W.; Yang, Z.; Zhai, P.; Liu, Y.; Wu, W.; Wang, J. A flexible, ion-conducting solid electrolyte with vertically bicontinuous transfer channels toward high performance all-solid-state lithium batteries. Chem. Eng. J. 2021, 404, 126517. [Google Scholar] [CrossRef]
- Mallakpour, S.; Barati, A. Efficient preparation of hybrid nanocomposite coatings based on poly(vinyl alcohol) and silane coupling agent modified TiO2 nanoparticles. Prog. Org. Coat. 2011, 71, 391–398. [Google Scholar] [CrossRef]
- Sabzi, M.; Mirabedini, S.; Zohuriaan-Mehr, J.; Atai, M. Surface modification of TiO2 nano-particles with silane coupling agent and investigation of its effect on the properties of polyurethane composite coating. Prog. Org. Coat. 2009, 65, 222–228. [Google Scholar] [CrossRef]
- Jia, M.; Bi, Z.; Shi, C.; Zhao, N.; Guo, X. Polydopamine Coated Lithium Lanthanum Titanate in Bilayer Membrane Electrolytes for Solid Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 46231–46238. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, R.; He, Y.-B.; Li, F.; Liu, M.; Li, B.; Yang, Q.-H.; Cai, Q.; Kang, F. SiO2 Hollow Nanosphere-Based Composite Solid Electrolyte for Lithium Metal Batteries to Suppress Lithium Dendrite Growth and Enhance Cycle Life. Adv. Energy Mater. 2016, 6, 2214. [Google Scholar] [CrossRef]
- Lin, D.; Liu, W.; Liu, Y.; Lee, H.R.; Hsu, P.-C.; Liu, K.; Cui, Y. High Ionic Conductivity of Composite Solid Polymer Electrolyte via In Situ Synthesis of Monodispersed SiO2 Nanospheres in Poly(ethylene oxide). Nano Lett. 2016, 16, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, F.; Zhang, C.; Wang, L.; Wang, M.; Qin, M.; Hu, G.; Kong, J. Enhanced dielectric tunability of Ba0.6Sr0.4TiO3/poly(vinylidene fluoride) composites via interface modification by silane coupling agent. Compos. Sci. Technol. 2016, 129, 93–100. [Google Scholar] [CrossRef]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.-H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Sun, J.; Hsu, P.-C.; Li, Y.; Lee, H.-W.; Cui, Y. Ionic Conductivity Enhancement of Polymer Electrolytes with Ceramic Nanowire Fillers. Nano Lett. 2015, 15, 2740–2745. [Google Scholar] [CrossRef]
- Chai, J.; Chen, B.; Xian, F.; Wang, P.; Du, H.; Zhang, J.; Liu, Z.; Zhang, H.; Dong, S.; Zhou, X.; et al. Dendrite-free lithium deposition via flexible-rigid coupling composite network for LiNi0.5Mn1.5O4/Li metal batteries. Small 2018, 14, 1802244. [Google Scholar] [CrossRef]
- Li, J.; Dong, S.; Wang, C.; Hu, Z.; Zhang, Z.; Zhang, H.; Cui, G. A study on the interfacial stability of the cathode/polycarbonate interface: Implication of overcharge and transition metal redox. J. Mater. Chem. A 2018, 6, 11846–11852. [Google Scholar] [CrossRef]
- Jia, M.; Zhao, N.; Bi, Z.; Fu, Z.; Xu, F.; Shi, C.; Guo, X. Polydopamine-Coated Garnet Particles Homogeneously Distributed in Poly(propylene carbonate) for the Conductive and Stable Membrane Electrolytes of Solid Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 46162–46169. [Google Scholar] [CrossRef]
- Chang, C.-H.; Liu, Y.-L. Gel Polymer Electrolytes Based on an Interconnected Porous Matrix Functionalized with Poly(ethylene glycol) Brushes Showing High Lithium Transference Numbers for High Charging-Rate Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 4904–4912. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Liu, W.; Yue, Z.; Wang, Y.; Wu, Y.; Li, Y.; Chen, Z. Effectively Elevating Ceramic Fillers’ Dispersity in Gel Hybrid Electrolyte through Bridge–Linked Construction for High–Performance Lithium Metal Batteries. Batteries 2023, 9, 270. https://doi.org/10.3390/batteries9050270
Chen M, Liu W, Yue Z, Wang Y, Wu Y, Li Y, Chen Z. Effectively Elevating Ceramic Fillers’ Dispersity in Gel Hybrid Electrolyte through Bridge–Linked Construction for High–Performance Lithium Metal Batteries. Batteries. 2023; 9(5):270. https://doi.org/10.3390/batteries9050270
Chicago/Turabian StyleChen, Minghua, Wannian Liu, Ziyu Yue, Yang Wang, Yixin Wu, Yu Li, and Zhen Chen. 2023. "Effectively Elevating Ceramic Fillers’ Dispersity in Gel Hybrid Electrolyte through Bridge–Linked Construction for High–Performance Lithium Metal Batteries" Batteries 9, no. 5: 270. https://doi.org/10.3390/batteries9050270




