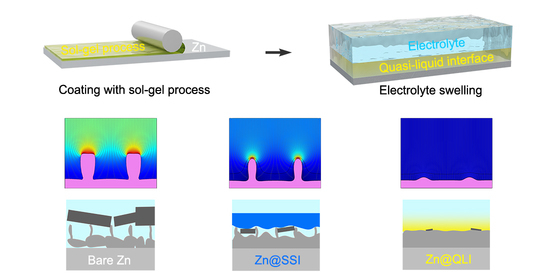
Constructing a Quasi-Liquid Interphase to Enable Highly Stable Zn-Metal Anode
Abstract
1. Introduction
2. Materials and Methods
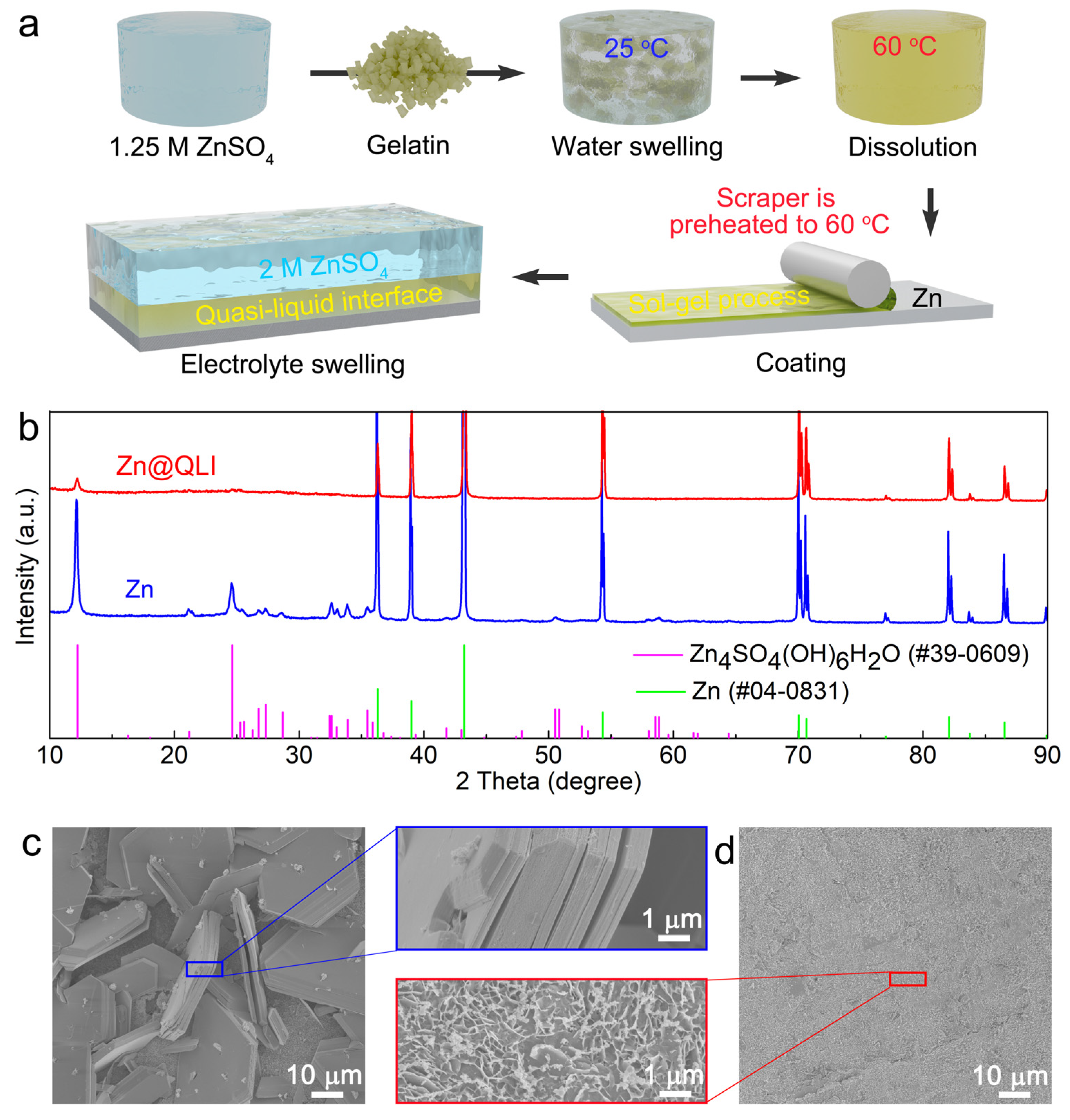
2.1. Zn@QLI Synthesis
2.2. V2O5·1.6H2O Cathode Synthesis
2.3. Material Characterization
2.4. Electrochemical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Li, H.; Wu, Y.; Li, H.; Zhong, B.; Sun, Y.; Wu, Z.; Guo, X. How to Promote the Industrial Application of SiOx Anode Prelithiation: Capability, Accuracy, Stability, Uniformity, Cost, and Safety. Adv. Energy Mater. 2022, 12, 2202342. [Google Scholar] [CrossRef]
- Wu, D.; Wu, F. Toward better batteries: Solid-state battery roadmap 2035+. eTransportation 2023, 16, 100224. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, N.; Feng, T.; Wu, F.; Zhao, T.; Chen, R. A copolymer microspheres-coated separator to enhance thermal stability of lithium-sulfur batteries. Chem. Eng. J. 2022, 430, 132678. [Google Scholar] [CrossRef]
- Yun, F.; Liu, S.; Gao, M.; Bi, X.; Zhao, W.; Chang, Z.; Yuan, M.; Li, J.; Shen, X.; Qi, X.; et al. Investigation on step overcharge to self-heating behavior and mechanism analysis of lithium ion batteries. J. Energy Chem. 2023, 79, 301–311. [Google Scholar] [CrossRef]
- Zhang, J.G.; Xu, W.; Xiao, J.; Cao, X.; Liu, J. Lithium Metal Anodes with Nonaqueous Electrolytes. Chem. Rev. 2020, 120, 13312–13348. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-H.; Bai, Z.; Li, M.; Yu, A.; Luo, D.; Liu, W.; Yang, L.; Lu, J.; Amine, K.; Chen, Z. Developing high safety Li-metal anodes for future high-energy Li-metal batteries: Strategies and perspectives. Chem. Soc. Rev. 2020, 49, 5407–5445. [Google Scholar] [CrossRef]
- Han, C.; Li, W.; Liu, H.K.; Dou, S.; Wang, J. Principals and strategies for constructing a highly reversible zinc metal anode in aqueous batteries. Nano Energy 2020, 74, 104880. [Google Scholar] [CrossRef]
- Yang, C.; Xia, J.; Cui, C.; Pollard, T.P.; Vatamanu, J.; Faraone, A.; Dura, J.A.; Tyagi, M.; Kattan, A.; Thimsen, E. All-temperature zinc batteries with high-entropy aqueous electrolyte. Nat. Sustain. 2023, 6, 1–11. [Google Scholar] [CrossRef]
- Lu, X.; Yang, P.; Xu, H.; Xiao, L.; Liu, L.; Li, R.; Alekseeva, E.; Zhang, J.; Levin, O.; An, M. Biomass derived robust Fe4N active sites supported on porous carbons as oxygen reduction reaction catalysts for durable Zn–air batteries. J. Mater. Chem. A 2023, 11, 3725–3734. [Google Scholar] [CrossRef]
- Karbak, M.; Baazizi, M.; Sayah, S.; Autret-Lambert, C.; Tison, Y.; Martinez, H.; Chafik, T.; Ghamouss, F. Unraveling high-performance oxygen-deficient amorphous manganese oxide as the cathode for advanced zinc ion batteries. J. Mater. Chem. A 2023, 11, 2634–2640. [Google Scholar] [CrossRef]
- Han, J.; Euchner, H.; Kuenzel, M.; Hosseini, S.M.; Groß, A.; Varzi, A.; Passerini, S. A Thin and Uniform Fluoride-Based Artificial Interphase for the Zinc Metal Anode Enabling Reversible Zn/MnO2 Batteries. ACS Energy Lett. 2021, 6, 3063–3071. [Google Scholar] [CrossRef]
- Cao, L.; Li, D.; Pollard, T.; Deng, T.; Zhang, B.; Yang, C.; Chen, L.; Vatamanu, J.; Hu, E.; Hourwitz, M.J. Fluorinated interphase enables reversible aqueous zinc battery chemistries. Nat. Nanotechnol. 2021, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dou, Q.; Yang, C.; Zang, L.; Yan, X. Polyiodide shuttle inhibition in ethylene glycol-added aqueous electrolytes for high energy and long-term cyclability of zinc–iodine batteries. J. Mater. Chem. A 2023, 11, 3632–3639. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Dong, S.; Xu, T.; Li, S.; An, Y.; Jiang, J.; Zhang, X. Pencil Drawing Stable Interface for Reversible and Durable Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2020, 31, 2006495. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Y.; Lu, H.; Zhang, Y.; Fu, C.; Usman, I.; Liu, Z.; Feng, M.; Fang, G.; Cao, X.; et al. Anti-Corrosive and Zn-Ion-Regulating Composite Interlayer Enabling Long-Life Zn Metal Anodes. Adv. Funct. Mater. 2021, 10, 2104361. [Google Scholar] [CrossRef]
- Chuai, M.; Yang, J.; Wang, M.; Yuan, Y.; Liu, Z.; Xu, Y.; Yin, Y.; Sun, J.; Zheng, X.; Chen, N.; et al. High-performance Zn battery with transition metal ions co-regulated electrolytic MnO2. eScience 2021, 1, 178–185. [Google Scholar] [CrossRef]
- Zhang, X.T.; Li, J.X.; Liu, D.Y.; Liu, M.K.; Zhou, T.S.; Qi, K.W.; Shi, L.; Zhu, Y.C.; Qian, Y.T. Ultra-long-life and highly reversible Zn metal anodes enabled by a desolvation and deanionization interface layer dagger. Energy Environ. Sci. 2021, 14, 3120–3129. [Google Scholar] [CrossRef]
- Leng, K.; Li, G.; Guo, J.; Zhang, X.; Wang, A.; Liu, X.; Luo, J. A Safe Polyzwitterionic Hydrogel Electrolyte for Long-Life Quasi-Solid State Zinc Metal Batteries. Adv. Funct. Mater. 2020, 30, 2001317. [Google Scholar] [CrossRef]
- Xu, L.; Meng, T.; Zheng, X.; Li, T.; Brozena, A.H.; Mao, Y.; Zhang, Q.; Clifford, B.C.; Rao, J.; Hu, L. Nanocellulose-Carboxymethylcellulose Electrolyte for Stable, High-Rate Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 2302098. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Xu, L.; Yang, C.; Hong, M.; Cui, M.; Clifford, B.C.; He, S.; Jing, S.; Yao, Y.; et al. A sustainable chitosan-zinc electrolyte for high-rate zinc-metal batteries. Matter 2022, 5, 3402–3416. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Hu, J.; Zhu, J.; Niu, C.; Zhang, H.; Li, C.; Wu, B.; Han, C.; Mai, L. Comprehensive H2O Molecules Regulation via Deep Eutectic Solvents for Ultra-Stable Zinc Metal Anode. Angew. Chem. Int. Ed. 2023, 62, 202215552. [Google Scholar]
- Wu, C.; Tan, H.T.; Huang, W.J.; Li, W.X.; Dinh, K.N.; Yan, C.S.; Wei, W.F.; Chen, L.B.; Yan, Q.Y. A New Scalable Preparation of Metal Nanosheets: Potential Applications for Aqueous Zn-Ion Batteries Anode. Adv. Funct. Mater. 2020, 30, 2003187. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, M.; Wu, F.; Mei, Y.; Hao, Y.; Li, L.; Chen, R. Encapsulation of Metallic Zn in a Hybrid MXene/Graphene Aerogel as a Stable Zn Anode for Foldable Zn-Ion Batteries. Adv. Mater. 2021, 34, 2106897. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Ou, Y.; Zhang, B.; Wang, J.; Fu, L.; Wan, M.; Li, G.; Wang, W.; Wang, L.; Jiang, J.; et al. A Replacement Reaction Enabled Interdigitated Metal/Solid Electrolyte Architecture for Battery Cycling at 20 mA cm−2 and 20 mAh cm−2. J. Am. Chem. Soc. 2021, 143, 3143–3152. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, S.; Wu, S.; Hu, Z.; Cui, G.; Luo, J. In situ built interphase with high interface energy and fast kinetics for high performance Zn metal anodes. Energy Environ. Sci. 2021, 14, 3609–3620. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.; Kim, Y.; Park, Y.; Kim, M.; Choi, J.W. Highly Reversible, Grain-Directed Zinc Deposition in Aqueous Zinc Ion Batteries. Adv. Energy Mater. 2021, 11, 2100676. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Li, J.; Luo, N.; Chai, G.-L.; Miller, T.S.; Lai, F.; Shearing, P.; Brett, D.J.L.; Han, D.; et al. Alleviation of Dendrite Formation on Zinc Anodes via Electrolyte Additives. ACS Energy Lett. 2021, 6, 395–403. [Google Scholar] [CrossRef]
- Cao, L.; Li, D.; Hu, E.; Xu, J.; Deng, T.; Ma, L.; Wang, Y.; Yang, X.Q.; Wang, C. Solvation Structure Design for Aqueous Zn Metal Batteries. J. Am. Chem. Soc. 2020, 142, 21404–21409. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Y.; Lu, Y.; Zhou, X.; Lin, L.; Li, L.; Yan, Z.; Zhao, Q.; Zhang, K.; Chen, J. Designing Anion-Type Water-Free Zn2+ Solvation Structure for Robust Zn Metal Anode. Angew. Chem. 2021, 60, 23357–23364. [Google Scholar] [CrossRef]
- Hu, K.; Guan, X.; Lv, R.; Li, G.; Hu, Z.; Ren, L.; Wang, A.; Liu, X.; Luo, J. Stabilizing zinc metal anodes by artificial solid electrolyte interphase through a surface ion-exchanging strategy. Chem. Eng. J. 2020, 396, 125363. [Google Scholar] [CrossRef]
- Liu, X.; Yang, F.; Xu, W.; Zeng, Y.; He, J.; Lu, X. Zeolitic Imidazolate Frameworks as Zn(2+) Modulation Layers to Enable Dendrite-Free Zn Anodes. Adv. Sci. (Weinh.) 2020, 7, 2002173. [Google Scholar] [CrossRef]
- Ma, L.; Li, Q.; Ying, Y.; Ma, F.; Chen, S.; Li, Y.; Huang, H.; Zhi, C. Toward Practical High-Areal-Capacity Aqueous Zinc-Metal Batteries: Quantifying Hydrogen Evolution and a Solid-Ion Conductor for Stable Zinc Anodes. Adv. Mater. 2021, 33, 2007406. [Google Scholar] [CrossRef]
- Xie, X.; Liang, S.; Gao, J.; Guo, S.; Guo, J.; Wang, C.; Xu, G.; Wu, X.; Chen, G.; Zhou, J. Manipulating the ion-transfer kinetics and interface stability for high-performance zinc metal anodes. Energy Environ. Sci. 2020, 13, 503–510. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, Y.; Liu, G.; Zhu, R.; Huang, J.; Chen, Z.; Gao, Z.; Chen, X.; Qie, L. Redirected Zn Electrodeposition by an Anti-Corrosion Elastic Constraint for Highly Reversible Zn Anodes. Adv. Funct. Mater. 2020, 31, 2001867. [Google Scholar] [CrossRef]
- Kang, L.; Cui, M.; Jiang, F.; Gao, Y.; Luo, H.; Liu, J.; Liang, W.; Zhi, C. Nanoporous CaCO3. Coatings Enabled Uniform Zn Stripping/Plating for Long-Life Zinc Rechargeable Aqueous Batteries. Adv. Energy Mater. 2018, 8, 1801090. [Google Scholar] [CrossRef]
- Liang, P.; Yi, J.; Liu, X.; Wu, K.; Wang, Z.; Cui, J.; Liu, Y.; Wang, Y.; Xia, Y.; Zhang, J. Highly Reversible Zn Anode Enabled by Controllable Formation of Nucleation Sites for Zn-Based Batteries. Adv. Funct. Mater. 2020, 30, 1908528. [Google Scholar] [CrossRef]
- Yang, H.; Chang, Z.; Qiao, Y.; Deng, H.; Mu, X.; He, P.; Zhou, H. Constructing a super-saturated electrolyte front surface for stable rechargeable aqueous zinc batteries. Angew. Chem. Int. Ed. 2020, 59, 9377–9381. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Chi, X.; Liu, Y.; Wang, L.; Du, Y.; Ren, Y.; Liu, Y. An inorganic salt reinforced Zn2+-conducting solid-state electrolyte for ultra-stable Zn metal batteries. J. Mater. Chem. A 2019, 7, 22287–22295. [Google Scholar] [CrossRef]
- Tan, L.; Lan, X.; Chen, J.; Zhang, H.; Hu, R.; Zhu, M. LiF-Induced Stable Solid Electrolyte Interphase for a Wide Temperature SnO2-Based Anode Extensible to −50 °C. Adv. Energy Mater. 2021, 11, 2101855. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, C.; Wang, J.; Yao, Z.; Wang, S.; Kumar, S.G.H.; Ganapathy, S.; Eustace, S.; Bai, X.; Li, B.; et al. High entropy liquid electrolytes for lithium batteries. Nat. Commun. 2023, 14, 440. [Google Scholar] [CrossRef]
- Feng, G.; Jia, H.; Shi, Y.; Yang, X.; Liang, Y.; Engelhard, M.H.; Zhang, Y.; Yang, C.; Xu, K.; Yao, Y.; et al. Imaging solid-electrolyte interphase dynamics using operando reflection interference microscopy. Nat. Nanotechnol. 2023, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Shen, X.; Wen, Z.; Wang, X.; Peng, L.; Zeng, J.; Zhao, J. Ultra-stable and highly reversible aqueous zinc metal anodes with high preferred orientation deposition achieved by a polyanionic hydrogel electrolyte. Energy Storage Mater. 2020, 35, 586–594. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 1–20. [Google Scholar] [CrossRef]
- Wang, J.; Fan, M.; Bian, X.; Yu, M.; Wang, T.; Liu, S.; Yang, Y.; Tian, Y.; Guan, R. Enhanced magnetic heating efficiency and thermal conductivity of magnetic nanofluids with FeZrB amorphous nanoparticles. J. Magn. Magn. Mater. 2018, 465, 480–488. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef]
- Han, Q.; Chi, X.; Zhang, S.; Liu, Y.; Zhou, B.; Yang, J.; Liu, Y. Durable, flexible self-standing hydrogel electrolytes enabling high-safety rechargeable solid-state zinc metal batteries. J. Mater. Chem. A 2018, 6, 23046–23054. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, X.; Xu, D.; Dong, P.; Chee, M.O.L.; Li, X.; Zhu, K.; Ye, M.; Shen, J. Eliminating Zn dendrites by commercial cyanoacrylate adhesive for zinc ion battery. Energy Storage Mater. 2021, 36, 132–138. [Google Scholar] [CrossRef]
- Zeng, X.; Xie, K.; Liu, S.; Zhang, S.; Hao, J.; Liu, J.; Pang, W.K.; Liu, J.; Rao, P.; Wang, Q. Bio-inspired design of an in situ multifunctional polymeric solid–electrolyte interphase for Zn metal anode cycling at 30 mA cm−2 and 30 mA h cm−2. Energy Environ. Sci. 2021, 14, 5947–5957. [Google Scholar] [CrossRef]
- Yan, M.; Dong, N.; Zhao, X.; Sun, Y.; Pan, H. Tailoring the Stability and Kinetics of Zn Anodes through Trace Organic Polymer Additives in Dilute Aqueous Electrolyte. ACS Energy Lett. 2021, 6, 3236–3243. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Chen, C.-H.; Pao, C.-W. Phase-field study of dendritic morphology in lithium metal batteries. J. Power Sources 2021, 484, 229203. [Google Scholar] [CrossRef]
- Liu, K.; Pei, A.; Lee, H.R.; Kong, B.; Liu, N.; Lin, D.; Liu, Y.; Liu, C.; Hsu, P.C.; Bao, Z.; et al. Lithium Metal Anodes with an Adaptive "Solid-Liquid" Interfacial Protective Layer. J. Am. Chem. Soc. 2017, 139, 4815–4820. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xu, Z.; Qin, T.; Wang, J.; Tian, R.; Guo, X.; Wang, Z.; Luo, Z.; Yang, H. Constructing a Quasi-Liquid Interphase to Enable Highly Stable Zn-Metal Anode. Batteries 2023, 9, 328. https://doi.org/10.3390/batteries9060328
Wang J, Xu Z, Qin T, Wang J, Tian R, Guo X, Wang Z, Luo Z, Yang H. Constructing a Quasi-Liquid Interphase to Enable Highly Stable Zn-Metal Anode. Batteries. 2023; 9(6):328. https://doi.org/10.3390/batteries9060328
Chicago/Turabian StyleWang, Junzhang, Zhou Xu, Tengteng Qin, Jintian Wang, Rui Tian, Xingzhong Guo, Zongrong Wang, Zhongkuan Luo, and Hui Yang. 2023. "Constructing a Quasi-Liquid Interphase to Enable Highly Stable Zn-Metal Anode" Batteries 9, no. 6: 328. https://doi.org/10.3390/batteries9060328
APA StyleWang, J., Xu, Z., Qin, T., Wang, J., Tian, R., Guo, X., Wang, Z., Luo, Z., & Yang, H. (2023). Constructing a Quasi-Liquid Interphase to Enable Highly Stable Zn-Metal Anode. Batteries, 9(6), 328. https://doi.org/10.3390/batteries9060328