A Polyacrylonitrile Shutdown Film for Prevention of Thermal Runaway in Lithium-Ion Cells
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Graphite Battery Electrode Preparation
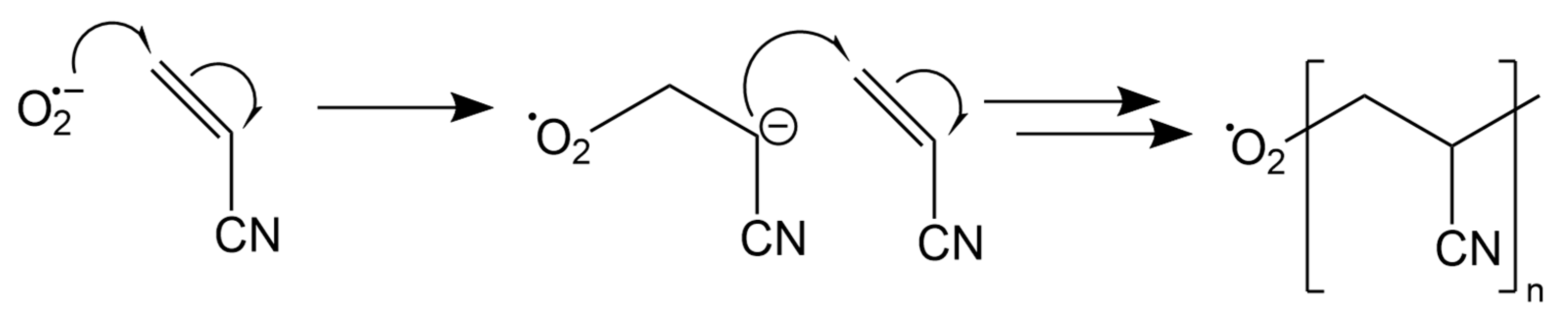
2.3. PAN Electrodeposition
2.4. Lithium Half-Cell Assembly and Electrochemical Testing
2.5. Characterisation
3. Results and Discussion
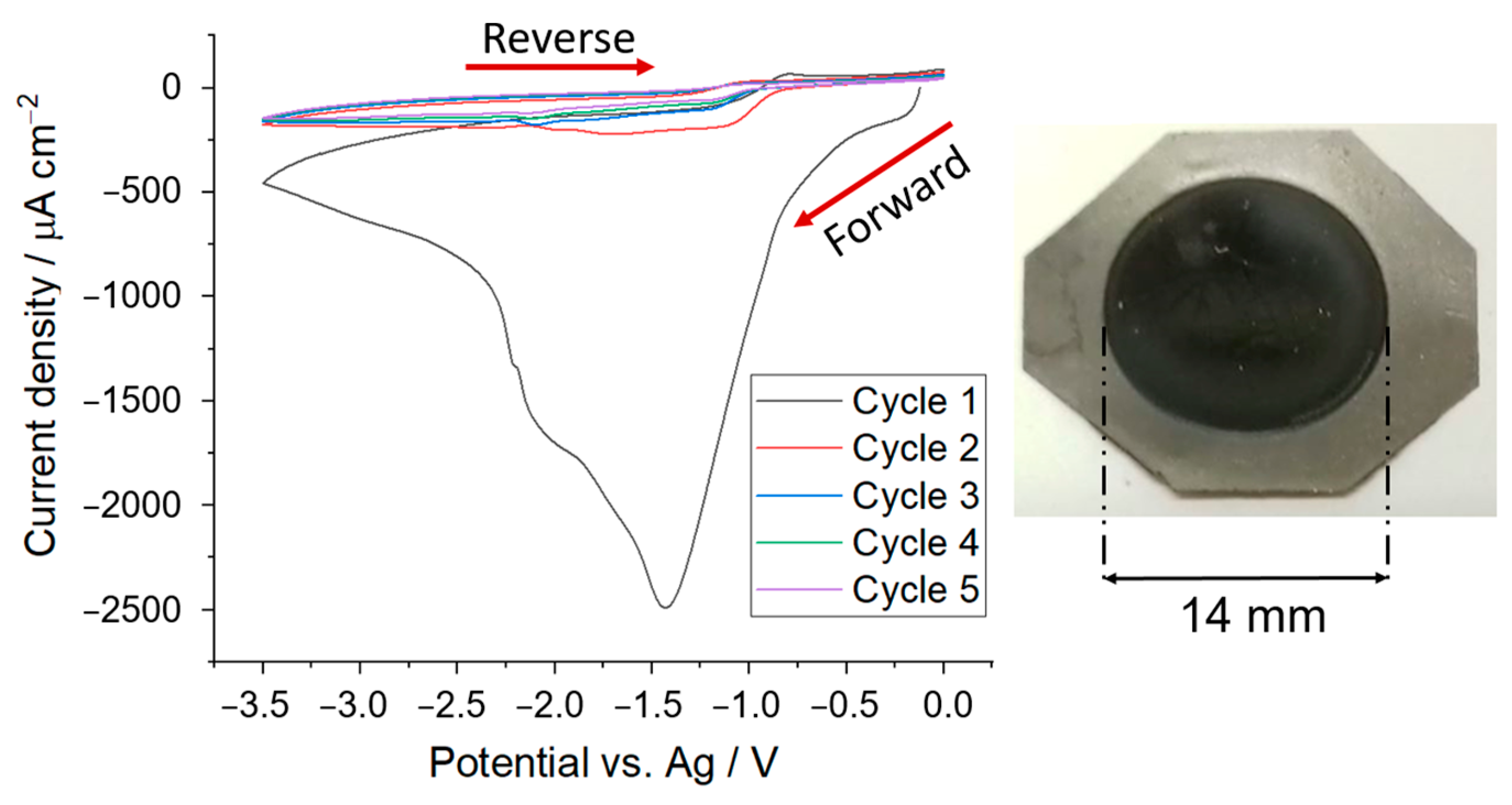
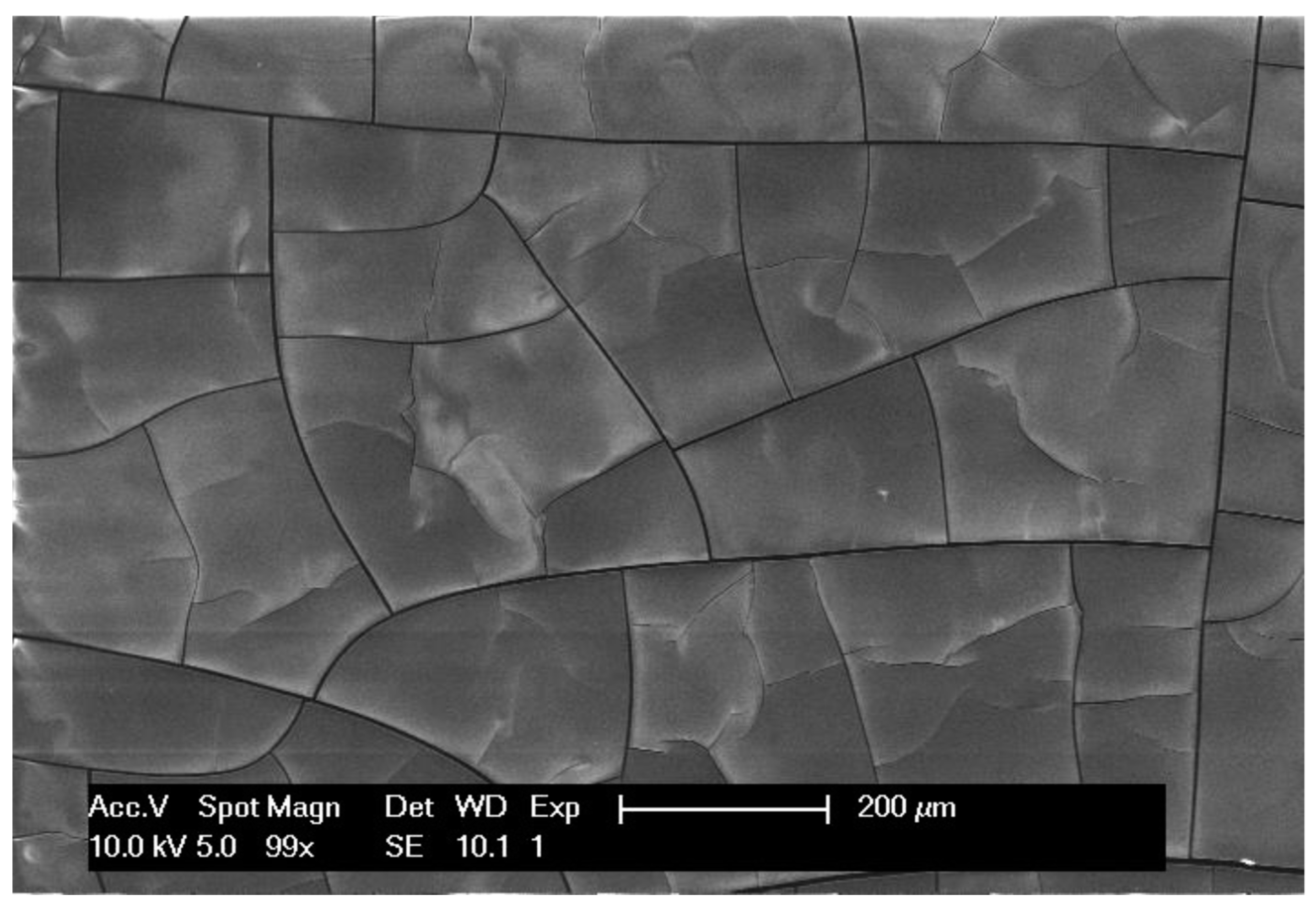
3.1. Formation of Polymer Coating on Graphite Battery Electrode by Electrodeposition
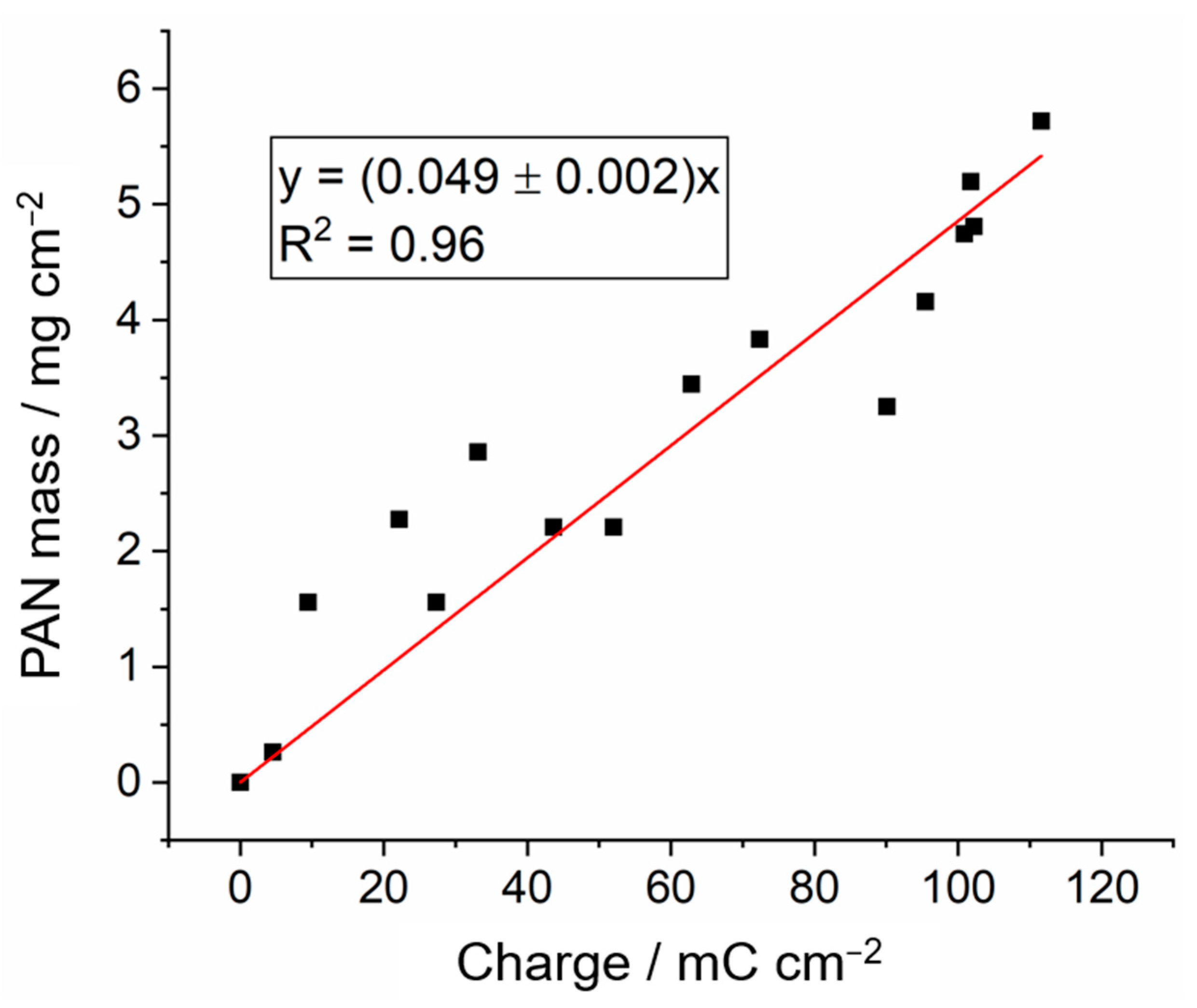
3.2. Controlling the Thickness of the Polymer Coating by Chronoamperometry
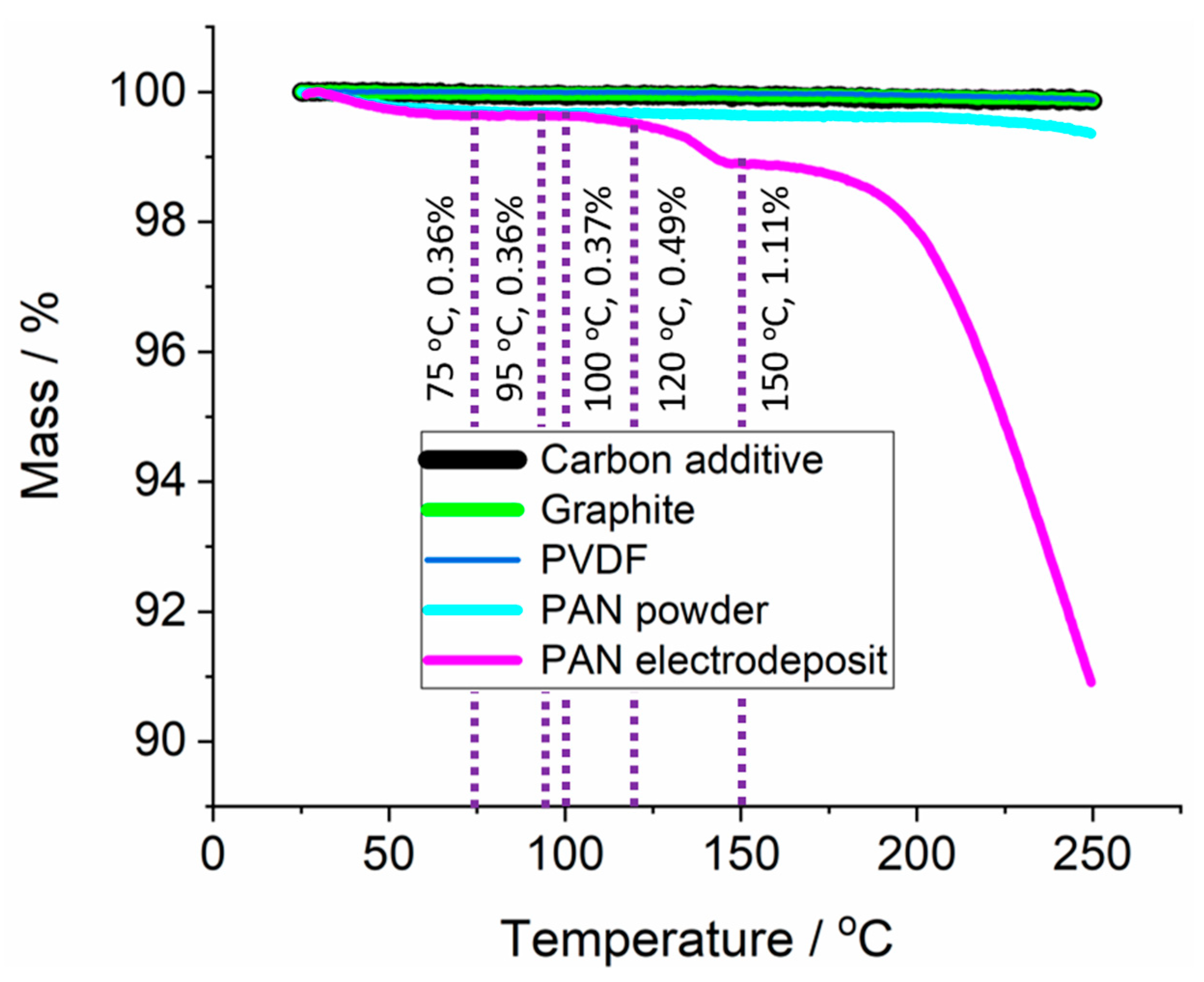
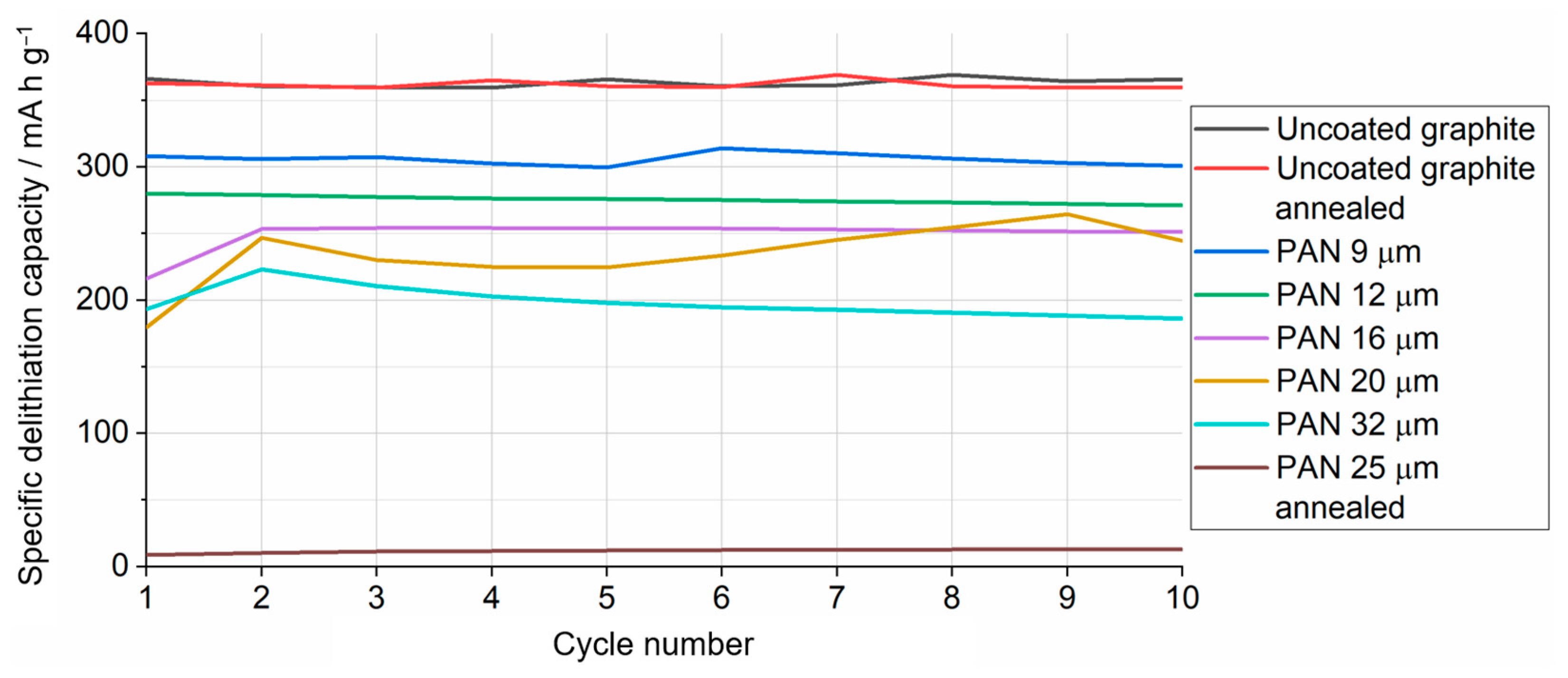
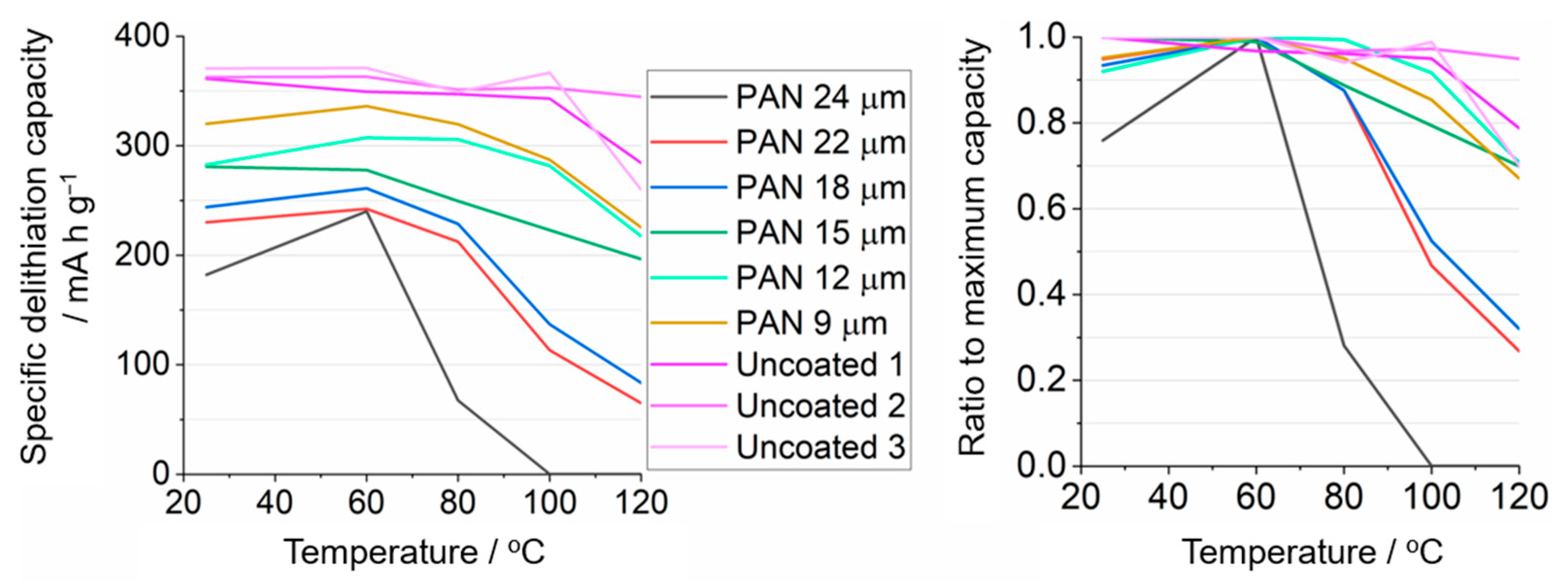
3.3. Application of PAN-Coated Graphite Electrodes for Thermal Runaway Prevention
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–247. [Google Scholar] [CrossRef]
- Sun, P.; Bisschop, R.; Niu, H.; Huang, X. A Review of Battery Fires in Electric Vehicles. Fire Technol. 2020, 56, 1361–1410. [Google Scholar] [CrossRef]
- Börger, A.; Mertens, J.; Wenzl, H. Thermal runaway and thermal runaway propagation in batteries: What do we talk about? J. Energy Storag. 2019, 24, 100649. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Y.; Yu, X.; Qin, Y.; Meng, T.; Hu, X. The pursuit of commercial silicon-based microparticle anodes for advanced lithium-ion batteries: A review. Nano Res. Energy 2022, 1, e9120037. [Google Scholar] [CrossRef]
- Sui, Y.; Guan, J.; Li, K.; Feng, Y.; Peng, S.; Maximov, M.; Liu, Q.; Yang, J.; Geng, H. Synergy of oxygen defects and structural modulation on titanium niobium oxide with a constructed conductive network for high-rate lithium-ion half/full batteries. Inorg. Chem. Front. 2023, 10, 2304–2313. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef]
- Kong, L.; Li, C.; Jiang, J.; Pecht, M. Li-Ion Battery Fire Hazards and Safety Strategies. Energies 2018, 11, 2191. [Google Scholar] [CrossRef]
- Chombo, P.; Laoonual, Y. A review of safety strategies of a Li-ion battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-safety separators for lithium-ion batteries and sodium-ion batteries: Advances and perspective. Energy Storag. Mater. 2021, 41, 522–545. [Google Scholar] [CrossRef]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Orendorff, C. The Role of Separators in Lithium-Ion Cell Safety. Electrochem. Soc. Interface 2012, 21, 61. [Google Scholar] [CrossRef]
- Zhang, M.; Fop, S.; Kramer, D.; Garcia-Araez, N.; Hector, A. A La and Nb co-doped BaTiO3 film with positive-temperature-coefficient of resistance for thermal protection of batteries. J. Mater. Chem. A 2022, 10, 11587–11599. [Google Scholar] [CrossRef]
- Xia, L.; Li, S.; Ai, X.; Yang, H.; Cao, Y. Temperature-sensitive cathode materials for safer lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2845–2848. [Google Scholar] [CrossRef]
- Zhong, H.; Kong, C.; Zhan, H.; Zhan, C.; Zhou, Y. Safe positive temperature coefficient composite cathode for lithium ion battery. J. Power Sources 2012, 216, 273–280. [Google Scholar] [CrossRef]
- Xia, L.; Zhu, L.; Zhang, H.; Ai, X. A positive-temperature-coefficient electrode with thermal protection mechanism for rechargeable lithium batteries. Chin. Sci. Bull. 2012, 57, 4205–4209. [Google Scholar] [CrossRef]
- Ji, W.; Wang, F.; Liu, D.; Qian, J.; Cao, Y.; Chen, Z.; Yang, H.; Ai, X. Building thermally stable Li-ion batteries using a temperature-responsive cathode. J. Mater. Chem. A 2016, 4, 11239–11246. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Zhang, C.; Ji, W.; Qian, J.; Cao, Y.; Yang, H.; Ai, X. A temperature-sensitive poly(3-octylpyrrole)/carbon composite as a conductive matrix of cathodes for building safer Li-ion batteries. Energy Storag. Mater. 2019, 17, 275–283. [Google Scholar] [CrossRef]
- Zhang, H.; Pang, J.; Ai, X.; Cao, Y.; Yang, H.; Lu, S. Poly(3-butylthiophene)-based positive-temperature-coefficient electrodes for safer lithium-ion batteries. Electrochim. Acta 2016, 187, 173–178. [Google Scholar] [CrossRef]
- Chen, Z.; Hsu, P.; Lopez, J.; Li, Y.; To, J.; Liu, N.; Wang, C.; Andrews, S.; Liu, J.; Cui, Y.; et al. Fast and reversible thermoresponsive polymer switching materials for safer batteries. Nat. Energy 2016, 1, 15009. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Zhang, C.; Cao, Y.; Yang, H.; Ai, X.; Zhong, F. Building a Thermal Shutdown Cathode for Li-Ion Batteries Using Temperature-Responsive Poly(3-Dodecylthiophene. Energy Technol. 2020, 8, 7. [Google Scholar] [CrossRef]
- Li, M.; Shi, Y.; Gao, H.; Chen, Z. Bio-Inspired Nanospiky Metal Particles Enable Thin, Flexible, and Thermo-Responsive Polymer Nanocomposites for Thermal Regulation. Adv. Funct. Mater. 2020, 30, 23. [Google Scholar] [CrossRef]
- Baginska, M.; Blaiszik, B.; Merriman, R.; Sottos, N.; Moore, J.; White, S. Autonomic shutdown of lithium-ion batteries using thermoresponsive microspheres. Adv. Energy Mater. 2012, 2, 583–590. [Google Scholar] [CrossRef]
- Baginska, M.; Blaiszik, B.; Rajh, T.; Sottos, N.; White, S. Enhanced autonomic shutdown of Li-ion batteries by polydopamine coated polyethylene microspheres. J. Power Sources 2014, 269, 735–739. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Wang, S.; Cao, Y.; Yang, H.; Ai, X.; Zhong, F. A polyethylene microsphere-coated separator with rapid thermal shutdown function for lithium-ion batteries. J. Energy Chem. 2020, 44, 33–40. [Google Scholar] [CrossRef]
- Allen, J.; Hector, A.; Garcia-Araez, N. Cell design for the electrodeposition of polyacrylonitrile onto graphite composite electrodes for use in lithium-ion cells. Energy Rep. 2021, 7, 15–19. [Google Scholar] [CrossRef]
- El-Enany, G.; Lacey, M.; Johns, P.; Owen, J. In situ growth of polymer electrolytes on lithium ion electrode surfaces. Electrochem. Commun. 2009, 11, 2320–2323. [Google Scholar] [CrossRef]
- Lacey, M.; Sosna, M.; Owen, J. An electrochemical quartz crystal microbalance study of poly(acrylonitrile) deposition initiated by electrogenerated superoxide. Electrochim. Acta 2013, 29, 23–26. [Google Scholar] [CrossRef]
- Lécayon, G.; Bouizem, Y.; Le Gressus, C.; Reynaud, C.; Boiziau, C.; Juret, C. Grafting and growing mechanisms of polymerised organic films onto metallic surfaces. Chem. Phys. Lett. 1982, 91, 506–510. [Google Scholar] [CrossRef]
- Boiziau, C.; Lécayon, G. Adhesion of polymers to metals: A review of the results obtained studying a model system. Surf. Interface Anal. 1988, 12, 475–485. [Google Scholar] [CrossRef]
- Boiziau, C.; Leroy, S.; Reynaud, C.; Lécayon, G.; Le Gressus, C.; Viel, P. Elementary Mechanisms in the Interaction of Organic Molecules with Mineral Surfaces. J. Adhes. 1987, 23, 21–44. [Google Scholar] [CrossRef]
- Abraham, K.; Alamgir, M. Li+-Conductive Solid Polymer Electrolytes with Liquid-Like Conductivity. J. Electrochem. Soc. 1990, 137, 1657. [Google Scholar] [CrossRef]
- Huang, B.; Wnag, Z.; Li, G.; Huang, H.; Xue, R.; Chen, L.; Wang, F. Lithium ion conduction in polymer electrolytes based on PAN. Solid State Ion. 1996, 85, 79–84. [Google Scholar] [CrossRef]
- Abraham, K.; Choe, H.; Pasquariello, D. Polyacrylonitrile electrolyte-based Li ion batteries. Electrochim. Acta 1998, 43, 2399–2412. [Google Scholar] [CrossRef]
- Watanabe, M.; Kanba, M.; Matsuda, H.; Tsunemi, K.; Mizoguchi, K.; Tsuchida, E.; Shinohara, I. High lithium ionic conductivity of polymeric solid electrolytes. Die Makromol. Chem. Rapid Commun. 1981, 2, 741–744. [Google Scholar] [CrossRef]
- Watanabe, M.; Kanba, M.; Nagaoka, K.; Shinohara, I. Ionic conductivity of hybrid films composed of polyacrylonitrile, ethylene carbonate, and LiClO4. J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 939–948. [Google Scholar] [CrossRef]
- Huang, Y.; Koenig, J. Raman Spectra of Polyacrylonitrile. Appl. Spectrosc. 1971, 25, 620–622. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126. [Google Scholar] [CrossRef]
- Krigbaum, W.; Kotliar, A. The molecular weight of polyacrylonitrile. J. Polym. Sci. 1958, 32, 323–341. [Google Scholar] [CrossRef]
- Beevers, R. The physical properties of polyacrylonitrile and its copolymers. J. Polym. Sci. Macromol. Rev. 1968, 3, 113–254. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Zhong, J.; Wang, T.; Wang, L.; Xu, H.; Cao, J.; Li, J.; Zhang, G.; Fei, H.; et al. Hierarchically Micro/Nanostructured Current Collectors Induced by Ultrafast Femtosecond Laser Strategy for High-Performance Lithium-ion Batteries. Energy Environ. Mater. 2022, 5, 969–976. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Q.; Zhong, J.; Chen, M.; Deng, H.; Cao, J.; Wang, L.; Peng, L.; Zhu, J.; Lu, B. 3D Holey Graphene/Polyacrylonitrile Sulfur Composite Architecture for High Loading Lithium Sulfur Batteries. Adv. Energy Mater. 2021, 11, 16. [Google Scholar] [CrossRef]




| Material | Usage | Supplier |
|---|---|---|
| Acrylonitrile (AN, ≥99%, contains the inhibitor monomethyl ether hydroquinone in 35–45 ppm) | Solvent, monomer for electrodeposition of PAN | Sigma-Aldrich, Gillingham, UK |
| Alumina powder (1 µm, 0.3 µm, and 0.05 µm) | Polishing powder | Buehler, Coventry, UK |
| Calcium hydride (95%) | Drying agent | Sigma-Aldrich, Gillingham, UK |
| Carbon powder, (99%, Super C65 carbon black) | Conductive additive | Timcal, Cambridge, UK |
| Copper metal foil (99.9%, 50 µm thickness) | Current collector | Advent, Oxford, UK |
| Glass fibre (50 µm thickness, GF/F, WhatmanTM) | Separator | GE Healthcare Life Sciences, Chicago IL, USA |
| Graphite powder (99%) | Active material | Hitachi Chemical, Tokyo, Japan |
| Lithium metal foil (99.9% purity, 120 µm thickness) | Counter-reference electrode | Goodfellow, Cambridge, UK |
| LP57 (1 mol dm−3 LiPF6 in 3:7 ethylene carbonate/ethyl methyl carbonate) | Lithium-ion electrolyte | Soulbrain MI, Northville MI, USA |
| N-methyl-2-pyrrolidone (NMP, 99.5%) | Solvent | Sigma-Aldrich, Gillingham, UK |
| PAN, Polyacrylonitrile (PAN, Mw 150,000) | Percolating polymer matrix | Sigma-Aldrich, Gillingham, UK |
| PVDF, Polyvinylidene difluoride (PVDF, Solef® 5130) | Electrode binder and percolating polymer matrix | Solef, Tavaux, France |
| Tetrabutylammonium perchlorate (TBAP, ≥99%) | Supporting electrolyte | Sigma-Aldrich, Gillingham, UK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, J.P.C.; Mierzwa, M.; Kramer, D.; Garcia-Araez, N.; Hector, A.L. A Polyacrylonitrile Shutdown Film for Prevention of Thermal Runaway in Lithium-Ion Cells. Batteries 2023, 9, 282. https://doi.org/10.3390/batteries9050282
Allen JPC, Mierzwa M, Kramer D, Garcia-Araez N, Hector AL. A Polyacrylonitrile Shutdown Film for Prevention of Thermal Runaway in Lithium-Ion Cells. Batteries. 2023; 9(5):282. https://doi.org/10.3390/batteries9050282
Chicago/Turabian StyleAllen, Jonathan Peter Charles, Marcin Mierzwa, Denis Kramer, Nuria Garcia-Araez, and Andrew L. Hector. 2023. "A Polyacrylonitrile Shutdown Film for Prevention of Thermal Runaway in Lithium-Ion Cells" Batteries 9, no. 5: 282. https://doi.org/10.3390/batteries9050282
APA StyleAllen, J. P. C., Mierzwa, M., Kramer, D., Garcia-Araez, N., & Hector, A. L. (2023). A Polyacrylonitrile Shutdown Film for Prevention of Thermal Runaway in Lithium-Ion Cells. Batteries, 9(5), 282. https://doi.org/10.3390/batteries9050282








