Multifunctional MXene–Fe3O4–Carbon Nanotube Composite Electrodes for High Active Mass Asymmetric Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Synthesis of Composite Materials
2.3. Fabrication of Electrodes
2.4. Characterization
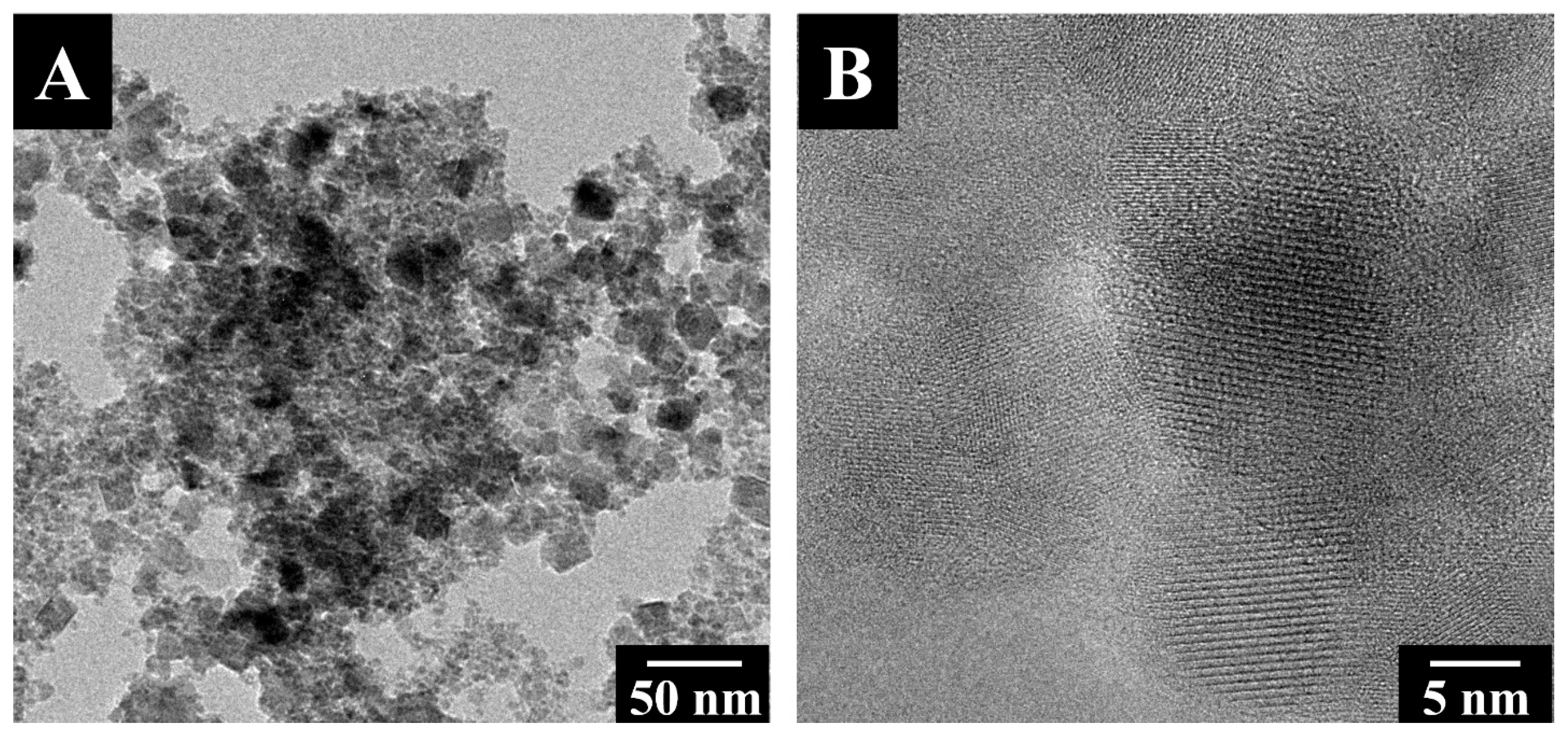
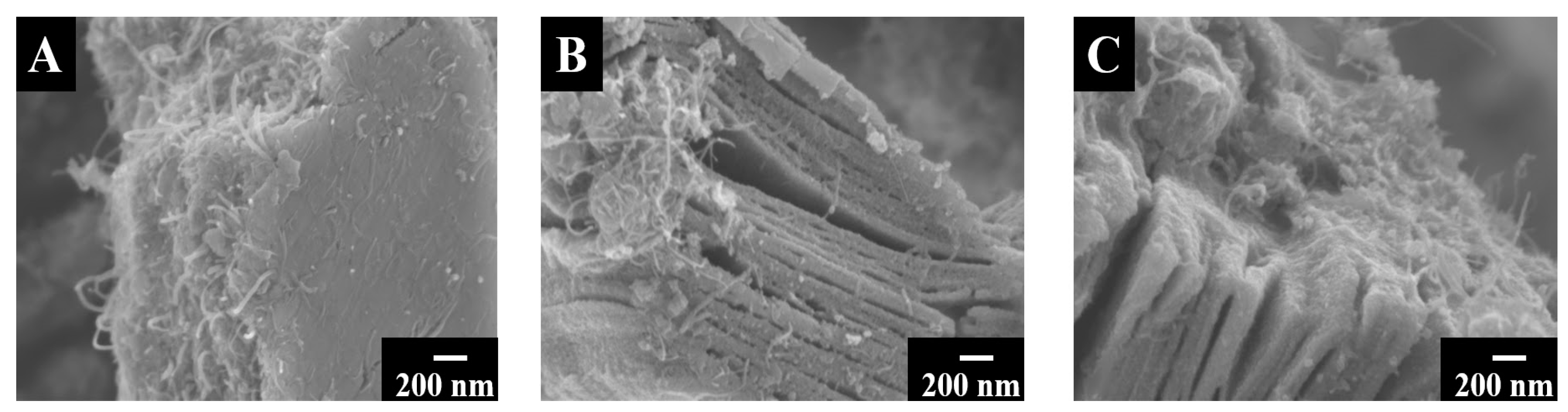
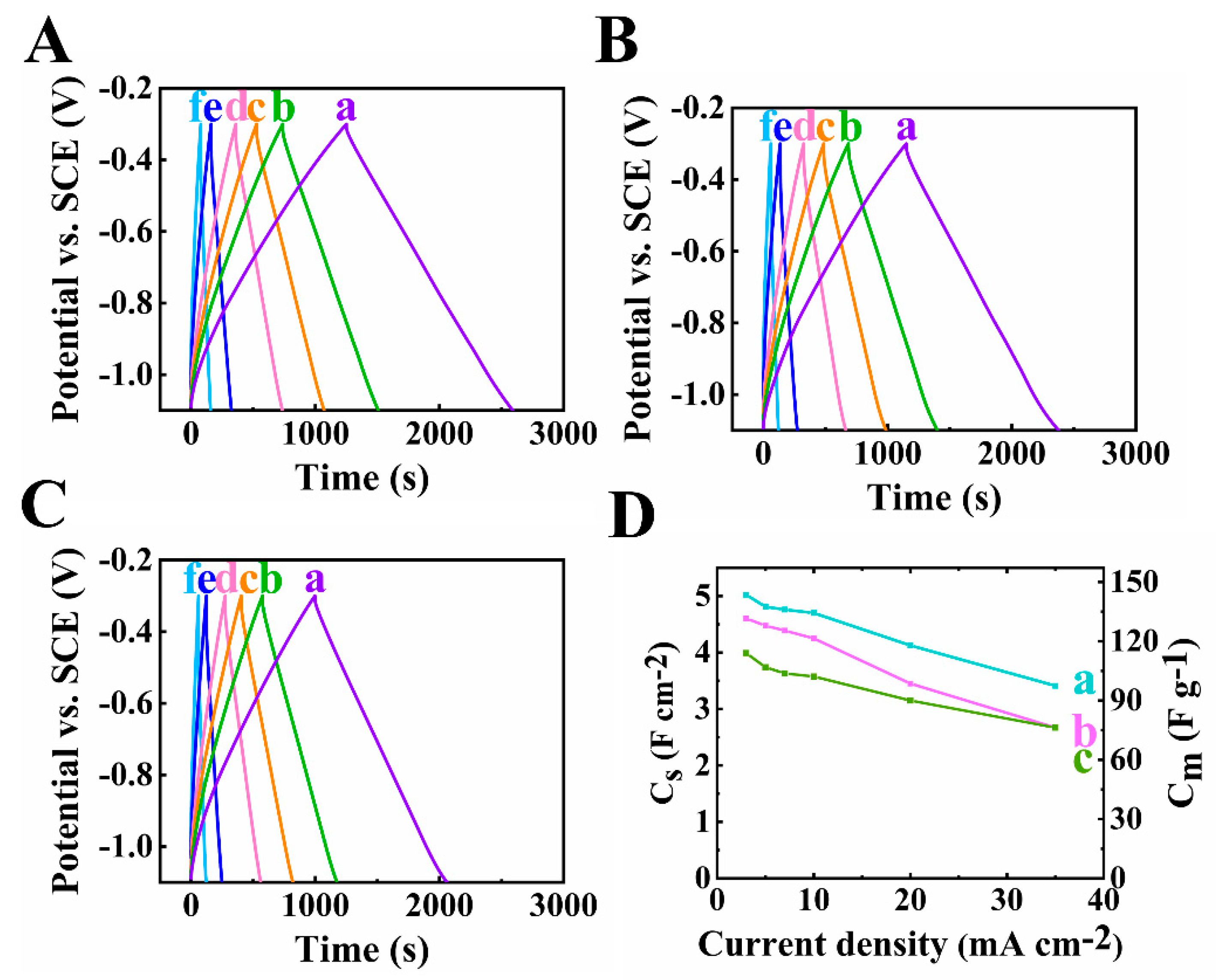
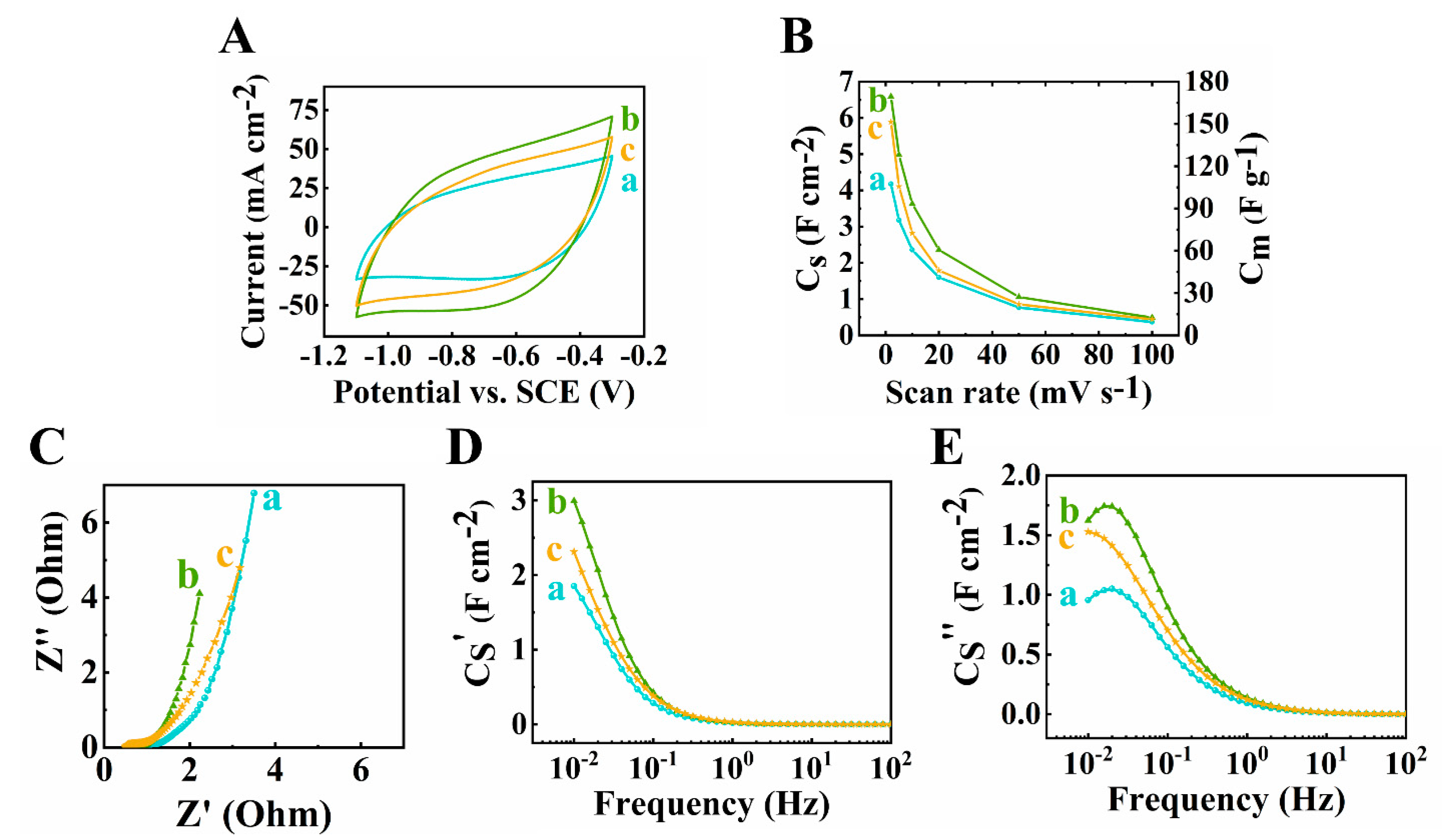
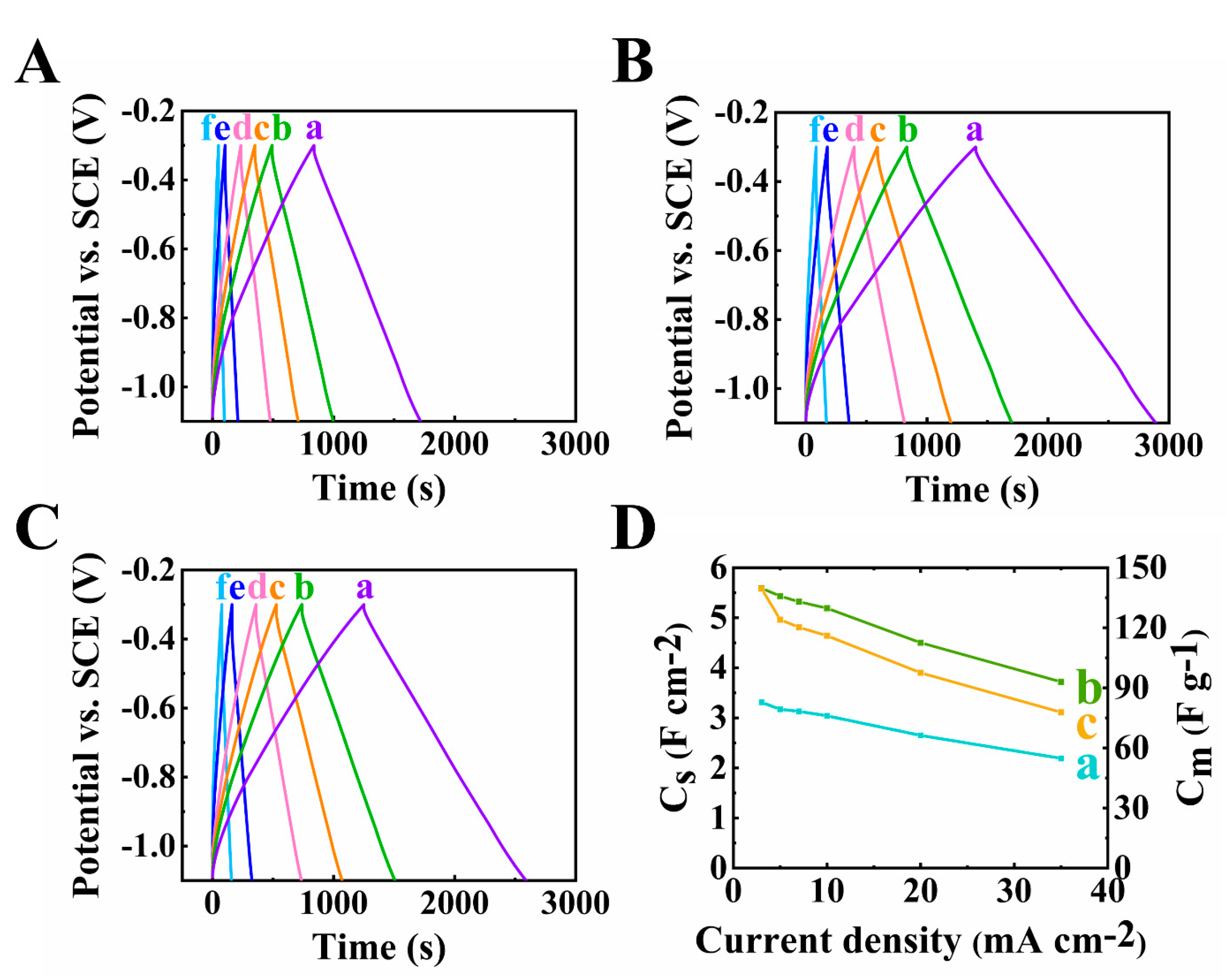
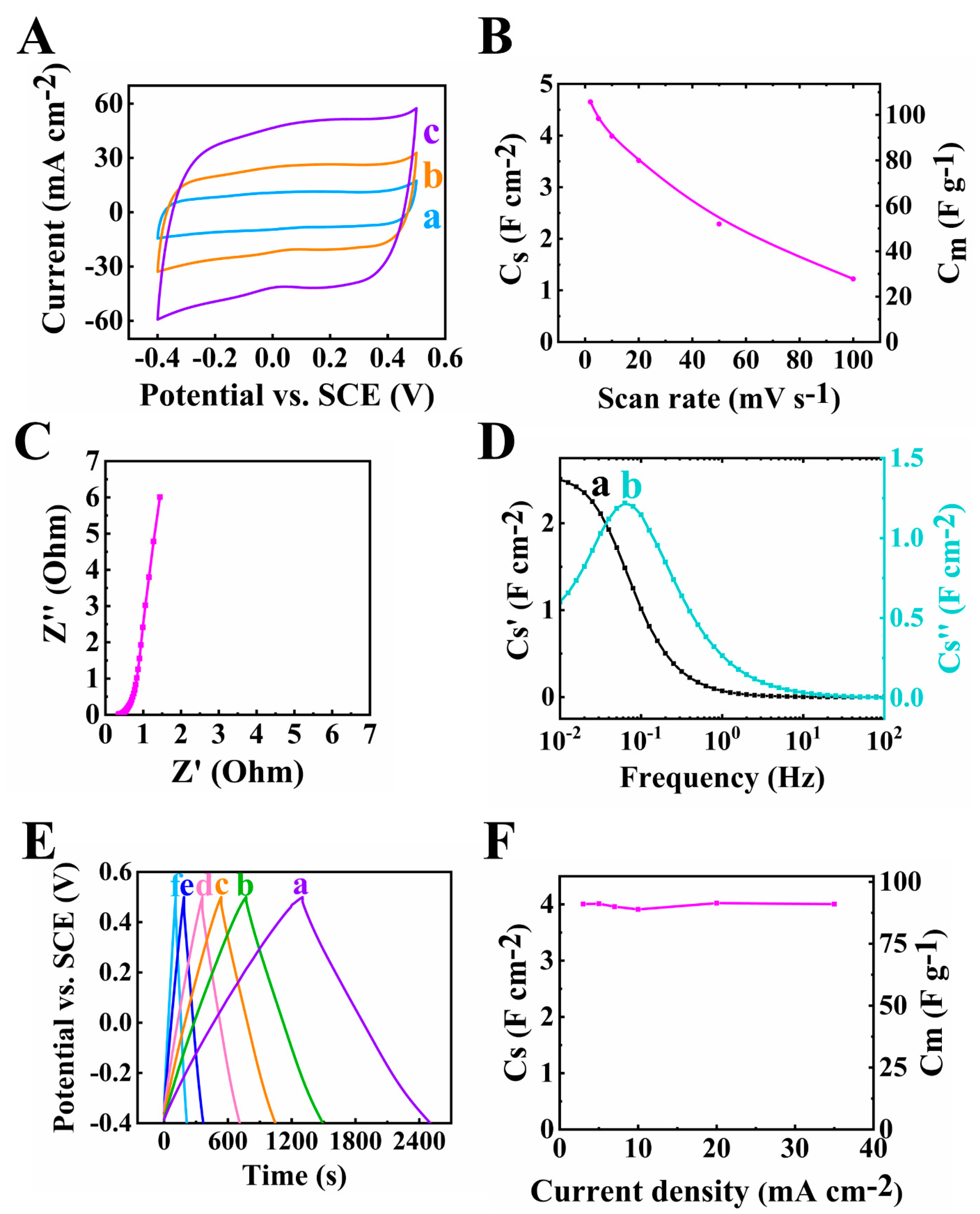
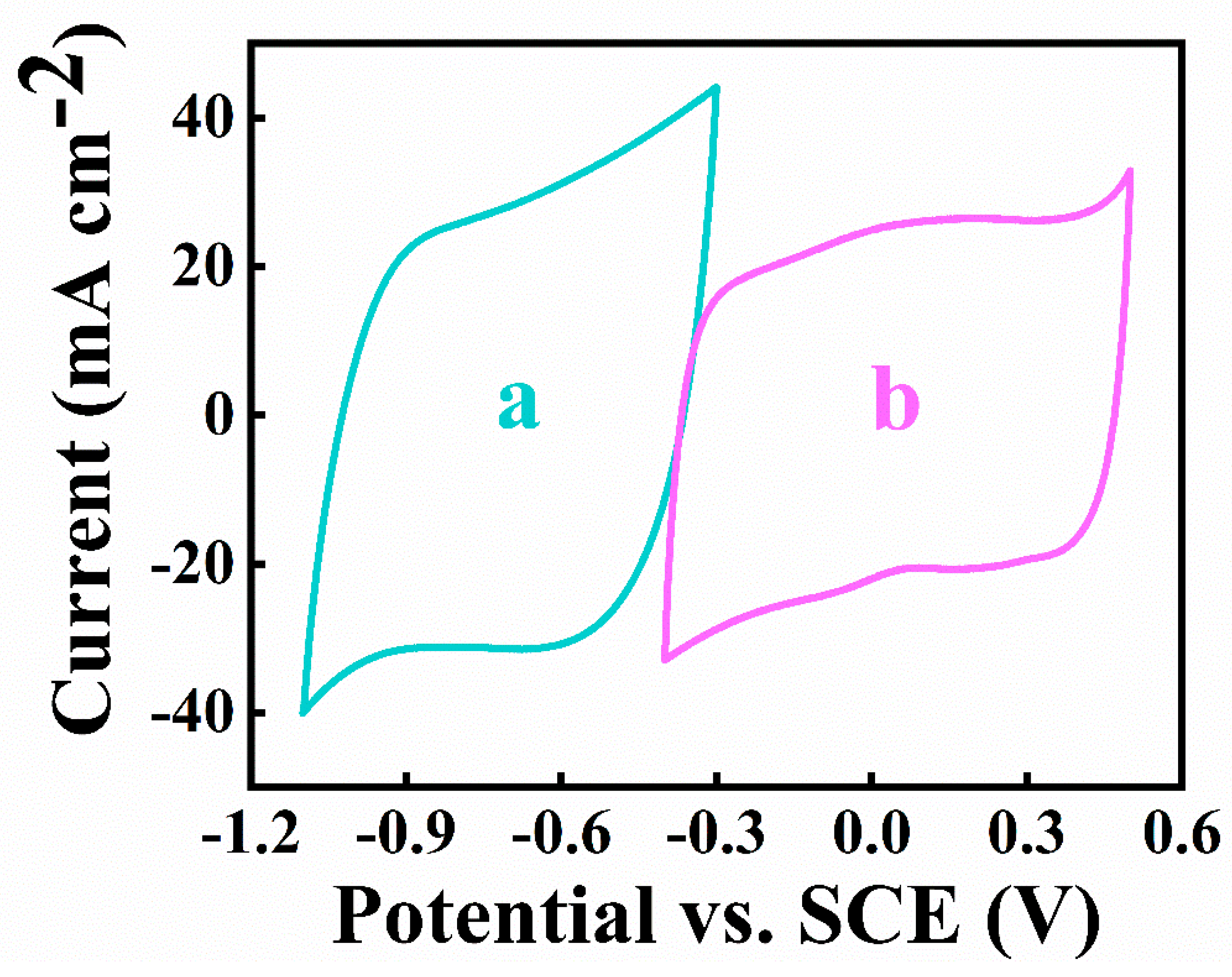
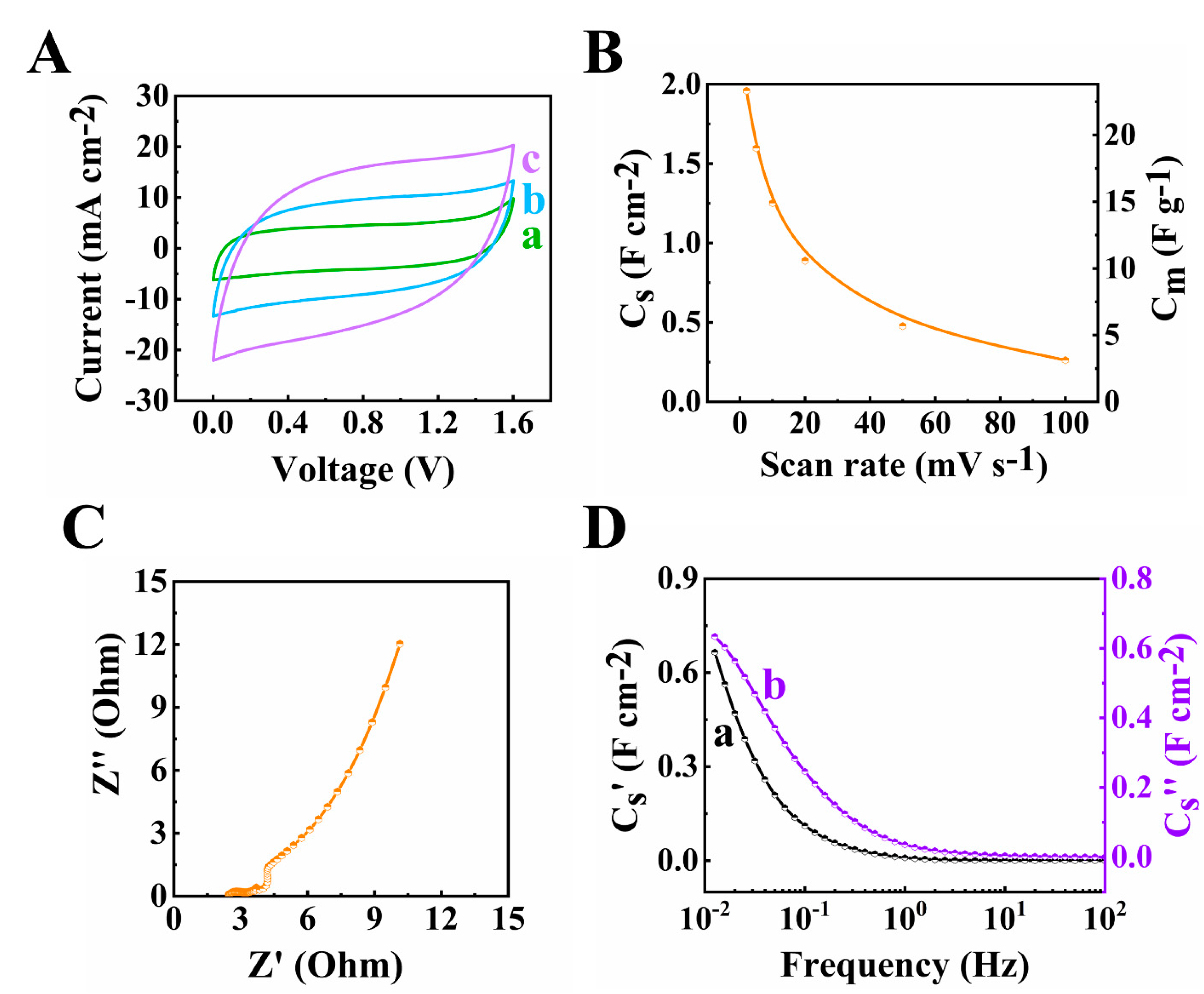
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariappan, V.K.; Krishnamoorthy, K.; Manoharan, S.; Pazhamalai, P.; Kim, S.J. Electrospun Polymer-Derived Carbyne Supercapacitor for Alternating Current Line Filtering. Small 2021, 17, 2102971. [Google Scholar] [CrossRef]
- Tian, J.; Cui, N.; Chen, P.; Guo, K.; Chen, X. High-Performance Wearable Supercapacitors Based on PANI/N-CNT@CNT Fiber with a Designed Hierarchical Core-Sheath Structure. J. Mater. Chem. A Mater. 2021, 9, 20635–20644. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; da Silva, M.I.; Toma, H.E.; Angnes, L.; Martins, P.R.; Araki, K. Trimetallic Oxides/Hydroxides as Hybrid Supercapacitor Electrode Materials: A Review. J. Mater. Chem. A Mater. 2020, 8, 10534–10570. [Google Scholar] [CrossRef]
- Yewale, M.A.; Jadhvar, A.A.; Kharade, R.B.; Kadam, R.A.; Kumar, V.; Nakate, U.T.; Shelke, P.B.; Bobade, D.H.; Teli, A.M.; Dhas, S.D.; et al. Hydrothermally Synthesized Ni3V2O8 Nanoparticles with Horny Surfaces for HER and Supercapacitor Application. Mater. Lett. 2023, 338, 134033. [Google Scholar] [CrossRef]
- Yewale, M.A.; Jadhavar, A.A.; Kadam, R.A.; Velhal, N.B.; Nakate, U.T.; Teli, A.M.; Shin, J.C.; Nguyen, L.N.; Shin, D.K.; Kaushik, N.K. Hydrothermal Synthesis of Manganese Oxide (Mn3O4) with Granule-like Morphology for Supercapacitor Application. Ceram. Int. 2022, 48, 29429–29437. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhou, Z. MXene-Based Materials for Electrochemical Energy Storage. J. Energy Chem. 2018, 27, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Nan, J.; Guo, X.; Xiao, J.; Li, X.; Chen, W.; Wu, W.; Liu, H.; Wang, Y.; Wu, M.; Wang, G. Nanoengineering of 2D MXene-Based Materials for Energy Storage Applications. Small 2021, 17, 1902085. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liang, M.; Wang, H.; Wang, X.; Huang, Y.; Coelho, J.; Pinilla, S.; Zhang, Y.; Qi, F.; Nicolosi, V.; et al. 3D MXene Architectures for Efficient Energy Storage and Conversion. Adv. Funct. Mater. 2020, 30, 2000842. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive Electrodes Produced by Oxidant-Free Polymerization of Pyrrole between the Layers of 2D Titanium Carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef]
- Xu, J.; Hu, X.; Wang, X.; Wang, X.; Ju, Y.; Ge, S.; Lu, X.; Ding, J.; Yuan, N.; Gogotsi, Y. Low-Temperature Pseudocapacitive Energy Storage in Ti3C2Tx MXene. Energy Storage Mater. 2020, 33, 382–389. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Ren, C.E.; Ling, Z.; Lukatskaya, M.R.; Zhang, C.; Van Aken, K.L.; Barsoum, M.W.; Gogotsi, Y. Flexible MXene/Carbon Nanotube Composite Paper with High Volumetric Capacitance. Adv. Mater. 2015, 27, 339–345. [Google Scholar] [CrossRef]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/Graphene Films for Ultrafast Supercapacitors with Outstanding Volumetric Capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Yan, J.; Ma, Y.; Zhang, C.; Li, X.; Liu, W.; Yao, X.; Yao, S.; Luo, S. Polypyrrole-MXene Coated Textile-Based Flexible Energy Storage Device. RSC Adv. 2018, 8, 39742–39748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Wei, D.; Zhu, J.; Niu, D.; Wang, F.; Wang, L.; Yang, L.; Yang, P.; Wang, C. Enhanced Electrochemical Performances of Organ-like Ti 3 C 2 MXenes/Polypyrrole Composites as Supercapacitors Electrode Materials. Ceram. Int. 2019, 45, 7328–7337. [Google Scholar] [CrossRef]
- Le, T.A.; Tran, N.Q.; Hong, Y.; Lee, H. Intertwined Titanium Carbide MXene within a 3 D Tangled Polypyrrole Nanowires Matrix for Enhanced Supercapacitor Performances. Chem.—A Eur. J. 2019, 25, 1037–1043. [Google Scholar] [CrossRef]
- Liang, W.; Zhitomirsky, I. MXene-Polypyrrole Electrodes for Asymmetric Supercapacitors. Electrochim. Acta 2022, 406, 139843. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, Y.; Yang, C.; Wang, F.; Cao, M. Composites of TiO 2 Nanoparticles Deposited on Ti 3 C 2 MXene Nanosheets with Enhanced Electrochemical Performance. J. Electrochem. Soc. 2016, 163, A785–A791. [Google Scholar] [CrossRef]
- Nagarajan, R.D.; Sundaramurthy, A.; Sundramoorthy, A.K. Synthesis and Characterization of MXene (Ti3C2Tx)/Iron Oxide Composite for Ultrasensitive Electrochemical Detection of Hydrogen Peroxide. Chemosphere 2022, 286, 131478. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, M.; Luo, W.; Dai, B.; Ren, J.; Ma, M.; Li, T.; Ma, Y. All Pseudocapacitive MXene-MnO2 Flexible Asymmetric Supercapacitor. J. Energy Storage 2022, 45, 103715. [Google Scholar] [CrossRef]
- Shen, B.; Huang, H.; Jiang, Y.; Xue, Y.; He, H. 3D Interweaving MXene–Graphene Network–Confined Ni–Fe Layered Double Hydroxide Nanosheets for Enhanced Hydrogen Evolution. Electrochim. Acta 2022, 407, 139913. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, J.; Zhang, W.; Ma, L.; Jiang, Z.; Wang, J.; Huang, Y. Synergistically Coupling of 3D FeNi-LDH Arrays with Ti3C2Tx-MXene Nanosheets toward Superior Symmetric Supercapacitor. Nano Energy 2022, 91, 106633. [Google Scholar] [CrossRef]
- Liang, W.; Zhitomirsky, I. MXene-Carbon Nanotube Composite Electrodes for High Active Mass Asymmetric Supercapacitors. J. Mater. Chem. A Mater. 2021, 9, 10335–10344. [Google Scholar] [CrossRef]
- Yi, H.; Pang, S.; Yang, G.; Yao, X.; Li, C.; Jiang, J.; Li, Y. MXene Modified Carbon Fiber Composites with Improved Mechanical Properties Based on Electrophoretic Deposition. Mater. Res. Bull. 2022, 150, 111761. [Google Scholar] [CrossRef]
- Wen, D.; Ying, G.; Liu, L.; Li, Y.; Sun, C.; Hu, C.; Zhao, Y.; Ji, Z.; Zhang, J.; Wang, X. Direct Inkjet Printing of Flexible MXene/Graphene Composite Films for Supercapacitor Electrodes. J. Alloy. Compd. 2022, 900, 163436. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, M.; Meng, Y.; Fang, P.; Lu, X.; Tong, Y. Iron-Based Supercapacitor Electrodes: Advances and Challenges. Adv. Energy Mater. 2016, 6, 1601053. [Google Scholar] [CrossRef]
- Li, Y.; Hu, N. Modification of the Elemental Composition of Iron(III) Oxide as an Asymmetric Supercapacitor Anode: A Minireview. Instrum. Sci. Technol. 2020, 48, 637–666. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Wu, Q. α-Fe2O3 Based Carbon Composite as Pure Negative Electrode for Application as Supercapacitor. Eur. J. Inorg. Chem. 2019, 2019, 1301–1312. [Google Scholar] [CrossRef]
- Tufa, L.T.; Gicha, B.B.; Wu, H.; Lee, J. Fe-Based Mesoporous Nanostructures for Electrochemical Conversion and Storage of Energy. Batter. Supercaps 2021, 4, 429–444. [Google Scholar] [CrossRef]
- Ma, J.; Guo, X.; Yan, Y.; Xue, H.; Pang, H. FeOx-Based Materials for Electrochemical Energy Storage. Adv. Sci. 2018, 5, 1700986. [Google Scholar] [CrossRef]
- Ma, Y.; Sheng, H.; Dou, W.; Su, Q.; Zhou, J.; Xie, E.; Lan, W. Fe2O3Nanoparticles Anchored on the Ti3C2TxMXene Paper for Flexible Supercapacitors with Ultrahigh Volumetric Capacitance. ACS Appl. Mater. Interfaces 2020, 12, 41410–41418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, H.; Zhu, C.; Lin, S.; Xu, Z.; Zhang, X. Free-Standing MXene Film Modified by Amorphous FeOOH Quantum Dots for High-Performance Asymmetric Supercapacitor. Electrochim. Acta 2019, 308, 1–8. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.L.; Wang, G.G.; Zhang, H.Y.; Zhang, B.; Li, G.Z.; Wu, Z.P.; Dang, L.Y.; Han, J.C. Few-Layered Ti3C2T: X MXenes Coupled with Fe2O3 Nanorod Arrays Grown on Carbon Cloth as Anodes for Flexible Asymmetric Supercapacitors. J. Mater. Chem. A Mater. 2019, 7, 22631–22641. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Xie, Z.; Xu, X.; Yuan, Y.; Cheng, Z.; Liu, Y. A Nanoporous MXene Film Enables Flexible Supercapacitors with High Energy Storage. Nanoscale 2018, 10, 9642–9652. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Lin, S.; Li, H.; Wu, Z.; Zhu, L.; Li, C.; Wang, X.; Zhu, X.; Sun, Y. Laser Crystallized Sandwich-like MXene/Fe3O4/MXene Thin Film Electrodes for Flexible Supercapacitors. J. Power Sources 2021, 497, 229882. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Qiu, Z.; Wu, X.; Zhou, P.; Zhou, T.; Zhao, J.; Miao, Z.; Zhou, J.; Zhuo, S. Fe3O4@Ti3C2 MXene Hybrids with Ultrahigh Volumetric Capacity as an Anode Material for Lithium-Ion Batteries. J. Mater. Chem. A Mater. 2018, 6, 11189–11197. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, S.; Cai, D.; Cao, J.; Wang, L.; Han, W. Planar Supercapacitor with High Areal Capacitance Based on Ti3C2/Polypyrrole Composite Film. Electrochim. Acta 2020, 330, 135277. [Google Scholar] [CrossRef]
- Wu, W.; Niu, D.; Zhu, J.; Gao, Y.; Wei, D.; Zhao, C.; Wang, C.; Wang, F.; Wang, L.; Yang, L. Hierarchical Architecture of Ti3C2@PDA/NiCo2S4 Composite Electrode as High-Performance Supercapacitors. Ceram. Int. 2019, 45, 16261–16269. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Momodu, D.Y.; Naguib, M.; Mirghni, A.A.; Masikhwa, T.M.; Khaleed, A.A.; Kebede, M.; Manyala, N. Electrochemical Performance of Two-Dimensional Ti3C2-Mn3O4 Nanocomposites and Carbonized Iron Cations for Hybrid Supercapacitor Electrodes. Electrochim. Acta 2019, 301, 487–499. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Li, H.; Lin, S.; Ding, W.; Zhu, X.; Sheng, Z.; Wang, H.; Zhu, X.; Sun, Y. 2D/2D 1T-MoS2/Ti3C2 MXene Heterostructure with Excellent Supercapacitor Performance. Adv. Funct. Mater. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, C.; Zhou, F.; Dong, Y.; Shi, X.; Nicolosi, V.; Wu, Z.S.; Bao, X. Ionic Liquid Pre-Intercalated MXene Films for Ionogel-Based Flexible Micro-Supercapacitors with High Volumetric Energy Density. J. Mater. Chem. A Mater. 2019, 7, 9478–9485. [Google Scholar] [CrossRef]
- Tian, Y.; Que, W.; Luo, Y.; Yang, C.; Yin, X.; Kong, L.B. Surface Nitrogen-Modified 2D Titanium Carbide (MXene) with High Energy Density for Aqueous Supercapacitor Applications. J. Mater. Chem. A Mater. 2019, 7, 5416–5425. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The Development of Pseudocapacitor Electrodes and Devices with High Active Mass Loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Simon, P. True Performance Metrics in Electrochemical Energy Storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Holt, C.M.B.; Li, Z.; Tan, X.; Amirkhiz, B.S.; Xu, Z.; Olsen, B.C.; Stephenson, T.; Mitlin, D. Graphene-Nickel Cobaltite Nanocomposite Asymmetrical Supercapacitor with Commercial Level Mass Loading. Nano Res. 2012, 5, 605–617. [Google Scholar] [CrossRef]
- Liao, J.; Li, Y.; Wang, Z.; Lv, L.; Chang, L. In-Situ Preparation of Fe3O4/Graphene Nanocomposites and Their Electrochemical Performances for Supercapacitor. Mater. Chem. Phys. 2021, 258, 123995. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Maiorov, M.; Krumina, A.; Skaudžius, R.; Zarkov, A.; Kareiva, A.; Popov, A.I. Impact of Gadolinium on the Structure and Magnetic Properties of Nanocrystalline Powders of Iron Oxides Produced by the Extraction-Pyrolytic Method. Materials 2020, 13, 4147. [Google Scholar] [CrossRef]
- Liang, W.; Zhitomirsky, I. Composite Fe3O4-Mxene-Carbon Nanotube Electrodes for Supercapacitors Prepared Using the New Colloidal Method. Materials 2021, 14, 2930. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, K.; Zhitomirsky, I. Polypyrrole Coated Carbon Nanotubes for Supercapacitor Devices with Enhanced Electrochemical Performance. J. Power Sources 2014, 268, 233–239. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.; Fu, J.; Liu, Y.; Wu, Y.; Zhou, J.; Zhang, Z.; Xie, E. Commercial-Level Mass-Loading MnO2with Ion Diffusion Channels for High-Performance Aqueous Energy Storage Devices. J. Mater. Chem. A Mater. 2021, 9, 17945–17954. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhitomirsky, I. Surface Modification of MnO2 and Carbon Nanotubes Using Organic Dyes for Nanotechnology of Electrochemical Supercapacitors. J. Mater. Chem. A Mater. 2013, 1, 12519–12526. [Google Scholar] [CrossRef]
- Zang, X.; Wang, J.; Qin, Y.; Wang, T.; He, C.; Shao, Q.; Zhu, H.; Cao, N. Enhancing Capacitance Performance of Ti3C2Tx MXene as Electrode Materials of Supercapacitor: From Controlled Preparation to Composite Structure Construction. Nanomicro Lett. 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Liu, J. Mechanistic Investigation of the Charge Storage Process of Pseudocapacitive Fe3O4 Nanorod Film. Electrochim. Acta 2014, 120, 52–56. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Nguyen, L.N.; Nakate, U.T.; Lingamdinne, L.P.; Koduru, J.R.; Auti, P.S.; Vattikuti, S.V.P.; Shin, D.K. Electrochemical Supercapacitor Performance of NiCo2O4 Nanoballs Structured Electrodes Prepared via Hydrothermal Route with Varying Reaction Time. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129901. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Linh, N.N.; Teli, A.M.; Shin, J.C.; Lingamdinne, L.P.; Koduru, J.R.; Shin, D.K. Mesoporous Hexagonal Nanorods of NiCo2O4 Nanoparticles via Hydrothermal Route for Supercapacitor Application. Chem. Phys. Lett. 2022, 800, 139654. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Koduru, J.R.; Velhal, N.B.; Nakate, U.T.; Jadhavar, A.A.; Sali, N.D.; Shin, D.K. Interconnected Plate-like NiCo2O4 Microstructures for Supercapacitor Application. Mater. Sci. Eng. B. Solid. State Mater. Adv. Technol. 2023, 287, 116072. [Google Scholar] [CrossRef]
- Mariappan, V.K.; Krishnamoorthy, K.; Pazhamalai, P.; Natarajan, S.; Sahoo, S.; Nardekar, S.S.; Kim, S.J. Antimonene Dendritic Nanostructures: Dual-Functional Material for High-Performance Energy Storage and Harvesting Devices. Nano Energy 2020, 77, 105248. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Graphene/Reduced Graphene Oxide-Carbon Nanotubes Composite Electrodes: From Capacitive to Battery-Type Behaviour. Nanomaterials 2021, 11, 1240. [Google Scholar] [CrossRef]
- Liang, W. Advanced Electrode Materials and Fabrication of Supercapacitors. 2022. Available online: http://hdl.net/11375/27386 (accessed on 1 December 2022).
- Zhang, L.; Wang, Z.; Chen, W.; Yuan, R.; Zhan, K.; Zhu, M.; Yang, J.; Zhao, B. Fe3O4nanoplates Anchored on Ti3C2T: XMXene with Enhanced Pseudocapacitive and Electrocatalytic Properties. Nanoscale 2021, 13, 15343–15351. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, K.; Li, Y.; Wang, Z.; Zhan, K.; Yang, J.; Zhao, B. Nanoparticles of Fe3O4 Anchored on Ti3C2Tx MXene/RGO Aero gels as Hybrid Negative Electrodes for Advanced Supercapacitors. ACS Appl. Nano Mater. 2023, 6, 482–491. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Xu, R.; Nawwar, M.; Zhitomirsky, I. Multifunctional MXene–Fe3O4–Carbon Nanotube Composite Electrodes for High Active Mass Asymmetric Supercapacitors. Batteries 2023, 9, 327. https://doi.org/10.3390/batteries9060327
Liang W, Xu R, Nawwar M, Zhitomirsky I. Multifunctional MXene–Fe3O4–Carbon Nanotube Composite Electrodes for High Active Mass Asymmetric Supercapacitors. Batteries. 2023; 9(6):327. https://doi.org/10.3390/batteries9060327
Chicago/Turabian StyleLiang, Wenyu, Rui Xu, Mohamed Nawwar, and Igor Zhitomirsky. 2023. "Multifunctional MXene–Fe3O4–Carbon Nanotube Composite Electrodes for High Active Mass Asymmetric Supercapacitors" Batteries 9, no. 6: 327. https://doi.org/10.3390/batteries9060327






