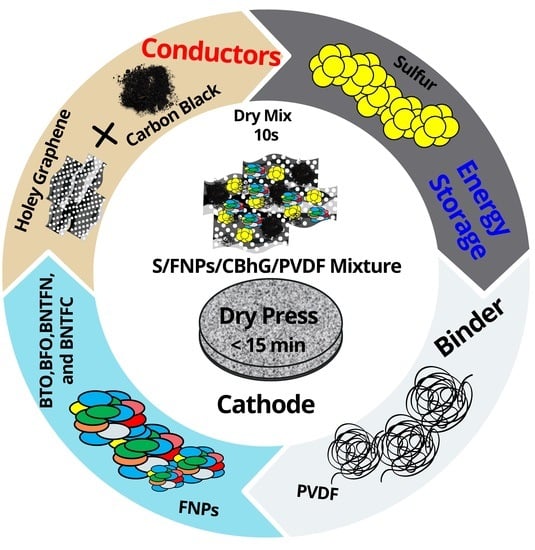
High Areal Capacity and Sustainable High Energy in Ferroelectric Doped Holey Graphene/Sulfur Composite Cathode for Lithium-Sulfur Batteries
Abstract
:1. Introduction
2. Materials, Methods, and Characterizations
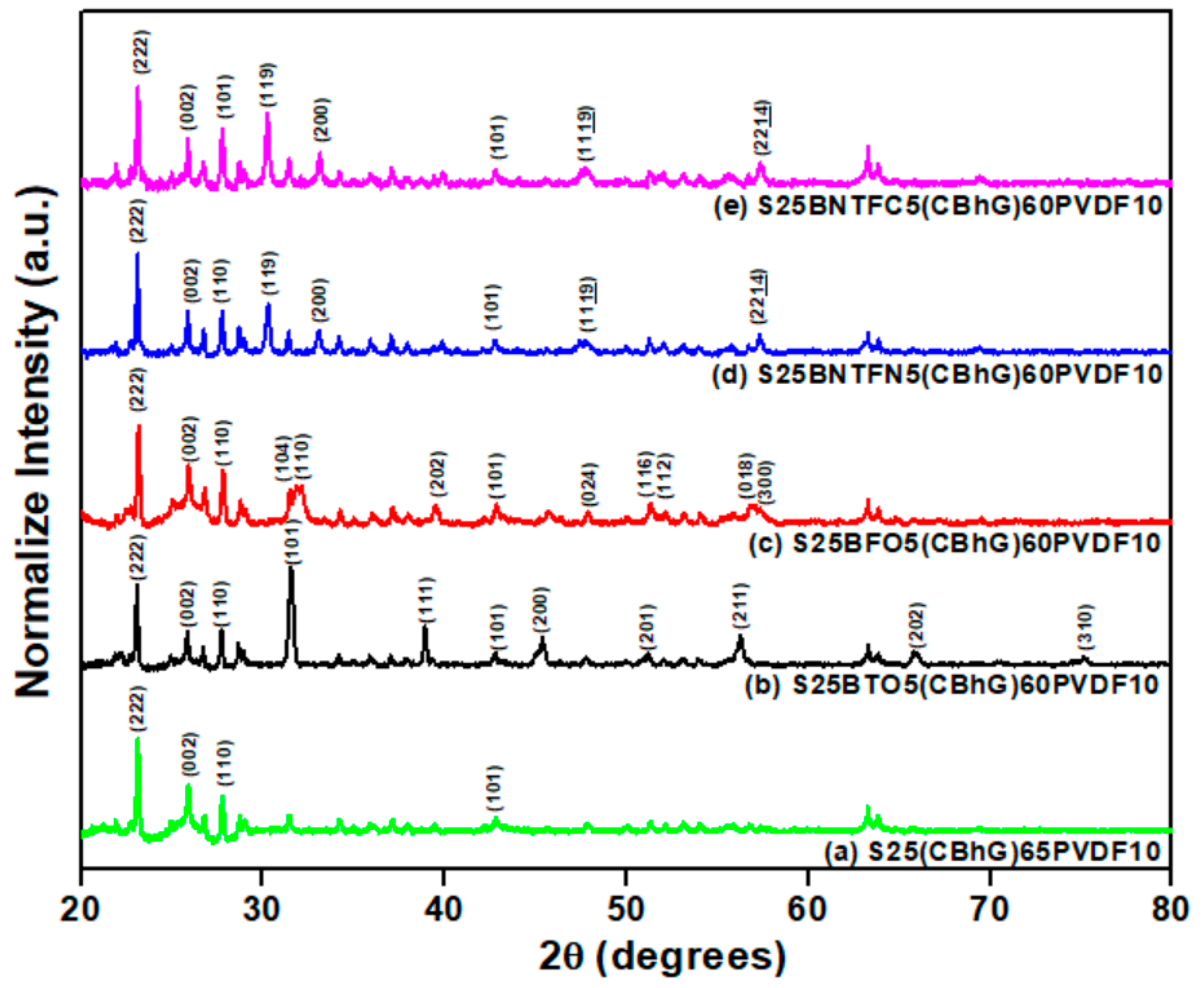
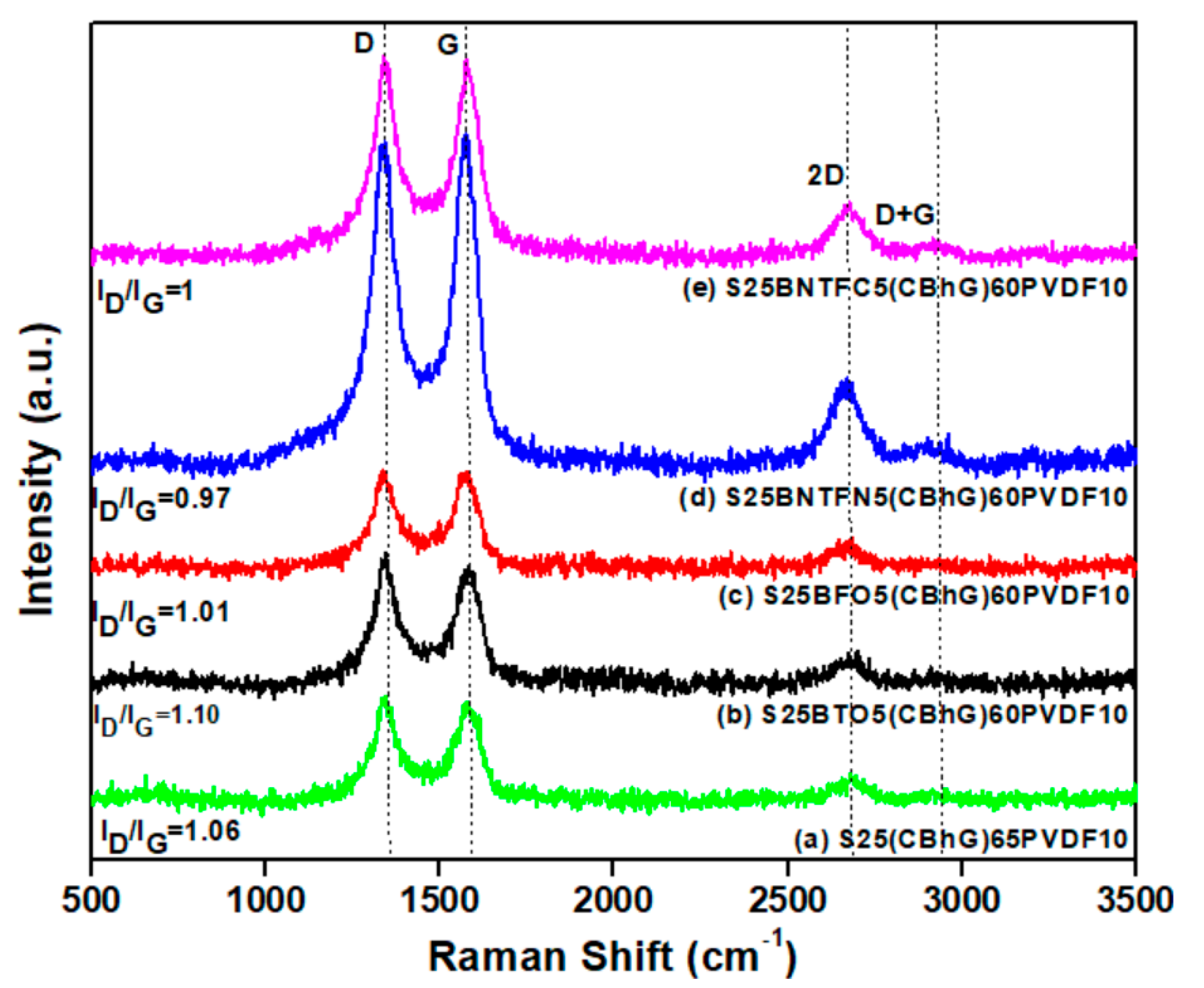
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manthiram, A.; Chung, S.-H.; Zu, C. Lithium-Sulfur Batteries: Progress and Prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Fang, R.; Wen, L.; Shi, Y.; Wang, S.; Li, F. A trilayer separator with dual function for high performance lithium–sulfur batteries. J. Power Sources 2016, 301, 179–186. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2011, 11, 19–29. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.-W.; Cheng, H.-M.; Li, F. More Reliable Lithium-Sulfur Batteries: Status, Solutions and Prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Zheng, J.; Li, Q.; Xie, X.; Ferrara, S.; Nie, Z.; Mehdi, L.B.; Browning, N.D.; Zhang, J.-G.; Graff, G.L.; et al. High Energy Density Lithium-Sulfur Batteries: Challenges of Thick Sulfur Cathodes. Adv. Energy Mater. 2015, 5, 1402290. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li-S Battery System. J. Ofthe Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Wang, D.-W.; Shan, X.-Y.; Pei, S.; Li, F.; Cheng, H.-M. A flexible sulfur-graphene-polypropylene separator integrated electrode for advanced Li-S batteries. Adv. Mater. 2015, 27, 641–647. [Google Scholar] [CrossRef]
- Liang, J.; Yin, L.; Tang, X.; Yang, H.; Yan, W.; Song, L.; Cheng, H.-M.; Li, F. Kinetically Enhanced Electrochemical Redox of Polysulfides on Polymeric Carbon Nitrides for Improved Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2016, 8, 25193–25201. [Google Scholar] [CrossRef]
- Han, X.; Yang, Z.; Zhao, B.; Zhu, S.; Zhou, L.; Dai, J.; Kim, J.-W.; Liu, B.; Connell, J.W.; Li, T.; et al. Compressible, Dense, Three-Dimensional Holey Graphene Monolithic Architecture. ACS Nano 2017, 11, 3189–3197. [Google Scholar] [CrossRef]
- Yen, Y.-J.; Chen, T.-H.; Wang, Y.-T.; Robles, A.; Đerić, M.; Miljanić, O.; Kaveevivitchai, W.; Chung, S.-H. Selective chemisorption of polysulfides by porous molecular crystal: Cathode host materials for lean-electrolyte lithium-sulfur cells with high electrochemical stability. J. Power Sources 2023, 565, 232891. [Google Scholar] [CrossRef]
- Shah, S.S.; Aziz, A.; Cevik, E.; Ali, M.; Gunday, S.T.; Bozkurt, A.; Yamani, Z.H. Sulfur nano-confinement in hierarchically porous jute derived activated carbon towards high-performance supercapacitor: Experimental and theoretical insights. J. Energy Storage 2022, 56, 105944. [Google Scholar] [CrossRef]
- Yan, S.-X.; Wang, Q.; Luo, S.-H.; Zhang, Y.-H.; Liu, X.; Liu, Y.-G.; Wang, Z.-Y.; Hao, A.-M.; Yi, T.-F. Coal-based S hybrid self-doped porous carbon for high-performance supercapacitors and potassium-ion batteries. J. Power Sources 2020, 461, 228151. [Google Scholar] [CrossRef]
- Zhang, M.; Song, Z.; Liu, H.; Wang, A.; Shao, S. MoO2 coated few layers of MoS2 and FeS2 nanoflower decorated S-doped graphene interoverlapped network for high-energy asymmetric supercapacitor. J. Colloid Interface Sci. 2021, 584, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Raj, F.R.M.S.; Jaya, N.V.; Boopathi, G.; Kalpana, D.; Pandurangan, A. S-doped activated mesoporous carbon derived from the Borassus flabellifer flower as active electrodes for supercapacitors. Mater. Chem. Phys. 2020, 240, 122151. [Google Scholar]
- Gu, W.; Sevilla, M.; Magasinski, A.; Fuertes, A.B.; Yushin, G. Sulfur-containing activated carbons with greatly reduced content of bottle neck pores for double-layer capacitors: A case study for pseudocapacitance detection. Energy Environ. Sci. 2013, 6, 2465–2476. [Google Scholar] [CrossRef]
- Cao, S.; Liu, D.; Ding, H.; Lu, H.; Gui, J. Towards understanding corrosion inhibition of sulfonate/carboxylate functionalized ionic liquids: An experimental and theoretical study. J. Colloid Interface Sci. 2020, 579, 315–329. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Singh, S.; Yun, J.M.; Kwon, S.H.; Kim, K.H. Sb2S3 Nanoparticles Anchored or Encapsulated by the Sulfur-Doped Carbon Sheet for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 33966–33977. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.-H.; Zhang, L.; Xi, R.; Jiang, D.-P.; Chen, Z.-Y.; Huang, H.; Ding, L.-Y.; Pan, G.-B. Natural bamboo leaves derived sulphur-doped mesoporous heteroatom enriched carbon for high-performance supercapacitors and gas sensors. J. Power Sources 2019, 443, 227183. [Google Scholar] [CrossRef]
- Zhou, Y.; Candelaria, S.L.; Liu, Q.; Huang, Y.; Uchaker, E.; CaO, G. Sulfur-rich carbon cryogels for supercapacitors with improved conductivity and wettability. J. Mater. Chem. A 2014, 2, 8472–8482. [Google Scholar] [CrossRef]
- Ma, X.; Song, X.; Ning, G.; Hou, L.; Kan, Y.; Xiao, Z.; Li, W.; Ma, G.; Gao, J.; Li, Y. S-Doped Porous Graphene Microspheres with Individual Robust Red-Blood-Cell-Like Microarchitecture for Capacitive Energy Storage. Ind. Eng. Chem. Res. 2017, 56, 9524–9532. [Google Scholar] [CrossRef]
- Salhabi, E.H.M.; Zhao, J.; Wang, J.; Yang, M.; Wang, B.; Wang, D. Hollow Multi-Shelled Structural TiO2-x with Multiple Spatial Confinement for Long-Life Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. Engl. 2019, 58, 9078–9082. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yu, Z.; Gordin, M.L.; Wang, D. Advanced Sulfur Cathode Enabled by Highly Crumpled Nitrogen-Doped Graphene Sheets for High-Energy-Density Lithium-Sulfur Batteries. Nano Lett. 2016, 16, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Noheda, B.; Kooi, B.J. Ferroelectric chalcogenides—Materials at the edge. Science 2016, 353, 221–222. [Google Scholar]
- Zhou, W.; Guo, B.; Gao, H.; Goodenough, J.B. Low-Cost Higher Loading of a Sulfur Cathode. Adv. Energy Mater. 2016, 6, 1502059. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, H.; Li, X.; Wang, Z.; Ma, J.; Zhao, H. N-doped carbon nanotubes as cathode material in Li–S batteries. J. Mater. Sci. Mater. Electron. 2015, 26, 7895–7900. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Cheng, B.; Hu, X.; Yu, J. Holey Graphene for Electrochemical Energy Storage. Cell Rep. Phys. Sci. 2020, 1, 100215. [Google Scholar] [CrossRef]
- Yim, T.; Han, S.H.; Park, N.H.; Park, M.-S.; Lee, J.H.; Shin, J.; Choi, J.W.; Jung, Y.; Jo, Y.N.; Yu, J.-S.; et al. Effective Polysulfide Rejection by Dipole-Aligned BaTiO3 Coated Separator in Lithium-Sulfur Batteries. Adv. Funct. Mater. 2016, 26, 7817–7823. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, H.; Jin, H.; Cai, N.; Gao, C.; Zhao, S.; Wang, M. Suppression of polysulfide shuttling with a separator modified using spontaneously polarized bismuth ferrite for high performance lithium–sulfur batteries. J. Mater. Chem. A 2020, 8, 16429–16436. [Google Scholar] [CrossRef]
- Xie, K.; You, Y.; Yuan, K.; Lu, W.; Zhang, K.; Xu, F.; Ye, M.; Ke, S.; Shen, C.; Zeng, X.; et al. Ferroelectric-Enhanced Polysulfide Trapping for Lithium-Sulfur Battery Improvement. Adv. Mater. 2016, 29, 1604724. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, G.; Wang, Z.; Feng, M.; Sun, M.; Xue, X.; Liu, R.; Jia, H.; Wang, Z.; Zhang, W.; et al. Black BaTiO3 as Multifunctional Sulfur Immobilizer for Superior Lithium Sulfur Batteries. J. Power Sources 2019, 434, 226729. [Google Scholar] [CrossRef]
- Tripathi, B.; Katiyar, R.K.; Morell, G.; Dixit, A.; Katiyar, R.S. BiFeO3 Coupled Polysulfide Trapping in C/S Composite Cathode Material for Li-S Batteries as Large Efficiency and High Rate Performance. Energies 2021, 14, 8362. [Google Scholar] [CrossRef]
- Półrolniczak, P.; Walkowiak, M.; Kaźmierczak-Raźna, J.; Kasprzak, D.; Mathew, D.E.; Kathiresan, M.; Stephan, A.M.; Angulakshmi, N. BaTiO3-g-GO as an efficient permselective material for lithium–sulfur batteries. Mater. Chem. Front. 2021, 5, 950–960. [Google Scholar] [CrossRef]
- Zuluaga-Gómez, C.C.; Plaza-Rivera, C.O.; Tripathi, B.; Katiyar, R.K.; Pradhan, D.K.; Morell, G.; Lin, Y.; Correa, M.; Katiyar, R.S. Holey Graphene/Ferroelectric/Sulfur Composite Cathodes for HighCapacity Lithium–Sulfur Batteries. ACS OMEGA 2023, 8, 13097–13108. [Google Scholar] [CrossRef]
- Lin, Y.; Han, X.; Campbell, C.J.; Kim, J.-W.; Zhao, B.; Luo, W.; Dai, J.; Hu, L.; Connell, J.W. Holey Graphene Nanomanufacturing: Structure, Composition, and Electrochemical Properties. Adv. Funct. Mater. 2015, 25, 2920–2927. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, W.; Chen, W.; Li, S. Preparation and properties of BaTiO3 ceramics from the fine ceramic powder. Ceram. Int. 2015, 41, S111–S116. [Google Scholar] [CrossRef]
- Bernardo, M.; Jardiel, T.; Peiteado, M.; Caballero, A.; Villegas, M. Reaction pathways in the solid state synthesis of multiferroic BiFeO3. J. Eur. Ceram. Soc. 2011, 31, 3047–3053. [Google Scholar] [CrossRef]
- Lin, Y.; Jones, K.J.; Greenburg, L.C.; Kim, J.-W.; Hu, L.; Connell, J.W. Facile, Solvent-Free Preparation of High Density, High Mass Loading Sulfur Cathodes Enabled by Dry-Pressable Holey Graphene Scaffolds. Batter. Supercaps 2019, 2, 774–783. [Google Scholar] [CrossRef]
- Das, S.R.; Choudhary, R.N.P.; Bhattacharya, P.; Katiyar, R.S.; Dutta, P.; Manivannan, A.; Seehra, M.S. Structural and Multiferroic Properties of La-Modified BiFeO3 Ceramics. J. Appl. Phys. 2007, 101, 034104. [Google Scholar] [CrossRef]
- Chen, X.; Xiao, J.; Yao, J.; Kang, Z.; Yang, F.; Zeng, X. Room Temperature Magnetoelectric Coupling Study in Multiferroic Bi4NdTi3Fe0.7Ni0.3O15 Prepared by a Multicalcination Procedure. Ceram. Int. 2014, 40, 6815–6819. [Google Scholar] [CrossRef]
- Yang, F.J.; Su, P.; Wei, C.; Chen, X.Q.; Yang, C.P.; Cao, W.Q. Large Magnetic Response in (Bi4Nd)Ti3(Fe0.5Co0.5)O15 Ceramic at Room-Temperature. J. Appl. Phys. 2011, 110, 126102. [Google Scholar] [CrossRef]
- Radhika, G.; Subadevi, R.; Krishnaveni, K.; Liu, W.R.; Sivakumar, M. Synthesis and Electrochemical Performance of PEG-MnO(2)-Sulfur Composites Cathode Materials for Lithium-Sulfur Batteries. J. Nanosci. Nanotechnol. 2018, 18, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Bai, Z.; Liu, W.W.; Liu, T.; Gim, J.; Jiang, G.; Yuan, Y.; Luo, D.; Feng, K.; et al. A Lithium-Sulfur Battery using a 2D Current Collector Architecture with a Large-Sized Sulfur Host Operated under High Areal Loading and Low E/S Ratio. Adv. Mater. 2018, 30, e1804271. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Breitkopf, C. Determination of Diffusion Coefficients Using Impedance Spectroscopy Data. J. Electrochem. Soc. 2018, 165, E826–E831. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Q.; Zhong, J.; Chen, M.; Deng, H.; Cao, J.; Wang, L.; Peng, L.; Zhu, J.; Lu, B. 3D Holey Graphene/Polyacrylonitrile Sulfur Composite Architecture for High Loading Lithium Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2100448. [Google Scholar] [CrossRef]
- Gaberscek, M. Understanding Li-based battery materials via electrochemical impedance spectroscopy. Nat. Commun. 2021, 12, 6513. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Ahad, S.A.; Li, M.; Luo, B.; Wang, L.; Gentle, I.; Knibbe, R. Review on areal capacities and long-term cycling performances of lithium sulfur battery at high sulfur loading. Energy Storage Mater. 2019, 18, 289–310. [Google Scholar] [CrossRef]
- Fan, C.-Y.; Yuan, H.-Y.; Li, H.-H.; Wang, H.-F.; Li, W.-L.; Sun, H.-Z.; Wu, X.-L.; Zhang, J.-P. The Effective Design of a Polysulfide-Trapped Separator at the Molecular Level for High Energy Density Li-S Batteries. ACS Appl Mater Interfaces 2016, 8, 16108–16115. [Google Scholar] [CrossRef] [PubMed]




| Reference | Preparation Method | Incorporated Ferroelectric Materials | Initial Specifics Capacity [mAh/g] | Cyclability | Capacity Retention | Coulombic Efficiency |
|---|---|---|---|---|---|---|
| 2016 [29] Separator | Were dispersed in acetone (80 mL) using a high-speed mixer (Primix) at room temperature for 2 hrs | A. PE B. PE-poled BTO C. PE–BTO | 997.2 1121.1 1124 | 50 cycles 50 cycles 50 cycles | 59.4% 82.8% 72.3% | 26.3% 79.6% 42.3% |
| 2016 [31] Cathodes | Slurry | A. C/S B. C/S + BTO C. Multi-rate (A and B) | 407 1143-0.2 C A. B. | 100 cycles 100 cycles 60 cycles | ------------ ---------- ----------- | ------------- ------------ ------------ |
| 2019 [32] Cathodes | Slurry | A. C/S B. C/S@B-BTO C. C/S@W-BTO D. Multi-rate (A, B, and C) | 1009.1 1129.5 928.2 A. 223.9, B. 607.6 C. 475.2 | 200 cycles 200 cycles 200 cycles 50 cycles 50 cycles 50 cycles | 71.3% 80.2% 42.5% --------- --------- --------- | ------------ ------------- ------------- ------------ ------------- -------------- |
| 2021 [34] | Separator | A. Celgard 2320 B. AC/GO C. AC/BTO D. AC/BTO-g-GO C. Multi-rate (B, C, and D) | 910 1200 950 1450-0.1 C ----------- | --------- --------- --------- 100 cycles 55 cycles | --------- --------- --------- -------- -------- | --------- --------- --------- --------- 75% |
| 2021 [33] Cathodes | Slurry | A. S60BFO30C10 B. S70BFO20C10 C. S80BFO10C10 | 1600 1525 1450 | 30 cycles 30 cycles 30 cycles | ~86% | 86% |
| ~62% | -------- | |||||
| 2023 [35] Cathodes | Dry pressable | A. S/hG B. S/BFO/hG C. S/BTO/hG D. S/BNTFN/hG E. S/BNTFC/hG | 1390 1316 1409 1069 1330 | 6 cycles 57 cycles 58 cycles 18 cycles 37 cycles | 57.7% 26% 34% 90% 53% | 25% 83.71% 82.65% 78.93% 86.92% |
| 2023 This work Cathodes | Dry pressable | A. S/CBhG/PVDF B. S/BTO/CBhG/PVDF C. S/BFO/CBhG/PVDF D. S/BNTFN/CBhG/PVDF E. S/BNTFC/CBhG/PVDF | 1123 1402 1430 1486 1509 | 134 cycles 110 cycles 116 cycles 158 cycles 107 cycles | 54.49% 46.72% 45.31% 43.40% 37.90% | 83% 87% 78% 93% 90% |
| Electrodes | Sulfur Content (wt%) [mgs] | Sulfur Loading [mgs/cm2] | Electrolyte-to-Sulfur Ratio [μL] |
|---|---|---|---|
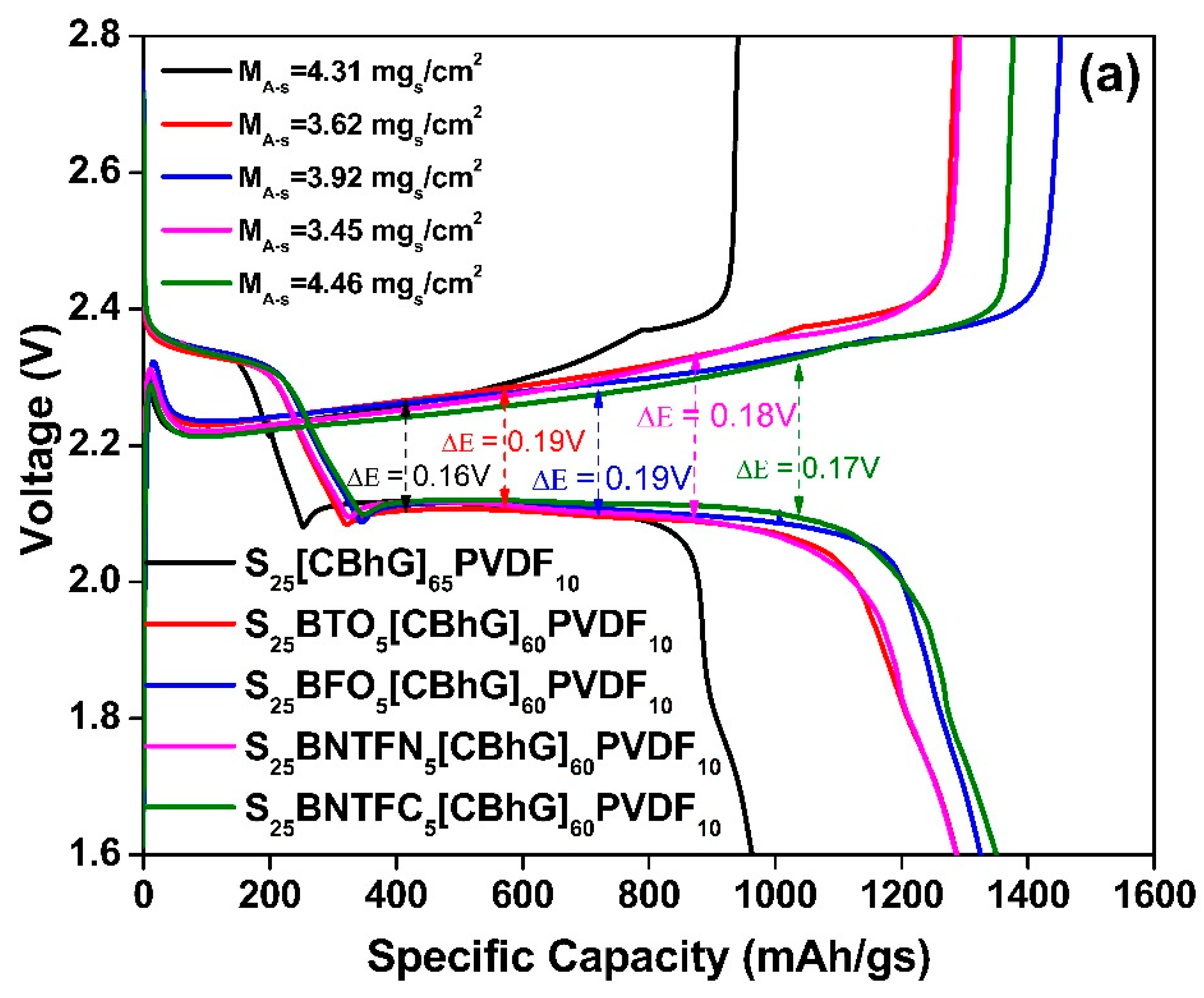
| S25(CBhG)65PVDF10 | 5.72 | 4.31 | 40 |
| S25BTO5(CBhG)60PVDF10 | 4.81 | 3.62 | 34 |
| S25BFO5(CBhG)60PVDF10 | 5.20 | 3.92 | 36 |
| S25BNTFN5(CBhG)60PVDF10 | 4.57 | 3.45 | 32 |
| S25BNTFCO5(CBhG)60PVDF10 | 5.92 | 4.46 | 42 |
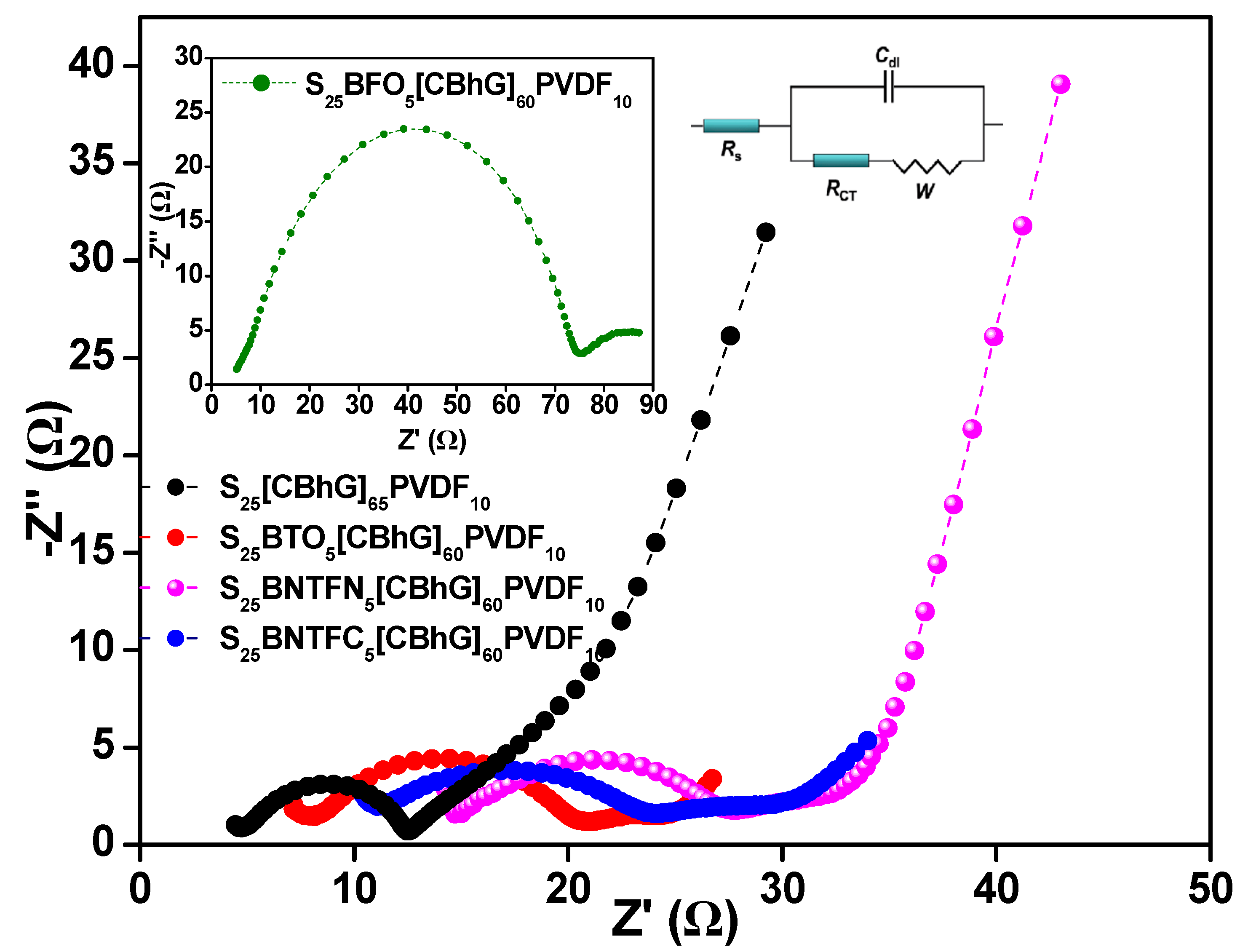
| Electrodes | Rs [Ω] | C | Rct [Ω] | Wsc | Dli [cm2/s] |
|---|---|---|---|---|---|
| S25(CBhG)65PVDF10 | 4.92 | 0.027 | 7.63 | 0.0320 | 2.17 × 10−16 |
| S25BTO5(CBhG)60PVDF10 | 8.15 | 1.459 × 10−8 | 12.67 | 0.1438 | 3.43 × 10−15 |
| S25BFO5(CBhG)60PVDF10 | 5.32 | 1.948 × 10−8 | 69.77 | 0.0907 | 4.15 × 10−15 |
| S25BNTFN5(CBhG)60PVDF10 | 14.85 | 0.179 | 12.82 | 0.0268 | 2.91 × 10−15 |
| S25BNTFC5(CBhG)60PVDF10 | 11.08 | 1.234 × 10−8 | 13.11 | 0.0705 | 4.11 × 10−15 |
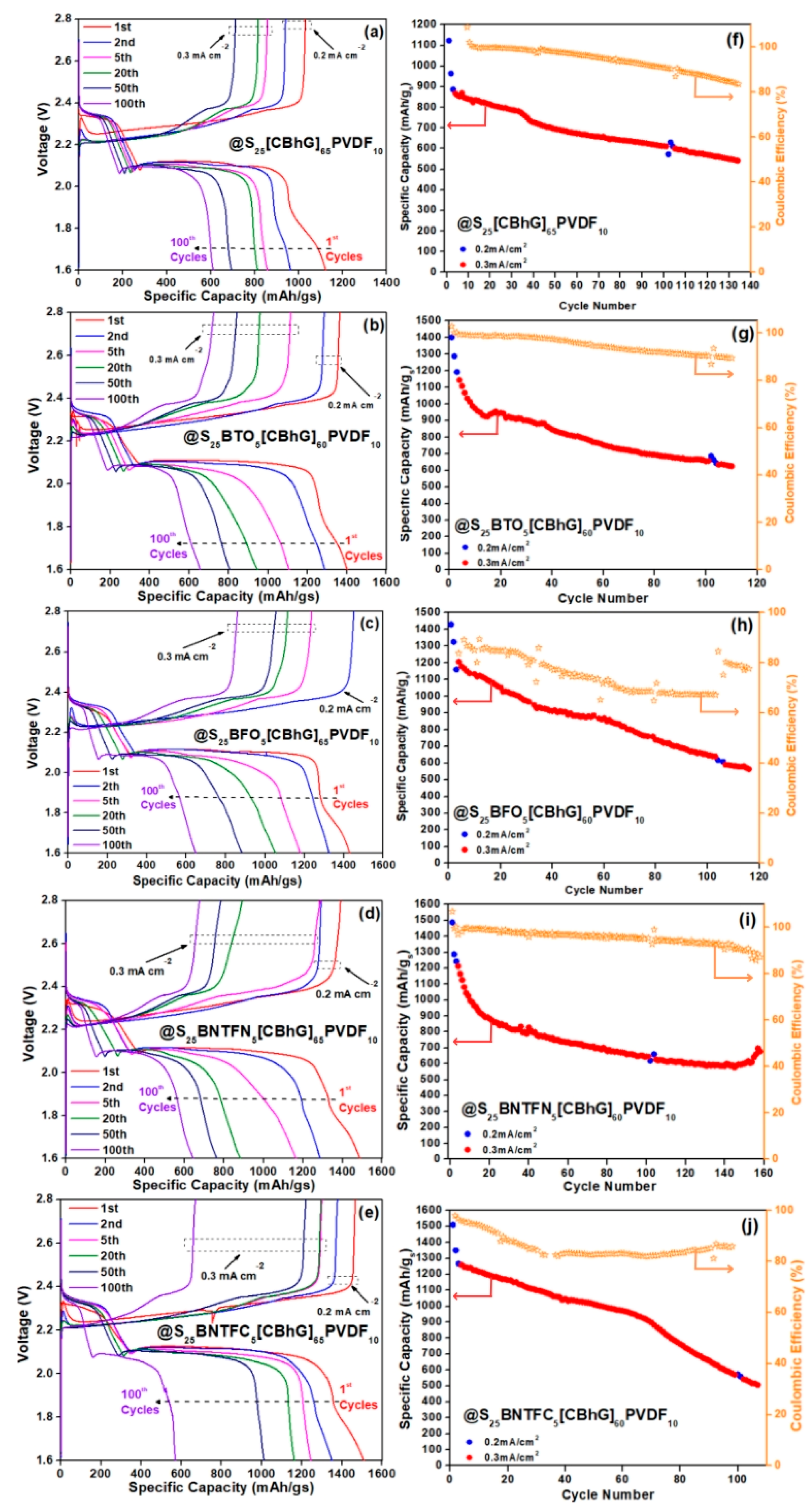
| Electrodes | Specific. Cap. 1st Cyc. [mAh/gs] | Specific. Cap. 100th Cyc. [mAh/gs] | Areal Cap. 1st Cyc. [mAh/cm−2] | Capacity Retention [%] |
|---|---|---|---|---|
| S25(CBhG)65PVDF10 | 1123 | 612 | 4.84 | 54.49 |
| S25BTO5(CBhG)60PVDF10 | 1402 | 655 | 5.08 | 46.72 |
| S25BFO5(CBhG)60PVDF10 | 1430 | 648 | 5.60 | 45.31 |
| S25BNTFN5(CBhG)60PVDF10 | 1486 | 645 | 5.12 | 43.40 |
| S25BNTFC5(CBhG)60PVDF10 | 1509 | 572 | 6.74 | 37.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuluaga-Gómez, C.C.; Tripathi, B.; Plaza-Rivera, C.O.; Katiyar, R.K.; Correa, M.; Pradhan, D.K.; Morell, G.; Katiyar, R.S. High Areal Capacity and Sustainable High Energy in Ferroelectric Doped Holey Graphene/Sulfur Composite Cathode for Lithium-Sulfur Batteries. Batteries 2023, 9, 293. https://doi.org/10.3390/batteries9060293
Zuluaga-Gómez CC, Tripathi B, Plaza-Rivera CO, Katiyar RK, Correa M, Pradhan DK, Morell G, Katiyar RS. High Areal Capacity and Sustainable High Energy in Ferroelectric Doped Holey Graphene/Sulfur Composite Cathode for Lithium-Sulfur Batteries. Batteries. 2023; 9(6):293. https://doi.org/10.3390/batteries9060293
Chicago/Turabian StyleZuluaga-Gómez, Claudia C., Balram Tripathi, Christian O. Plaza-Rivera, Rajesh K. Katiyar, Margarita Correa, Dhiren K. Pradhan, Gerardo Morell, and Ram S. Katiyar. 2023. "High Areal Capacity and Sustainable High Energy in Ferroelectric Doped Holey Graphene/Sulfur Composite Cathode for Lithium-Sulfur Batteries" Batteries 9, no. 6: 293. https://doi.org/10.3390/batteries9060293
APA StyleZuluaga-Gómez, C. C., Tripathi, B., Plaza-Rivera, C. O., Katiyar, R. K., Correa, M., Pradhan, D. K., Morell, G., & Katiyar, R. S. (2023). High Areal Capacity and Sustainable High Energy in Ferroelectric Doped Holey Graphene/Sulfur Composite Cathode for Lithium-Sulfur Batteries. Batteries, 9(6), 293. https://doi.org/10.3390/batteries9060293