Li3BO3-Li3PO4 Composites for Efficient Buffer Layer of Sulphide-Based All-Solid-State Batteries
Abstract
:1. Introduction
2. Materials and Methods
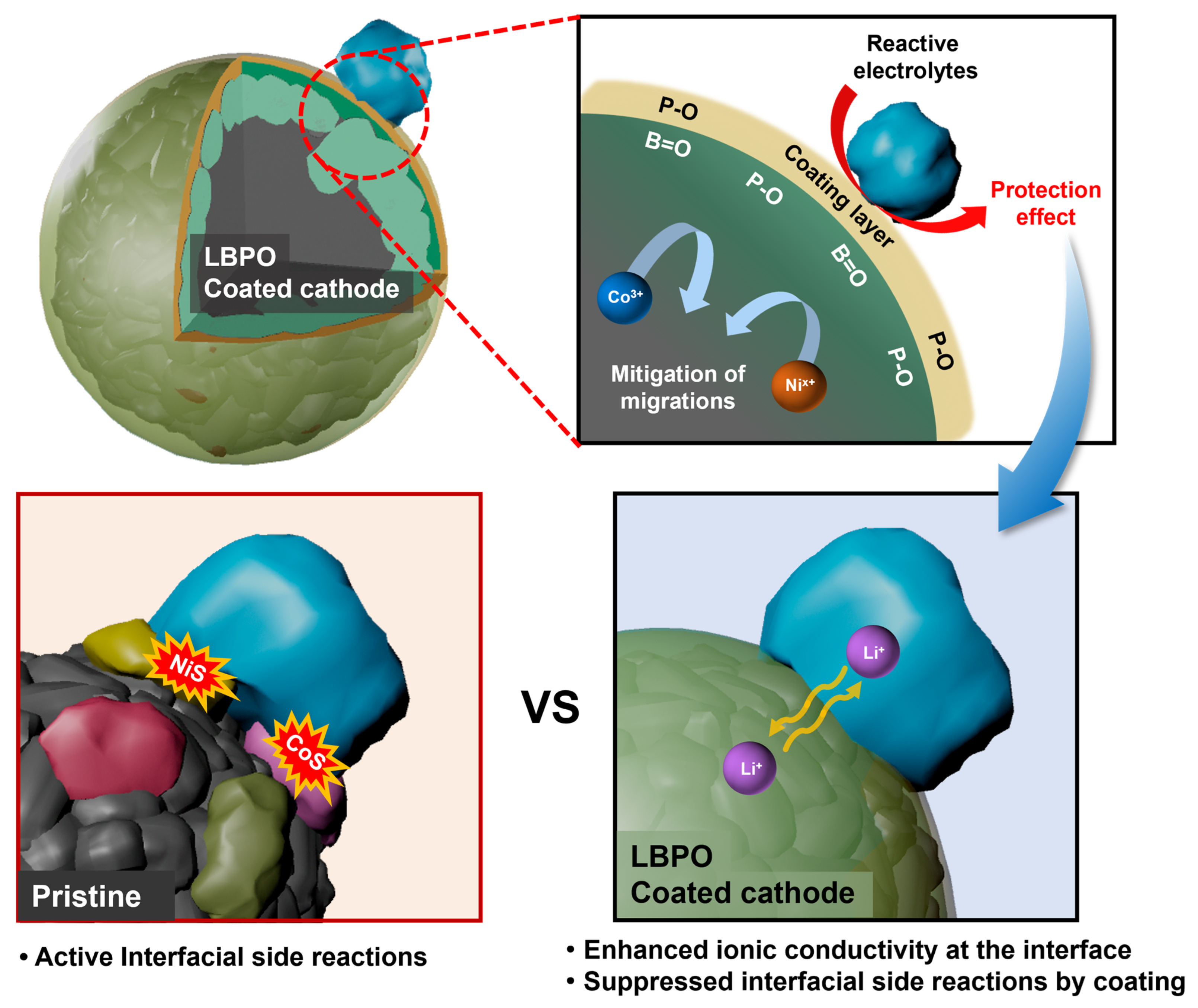
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, L.; Chen, M.; Nam, K.W.; Kang, Y.M. Redox Evolution of Li-Rich Layered Cathode Materials. Batteries 2022, 8, 132. [Google Scholar] [CrossRef]
- Teo, J.H.; Strauss, F.; Walther, F.; Ma, Y.; Payandeh, S.; Scherer, T.; Bianchini, M.; Janek, J.; Brezesinski, T. The Interplay between (Electro)Chemical and (Chemo)Mechanical Effects in The Cycling Performance of Thiophosphate-Based Solid-State Batteries. Mater. Futures 2022, 1, 015102. [Google Scholar] [CrossRef]
- Zhao, B.; Ma, L.; Wu, K.; Cao, M.; Xu, M.; Zhang, X.; Liu, W.; Chen, J. Asymmetric Double-Layer Composite Electrolyte with Enhanced Ionic Conductivity and Interface Stability for All-Solid-State Lithium Metal Batteries. Chin. Chem. Lett. 2020, 32, 125–131. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, M.; Wu, T.; Zhang, J.; Wang, P.; Zhang, L.; Yang, C.; Peng, C.; Lu, H. A Bifunctional-Modulated Conformal Li/Mn-Rich Layered Cathode for Fast-Charging, High Volumetric Density and Durable Li-Ion Full Cells. Nano-Micro Lett. 2021, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.I.; Yoon, W.S. Enhancing Electrochemical Performance of Co(OH)2 Anode Materials by Introducing Graphene for Next-Generation Li-Ion Batteries. J. Electrochem. Sci. Technol. 2022, 13, 398–406. [Google Scholar] [CrossRef]
- Wang, Q.; Kang, L.; Xing, Z.; Nie, C.; Hong, H.; Zhou, X.; Yun, Q.; Ju, Z.; Chen, B. Prussian Blue Analogue-Derived ZnO/ZnFe2O4 Core-Shell Nanospheres as High-Performance Anodes for Lithium-Ion and Potassium-Ion Batteries. Batter. Supercaps 2023, 6, e202200411. [Google Scholar] [CrossRef]
- Weng, W.; Zhou, D.; Liu, G.; Shen, L.; Li, M.; Chang, X.; Yao, X. Air Exposure Towards Stable Li/Li10GeP2S12 Interface for All-Solid-State Lithium Batteries. Mater. Futures 2022, 1, 021001. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C.; Cheng, Z.; Yao, Q.; Su, J.; Chen, B.; Yang, J.; Qian, Y. Bimetallic Bi-Sn Microspheres as High Initial Coulombic Efficiency and Long Lifespan Anodes for Sodium-Ion Batteries. Chem. Commun. 2022, 58, 5140–5143. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, Challenges, and Recent Progress of Inorganic Solid-State Electrolytes for All-Solid-State Lithium Batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef]
- Pervez, S.A.; Cambaz, M.A.; Thangadurai, V.; Fichtner, M. Interface in Solid-State Lithium Battery: Challenges, Progress, and Outlook. ACS Appl. Mater. Interfaces 2019, 11, 22029–22050. [Google Scholar] [CrossRef]
- Chong, P.; Zhou, Z.; Wang, K.; Zhai, W.; Li, Y.; Wang, J.; Wei, M. The Stabilizing of 1T-MoS2 for All-Solid-State Lithium-Ion Batteries. Batteries 2022, 9, 26. [Google Scholar] [CrossRef]
- Bubulinca, B.; Kazantseva, N.E.; Pechancova, V.; Joseph, N.; Fei, H.; Venher, M.; Ivanichenko, A.; Saha, P. Development of All-Solid-State Li-Ion Batteries: From Key Technical Areas to Commercial Use. Batteries 2023, 9, 157. [Google Scholar] [CrossRef]
- Stenina, I.; Pyrkova, A.; Yaroslavtsev, A. NASICON-Type Li1+xAlxZryTi2−x−y(PO4)3 Solid Electrolytes: Effect of Al, Zr Co-Doping and Synthesis Method. Batteries 2023, 9, 59. [Google Scholar] [CrossRef]
- Karabelli, D.; Birke, K.P.; Weeber, M. A Performance and Cost Overview of Selected Solid-State Electrolytes: Race between Polymer Electrolytes and Inorganic Sulfide Electrolytes. Batteries 2021, 7, 18. [Google Scholar] [CrossRef]
- Giraldo, S.; Nakagawa, K.; Vásquez, F.A.; Fujii, Y.; Wang, Y.; Miura, A.; Calderón, J.A.; Rosero-Navarro, N.C.; Tadanaga, K. Preparation of Composite Electrodes for All-Solid-State Batteries Based on Sulfide Electrolytes: An Electrochemical Point of View. Batteries 2021, 7, 77. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent Advances in All-Solid-State Rechargeable Lithium Batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Nakamura, T.; Amezawa, K.; Kulisch, J.; Zeier, W.G.; Janek, J. Guidelines for All-Solid-State Battery Design and Electrode Buffer Layers Based on Chemical Potential Profile Calculation. ACS Appl. Mater. Interfaces 2019, 11, 19968–19976. [Google Scholar] [CrossRef]
- Kato, Y.; Shiotani, S.; Morita, K.; Suzuki, K.; Hirayama, M.; Kanno, R. All-Solid-State Batteries with Thick Electrode Configurations. J. Phys. Chem. Lett. 2018, 9, 607–613. [Google Scholar] [CrossRef]
- Yoon, D.H.; Park, Y.J. Effects of Lithium Bis(Oxalato)Borate-Derived Surface Coating Layers on the Performances of High-Ni Cathodes for All-Solid-State Batteries. Appl. Energy 2022, 326, 119991. [Google Scholar] [CrossRef]
- Culver, S.P.; Koerver, R.; Zeier, W.G.; Janek, J. On the Functionality of Coatings for Cathode Active Materials in Thiophosphate-Based All-Solid-State Batteries. Adv. Energy Mater. 2019, 9, 1900626. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of Inorganic Solid-State Electrolytes for Batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Kerman, K.; Luntz, A.; Viswanathan, V.; Chiang, Y.-M.; Chen, Z. Review—Practical Challenges Hindering the Development of Solid State Li Ion Batteries. J. Electrochem. Soc. 2017, 164, A1731–A1744. [Google Scholar] [CrossRef]
- Sumita, M.; Tanaka, Y.; Ikeda, M.; Ohno, T. Charged and Discharged States of Cathode/Sulfide Electrolyte Interfaces in All-Solid-State Lithium Ion Batteries. J. Phys. Chem. C 2016, 120, 13332–13339. [Google Scholar] [CrossRef]
- Koerver, R.; Aygün, I.; Leichtweiß, T.; Dietrich, C.; Zhang, W.; Binder, J.O.; Hartmann, P.; Zeier, W.G.; Janek, J. Capacity Fade in Solid-State Batteries: Interphase Formation and Chemomechanical Processes in Nickel-Rich Layered Oxide Cathodes and Lithium Thiophosphate Solid Electrolytes. Chem. Mater. 2017, 29, 5574–5582. [Google Scholar] [CrossRef]
- Ohta, N.; Takada, K.; Zhang, L.; Ma, R.; Osada, M.; Sasaki, T. Enhancement of the High-Rate Capability of Solid-State Lithium Batteries by Nanoscale Interfacial Modification. Adv. Mater. 2006, 18, 2226–2229. [Google Scholar] [CrossRef]
- Auvergniot, J.; Cassel, A.; Ledeuil, J.B.; Viallet, V.; Seznec, V.; Dedryvère, R. Interface Stability of Argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in Bulk All-Solid-State Batteries. Chem. Mater. 2017, 29, 3883–3890. [Google Scholar] [CrossRef]
- Banerjee, A.; Tang, H.; Wang, X.; Cheng, J.H.; Nguyen, H.; Zhang, M.; Tan, D.H.S.; Wynn, T.A.; Wu, E.A.; Doux, J.M.; et al. Revealing Nanoscale Solid-Solid Interfacial Phenomena for Long-Life and High-Energy All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2019, 11, 43138–43145. [Google Scholar] [CrossRef]
- Xiao, Y.; Miara, L.J.; Wang, Y.; Ceder, G. Computational Screening of Cathode Coatings for Solid-State Batteries. Joule 2019, 3, 1252–1275. [Google Scholar] [CrossRef]
- Banerjee, A.; Wang, X.; Fang, C.; Wu, E.A.; Meng, Y.S. Interfaces and Interphases in All-Solid-State Batteries with Inorganic Solid Electrolytes. Chem. Rev. 2020, 120, 6878–6933. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Bo, S.H.; Kim, J.C.; Miara, L.J.; Ceder, G. Understanding Interface Stability in Solid-State Batteries. Nat. Rev. Mater. 2020, 5, 105–126. [Google Scholar] [CrossRef]
- Walther, F.; Strauss, F.; Wu, X.; Mogwitz, B.; Hertle, J.; Sann, J.; Rohnke, M.; Brezesinski, T.; Janek, J. The Working Principle of a Li2CO3/LiNbO3Coating on NCM for Thiophosphate-Based All-Solid-State Batteries. Chem. Mater. 2021, 33, 2110–2125. [Google Scholar] [CrossRef]
- Payandeh, S.; Strauss, F.; Mazilkin, A.; Kondrakov, A.; Brezesinski, T. Tailoring the LiNbO3 Coating of Ni-Rich Cathode Materials for Stable and High-Performance All-Solid-State Batteries. Nano Res. Energy 2022, 1, e9120016. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Interfacial Observation between LiCoO2 Electrode and Li 2S-P2S5 Solid Electrolytes of All-Solid-State Lithium Secondary Batteries Using Transmission Electron Microscopy. Chem. Mater. 2010, 22, 949–956. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, Y.J. Comparison of LiTaO3 and LiNbO3 Surface Layers Prepared by Post- And Precursor-Based Coating Methods for Ni-Rich Cathodes of All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2021, 13. [Google Scholar] [CrossRef]
- Lim, C.B.; Park, Y.J. Precursor-Based Surface Modification of Cathodes Using Ta and W for Sulfide-Based All-Solid-State Batteries. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Strauss, F.; Teo, J.H.; Maibach, J.; Kim, A.Y.; Mazilkin, A.; Janek, J.; Brezesinski, T. Li2ZrO3-Coated NCM622 for Application in Inorganic Solid-State Batteries: Role of Surface Carbonates in the Cycling Performance. ACS Appl. Mater. Interfaces 2020, 12, 57146–57154. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Rajagopal, R.; Kang, S.; Ryu, K.S. Novel Dry Deposition of LiNbO3 or Li2ZrO3 on LiNi0.6Co0.2Mn0.2O2 for High Performance All-Solid-State Lithium Batteries. Chem. Eng. J. 2020, 386. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, Y.J. Li3PO4 Coated Li[Ni0.75Co0.1Mn0.15]O2 Cathode for All-Solid-State Batteries Based on Sulfide Electrolyte. J. Electrochem. Sci. Technol. 2022, 13, 407–415. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, Y.J. Suppressing Unfavorable Interfacial Reactions Using Polyanionic Oxides as Efficient Buffer Layers: Low-Cost Li3PO4 Coatings for Sulfide-Electrolyte-based All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2023, 15, 12998–13011. [Google Scholar] [CrossRef]
- Zhu, Y.; He, X.; Mo, Y. First Principles Study on Electrochemical and Chemical Stability of Solid Electrolyte-Electrode Interfaces in All-Solid-State Li-Ion Batteries. J. Mater. Chem. A 2016, 4, 3253–3266. [Google Scholar] [CrossRef]
- Homma, K.; Liu, Y.; Sumita, M.; Tamura, R.; Fushimi, N.; Iwata, J.; Tsuda, K.; Kaneta, C. Optimization of a Heterogeneous Ternary Li3PO4-Li3BO3-Li2SO4Mixture for Li-Ion Conductivity by Machine Learning. J. Phys. Chem. C 2020, 124, 12865–12870. [Google Scholar] [CrossRef]
- Glass, A.M.; Nassau, K.; Negran, T.J. Ionic Conductivity of Quenched Alkali Niobate and Tantalate Glasses. J. Appl. Phys. 1978, 49, 4808–4811. [Google Scholar] [CrossRef]
- Jung, S.H.; Oh, K.; Nam, Y.J.; Oh, D.Y.; Brüner, P.; Kang, K.; Jung, Y.S. Li3BO3-Li2CO3: Rationally Designed Buffering Phase for Sulfide All-Solid-State Li-Ion Batteries. Chem. Mater. 2018, 30, 8190–8200. [Google Scholar] [CrossRef]
- Kim, A.Y.; Strauss, F.; Bartsch, T.; Teo, J.H.; Hatsukade, T.; Mazilkin, A.; Janek, J.; Hartmann, P.; Brezesinski, T. Stabilizing Effect of a Hybrid Surface Coating on a Ni-Rich NCM Cathode Material in All-Solid-State Batteries. Chem. Mater. 2019, 31, 9664–9672. [Google Scholar] [CrossRef]
- Vadhva, P.; Hu, J.; Johnson, M.J.; Stocker, R.; Braglia, M.; Brett, D.J.L.; Rettie, A.J.E. Electrochemical Impedance Spectroscopy for All-Solid-State Batteries: Theory, Methods and Future Outlook. ChemElectroChem 2021, 8, 1930–1947. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Song, D.; Zhang, H.; Shi, X.; Wang, Z.; Zhang, L.; Zhu, L. LiNbO3-Coated LiNi0.8Co0.1Mn0.1O2 Cathode with High Discharge Capacity and Rate Performance for All-Solid-State Lithium Battery. J. Energy Chem. 2020, 40, 39–45. [Google Scholar] [CrossRef]
- Riphaus, N.; Stiaszny, B.; Beyer, H.; Indris, S.; Gasteiger, H.A.; Sedlmaier, S.J. Editors’ Choice—Understanding Chemical Stability Issues between Different Solid Electrolytes in All-Solid-State Batteries. J. Electrochem. Soc. 2019, 166, A975–A983. [Google Scholar] [CrossRef]
- Kato, A.; Kowada, H.; Deguchi, M.; Hotehama, C.; Hayashi, A.; Tatsumisago, M. XPS and SEM Analysis between Li/Li3PS4 Interface with Au Thin Film for All-Solid-State Lithium Batteries. Solid State Ion. 2018, 322, 1–4. [Google Scholar] [CrossRef]
- Wu, E.A.; Jo, C.; Tand, D.H.S.; Zhang, M.; Doux, J.M.; Chen, Y.T.; Deysher, G.; Meng, Y.S. A Facile, Dry-Processed Lithium Borate-Based Cathode Coating for Improved All-Solid-State Battery Performance. J. Electrochem. Soc. 2020, 167, 130516. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, J.; Jeong, J.; Lee, H.J.; Kim, S.C.; Hah, H.J.; Oh, K.; Lee, S.H. Effect of Amorphous LiPON Coating on Electrochemical Performance of LiNi0.8Mn0.1Co0.1O2 (NCM811) in All Solid-State Batteries. J. Electrochem. Soc. 2021, 168, 060537. [Google Scholar] [CrossRef]
- Pearse, A.J.; Schmitt, T.E.; Fuller, E.J.; El-Gabaly, F.; Lin, C.F.; Gerasopoulos, K.; Kozen, A.C.; Talin, A.A.; Rubloff, G.; Gregorcyzck, K.E. Nanoscale Solid State Batteries Enabled by Thermal Atomic Layer Deposition of a Lithium Polyphosphazene Soli State Electrolyte. Chem. Mater. 2017, 29, 3740–3753. [Google Scholar] [CrossRef]
- Nisula, M.; Shindo, Y.; Koga, H.; Karppinen, M. Atomic Layer Deposition of Lithium Phosphorus Oxynitride. Chem. Mater. 2015, 27, 6987–6993. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Z.; Wang, X.; Meng, M.; Sun, Y.; Jing, M.; Liu, H.; Lu, F.; Wang, W.; Cheng, Y.; et al. Inhibitory Property of Lithium Phosphorus Oxynitride Surface Grown by Atomic Layer Deposition. Surf. Interfaces 2022, 33, 102280. [Google Scholar] [CrossRef]
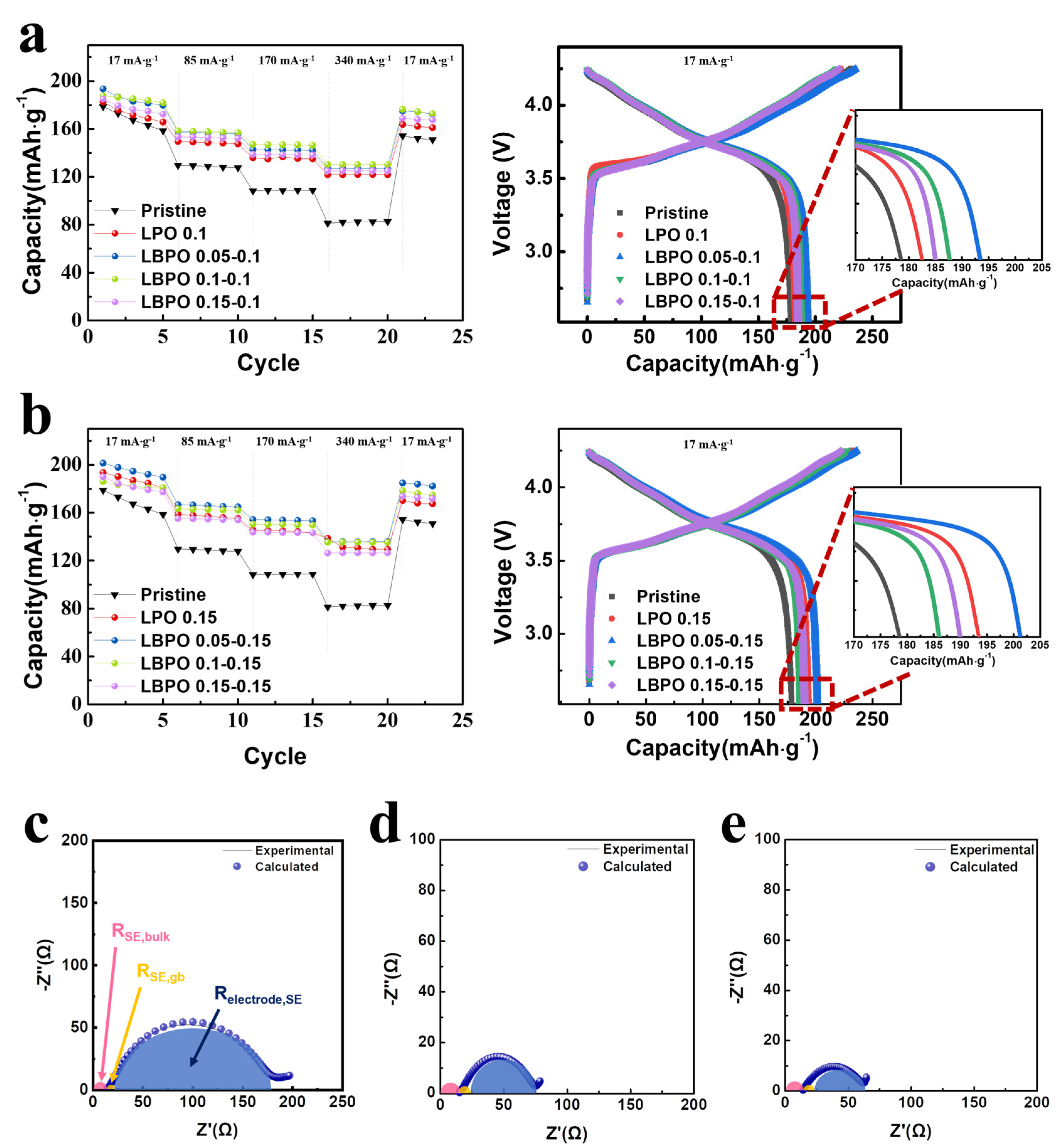


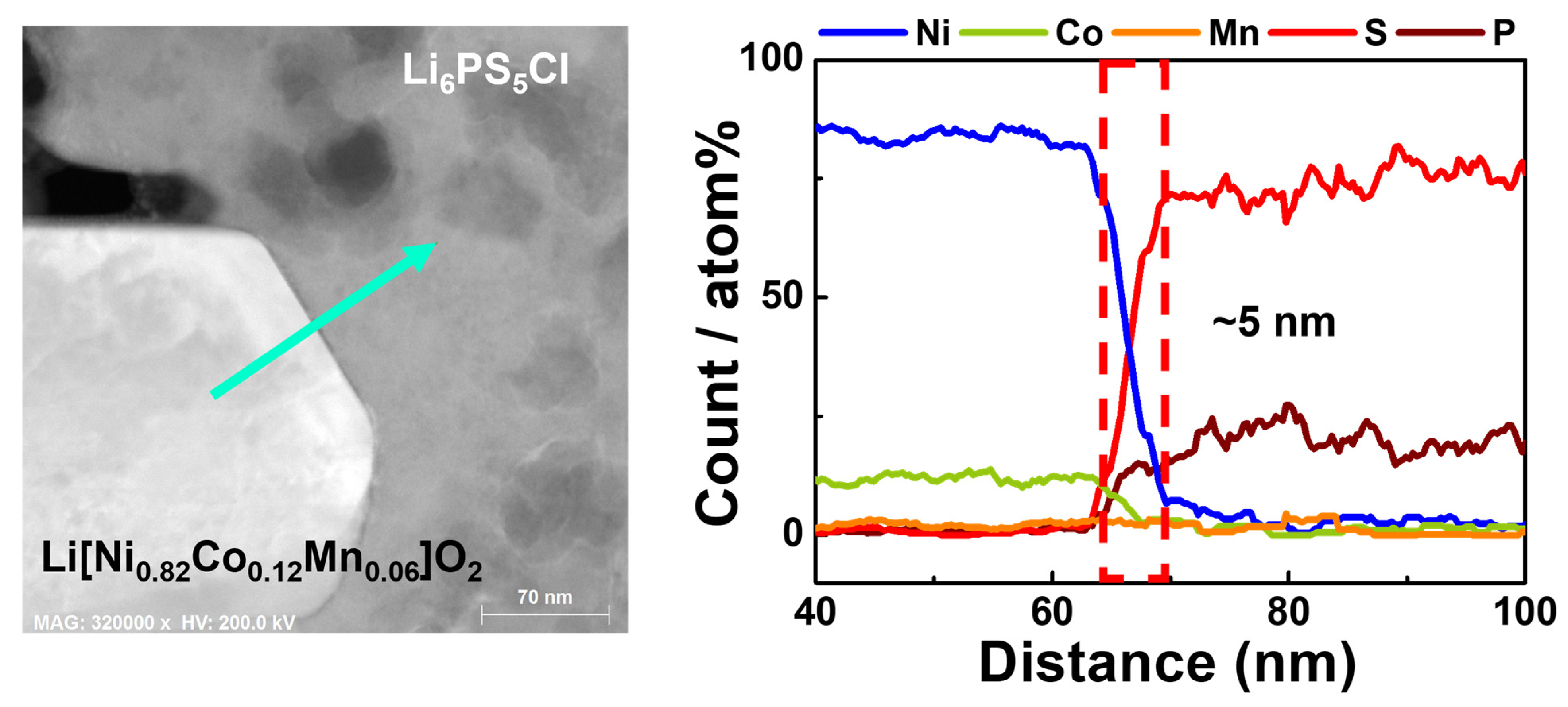
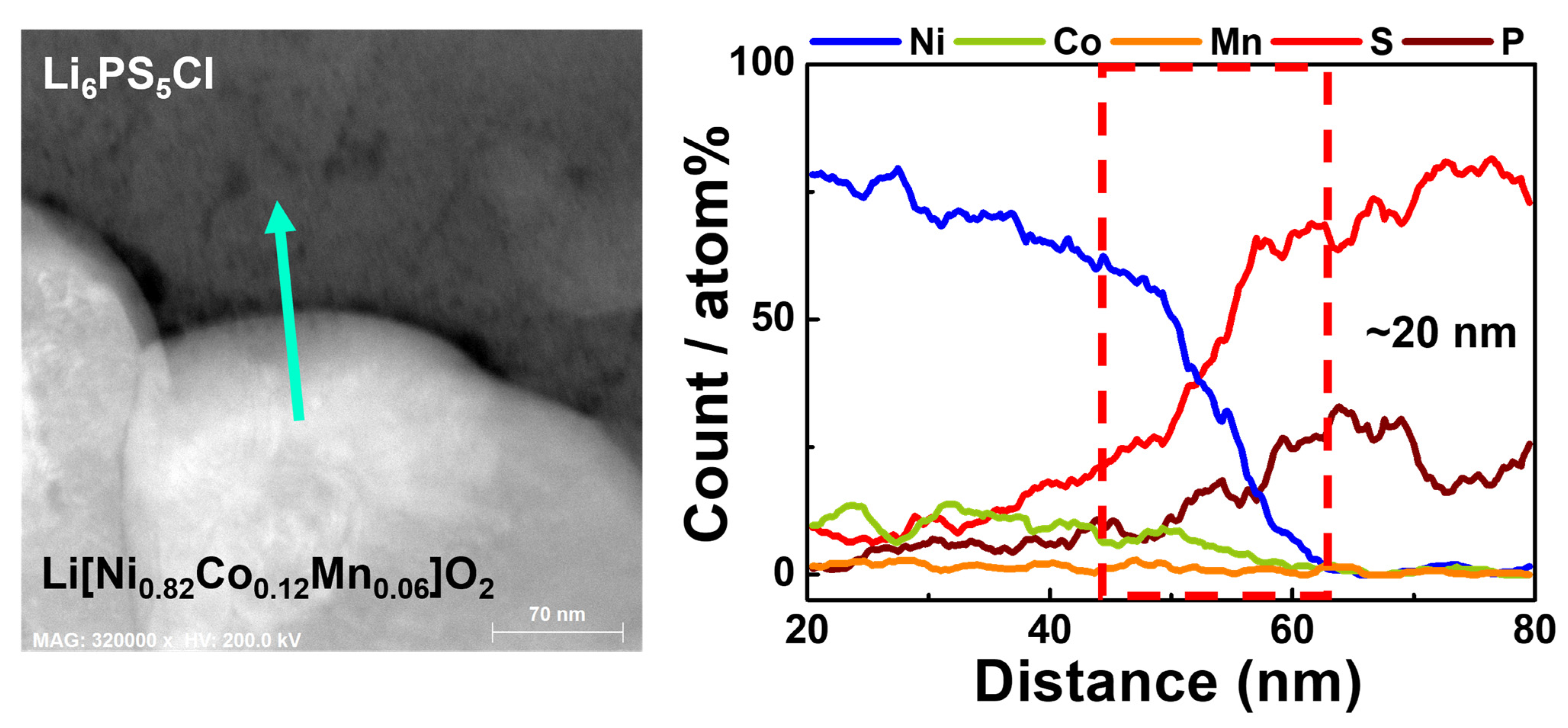
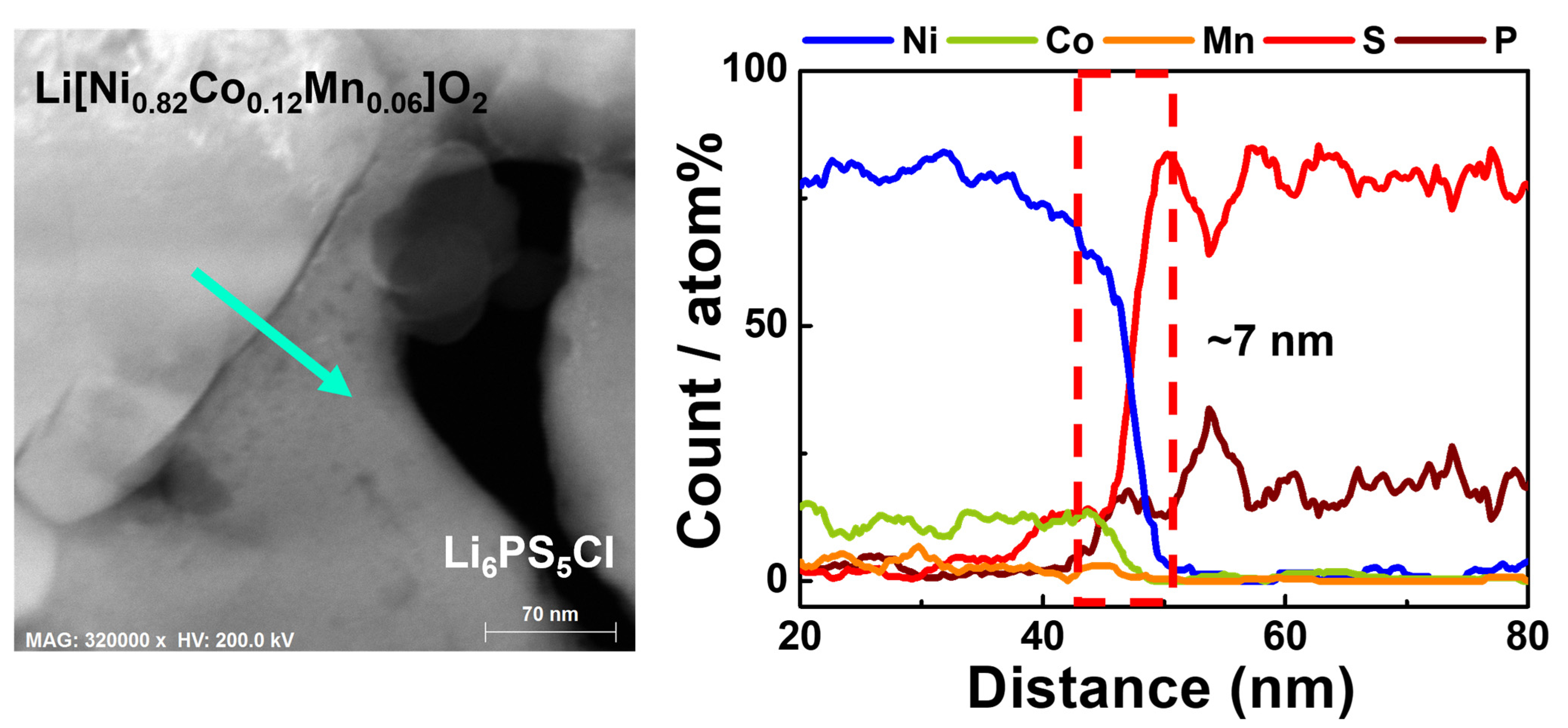
| Samples | Discharge Capacity (mAh·g−1) | Coulombic Efficiency, η (%) | Capacity Retention (%) * | |||
|---|---|---|---|---|---|---|
| 17 mA·g−1 (1st Cycle) | 85 mA·g−1 (7th Cycle) | 170 mA·g−1 (12th Cycle) | 340 mA·g−1 (17th Cycle) | |||
| Pristine | 178.7 | 129.3 | 108.8 | 82.1 | 76.37 | 45.94 |
| LPO 0.1 coated | 182.6 | 149.1 | 134.9 | 121.8 | 82.22 | 66.70 |
| LPO 0.15 coated | 193.6 | 157.8 | 145.1 | 131.2 | 82.74 | 67.77 |
| LBPO 0.05–0.1 coated | 193.6 | 157.4 | 142.7 | 126.9 | 82.28 | 65.55 |
| LBPO 0.1–0.1 coated | 187.7 | 158.2 | 147.0 | 130.4 | 85.63 | 69.47 |
| LBPO 0.15–0.1 coated | 185.1 | 153.2 | 138.8 | 125.2 | 83.79 | 67.64 |
| LBPO 0.05–0.15 coated | 201.4 | 166.4 | 154.1 | 135.8 | 85.27 | 67.43 |
| LBPO 0.1–0.15 coated | 186.0 | 162.9 | 150.2 | 135.4 | 81.79 | 72.80 |
| LBPO 0.15–0.15 coated | 190.0 | 154.9 | 143.7 | 126.5 | 85.55 | 66.58 |
| Samples | Impedance Values | |||
|---|---|---|---|---|
| RSE,bulk (Ω) | RSE,gb (Ω) | Relectrode/SE (Ω) | Rtotal (Ω) | |
| Pristine | 14.5 | 9.4 | 165.3 | 189.2 |
| LPO 0.15 coated | 15.3 | 9.2 | 51.7 | 76.2 |
| LBPO 0.05–0.15 coated | 14.3 | 8.3 | 40.1 | 62.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.J.; Noh, S.; Seong, J.Y.; Lee, S.; Park, Y.J. Li3BO3-Li3PO4 Composites for Efficient Buffer Layer of Sulphide-Based All-Solid-State Batteries. Batteries 2023, 9, 292. https://doi.org/10.3390/batteries9060292
Ji YJ, Noh S, Seong JY, Lee S, Park YJ. Li3BO3-Li3PO4 Composites for Efficient Buffer Layer of Sulphide-Based All-Solid-State Batteries. Batteries. 2023; 9(6):292. https://doi.org/10.3390/batteries9060292
Chicago/Turabian StyleJi, Yong Jun, Sungwoo Noh, Ju Yeong Seong, Sangheon Lee, and Yong Joon Park. 2023. "Li3BO3-Li3PO4 Composites for Efficient Buffer Layer of Sulphide-Based All-Solid-State Batteries" Batteries 9, no. 6: 292. https://doi.org/10.3390/batteries9060292
APA StyleJi, Y. J., Noh, S., Seong, J. Y., Lee, S., & Park, Y. J. (2023). Li3BO3-Li3PO4 Composites for Efficient Buffer Layer of Sulphide-Based All-Solid-State Batteries. Batteries, 9(6), 292. https://doi.org/10.3390/batteries9060292








