Yeast-Derived Sulfur Host for the Application of Sustainable Li–S Battery Cathode
Abstract
:1. Introduction
2. Materials and Methods
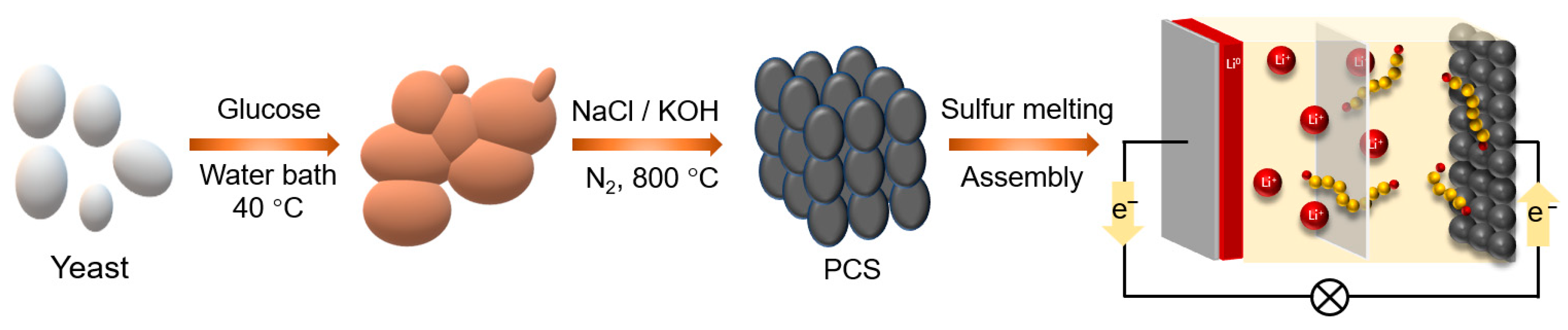
2.1. Preparation of Porous Carbon Materials
2.2. Preparation of PCS/S Composites
2.3. Assembly of Li–S Battery
2.4. Characterization
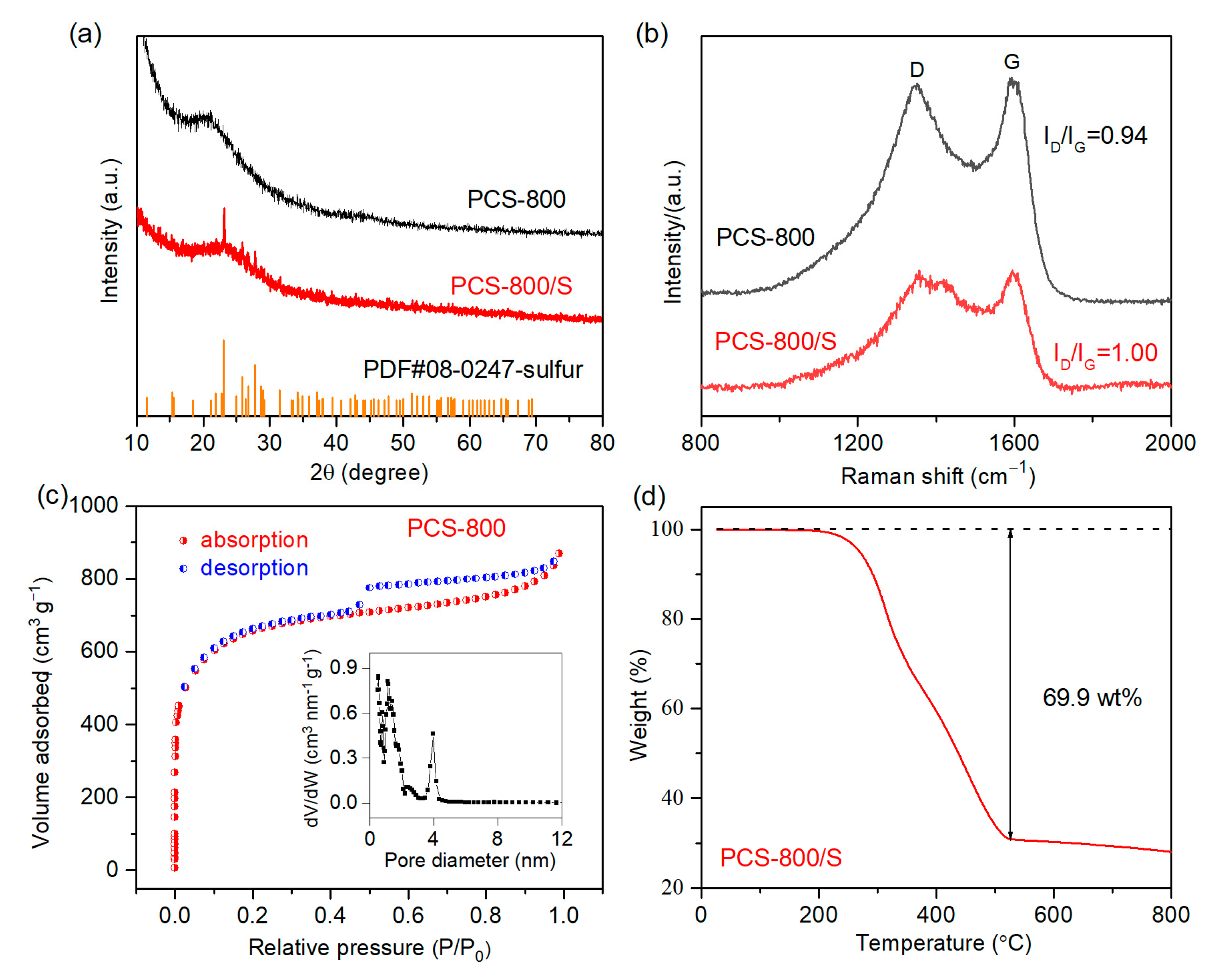
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feng, S.; Fu, Z.-H.; Chen, X.; Zhang, Q. A review on theoretical models for lithium–sulfur battery cathodes. InfoMat 2022, 4, e12304. [Google Scholar] [CrossRef]
- Park, J.W.; Jo, S.C.; Kim, M.J.; Choi, I.H.; Kim, B.G.; Lee, Y.J.; Choi, H.Y.; Kang, S.; Kim, T.; Baeg, K.J. Flexible high-energy-density lithium-sulfur batteries using nanocarbon-embedded fibrous sulfur cathodes and membrane separators. NPG Asia Mater. 2021, 13, 30. [Google Scholar] [CrossRef]
- Hong, H.; Che Mohamad, N.A.R.; Chae, K.; Marques Mota, F.; Kim, D.H. The lithium metal anode in Li–S batteries: Challenges and recent progress. J. Mater. Chem. A 2021, 9, 10012–10038. [Google Scholar] [CrossRef]
- Lim, W.-G.; Kim, S.; Jo, C.; Lee, J. A Comprehensive Review of Materials with Catalytic Effects in Li–S Batteries: Enhanced Redox Kinetics. Angew. Chem. Int. Ed. 2019, 58, 18746–18757. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Sun, W.; Meng, F.; Xing, A.; Liu, J. Advanced chemical strategies for lithium–sulfur batteries: A review. Green Energy Environ. 2018, 3, 2–19. [Google Scholar] [CrossRef]
- Zhou, G.M.; Chen, H.; Cui, Y. Formulating energy density for designing practical lithium-sulfur batteries. Nat. Energy 2022, 7, 312–319. [Google Scholar] [CrossRef]
- Ye, Z.; Jiang, Y.; Li, L.; Wu, F.; Chen, R. A High-Efficiency CoSe Electrocatalyst with Hierarchical Porous Polyhedron Nanoarchitecture for Accelerating Polysulfides Conversion in Li-S Batteries. Adv. Mater. 2020, 32, 2002168. [Google Scholar] [CrossRef]
- Xu, B.; Zhao, Y.; Liu, H.; Cheng, S.; Liu, J.; Meng, F. Nanolayer GO coated CNT film interlayer for lithium-sulfur batteries with enhanced cycling stability. Mater. Lett. 2021, 305, 130753. [Google Scholar] [CrossRef]
- Qi, C.; Zu, S.; Zhu, X.; Zhang, T.; Li, L.; Song, L.; Jin, Y.; Zhang, M. Bamboo-shaped Co@NCNTs as superior sulfur host for Li-S batteries. Appl. Surf. Sci. 2022, 601, 154245. [Google Scholar] [CrossRef]
- Jiang, J.C.; Fan, Q.N.; Chou, S.L.; Guo, Z.P.; Konstantinov, K.; Liu, H.K.; Wang, J.Z. Li2S-Based Li-Ion Sulfur Batteries: Progress and Prospects. Small 2021, 17, 1903934. [Google Scholar] [CrossRef]
- He, J.R.; Bhargav, A.; Manthiram, A. High-Performance Anode-Free Li-S Batteries with an Integrated Li2S-Electrocatalyst Cathode. ACS Energy Lett. 2022, 7, 583–590. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Wang, Y.; Chen, Y.; Guo, X.; Wu, Z.; Zhong, B. Review of the application of biomass-derived porous carbon in lithium-sulfur batteries. Ionics 2020, 26, 4765–4781. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.S.; Yang, J. Sulfur-based composite cathode materials for high-energy rechargeable lithium batteries. Adv. Mater. 2015, 27, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Puthirath, A.B.; Baburaj, A.; Kato, K.; Salpekar, D.; Chakingal, N.; Cao, Y.; Babu, G.; Ajayan, P.M. High sulfur content multifunctional conducting polymer composite electrodes for stable Li-S battery. Electrochim. Acta 2019, 306, 489–497. [Google Scholar] [CrossRef]
- Jiang, M.; Gan, B.; Deng, Y.; Xiong, Y.; Tan, R. Suppressing Self-Discharge with Polymeric Sulfur in Li-S Batteries. Materials 2018, 12, 64. [Google Scholar] [CrossRef]
- Li, H.; Shao, F.; Wen, X.; Ding, Y.; Zhou, C.; Zhang, Y.; Wei, H.; Hu, N. Graphene/MXene fibers-enveloped sulfur cathodes for high-performance Li-S batteries. Electrochim. Acta 2021, 371, 137838. [Google Scholar] [CrossRef]
- Cheng, M.; Zhao, H.; Zhao, Z.; Wang, J.; Cao, L.; Zhang, H.; Duan, X.; Wang, C.; Wang, J.; Wang, J.; et al. The improvement of pitch activation by graphene for long-cycle Li–S batteries. Green Chem. 2018, 20, 4675–4683. [Google Scholar] [CrossRef]
- Gueon, D.; Yoon, J.; Hwang, J.T.; Moon, J.H. Microdomain sulfur-impregnated CeO2-coated CNT particles for high-performance Li-S batteries. Chem. Eng. J. 2020, 390, 124548. [Google Scholar] [CrossRef]
- Wang, B.; Guo, W.; Fu, Y. Anodized Aluminum Oxide Separators with Aligned Channels for High-Performance Li-S Batteries. ACS Appl. Mater. Interfaces 2020, 12, 5831–5837. [Google Scholar] [CrossRef]
- Yanilmaz, M. TiO2-decorated porous carbon nanofiber interlayer for Li-S batteries. RSC Adv. 2020, 10, 16570–16575. [Google Scholar] [CrossRef]
- Meng, F.; Xu, B.; Long, T.; Cheng, S.; Li, Y.; Zhang, Y.; Liu, J. ZnS nanolayer coated hollow carbon spheres with enhanced rate and cycling performance for Li-S batteries. Sci. China Technol. Sci. 2021, 65, 272–281. [Google Scholar] [CrossRef]
- Xu, C.Y.; Du, R.; Yu, C.B.; Shi, Z.Y.; Wang, J.L.; Li, T.R. Glucose-Derived Micro–Mesoporous Carbon Spheres for High-Performance Lithium–Sulfur Batteries. Energy Fuel 2022, 37, 1318–1326. [Google Scholar] [CrossRef]
- He, J.; Gao, Z.; Li, X. Yeast-Derived Carbon Nanotube-Coated Separator for High Performance Lithium-Sulfur Batteries. JOM 2021, 73, 2516–2524. [Google Scholar] [CrossRef]
- Vijaya Kumar Saroja, A.P.; Arjunan, A.; Muthusamy, K.; Balasubramanian, V.; Sundara, R. Chemically bonded amorphous red phosphorous with disordered carbon nanosheet as high voltage cathode for rechargeable aluminium ion battery. J. Alloy Compd. 2020, 830, 154693. [Google Scholar] [CrossRef]
- Arjunan, A.; Subbiah, M.; Sekar, M.; Vs, A.P.; Balasubramanian, V.; Sundara, R. Biomass derived hierarchically porous carbon inherent structure as an effective metal free cathode for Li-O2/air battery. Electrochem. Sci. Adv. 2021, 1, e202000037. [Google Scholar] [CrossRef]
- Guo, X.T.; Wang, S.B.; Yang, B.; Xu, Y.X.; Liu, Y.; Pang, H. Porous pyrrhotite Fe7S8 nanowire/SiOx/nitrogen-doped carbon matrix for high-performance Li-ion-battery anodes. J. Colloid Interf. Sci. 2020, 561, 801–807. [Google Scholar] [CrossRef]
- Razzaq, A.A.; Yao, Y.Z.; Shah, R.; Qi, P.W.; Miao, L.X.; Chen, M.Z.; Zhao, X.H.; Peng, Y.; Deng, Z. High-performance lithium sulfur batteries enabled by a synergy between sulfur and carbon nanotubes. Energy Storage Mater. 2019, 16, 194–202. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Deng, H.H.; Su, Q.M.; Du, G.H.; Yu, Y.; Ma, S.F.; Xu, B.S. A free-standing nitrogen-doped porous carbon foam electrode derived from melaleuca bark for lithium-sulfur batteries. Electrochim. Acta 2019, 293, 19–24. [Google Scholar] [CrossRef]
- Nurhilal, O.; Hidayat, S.; Sumiarsa, D.; Risdiana, R. Natural Biomass-Derived Porous Carbon from Water Hyacinth Used as Composite Cathode for Lithium Sulfur Batteries. Sustainability 2023, 15, 1039. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Liu, J. Biomass-derived porous carbon materials for advanced lithium sulfur batteries. J. Energy Chem. 2019, 34, 171–185. [Google Scholar] [CrossRef]
- Imtiaz, S.; Zhang, J.; Zafar, Z.A.; Ji, S.; Huang, T.; Anderson, J.A.; Zhang, Z.; Huang, Y. Biomass-derived nanostructured porous carbons for lithium-sulfur batteries. Sci. China Mater. 2016, 59, 389–407. [Google Scholar] [CrossRef]
- Tian, Y.H.; Ren, Q.X.; Chen, X.Y.; Li, L.B.; Lan, X.Z. Yeast-Based Porous Carbon with Superior Electrochemical Properties. ACS Omega 2022, 7, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Park, S.K.; Kang, Y.C. A Salt-Templated Strategy toward Hollow Iron Selenides-Graphitic Carbon Composite Microspheres with Interconnected Multicavities as High-Performance Anode Materials for Sodium-Ion Batteries. Small 2019, 15, 1803043. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Liu, H.X.; Yu, Z.Y. Novel and multifunctional inorganic mixing salt-templated 2D ultrathin Fe/Co-N/S-carbon nanosheets as effectively bifunctional electrocatalysts for Zn-air batteries. Appl. Catal. B Environ. 2019, 241, 95–103. [Google Scholar] [CrossRef]
- Tan, Y.T.; Ren, J.N.; Li, X.M.; He, L.J.; Chen, C.M.; Li, H.B. Highly graphitic porous carbon prepared via K2FeO4-assisted KOH activation for supercapacitors. New J. Chem. 2022, 46, 14338–14345. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Xu, J.Y.; Liu, L.C.; Zhou, J.C.; Cai, J.J. High-surface-area porous carbons produced by the mild KOH activation of a chitosan hydrochar and their CO2 capture. New Carbon Mater. 2021, 36, 1081–1093. [Google Scholar] [CrossRef]
- Wang, D.; Liu, H.; Shan, Z.; Xia, D.; Na, R.; Liu, H.; Wang, B.; Tian, J. Nitrogen, sulfur Co-doped porous graphene boosting Li4Ti5O12 anode performance for high-rate and long-life lithium ion batteries. Energy Storage Mater. 2020, 27, 387–395. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.R.; Li, Y.Y.; Xiang, M.W.; Guo, J.M.; Bai, H.L.; Liu, X.F.; Yang, X.Z.; Su, C.W. Waste-honeycomb-derived in situ N-doped Hierarchical porous carbon as sulfur host in lithium-sulfur battery. Dalton Trans. 2022, 51, 1502–1512. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.L.; Meng, F.C.; Jin, L.Y.; Li, S.L.; Cheng, S.; Jiang, S.D.; Zhou, R.L.; Liu, J.H. Natural nori-based porous carbon composite for sustainable lithium-sulfur batteries. Sci. China Technol. Sci. 2022, 65, 2380–2387. [Google Scholar] [CrossRef]
- Peng, H.-J.; Huang, J.-Q.; Zhang, Q. A review of flexible lithium–sulfur and analogous alkali metal–chalcogen rechargeable batteries. Chem. Soc. Rev. 2017, 46, 5237–5288. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, M.; Wang, X.; Zhang, Y.; Huang, C.; Zhang, Y.; Wang, Y. Honeycomb-like nitrogen and sulfur dual-doped hierarchical porous biomass carbon bifunctional interlayer for advanced lithium-sulfur batteries. Chem. Eng. J. 2019, 355, 478–486. [Google Scholar] [CrossRef]
- Hou, J.; Tu, X.; Wu, X.; Shen, M.; Wang, X.; Wang, C.; Cao, C.; Pang, H.; Wang, G. Remarkable cycling durability of lithium-sulfur batteries with interconnected mesoporous hollow carbon nanospheres as high sulfur content host. Chem. Eng. J. 2020, 401, 126141. [Google Scholar] [CrossRef]
- Wu, D.; Chen, J.; Zhang, W.; Liu, W.; Li, J.; Cao, K.; Gao, Z.; Xu, F.; Jiang, K. Sealed pre-carbonization to regulate the porosity and heteroatom sites of biomass derived carbons for lithium-sulfur batteries. J. Colloid Interf. Sci. 2020, 579, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Hannauer, J.; Scheers, J.; Fullenwarth, J.; Fraisse, B.; Stievano, L.; Johansson, P. The Quest for Polysulfides in Lithium–Sulfur Battery Electrolytes: An Operando Confocal Raman Spectroscopy Study. ChemPhysChem 2015, 16, 2755–2759. [Google Scholar] [CrossRef]
- Zhou, L.; Danilov, D.L.; Eichel, R.A.; Notten, P.H. Host materials anchoring polysulfides in Li–S batteries reviewed. Adv. Energy Mater. 2021, 11, 2001304. [Google Scholar] [CrossRef]
- Du, Y.; Huang, R.; Lin, X.; Khan, S.; Zheng, B.; Fu, R. Template-Free Preparation of Hierarchical Porous Carbon Nanosheets for Lithium-Sulfur Battery. Langmuir 2020, 36, 14507–14513. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Cao, T.; Zhang, J.; Zhang, S.-W.; Zheng, D.; Wu, H.; Lv, W.; Kang, F.; Yang, Q.-H. Precise carbon structure control by salt template for high performance sodium-ion storage. J. Energy Chem. 2019, 31, 101–106. [Google Scholar] [CrossRef]
- Ponomarev, N.; Sillanpää, M. Combined chemical-templated activation of hydrolytic lignin for producing porous carbon. Ind. Crop. Prod. 2019, 135, 30–38. [Google Scholar] [CrossRef]
- Liu, W.; Fan, X.; Xu, B.; Chen, P.; Tang, D.; Meng, F.; Zhou, R.; Liu, J. MnO-Inlaid hierarchically porous carbon hybrid for lithium-sulfur batteries. Nano Sel. 2020, 2, 573–580. [Google Scholar] [CrossRef]
- Shi, Z.; Feng, W.; Wang, X.; Li, M.; Song, C.; Chen, L. Catalytic cobalt phosphide Co2P/carbon nanotube nanocomposite as host material for high performance lithium-sulfur battery cathode. J. Alloys Compd. 2021, 851, 156289. [Google Scholar] [CrossRef]
- Yan, Y.; Shi, M.; Wei, Y.; Zhao, C.; Chen, L.; Fan, C.; Yang, R.; Xu, Y. The hierarchical porous structure of carbon aerogels as matrix in cathode materials for Li-S batteries. J. Nanoparticle Res. 2018, 20, 260. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Liao, Y.; Li, X.; Li, W. Sulfur loaded in micropore-rich carbon aerogel as cathode of lithium-sulfur battery with improved cyclic stability. J. Power Sources 2016, 334, 23–30. [Google Scholar] [CrossRef]
- Liu, N.N.; Wu, X.; Zhang, Y.; Yin, Y.Y.; Sun, C.Z.; Mao, Y.C.; Fan, L.S.; Zhang, N.Q. Building High Rate Capability and Ultrastable Dendrite-Free Organic Anode for Rechargeable Aqueous Zinc Batteries. Adv. Sci. 2020, 7, 2000146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Wu, D.; Niu, Q.; Lu, P.; Ma, T.; Su, Y.; Chen, L.; Li, H.; Wu, F. Stable Ni-rich layered oxide cathode for sulfide-based all-solid-state lithium battery. eScience 2022, 2, 537–545. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. The same chemical state of carbon gives rise to two peaks in X-ray photoelectron spectroscopy. Sci. Rep. 2021, 11, 11195. [Google Scholar] [CrossRef]
- Wang, H.T.; Qiu, X.Y.; Wang, W.; Jiang, L.P.; Liu, H.F. Iron Sulfide Nanoparticles Embedded Into a Nitrogen and Sulfur Co-doped Carbon Sphere as a Highly Active Oxygen Reduction Electrocatalyst. Front. Chem. 2019, 7, 855. [Google Scholar] [CrossRef]
- Song, W.; Xu, C.; Li, M.; Cheng, Z.; Liu, Y.; Wang, P.; Liu, Z. Cobalt Nanocluster-Doped Carbon Micro-Spheres with Multilevel Porous Structure for High-Performance Lithium-Sulfur Batteries. Energies 2023, 16, 247. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, K.; Qian, J.; Guo, Y.; Song, X.; Pan, H.; Wang, D.; Li, X. Boosting Lithium–Sulfur Battery Performance by Integrating a Redox-Active Covalent Organic Framework in the Separator. ACS Appl. Energy Mater. 2019, 2, 5793–5798. [Google Scholar] [CrossRef]
- Long, T.; Meng, F.; Xu, B.; Zhao, Y.; Liu, W.; Wei, X.; Zheng, L.; Liu, J. Nitrogen-doped carbon nanotubes intertwined with porous carbon with enhanced cathode performance in lithium–sulfur batteries. Sustain. Energy Fuel 2020, 4, 3926–3933. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Wu, J.; Xuan, C.; Wu, Z.; Guo, X.; Lai, C.; Zhu, Y.; Wang, D. Coordination effect of network NiO nanosheet and a carbon layer on the cathode side in constructing a high-performance lithium–sulfur battery. J. Mater. Chem. A 2018, 6, 6503–6509. [Google Scholar] [CrossRef]
- Qiu, X.; Hua, Q.; Zheng, L.; Dai, Z. Study of the discharge/charge process of lithium–sulfur batteries by electrochemical impedance spectroscopy. RSC Adv. 2020, 10, 5283–5293. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, J.; Wei, Z.; Yang, H.; Ma, Z.; Ma, R.; Zhou, J.; Yang, Y.; Peng, L.; Fei, H.; et al. Bacteria-Derived Biological Carbon Building Robust Li-S Batteries. Nano Lett. 2019, 19, 4384–4390. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Dou, X.; Liu, W.; Zhang, L.; Lv, L.; Liu, J.; Meng, F. Yeast-Derived Sulfur Host for the Application of Sustainable Li–S Battery Cathode. Batteries 2023, 9, 289. https://doi.org/10.3390/batteries9060289
He Z, Dou X, Liu W, Zhang L, Lv L, Liu J, Meng F. Yeast-Derived Sulfur Host for the Application of Sustainable Li–S Battery Cathode. Batteries. 2023; 9(6):289. https://doi.org/10.3390/batteries9060289
Chicago/Turabian StyleHe, Zhanhui, Xinyi Dou, Weilin Liu, Luxian Zhang, Laixi Lv, Jiehua Liu, and Fancheng Meng. 2023. "Yeast-Derived Sulfur Host for the Application of Sustainable Li–S Battery Cathode" Batteries 9, no. 6: 289. https://doi.org/10.3390/batteries9060289





