A Tale of Nickel-Iron Batteries: Its Resurgence in the Age of Modern Batteries
Abstract
:1. Introduction
2. Nickel-Iron Battery Chemistry
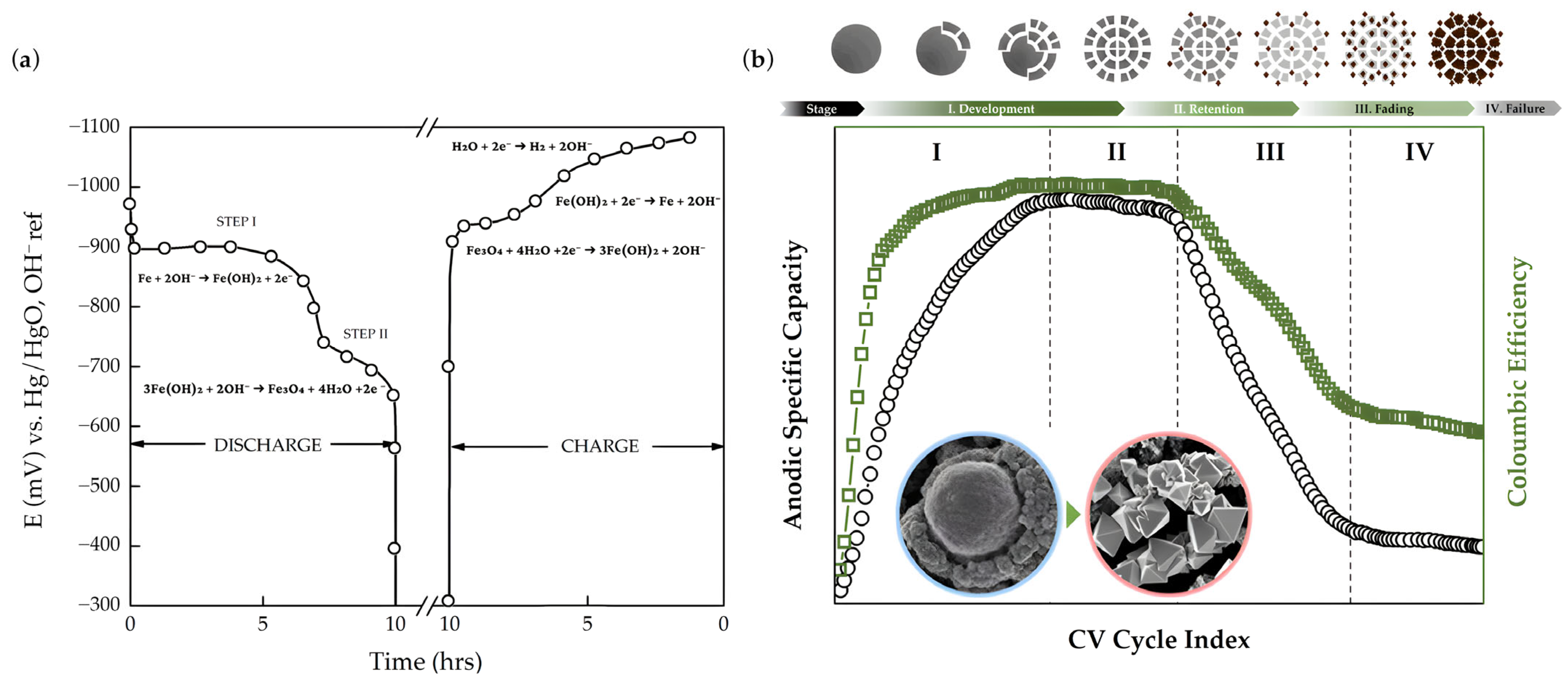
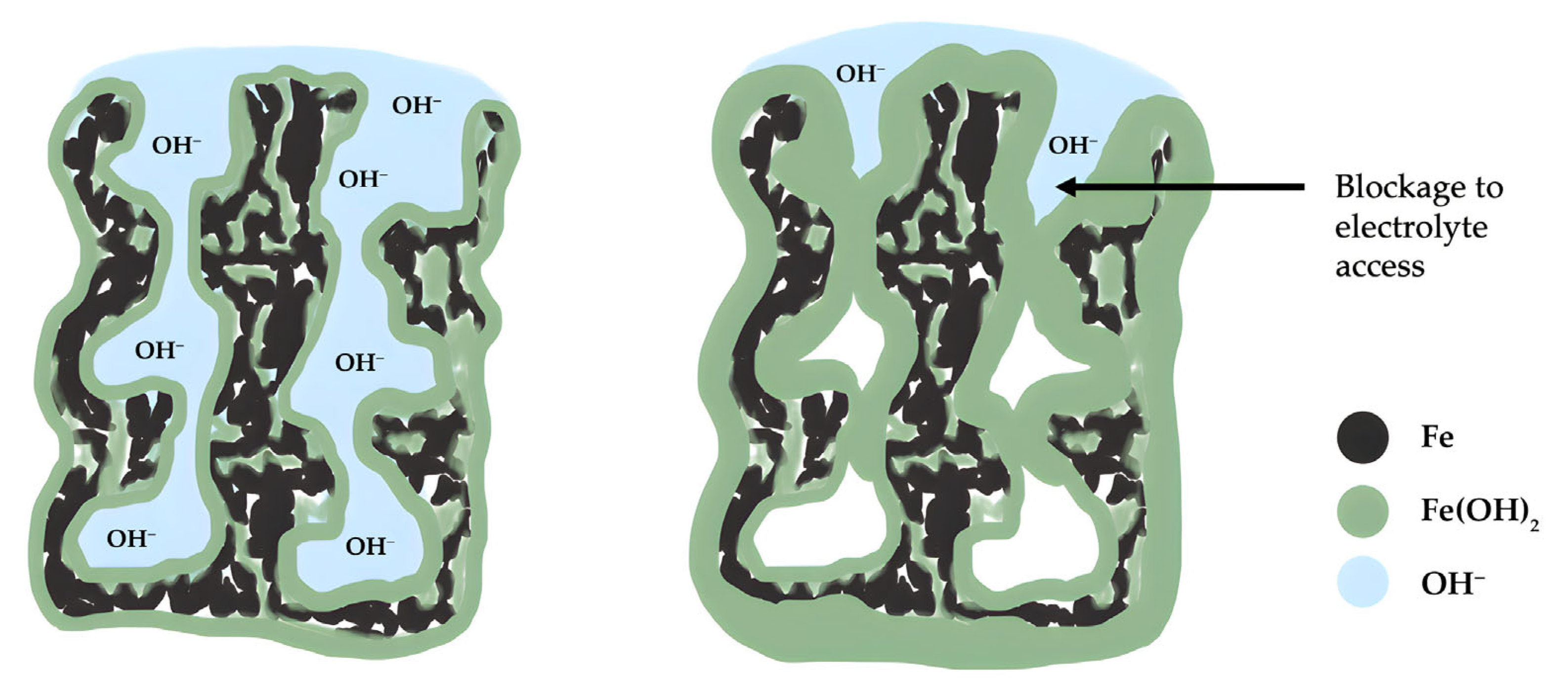
2.1. Negative Electrode
2.2. Positive Electrode
3. Negative Half-Cell Design Improvements
3.1. Material Morphology
3.2. Introduction of Defects
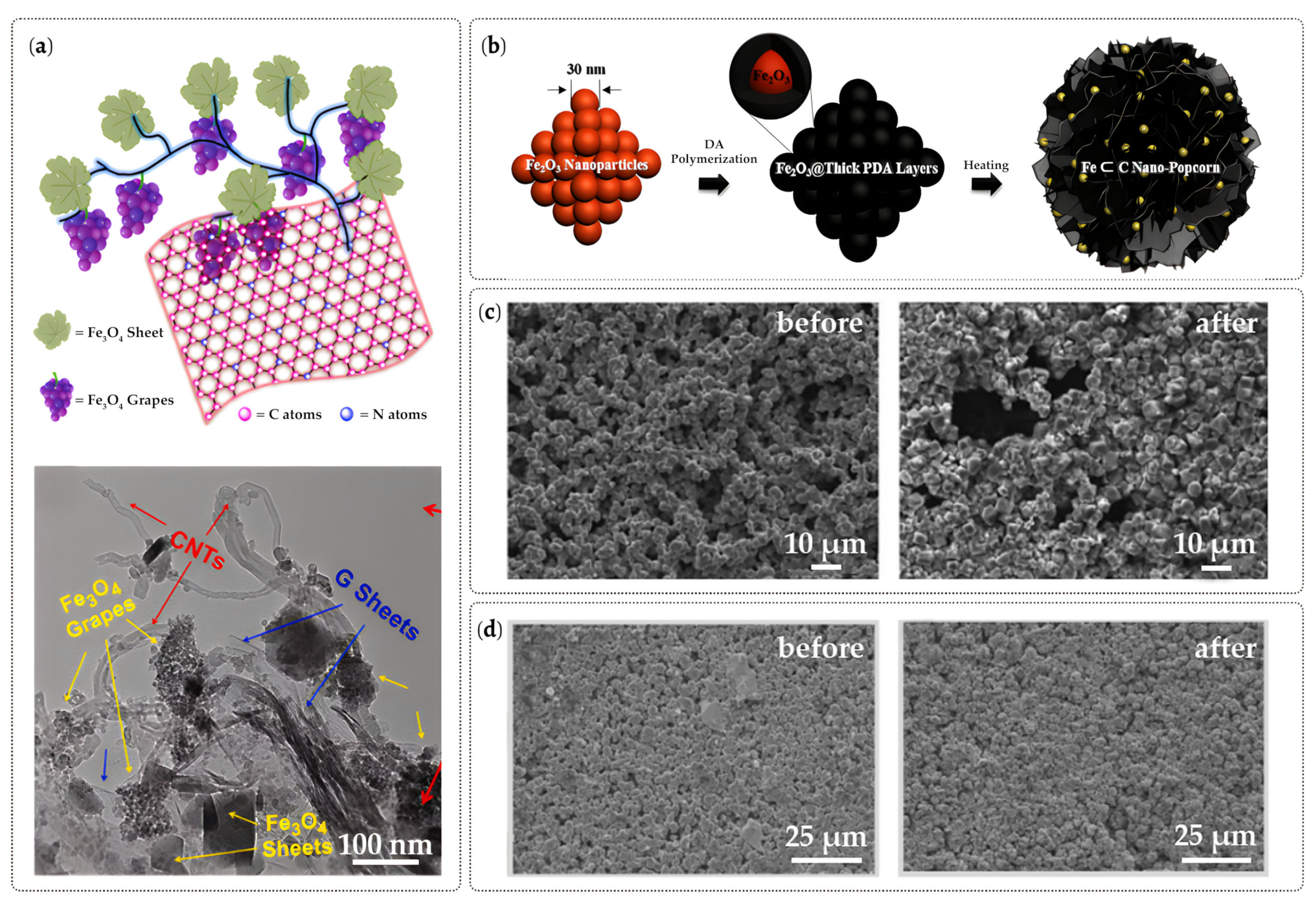
3.3. Material Composites
3.4. Electrode Additives
3.5. Electrolyte Composition
4. Positive Half-Cell Design Improvements
4.1. Material Morphology and Structure Modifications
4.2. Introduction of Defects
4.3. Material Composites
4.4. Stabilization of α-Ni(OH)2 Phase
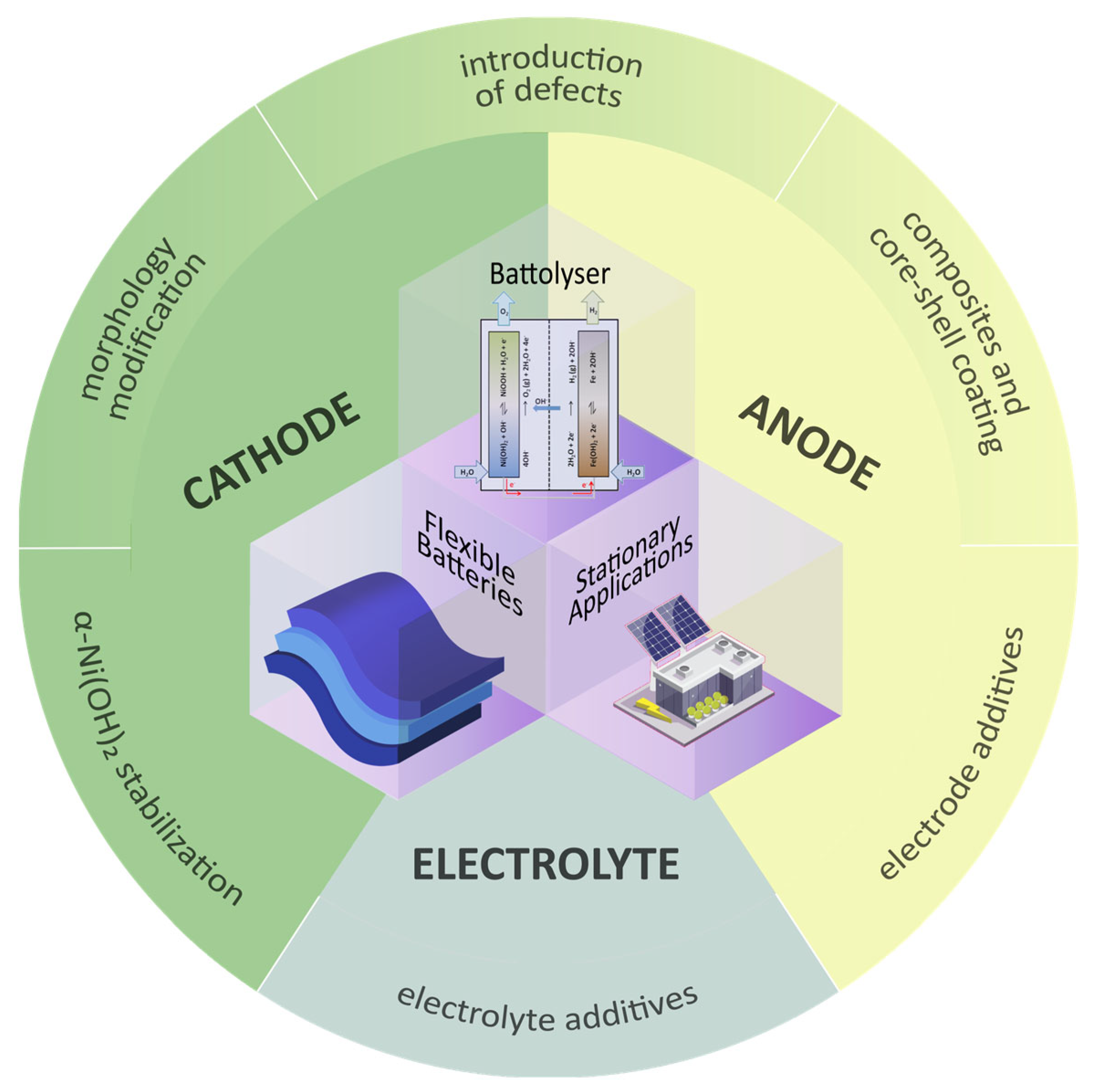
5. Full Cell Ni-Fe Improvements
5.1. Flexible Batteries
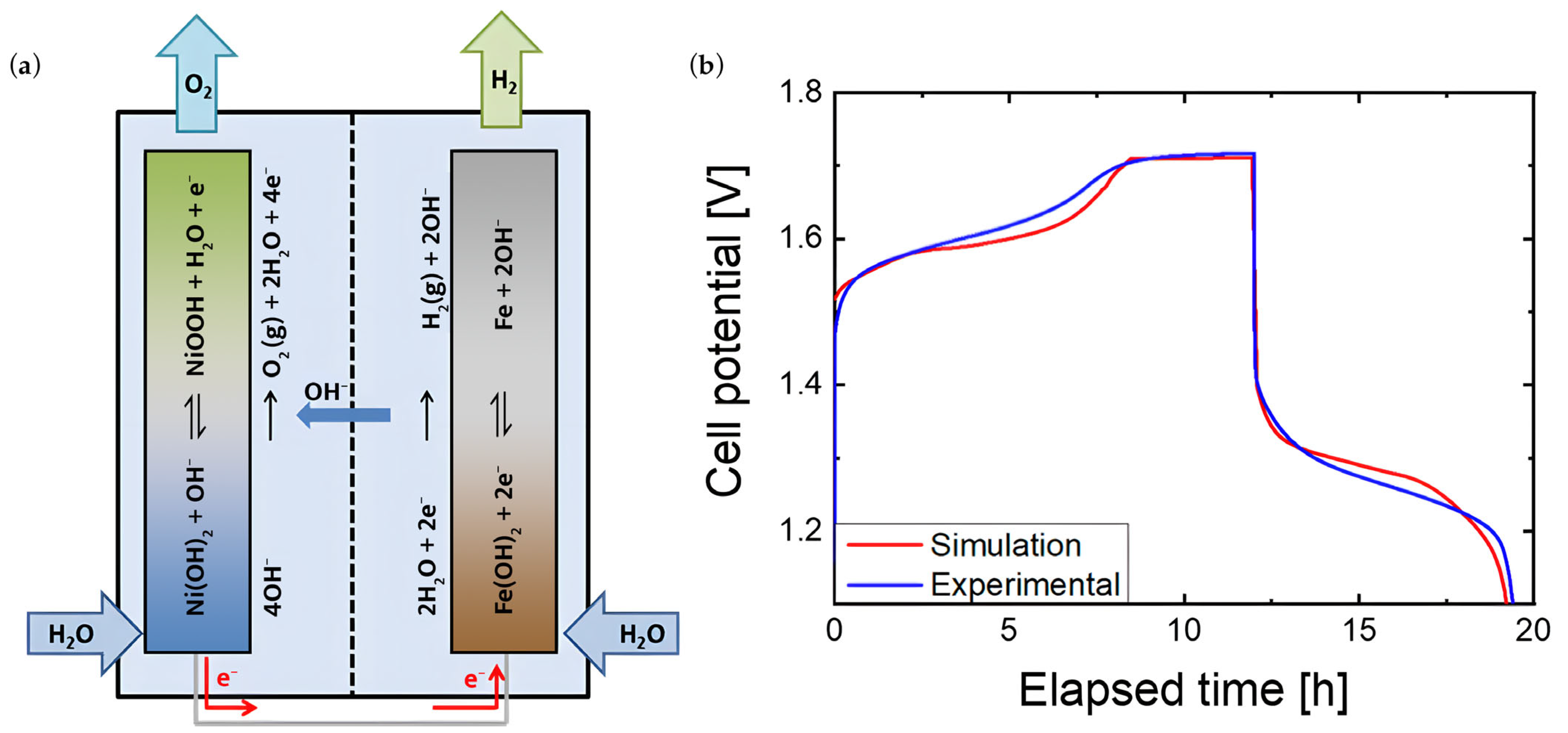
5.2. Battolyser
5.3. Stationary Applications
6. Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatzivasileiadi, A.; Ampatzi, E.; Knight, I. Characteristics of Electrical Energy Storage Technologies and Their Applications in Buildings. Renew. Sustain. Energy Rev. 2013, 25, 814–830. [Google Scholar] [CrossRef]
- Maeyaert, L.; Vandevelde, L.; Döring, T. Battery Storage for Ancillary Services in Smart Distribution Grids. J. Energy Storage 2020, 30, 101524. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef] [Green Version]
- US Geological Survey Mineral Commodity Summaries 2022. Diatomite. 2022, pp. 10–27. Available online: https://pubs.usgs.gov/periodicals/mcs2022/mcs2022.pdf (accessed on 11 July 2023).
- Huang, Z.; Du, G. Nickel-Based Batteries for Medium- and Large-Scale Energy Storage. In Advances in Batteries for Medium and Large-Scale Energy Storage: Types and Applications; Woodhead Publishing: Sawston, UK, 2015; pp. 73–90. ISBN 9781782420224. [Google Scholar]
- Moradzadeh, A.; Nazari-Heris, M.; Mohammadi-Ivatloo, B. Energy Storage Fundamentals and Components. In Energy Storage in Energy Markets: Uncertainties, Modelling, Analysis and Optimization; Academic Press: Cambridge, MA, USA, 2021; pp. 23–39. ISBN 9780128200957. [Google Scholar]
- Walawalkar, R.; Apt, J. Market Analysis of Emerging Electric Energy Storage Systems; National Energy Technology Laboratory: Albany, OR, USA, 2008; 118p. [Google Scholar]
- Omar, N.; Firouz, Y.; Monem, M.A.; Samba, A.; Gualous, H.; Coosemans, T.; Van den Bossche, P.; Van Mierlo, J. Analysis of Nickel-Based Battery Technologies for Hybrid and Electric Vehicles. Ref. Modul. Chem. Mol. Sci. Chem. Eng. 2014. [Google Scholar] [CrossRef]
- Chakkaravarthy, C.; Periasamy, P.; Jegannathan, S.; Vasu, K.I. The Nickel/Iron Battery. J. Power Sources 1991, 35, 21–35. [Google Scholar] [CrossRef]
- Chen, W.; Jin, Y.; Zhao, J.; Liu, N.; Cui, Y. Nickel-Hydrogen Batteries for Large-Scale Energy Storage. Proc. Natl. Acad. Sci. USA 2018, 115, 11694–11699. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, A.H.; Oseghale, C.I.; GilPosada, J.O.; Hall, P.J. Rechargeable Nickel-Iron Batteries for Largescale Energy Storage. IET Renew. Power Gener. 2016, 10, 1529–1534. [Google Scholar] [CrossRef] [Green Version]
- Barakat, S.; Emam, A.; Samy, M.M. Investigating Grid-Connected Green Power Systems’ Energy Storage Solutions in the Event of Frequent Blackouts. Energy Rep. 2022, 8, 5177–5191. [Google Scholar] [CrossRef]
- Linden, D., III. Basic Concepts. In Handbook of Batteries; McGraw-Hill Professional: New York, NY, USA, 2001; pp. 1–13. ISBN 0-07-135978-8. [Google Scholar]
- Jackovitz, J.F.; Brodd, R.J. Iron Electrode Batteries. In Handbook of Batteries; McGraw Hill Professional: New York, NY, USA, 2001; pp. 25.1–25.26. ISBN 9780071414753. [Google Scholar]
- Wang, H.; Liang, Y.; Gong, M.; Li, Y.; Chang, W.; Mefford, T.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; et al. An Ultrafast Nickel-Iron Battery from Strongly Coupled Inorganic Nanoparticle/Nanocarbon Hybrid Materials. Nat. Commun. 2012, 3, 917–918. [Google Scholar] [CrossRef] [Green Version]
- Mulder, F.M.; Weninger, B.M.H.; Middelkoop, J.; Ooms, F.G.B.; Schreuders, H. Efficient Electricity Storage with a Battolyser, an Integrated Ni-Fe Battery and Electrolyser. Energy Environ. Sci. 2017, 10, 756–764. [Google Scholar] [CrossRef] [Green Version]
- Iranzo, A.; Mulder, F.M. Nickel-Iron Layered Double Hydroxides for an Improved Ni/Fe Hybrid Battery-Electrolyser. Mater. Adv. 2021, 2, 5076–5088. [Google Scholar] [CrossRef]
- Lei, D.; Lee, D.C.; Magasinski, A.; Zhao, E.; Steingart, D.; Yushin, G. Performance Enhancement and Side Reactions in Rechargeable Nickel-Iron Batteries with Nanostructured Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 2088–2096. [Google Scholar] [CrossRef]
- Manohar, A.K.; Yang, C.; Malkhandi, S.; Yang, B.; Surya Prakash, G.K.; Narayanan, S.R. Understanding the Factors Affecting the Formation of Carbonyl Iron Electrodes in Rechargeable Alkaline Iron Batteries. J. Electrochem. Soc. 2012, 159, A2148–A2155. [Google Scholar] [CrossRef]
- Tang, H.; Sun, Z.; Chang, K.; Hou, Y.; Li, B.; Hou, Y.; Chang, Z. Uniform Carbon Coating Drastically Enhances the Electrochemical Performance of a Fe₃O₄ Electrode for Alkaline Nickel–Iron Rechargeable Batteries. Int. J. Hydrogen Energy 2019, 44, 24895–24904. [Google Scholar] [CrossRef]
- Lv, S.; Zhao, D.; Li, Y.; Liu, J. A Homogenous Mixed Coating Enabled Significant Stability and Capacity Enhancement of Iron Oxide Anodes for Aqueous Nickel–Iron Batteries. Chem. Commun. 2019, 55, 10308–10311. [Google Scholar] [CrossRef]
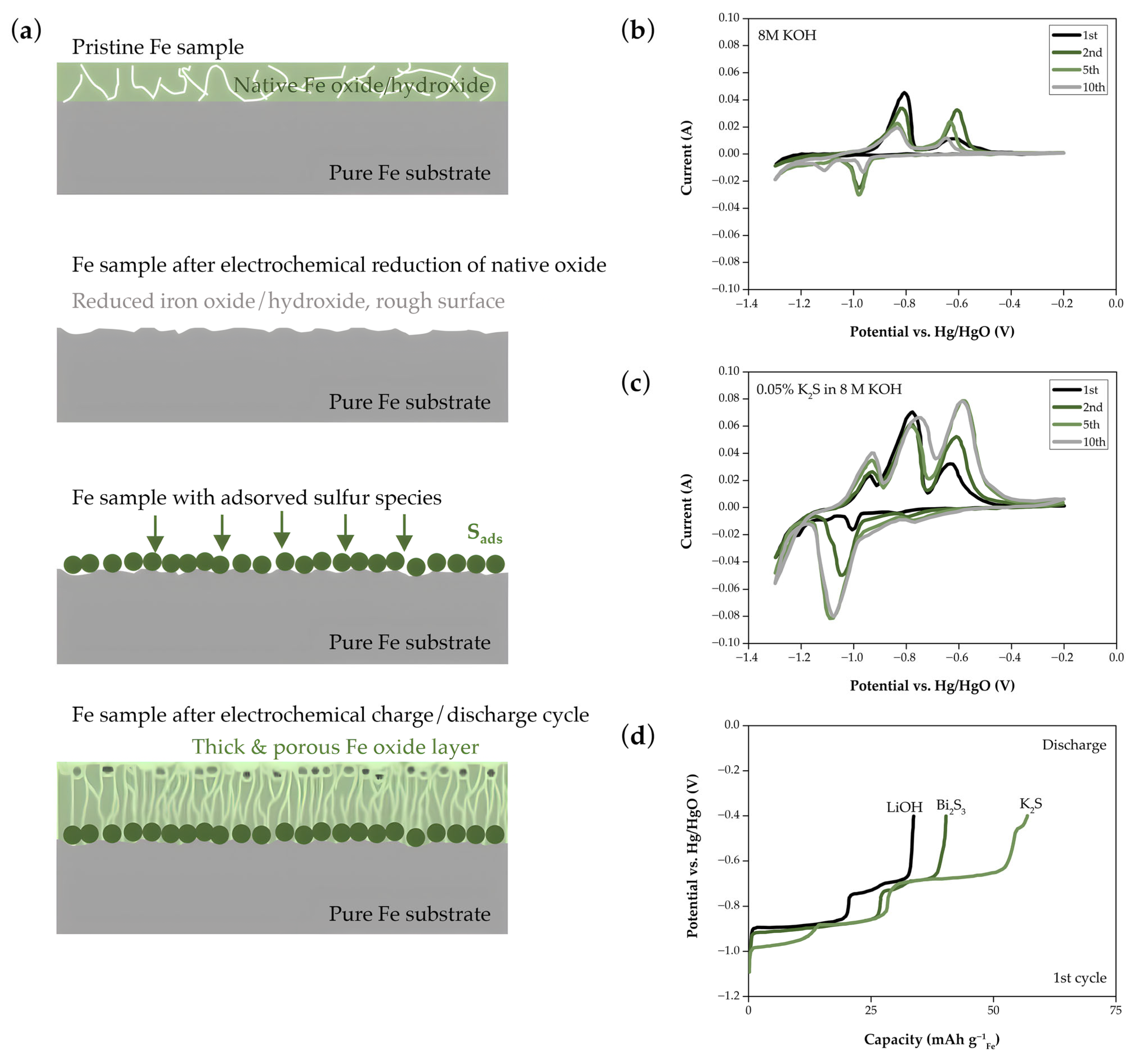
- Manohar, A.K.; Yang, C.; Narayanan, S.R. The Role of Sulfide Additives in Achieving Long Cycle Life Rechargeable Iron Electrodes in Alkaline Batteries. J. Electrochem. Soc. 2015, 162, A1864–A1872. [Google Scholar] [CrossRef]
- Hang, B.T.; Watanabe, T.; Egashira, M.; Watanabe, I.; Okada, S.; Yamaki, J.-I. The Effect of Additives on the Electrochemical Properties of Fe/C Composite for Fe/Air Battery Anode. J. Power Sources 2006, 155, 461–469. [Google Scholar] [CrossRef]
- Wang, H.; Dai, H. Strongly Coupled Inorganic–Nano-Carbon Hybrid Materials for Energy Storage. Chem. Soc. Rev. 2013, 42, 3088–3113. [Google Scholar] [CrossRef]
- Hang, B.T.; Watanabe, T.; Eashira, M.; Okada, S.; Yamaki, J.I.; Hata, S.; Yoon, S.H.; Mochida, I. The Electrochemical Properties of Fe2O3-Loaded Carbon Electrodes for Iron-Air Battery Anodes. J. Power Sources 2005, 150, 261–271. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Huang, K.J.; Chen, Z. Stabilizing Metallic Iron Nanoparticles by Conformal Graphitic Carbon Coating for High-Rate Anode in Ni-Fe Batteries. Nano Lett. 2020, 20, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, H.B.; Xiong, W.; Le, Z.; Sun, F.; Liu, F.; Chen, J.; Zhu, Z.; Lu, Y. Robust Iron Nanoparticles with Graphitic Shells for High-Performance Ni-Fe Battery. Nano Energy 2016, 30, 217–224. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Cao, J.; Zhang, Q.; Man, P.; Zhou, Z.; Li, C.; Yao, Y. Superstructured α-Fe₂O₃ Nanorods as Novel Binder-Free Anodes for High-Performing Fiber-Shaped Ni/Fe Battery. Sci. Bull. 2020, 65, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, C.; Sun, S.; Huang, Y.; Meng, G.; Han, A.; Liu, J. Mesoporous Fe₃O₄@C Nanoarrays as High-Performance Anode for Rechargeable Ni/Fe Battery. Sci. China Mater. 2020, 64, 1105–1113. [Google Scholar] [CrossRef]
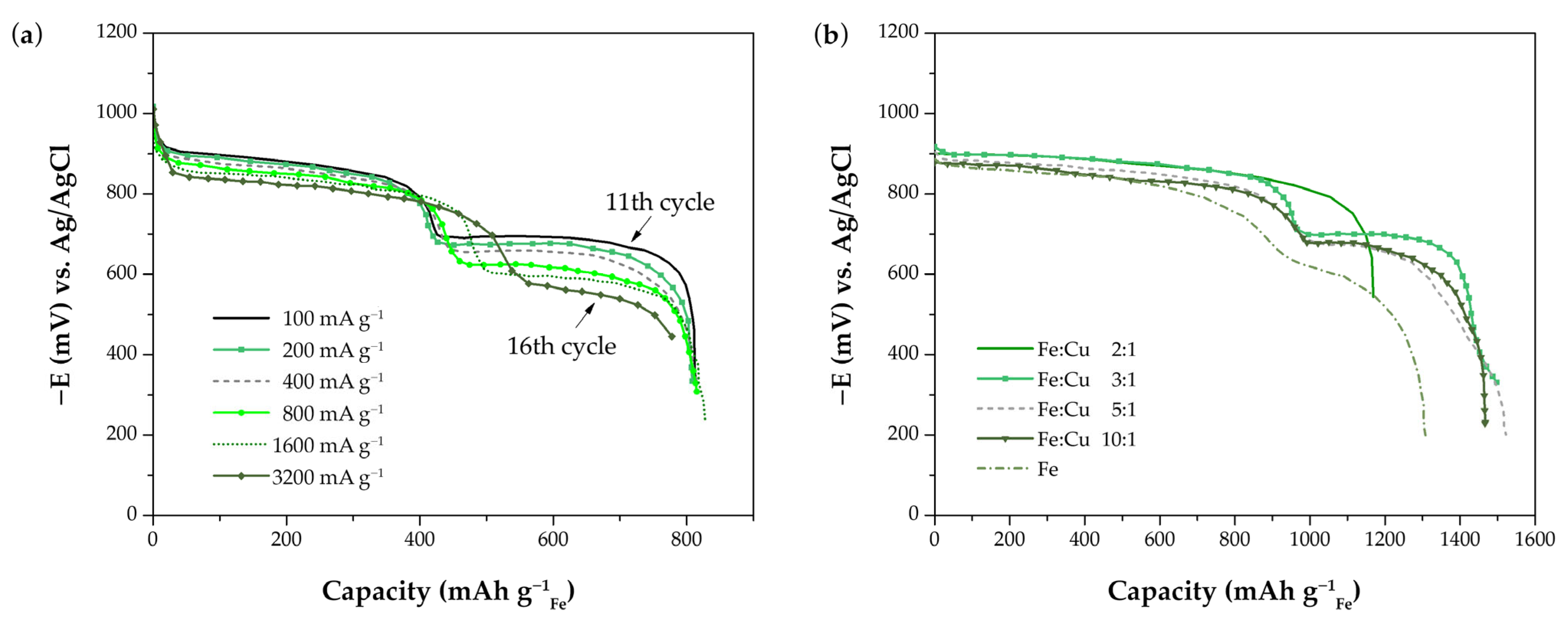
- Liu, P.; Zhu, D.; Yang, J.; Huang, L.X.; Chen, Y.G. Electrochemically Self-Assembled Fe/Cu Nanocomposite with Improved High-Rate and Low-Temperature Performances for Nickel-Iron Alkaline Battery. Chin. J. Inorg. Chem. 2017, 33, 779–786. [Google Scholar] [CrossRef]
- He, Z.; Xiong, F.; Tan, S.; Yao, X.; Zhang, C.; An, Q. Iron Metal Anode for Aqueous Rechargeable Batteries. Mater. Today Adv. 2021, 11, 100156. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, J. Iron Anode-Based Aqueous Electrochemical Energy Storage Devices: Recent Advances and Future Perspectives. Interdiscip. Mater. 2022, 1, 116–139. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Wang, Z.; Wang, Z.; Zhang, Q.; He, B.; Zhang, T.; Gong, W.; Chen, M.; Qi, M.; et al. High-Capacity Iron-Based Anodes for Aqueous Secondary Nickel−Iron Batteries: Recent Progress and Prospects. ChemElectroChem 2021, 8, 274–290. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Fan, X.; Ding, J.; Hu, W.; Zhong, C. 120 Years of Nickel-Based Cathodes for Alkaline Batteries. J. Alloys Compd. 2020, 834, 155185. [Google Scholar] [CrossRef]
- Raventos, A.M.; Kluivers, G.; Haverkort, J.W.; De Jong, W.; Mulder, F.M.; Kortlever, R. Modeling the Performance of an Integrated Battery and Electrolyzer System. Ind. Eng. Chem. Res. 2021, 60, 10988–10996. [Google Scholar] [CrossRef]
- Muralidharan, V.S.; Veerashanmugamani, M. Electrochemical Behaviour of Pure Iron in Concentrated Sodium Hydroxide Solutions at Different Temperatures: A Triangular Potential Sweep Voltammetric Study. J. Appl. Electrochem. 1985, 15, 675–683. [Google Scholar] [CrossRef]
- Öjefors, L.; Carlsson, L. An Iron—Air Vehicle Battery. J. Power Sources 1978, 2, 287–296. [Google Scholar] [CrossRef]
- Lee, D.C.; Lei, D.; Yushin, G. Morphology and Phase Changes in Iron Anodes Affecting Their Capacity and Stability in Rechargeable Alkaline Batteries. ACS Energy Lett. 2018, 3, 794–801. [Google Scholar] [CrossRef]
- Periasamy, P.; Babu, B.R.; Iyer, S.V. Cyclic Voltammetry Studies of Porous Iron Electrodes in Alkaline Solutions Used for Alkaline Batterues. J. Power Sources 1996, 58, 35–40. [Google Scholar] [CrossRef]
- Vijayamohanan, K.; Balasubramanian, T.S.; Shukla, A.K. Rechargeable Alkaline Iron Electrodes. J. Power Sources 1991, 34, 269–285. [Google Scholar] [CrossRef]
- Demidov, A.I.; Kokhatskaya, M.S.; Chernovets, B.V. Thermodynamics of Discharge of the Negative Electrode of a Nickel-Iron Battery. Russ. J. Appl. Chem. 2006, 79, 677–679. [Google Scholar] [CrossRef]
- Bryant, W.A. The Structure and Performance of Sintered Iron Electrodes. Electrochim. Acta 1979, 24, 1057–1060. [Google Scholar] [CrossRef]
- Manohar, A.K.; Yang, C.; Malkhandi, S.; Prakash, G.K.S.; Narayanan, S.R. Enhancing the Performance of the Rechargeable Iron Electrode in Alkaline Batteries with Bismuth Oxide and Iron Sulfide Additives. J. Electrochem. Soc. 2013, 160, A2078–A2084. [Google Scholar] [CrossRef]
- Balasubramanian, T.S.; Shukla, A.K. Effect of Metal-Sulfide Additives on Charge/Discharge Reactions of the Alkaline Iron Electrode. J. Power Sources 1993, 41, 99–105. [Google Scholar] [CrossRef]
- Yang, C.; Manohar, A.K.; Narayanan, S.R. A High-Performance Sintered Iron Electrode for Rechargeable Alkaline Batteries to Enable Large-Scale Energy Storage. J. Electrochem. Soc. 2017, 164, A418–A429. [Google Scholar] [CrossRef]
- McKerracher, R.D.; Figueredo-Rodriguez, H.A.; Dimogiannis, K.; Alegre, C.; Villanueva-Martinez, N.I.; Lázaro, M.J.; Baglio, V.; Aricò, A.S.; Ponce de Leόn, C. Effect of 1-Octanethiol as an Electrolyte Additive on the Performance of the Iron-Air Battery Electrodes. J. Solid State Electrochem. 2021, 25, 225–230. [Google Scholar] [CrossRef]
- Comisso, N.; Mengoli, G. Electrochemical Investigations on Composite Iron Electrodes. J. Appl. Electrochem. 2007, 37, 949–959. [Google Scholar] [CrossRef]
- Sui, Y.; Ji, X. Anticatalytic Strategies to Suppress Water Electrolysis in Aqueous Batteries. Chem. Rev. 2021, 121, 6654–6695. [Google Scholar] [CrossRef]
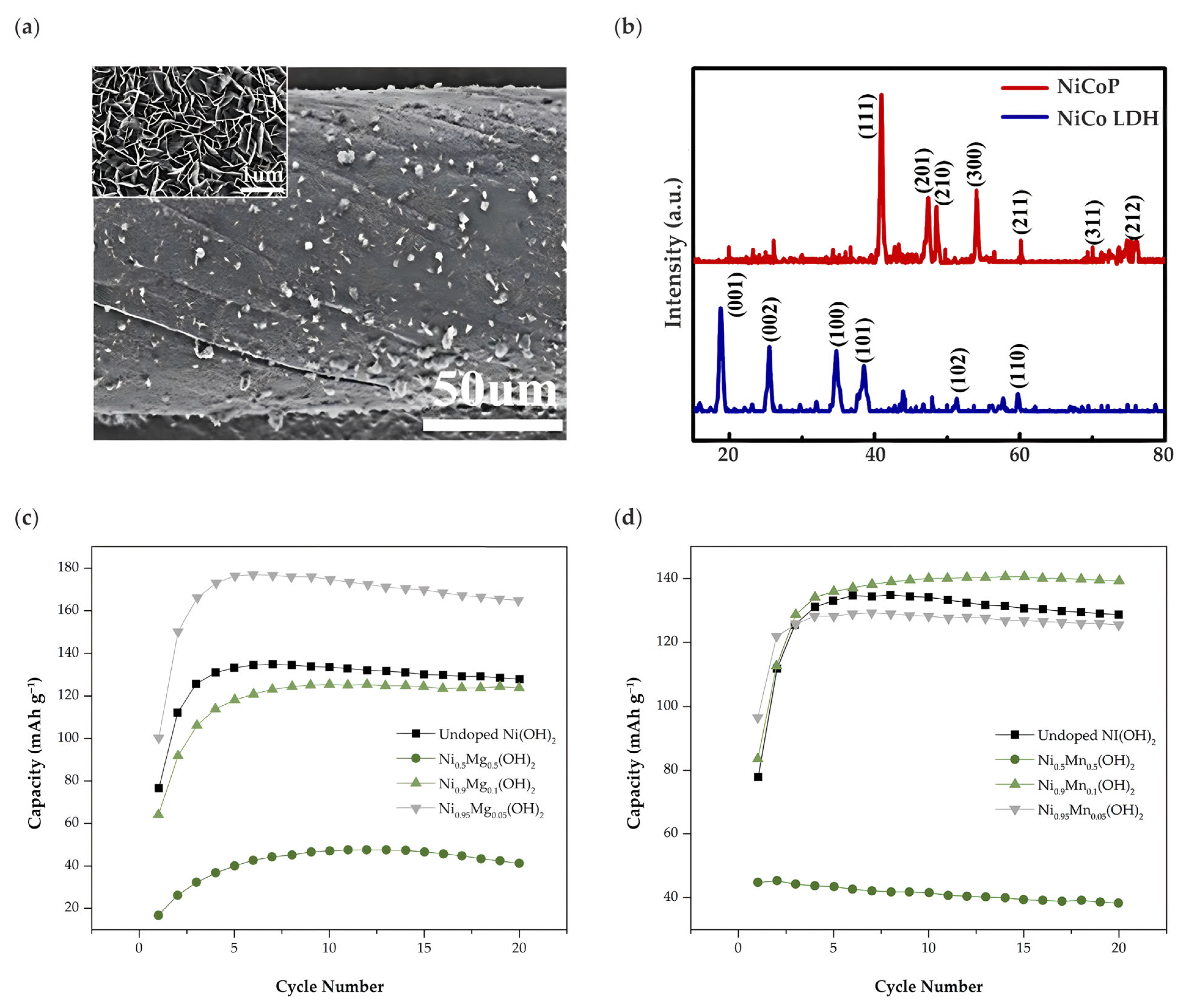
- Zide, D.; Felix, C.; Oosthuysen, T.; Bladergroen, B.J. Synthesis, Structural Characterization, and Electrochemical Properties of the Mg and Mn Doped-Ni(OH)₂ for Use as Active Cathode Materials in NiFe Batteries. J. Electroanal. Chem. 2021, 895, 115418. [Google Scholar] [CrossRef]
- Corrigan, D.A. The Catalysis of the Oxygen Evolution Reaction by Iron Impurities in Thin Film Nickel Oxide Electrodes. J. Electrochem. Soc. 1987, 134, 377–384. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H. A Mini Review of NiFe-Based Materials as Highly Active Oxygen Evolution Reaction Electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Thakur, N.; Kumar, M.; Mandal, D.; Nagaiah, T.C. Nickel Iron Phosphide/Phosphate as an Oxygen Bifunctional Electrocatalyst for High-Power-Density Rechargeable Zn-Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 52487–52497. [Google Scholar] [CrossRef]
- Hunter, B.M.; Winkler, J.R.; Gray, H.B.; Åkermark, B.; Johnston, E.V.; Kärkäs, M.D. Iron Is the Active Site in Nickel/Iron Water Oxidation Electrocatalysts. Molecules 2018, 23, 903. [Google Scholar] [CrossRef] [Green Version]
- Stevens, M.B.; Trang, C.D.M.; Enman, L.J.; Deng, J.; Boettcher, S.W. Reactive Fe-Sites in Ni/Fe (Oxy)Hydroxide Are Responsible for Exceptional Oxygen Electrocatalysis Activity. J. Am. Chem. Soc. 2017, 139, 11361–11364. [Google Scholar] [CrossRef]
- Yao, J.; Li, Y.; Huang, R.; Jiang, J.; Xiao, S.; Yang, J. Crucial Role of Water Content on the Electrochemical Performance of α-Ni(OH)2 as an Anode Material for Lithium-Ion Batteries. Ionics 2021, 27, 65–74. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.M.; Pan, T.; Zhao, Y.L.; Zhang, J.Q.; Cao, C.N. The Structure and Electrochemical Performance of Spherical Al-Substituted α-Ni(OH)₂ for Alkaline Rechargeable Batteries. J. Power Sources 2005, 143, 243–255. [Google Scholar] [CrossRef]
- Kamath, P.V.; Dixit, M.; Indira, L.; Shukla, A.K.; Kumar, V.G.; Munichandraiah, N. Stabilized A-Ni(OH)2 as Electrode Material for Alkaline Secondary Cells. J. Electrochem. Soc. 1994, 141, 2956–2959. [Google Scholar] [CrossRef]
- Aiyejuro, V.O. Interconversion of Nickel Hydroxides Studied Using Dynamic Electrochemical Impedance by Master of Science Interconversion of Nickel Hydroxides Studied Using Dynamic Electrochemical Impedance. Master’s Thesis, University of Victoria, Victoria, BC, Canada, 2020. [Google Scholar]
- Motori, A.; Sandrolini, F.; Davolio, G. Electrical Properties of Nickel Hydroxide for Alkaline Cell Systems. J. Power Sources 1994, 48, 361–370. [Google Scholar] [CrossRef]
- Kimmel, S.W.; Hopkins, B.J.; Chervin, C.N.; Skeele, N.L.; Ko, J.S.; Deblock, R.H.; Long, J.W.; Parker, J.F.; Hudak, B.M.; Stroud, R.M.; et al. Capacity and Phase Stability of Metal-Substituted α-Ni(OH)2 Nanosheets in Aqueous Ni-Zn Batteries. Mater. Adv. 2021, 2, 3060–3074. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J.; Jiang, M.; Hou, J.; Zhu, Y.; Zhang, J.; Ba, Z.; Li, L. A More Economical Choice for the Cathode Material of Ni-MH Batteries with High Electrochemical Performances: 3D Flower-like Ni–Fe LDHs. Int. J. Hydrogen Energy 2022, 47, 41087–41096. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Gao, T.; Li, P.; Jin, Z.; Xiao, D. Interconnecting 3D Conductive Networks with Nanostructured Iron/Iron Oxide Enables a High-Performance Flexible Battery. ACS Appl. Mater. Interfaces 2021, 13, 57411–57421. [Google Scholar] [CrossRef]
- Xue, L.; Li, S.; Shen, T.; Ni, M.; Qiu, C.; Sun, S.; Geng, H.; Zhu, X.; Xia, H. Two-Dimensional Metal (Oxy)Hydroxide and Oxide Ultrathin Nanosheets via Liquid Phase Epitaxy. Energy Storage Mater. 2020, 32, 272–280. [Google Scholar] [CrossRef]
- Lai, C.; Cheng, L.; Sun, Y.; Lee, K.; Lin, B. Alkaline Aqueous Rechargeable Ni-Fe Batteries with High-Performance Based on Flower-like Hierarchical NiCo2O4 Microspheres and Vines-Grapes-like Fe3O4-NGC Composites. Appl. Surf. Sci. 2021, 563, 150411. [Google Scholar] [CrossRef]
- Li, X.; Gao, T.; Liu, Q.; Xu, Y.; Li, J.; Xiao, D. Designing a High-Performance Anode Composed of Carbon Nanotubes and Fe-Fe₃C Nanoparticles for Quasi-Solid-State Fibrous Ni/Fe Batteries. Mater. Chem. Front. 2021, 5, 3636–3645. [Google Scholar] [CrossRef]
- Li, J.; Guo, L.; Shangguan, E.; Yue, M.; Xu, M.; Wang, D.; Chang, Z.; Li, Q. Synthesis of Novel Spherical Fe3O4@Ni3S2 Composite as Improved Anode Material for Rechargeable Nickel-Iron Batteries. Electrochim. Acta 2017, 240, 456–465. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L. Influence of Nanosized Ni(OH)₂ Addition on the Electrochemical Performance of Nickel Hydroxide Electrode. J. Power Sources 2004, 128, 326–330. [Google Scholar] [CrossRef]
- Kiani, M.A.; Mousavi, M.F.; Ghasemi, S. Size Effect Investigation on Battery Performance: Comparison between Micro- and Nano-Particles of β-Ni(OH)2 as Nickel Battery Cathode Material. J. Power Sources 2010, 195, 5794–5800. [Google Scholar] [CrossRef]
- Hu, W.K.; Gao, X.P.; Noréus, D.; Burchardt, T.; Nakstad, N.K. Evaluation of Nano-Crystal Sized α-Nickel Hydroxide as an Electrode Material for Alkaline Rechargeable Cells. J. Power Sources 2006, 160, 704–710. [Google Scholar] [CrossRef]
- Song, Q.S.; Chiu, C.H.; Chan, S.L.I. Effects of Ball Milling on the Physical and Electrochemical Characteristics of Nickel Hydroxide Powder. J. Appl. Electrochem. 2006, 36, 97–103. [Google Scholar] [CrossRef]
- Wang, H.; Tang, Z.Y.; Liu, Y.G.; Lee, C. sheng Synthesis and Behavior of Al-Stabilized α-Ni(OH)2. Trans. Nonferrous Met. Soc. China Engl. Ed. 2009, 19, 170–175. [Google Scholar] [CrossRef]
- He, X.; Pu, W.; Cheng, H.; Jiang, C.; Wan, C. Granulation of Nano-Scale Ni(OH)2 Cathode Materials for High Power Ni-MH Batteries. Energy Convers. Manag. 2006, 47, 1879–1883. [Google Scholar] [CrossRef]
- Ying, T. Surface Modification of Nickel Hydroxide Particles by Micro-Sized Cobalt Oxide Hydroxide and Properties as Electrode Materials. Surf. Coat. Technol. 2005, 200, 2376–2379. [Google Scholar] [CrossRef]
- Raventos, A.M.; Kortlever, R. Effect of Different Alkali Metal Cations on the Oxygen Evolution Activity and Battery Capacity of Nickel Electrodes in Concentrated Hydroxide Electrolytes. Electrochim. Acta 2022, 415, 140255. [Google Scholar] [CrossRef]
- Wang, X.; Ding, R.; Ren, X.; Shi, L.; Li, Q.; Yang, Y.; Wang, H.; Wang, M.; Wang, L.; Lv, B. Micron Iron Oxide Particles with Thickness-Controllable Carbon Coating for Ni-Fe Battery. Electrochim. Acta 2019, 299, 800–808. [Google Scholar] [CrossRef]
- Qiu, W.; Xiao, H.; He, W.; Li, Y.; Tong, Y. A Flexible Rechargeable Quasi-Solid-State Ni-Fe Battery Based on Surface Engineering Exhibits High Energy and Long Durability. Inorg. Chem. Front. 2018, 5, 1805–1815. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Liu, Y.; Meng, T.; Ma, L.; Xu, M.; Zhu, J.; Li, C.M.; Jiang, J. Phase Transition Triggers Explosion-like Puffing Process to Make Popcorn-Inspired All-Conductive Anodes for Superb Aqueous Rechargeable Batteries. ACS Appl. Mater. Interfaces 2019, 11, 42365–42374. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liu, Z.; Zhang, Z.; Xiao, B.; Jin, Y. Metal Oxide Nanostructures Generated from In Situ Sacrifice of Zinc in Bimetallic Textures as Flexible Ni/Fe Fast Battery Electrodes. Chem.—Asian J. 2017, 12, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lu, X.; Deng, P.; Hu, W.; Sun, Z.; Liu, X.X.; Sun, X. Morphology Engineering of Electro-Deposited Iron Oxides for Aqueous Rechargeable Ni/Fe Battery Applications. Chem. Eng. J. 2018, 354, 672–679. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Y.; Huang, S.; Zhang, B.; Lim, Y.V.; Sim, G.J.; Valdivia, Y.; Alvarado, P.; Ge, Q.; Yang, H.Y. 3D Printed Compressible Quasi-Solid-State Nickel-Iron Battery. ACS Nano 2020, 14, 9675–9686. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, D. Modification of Corroded Metal (Ni or Fe) Foam for High-Performance Rechargeable Alkaline Ni/Fe Batteries. ChemElectroChem 2020, 7, 3098–3105. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Wang, Z.; Zhang, J.; Zhang, Q.; Shum, P.P.; Wei, L. All-Metal Phosphide Electrodes for High-Performance Quasi-Solid-State Fiber-Shaped Aqueous Rechargeable Ni-Fe Batteries. ACS Appl. Mater. Interfaces 2020, 12, 12801–12808. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Chen, X.; Xiao, T.; Tan, X.; Xiang, P.; Jiang, L. Enhancing Electrochemical Performance of Fe2O3 via in Situ Sulfurization and Carbon Coating Modification for Nickel-Iron Rechargeable Batteries. Electrochim. Acta 2018, 290, 332–338. [Google Scholar] [CrossRef]
- Guo, C.X.; Li, C.M. Molecule-Confined FeOx Nanocrystals Mounted on Carbon as Stable Anode Material for High Energy Density Nickel-Iron Batteries. Nano Energy 2017, 42, 166–172. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Mao, X.; Shen, P.K.; MacFarlane, D.R. High-Capacity and High-Rate Ni-Fe Batteries Based on Mesostructured Quaternary Carbon/Fe/FeO/Fe3O4 Hybrid Material. iScience 2021, 24, 102547. [Google Scholar] [CrossRef]
- Xiao, Y.; Ding, R.; Cui, X.; Wang, H.; Wang, L.; Wang, M.C.; Niu, B.; Lv, B. Micron-Sized Iron Oxide Functionalized with Hydrophobic Mesoporous Sheets for the Ni-Fe Battery. Sustain. Energy Fuels 2021, 5, 1756–1766. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Wang, Z.; Wang, Z.; Kang, L.; Qi, M.; Chen, M.; Liu, W.; Gong, W.; Lu, W.; et al. Rational Construction of Self-Standing Sulfur-Doped Fe2O3 Anodes with Promoted Energy Storage Capability for Wearable Aqueous Rechargeable NiCo-Fe Batteries. Adv. Energy Mater. 2020, 10, 2001064. [Google Scholar] [CrossRef]
- Li, X.; Dong, H.; Song, Y.; Zhang, L.; Wang, S. Copper Sulfate as Additive for Fe3O4 Electrodes of Rechargeable Ni–Fe Batteries. Asia-Pac. J. Chem. Eng. 2020, 15, e2475. [Google Scholar] [CrossRef]
- Tawonezvi, T.; Bladergroen, B.J.; John, J. Development of FeCuₓ/FeS/Graphite Composite Electrode Materials for Iron-Based Alkaline Batteries. Int. J. Electrochem. Sci. 2020, 15, 12428–12446. [Google Scholar] [CrossRef]
- Ma, L.; Xu, Y.; Liu, Y.; Zhang, H.; Yao, J.; Li, N.; Li, C.M.; Zhou, W.; Jiang, J. Smart Colloid-Assisted Technique Prompts the Evolution of Bamboo Wastes into Nanometal-Inlaid Carbon Microfibers for Sustainable Ni-Fe Batteries. ACS Sustain. Chem. Eng. 2019, 7, 17919–17928. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Mi, M.; Kong, W.; Ge, Y.; Ma, J.; Hu, J. Fe1−xS/Reduced Graphene Oxide Composite as Anode Material for Aqueous Rechargeable Ni/Fe Batteries. J. Alloys Compd. 2019, 800, 99–106. [Google Scholar] [CrossRef]
- Ye, L.; Feng, L.; Zhao, L.; Yang, X.; Zhao, Y.; Guo, Z.; Liu, X.; He, D. Constructing Efficient Quasi-Solid-State Alkaline Ni–Fe Battery Based on Ni–Mn Hydroxides/Ni3S2 and FeOOH@RGO Electrodes. J. Mater. Sci. Mater. Electron. 2019, 30, 13076–13089. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Meng, T.; Ma, L.; Zhu, J.; Xu, M.; Li, C.M.; Zhou, W.; Jiang, J. Mass Production of Metallic Fe@Carbon Nanoparticles with Plastic and Rusty Wastes for High-Capacity Anodes of Ni–Fe Batteries. ACS Sustain. Chem. Eng. 2019, 7, 10995–11003. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Liu, C.; Sun, J.; Guo, J.; Zhang, J.; Zhou, Z.; He, B.; Pan, Z.; Yao, Y. Flexible All-Solid-State Fiber-Shaped Ni-Fe Batteries with High Electrochemical Performance. J. Mater. Chem. A 2019, 7, 520–530. [Google Scholar] [CrossRef]
- Shangguan, E.; Fu, S.; Wu, S.; Wang, Q.; Wu, C.; Li, J.; Cai, X.; Chang, Z.; Wang, Z.; Li, Q.; et al. Evolution of Spent LiFePO₄ Powders into LiFePO4/C/FeS Composites: A Facile and Smart Approach to Make Sustainable Anodes for Alkaline Ni-Fe Secondary Batteries. J. Power Sources 2018, 403, 38–48. [Google Scholar] [CrossRef]
- Huang, L.; Yang, J.; Liu, P.; Zhu, D.; Chen, Y. Copper/Iron Composite Anode Prepared by in Situ Co-Precipitation with Excellent High-Rate and Low-Temperature Performance for Rechargeable Nickel-Iron Battery. Int. J. Electrochem. Sci. 2018, 13, 7045–7056. [Google Scholar] [CrossRef]
- Jin, Z.; Li, P.; Jin, Y.; Xiao, D. Superficial-Defect Engineered Nickel/Iron Oxide Nanocrystals Enable High-Efficient Flexible Fiber Battery. Energy Storage Mater. 2018, 13, 160–167. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, Y.; Li, L.; Zhu, J.; Xu, M.; Li, C.M. Smart Magnetic Interaction Promotes Efficient and Green Production of High-Quality Fe3O4@Carbon Nanoactives for Sustainable Aqueous Batteries. ACS Sustain. Chem. Eng. 2018, 6, 757–765. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, C.; Chang, K.; Shangguan, E.; Li, B.; Chang, Z. Synthesis of NiS Coated Fe3O4 Nanoparticles as High-Performance Positive Materials for Alkaline Nickel-Iron Rechargeable Batteries. Int. J. Hydrogen Energy 2017, 42, 24939–24947. [Google Scholar] [CrossRef]
- Luo, H.; Wang, B.; Li, Y.; Liu, T.; You, W.; Wang, D. Core-Shell Structured Fe3O4@NiS Nanocomposite as High-Performance Anode Material for Alkaline Nickel-Iron Rechargeable Batteries. Electrochim. Acta 2017, 231, 479–486. [Google Scholar] [CrossRef]
- Li, L.; Zhu, J.; Niu, Y.; Xiong, Z.; Jiang, J. Metallic Fe Nanoparticles Trapped in Self-Adapting Nanoreactors: A Novel High-Capacity Anode for Aqueous Ni-Fe Batteries. Chem. Commun. 2017, 53, 12661–12664. [Google Scholar] [CrossRef]
- Li, F.; Pan, Y.; Wang, H.; Huang, X.; Zhang, Q.; Peng, Z.; Tang, Y. Core-Bishell Fe-Ni@Fe3O4@C Nanoparticles as an Advanced Anode for Rechargeable Nickel-Iron Battery. J. Electrochem. Soc. 2017, 164, A1333–A1338. [Google Scholar] [CrossRef]
- Li, P.; Jin, Z.; Xiao, D. A Phytic Acid Etched Ni/Fe Nanostructure Based Flexible Network as a High-Performance Wearable Hybrid Energy Storage Device. J. Mater. Chem. A 2017, 5, 3274–3283. [Google Scholar] [CrossRef]
- Guan, C.; Zhao, W.; Hu, Y.; Ke, Q.; Li, X.; Zhang, H.; Wang, J. High-Performance Flexible Solid-State Ni/Fe Battery Consisting of Metal Oxides Coated Carbon Cloth/Carbon Nanofiber Electrodes. Adv. Energy Mater. 2016, 6, 1601034. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, F.; Wang, J.; Su, L.; Wu, Y.; Wang, L. Enhanced Electrochemical Performances of FeOx-Graphene Nanocomposites as Anode Materials for Alkaline Nickel-Iron Batteries. RSC Adv. 2014, 4, 15394–15399. [Google Scholar] [CrossRef]
- Kao, C.Y.; Chou, K. Sen Iron/Carbon-Black Composite Nanoparticles as an Iron Electrode Material in a Paste Type Rechargeable Alkaline Battery. J. Power Sources 2010, 195, 2399–2404. [Google Scholar] [CrossRef]
- Arunkumar, P.S.; Maiyalagan, T.; Kheawhom, S.; Mao, S.; Jiang, Z. Effect of Carbon Material Additives on Hydrogen Evolution at Rechargeable Alkaline Iron Battery Electrodes. Mater. Sci. Energy Technol. 2021, 4, 236–241. [Google Scholar] [CrossRef]
- Kao, C.Y.; Tsai, Y.R.; Chou, K. Sen Synthesis and Characterization of the Iron/Copper Composite as an Electrode Material for the Rechargeable Alkaline Battery. J. Power Sources 2011, 196, 5746–5750. [Google Scholar] [CrossRef]
- Liu, J.; Chen, M.; Zhang, L.; Jiang, J.; Yan, J.; Huang, Y.; Lin, J.; Fan, H.J.; Shen, Z.X. A Flexible Alkaline Rechargeable Ni/Fe Battery Based on Graphene Foam/Carbon Nanotubes Hybrid Film. Nano Lett. 2014, 14, 7180–7187. [Google Scholar] [CrossRef]
- Qin, Z.; Song, Y.; Shi, H.Y.; Li, C.; Guo, D.; Sun, X.; Liu, X.X. Heterojunction Induced Activation of Iron Oxide Anode for High-Power Aqueous Batteries. Chem. Eng. J. 2020, 400, 125874. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Yang, S.; Chen, L.; Zhao, P.; Yan, Q. Anode Electrodeposition of Fe/Fe3O4 Composite on Carbon Fabric as a Negative Electrode for Flexible Ni−Fe Batteries. ChemElectroChem 2021, 8, 4817–4825. [Google Scholar] [CrossRef]
- Hao, S.; Xing, Y.; Hou, P.; Zhao, G.; Huang, J.; Qiu, S.; Xu, X. Rational Construction of Phosphate Layer to Optimize Cu-Regulated Fe3O4 as Anode Material with Promoted Energy Storage Performance for Rechargeable Ni-Fe Batteries. J. Mater. Sci. Technol. 2022, 108, 133–141. [Google Scholar] [CrossRef]
- Tang, H.; Liu, M.; Kong, L.; Wang, X.; Lei, Y.; Li, X.; Hou, Y.; Chang, K.; Chang, Z. The Synergistic Effect of MoS2 and NiS on the Electrical Properties of Iron Anodes for Ni-Fe Batteries. Nanomaterials 2022, 12, 3472. [Google Scholar] [CrossRef]
- Li, W.; Xu, Q.; Kong, D.; Yang, H.; Xu, T.; Wang, H.; Zang, J.; Huang, S.; Li, X.; Wang, Y. High-Performance Flexible Quasi-Solid-State Aqueous Nickel-Iron Battery Enabled by MOF-Derived N-Doped Carbon Hollow Nanowall Arrays. Chem. Eng. J. 2023, 452, 139251. [Google Scholar] [CrossRef]
- Rubel, R.I.; Ali, M.H.; Jafor, M.A.; Alam, M.M.; Rubel, R.I.; Ali, M.H.; Jafor, M.A.; Alam, M.M. Carbon Nanotubes Agglomeration in Reinforced Composites: A Review. AIMS Mater. Sci. 2019, 6, 756–780. [Google Scholar] [CrossRef]
- Weinrich, H.; Gehring, M.; Tempel, H.; Kungl, H.; Eichel, R.A. Impact of the Charging Conditions on the Discharge Performance of Rechargeable Iron-Anodes for Alkaline Iron–Air Batteries. J. Appl. Electrochem. 2018, 48, 451–462. [Google Scholar] [CrossRef]
- Vijayamohanan, K.; Shukla, A.K.; Sathyanarayana, S. Formation Mechanism of Porous Alkaline Iron Electrodes. J. Power Sources 1990, 32, 329–339. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L. Synthesis of Highly Substitutional Nitrogen Doped TiO2 via Oxygen Vacancy Mediated Strategy for Ultrafast-Charging Lithium Ion Storage. Chem. Eng. J. 2022, 431, 134164. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Z.; Yu, H.; Zhang, X.; Liu, T.; Xia, M.; Zheng, R.; Shui, M.; Shu, J. Heteroatom-Doped Carbon-Based Materials for Lithium and Sodium Ion Batteries. Energy Storage Mater. 2020, 32, 65–90. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, M.; Ni, J.; Li, L. Ultrastable Sodium Storage in MoO3 Nanotube Arrays Enabled by Surface Phosphorylation. ACS Appl. Mater. Interfaces 2019, 11, 37761–37767. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Deng, Y.; Yu, J.; Zheng, L.; Du, L.; Song, H.; Liao, S. From Chlorella to Nestlike Framework Constructed with Doped Carbon Nanotubes: A Biomass-Derived, High-Performance, Bifunctional Oxygen Reduction/Evolution Catalyst. ACS Appl. Mater. Interfaces 2017, 9, 32168–32178. [Google Scholar] [CrossRef]
- Li, X.; Guan, G.; Zhang, K.; Gao, G.; Xiang, J. Flexible CoFe2O4 Nanoparticles/N-Doped Carbon Nanofibers Membrane as Self-Standing Anode for Lithium-Ion Batteries. J. Alloys Compd. 2023, 946, 169397. [Google Scholar] [CrossRef]
- Li, T.; Dong, H.; Shi, Z.; Liu, W.; Li, X.; Yue, H.; Yin, Y.; Li, B.; Yang, S. Fabrication of FeP-Based Composite via N-Doping into Amorphous Carbon and Graphene-Protecting Strategy for Lithium-Ion Batteries. J. Solid State Chem. 2023, 320, 123831. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Wu, K.; Wang, X.; Xiong, D.; He, M. Fe₃C Encapsulated in N-Doped Carbon as Potassium Ion Battery Anode with High Capacity and Long-Term Cycling Performance. J. Alloys Compd. 2022, 910, 164845. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, W.; Huang, M.; Xu, Y.; Xie, W.; Sun, H.; Zhao, Y. Iron Vacancies Engineering of FexC@NC Hybrids toward Enhanced Lithium-Ion Storage Properties. Nanotechnology 2022, 33, 135401. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, G.; Lei, L.; Yuan, H.; Nan, Y.; Zhou, Y. One-Step Synthesis of Fe Nanoparticles Wrapped in N-Doped Carbon Nanohorn Microspheres as High-Performance Electromagnetic Wave Absorber. Ceram. Int. 2022, 48, 18338–18347. [Google Scholar] [CrossRef]
- Liang, X.; Xiao, H.; Zhang, T.; Zhang, F.; Gao, Q. A Unique Nanocomposite with FeCo Nanoalloy Anchored on S, N Co-Doped Carbonaceous Matrix for High Bifunctional Oxygen Reduction Reaction/Oxygen Evolution Reaction Electrocatalytic Property in Zn-Air Battery. J. Colloid Interface Sci. 2023, 630, 170–181. [Google Scholar] [CrossRef]
- McFarland, E.W.; Metiu, H. Catalysis by Doped Oxides. Chem. Rev. 2013, 113, 4391–4427. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Xiao, J.; Tian, X.; Yuan, S. Enhancing Electrochemical Performance of Ultrasmall Fe2O3-Embedded Carbon Nanotubes via Combusting-Induced High-Valence Dopants. J. Mater. Sci. Technol. 2023, 134, 142–150. [Google Scholar] [CrossRef]
- Li, C.; Lin, Y.; Li, X.; Li, Z.; Luo, P.; Jin, Y.; Li, Z. Effect of Co-Doping Concentration on α-Fe2O3/Graphene as Anode Materials for Lithium Ion Batteries. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 660, 130681. [Google Scholar] [CrossRef]
- Cai, J.; Xu, L.; Tang, X.; Kong, L.; Wang, J.; Wang, R.; Li, X.; Xie, Q.; Mao, K.; Pan, H. Role of Lithium Doping on α-Fe₂O₃ Photoanode for Enhanced Photoelectrochemical Water Oxidation. J. Alloys Compd. 2022, 915, 165349. [Google Scholar] [CrossRef]
- Chen, J.; Wang, K.; Sun, M.; Ni, W.; Wang, M.; Yu, M.; Yu, D.; Ling, M.; Liang, C. Superior Lithium Storage in Fe2O3 Nanoporous Arrays Endowed by Surface Phosphorylation and Bulk Phosphorous Doping. Appl. Surf. Sci. 2022, 604, 154668. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Q.; Gong, J.; Liu, Z.; Tao, G.; Zhao, J.; Chen, L.; Li, W.; Wei, X.; Ni, L.; et al. Confine, Defect, and Interface Manipulation of Fe₃Se₄/3D Graphene Targeting Fast and Stable Potassium-Ion Storage. Small 2023, 19, 2206400. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, K.; Wang, D.; Zhang, Y.; Li, L.; Liang, C. Core-Shell Structured Fe₂P@TiO2/CNF Anode Nanocomposite Fibers for Efficient Lithium/Sodium-Ion Storage. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 653, 129953. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, S.; Yang, C.; Lei, Z. Ni, Co, and Yb Cation Co-Doping and Defect Engineering of FeOOH Nanorods as an Electrocatalyst for the Oxygen Evolution Reaction. Inorg. Chem. 2023, 62, 1719–1727. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C. jun A Comprehensive Review of Pt Electrocatalysts for the Oxygen Reduction Reaction: Nanostructure, Activity, Mechanism and Carbon Support in PEM Fuel Cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Hu, C.; Liu, D.; Xiao, Y.; Dai, L. Functionalization of Graphene Materials by Heteroatom-Doping for Energy Conversion and Storage. Prog. Nat. Sci. Mater. Int. 2018, 28, 121–132. [Google Scholar] [CrossRef]
- Hirsch, A.; Vostrowsky, O. Functionalization of Carbon Nanotubes. In Functional Molecular Nanostructures; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; Volume 245, pp. 193–237. [Google Scholar] [CrossRef]
- Tserengombo, B.; Jeong, H.; Dolgor, E.; Delgado, A.; Kim, S. Effects of Functionalization in Different Conditions and Ball Milling on the Dispersion and Thermal and Electrical Conductivity of MWCNTs in Aqueous Solution. Nanomaterials 2021, 11, 1323. [Google Scholar] [CrossRef] [PubMed]
- Vacchi, I.A.; Ménard-Moyon, C.; Bianco, A. Chemical Functionalization of Graphene Family Members. Phys. Sci. Rev. 2017, 2, 20160103. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Veisi, P.; Samiei, E.; Saeidfirozeh, H.; Tourchi Moghadam, M.T.; Di Bartolomeo, A. Transition-Metal Dichalcogenides in Electrochemical Batteries and Solar Cells. Micromachines 2023, 14, 691. [Google Scholar] [CrossRef]
- Chen, B.; Chao, D.; Liu, E.; Jaroniec, M.; Zhao, N.; Qiao, S.Z. Transition Metal Dichalcogenides for Alkali Metal Ion Batteries: Engineering Strategies at the Atomic Level. Energy Environ. Sci. 2020, 13, 1096–1131. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ren, Z.; Singh, G. Beyond Graphene Anode Materials for Emerging Metal Ion Batteries and Supercapacitors. Nano-Micro Lett. 2018, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Batteries. Joule 2018, 2, 2235–2259. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Zhou, Y.; Qiao, S.Z.; Fan, H.J. Metal Organic Framework (MOF) in Aqueous Energy Devices. Mater. Today 2021, 48, 270–284. [Google Scholar] [CrossRef]
- Posada, J.O.G.; Hall, P.J. Towards the Development of Safe and Commercially Viable Nickel–Iron Batteries: Improvements to Coulombic Efficiency at High Iron Sulphide Electrode Formulations. J. Appl. Electrochem. 2016, 46, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Wada, Y.; Maeda, Y.; Suzuki, T.; Sakamoto, H.; Tan, W.K.; Kawamura, G.; Muto, H.; Matsuda, A. Electrochemical Performance of Sintered Porous Negative Electrodes Fabricated with Atomized Powders for Iron-Based Alkaline Rechargeable Batteries. J. Electrochem. Soc. 2017, 164, A2049–A2055. [Google Scholar] [CrossRef]
- Zhang, E.Q.; Tang, L. Rechargeable Concrete Battery. Buildings 2021, 11, 103. [Google Scholar] [CrossRef]
- Shangguan, E.; Li, F.; Li, J.; Chang, Z.; Li, Q.; Yuan, X.Z.; Wang, H. FeS/C Composite as High-Performance Anode Material for Alkaline Nickel-Iron Rechargeable Batteries. J. Power Sources 2015, 291, 29–39. [Google Scholar] [CrossRef]
- Yang, B.; Malkhandi, S.; Manohar, A.K.; Surya Prakash, G.K.; Narayanan, S.R. Organo-Sulfur Molecules Enable Iron-Based Battery Electrodes to Meet the Challenges of Large-Scale Electrical Energy Storage. Energy Environ. Sci. 2014, 7, 2753–2763. [Google Scholar] [CrossRef]
- Mitra, D.; Rajan, A.S.; Irshad, A.; Narayanan, S.R. High Performance Iron Electrodes with Metal Sulfide Additives. J. Electrochem. Soc. 2021, 168, 030518. [Google Scholar] [CrossRef]
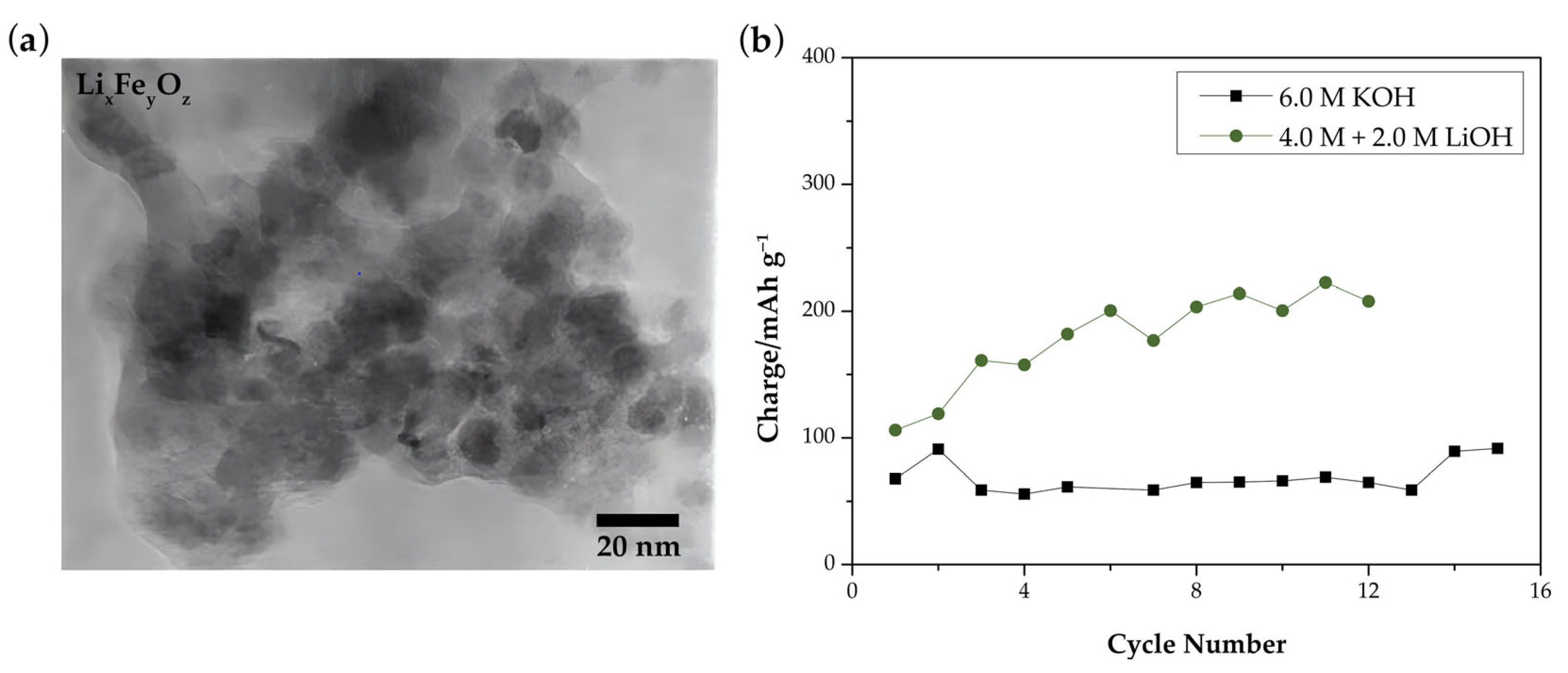
- Casellato, U.; Comisso, N.; Mengoli, G. Effect of Li Ions on Reduction of Fe Oxides in Aqueous Alkaline Medium. Electrochim. Acta 2006, 51, 5669–5681. [Google Scholar] [CrossRef]
- Hang, B.T.; Thang, D.H. Effect of Additives on the Electrochemical Properties of Fe2O3/C Nanocomposite for Fe/Air Battery Anode. J. Electroanal. Chem. 2016, 762, 59–65. [Google Scholar] [CrossRef]
- El Haleem, S.M.A.; El Aal, E.E.A. Electrochemical Behaviour of Iron in Alkaline Sulphide Solutions. Corros. Eng. Sci. Technol. 2008, 43, 173–178. [Google Scholar] [CrossRef]
- Tian, B.; Światowska, J.; Maurice, V.; Zanna, S.; Seyeux, A.; Marcus, P. The Effect of Na2S Additive in Alkaline Electrolyte on Improved Performances of Fe-Based Air Batteries. Electrochim. Acta 2018, 259, 196–203. [Google Scholar] [CrossRef]
- Malkhandi, S.; Yang, B.; Manohar, A.K.; Prakash, G.K.S.; Narayanan, S.R. Self-Assembled Monolayers of n-Alkanethiols Suppress Hydrogen Evolution and Increase the Efficiency of Rechargeable Iron Battery Electrodes. J. Am. Chem. Soc. 2013, 135, 347–353. [Google Scholar] [CrossRef]
- McKerracher, R.D.; Figueredo-Rodriguez, H.A.; Alegre, C.; Aricò, A.S.; Baglio, V.; Ponce de León, C. Improving the Stability and Discharge Capacity of Nanostructured Fe2O3/C Anodes for Iron-Air Batteries and Investigation of 1-Octhanethiol as an Electrolyte Additive. Electrochim. Acta 2019, 318, 625–634. [Google Scholar] [CrossRef]
- Deyab, M.A.; Mohsen, Q. Improved Battery Capacity and Cycle Life in Iron-Air Batteries with Ionic Liquid. Renew. Sustain. Energy Rev. 2021, 139, 110729. [Google Scholar] [CrossRef]
- Chamoun, M.; Skårman, B.; Vidarsson, H.; Smith, R.I.; Hull, S.; Lelis, M.; Milcius, D.; Noréus, D. Stannate Increases Hydrogen Evolution Overpotential on Rechargeable Alkaline Iron Electrodes. J. Electrochem. Soc. 2017, 164, A1251–A1257. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.T.; Yoon, C.S.; Lee, Y.S.; Sun, Y.K. Synthesis and Structural Changes of LixFeyOz Material Prepared by a Solid-State Method. J. Power Sources 2004, 134, 88–94. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of SiB4. Am. Chem. Soc. 1996, 43, 1533–1554. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Laibinis, P.E. Wet Chemical Approaches to The Characterization of Organic Surfaces: Self-Assembled Monolayers, Wetting, and The Physical-Organic Chemistry of The Solid-Liquid Interface. Langmuir 1990, 6, 87–96. [Google Scholar] [CrossRef]
- Qi, H.; Ren, Y.; Guo, S.; Wang, Y.; Li, S.; Hu, Y.; Yan, F. High-Voltage Resistant Ionic Liquids for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 591–600. [Google Scholar] [CrossRef]
- Kong, L.; Tang, H.; Wang, X.; Lei, Y.; Li, B.; Chang, K.; Chang, Z. Study on the in Situ Sulfidation and Electrochemical Performance of Spherical Nickel Hydroxide. Int. J. Hydrogen Energy 2021, 46, 30079–30089. [Google Scholar] [CrossRef]
- Yu, D.; Chen, C.; Si, Y.; Zhou, S.; Wang, L. Study on the Electronic Structure of Nickel Hydroxide by Quantum Chemical DV-Xα Calculation. Chin. Sci. Bull. 2008, 53, 40–45. [Google Scholar] [CrossRef]
- Chen, J.; Bradhurst, D.H.; Dou, S.X.; Liu, H.K. Nickel Hydroxide as an Active Material for the Positive Electrode in Rechargeable Alkaline Batteries. J. Electrochem. Soc. 1999, 146, 3606–3612. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhang, Y.; Wang, B.; Peng, H. The Recent Progress of Nitrogen-Doped Carbon Nanomaterials for Electrochemical Batteries. J. Mater. Chem. A 2018, 6, 12932–12944. [Google Scholar] [CrossRef]
- Tian, R.; Duan, H. Graphene-Based Materials for Advanced Lithium-Ion Batteries. In Handbook of Graphene: Energy, Healthcare, and Environmental Applications; Ozkan, C., Ozkan, U., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; Volume 5. [Google Scholar]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Indira, L.; Dixit, M.; Kamath, P.V. Electrosynthesis of Layered Double Hydroxides of Nickel with Trivalent Cations. J. Power Sources 1994, 52, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Freitas, M.B.J.G. Nickel Hydroxide Powder for NiO·OH/Ni(OH)2 Electrodes of the Alkaline Batteries. J. Power Sources 2001, 93, 163–173. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, T.; Kim, K. A Study of the Electrochemical Redox Behavior of Electrochemically Precipitated Nickel Hydroxides Using Electrochemical Quartz Crystal Microbalance. J. Electrochem. Soc. 1997, 144, 1537–1543. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, T.; Zhang, S.; Li, D.; Tang, P.; Alonso-Vante, N.; Feng, Y. Template-Free Synthesis of Three-Dimensional NiFe-LDH Hollow Microsphere with Enhanced OER Performance in Alkaline Media. J. Energy Chem. 2019, 33, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Zhu, C.; Li, C.; Zhang, S.; Zhang, X.; Chen, Y. Highly Stable Three-Dimensional Nickel–Iron Oxyhydroxide Catalysts for Oxygen Evolution Reaction at High Current Densities. Electrochim. Acta 2017, 245, 770–779. [Google Scholar] [CrossRef]
- Rosey, R. Westinghouse Nickel-Iron Battery Performance; Westinghouse Electric Corporation: Pittsburgh, PA, USA, 1981. [Google Scholar]
- Samy, M.M.; Emam, A.; Tag-Eldin, E.; Barakat, S. Exploring Energy Storage Methods for Grid-Connected Clean Power Plants in Case of Repetitive Outages. J. Energy Storage 2022, 54, 105307. [Google Scholar] [CrossRef]
- Kumar, P.P.; Saini, R.P. Optimization of an Off-Grid Integrated Hybrid Renewable Energy System with Different Battery Technologies for Rural Electrification in India. J. Energy Storage 2020, 32, 101912. [Google Scholar] [CrossRef]
- Kumar, P.P.; Suresh, V.; Jasinski, M.; Leonowicz, Z. Off-grid Rural Electrification in India Using Renewable Energy Resources and Different Battery Technologies with a Dynamic Differential Annealed Optimization. Energies 2021, 14, 5866. [Google Scholar] [CrossRef]
- Kumar, P.P.; Nuvvula, R.S.S.; Hossain, M.A.; Shezan, S.K.A.; Suresh, V.; Jasinski, M.; Gono, R.; Leonowicz, Z. Optimal Operation of an Integrated Hybrid Renewable Energy System with Demand-Side Management in a Rural Context. Energies 2022, 15, 5176. [Google Scholar] [CrossRef]
- Kumar, P.P.; Saini, R.P. Optimization of an Off-Grid Integrated Hybrid Renewable Energy System with Various Energy Storage Technologies Using Different Dispatch Strategies. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 1–29. [Google Scholar] [CrossRef]
- Barakat, S.; Ibrahim, H.; Elbaset, A.A. Multi-Objective Optimization of Grid-Connected PV-Wind Hybrid System Considering Reliability, Cost, and Environmental Aspects. Sustain. Cities Soc. 2020, 60, 102178. [Google Scholar] [CrossRef]
- Eteiba, M.B.; Barakat, S.; Samy, M.M.; Wahba, W.I. Optimization of an Off-Grid PV/Biomass Hybrid System with Different Battery Technologies. Sustain. Cities Soc. 2018, 40, 713–727. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, X.; Li, X.; Zhu, Y.; Liang, J.; Qian, Y. Surfactant Widens the Electrochemical Window of an Aqueous Electrolyte for Better Rechargeable Aqueous Sodium/Zinc Battery. J. Mater. Chem. A 2017, 5, 730–738. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.; Lee, T.; Kim, E.; An, M.; Park, J.; Cho, J.; Son, Y. Investigation of Mass Loading of Cathode Materials for High Energy Lithium-Ion Batteries. Electrochem. Commun. 2023, 147, 107437. [Google Scholar] [CrossRef]
- Manohar, A.K.; Malkhandi, S.; Yang, B.; Yang, C.; Surya Prakash, G.K.; Narayanan, S.R. A High-Performance Rechargeable Iron Electrode for Large-Scale Battery-Based Energy Storage. J. Electrochem. Soc. 2012, 159, A1209–A1214. [Google Scholar] [CrossRef]






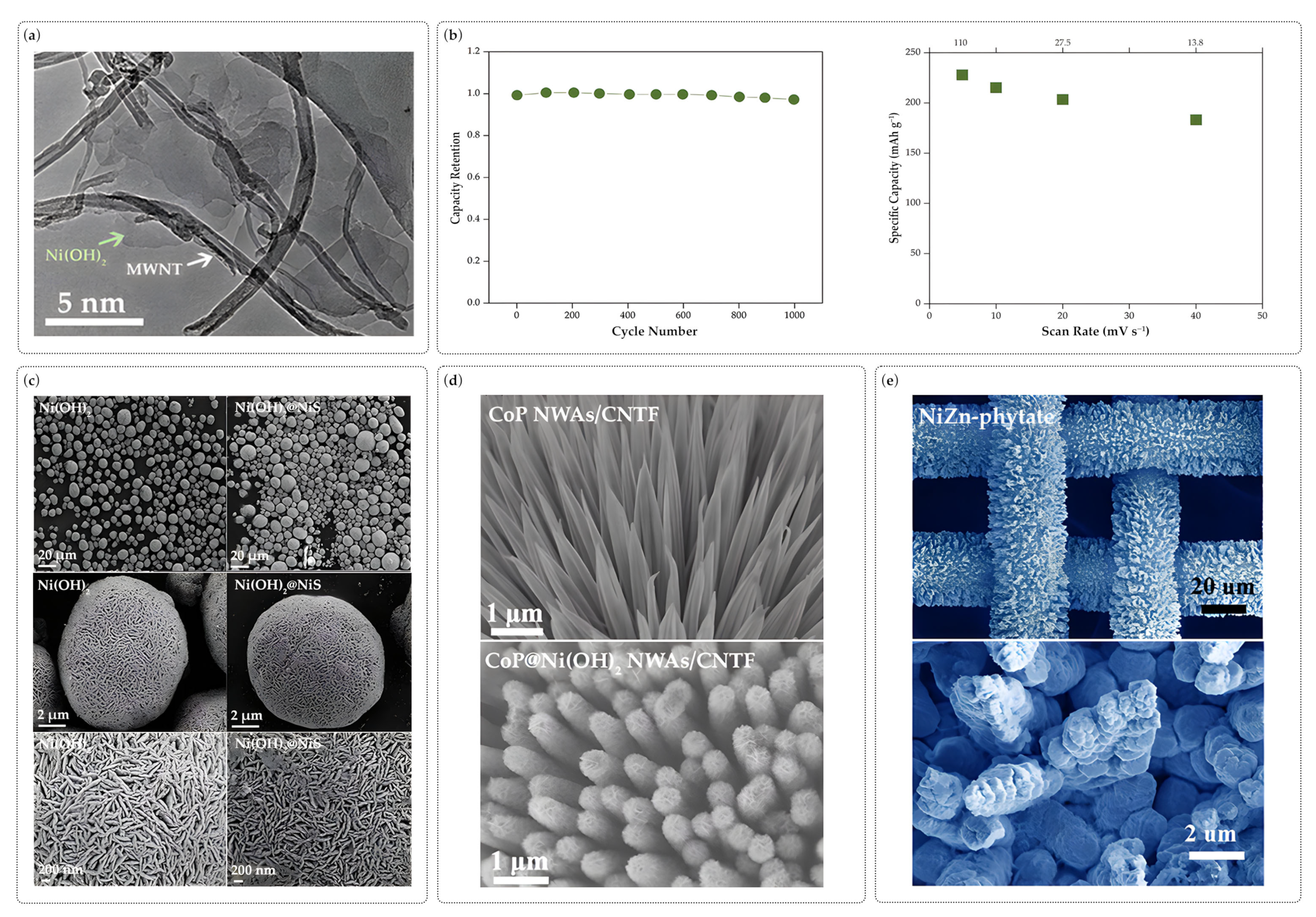

| Anode Material | Preparation | Electrolyte | Voltage Window | Specific Capacity | Capacity Retention | Ref. |
|---|---|---|---|---|---|---|
| FeOx/graphene nanocomposites | Solution phase reaction and gas phase annealing | 1 M KOH | −1.3 to −0.5 V vs. SCE | 377 mAh g−1 at 5 mV s−1 | - | [16] |
| Fe3O4@C microspheres | Spray drying | 6 M KOH +15 g L−1 LiOH +0.1% Na2S | −1.2 to −0.4 V vs. Hg/HgO | 556.7 mAh g−1 at 1.2 A g−1 | 92% after 100 cycles at 0.3 A g−1 | [21] |
| Core–shell Fe3O4@MoO2 -C | Electrodeposition | 3 M KOH | −1.2 to 0 V vs. Hg/HgO | 155.6 mAh g−1 at 1.1 A g−1 | - | [22] |
| Core–shell C-Fe | Aerosol-assisted spray pyrolysis | 1 M KOH | −1.6 to 0 V vs. SCE | 208 mAh g−1 at 1 A g−1 | 93% after 2000 cycles at 4 A g−1 | [27] |
| Core–shell Fe@C | Pyrolysis | 1 M KOH | −1.6 to 0 V vs. SCE | 314 mAh g−1 at 1 A g−1 | 90% after 1000 cycles at 10 mV s−1 | [28] |
| α-Fe2O3@PPy nanorods/CNTF | Hydrothermal synthesis and annealing | 3 M KOH | −1.6 to 0 V vs. Ag/AgCl | 0.62 Ah cm−3 at 1 A cm−3 | - | [29] |
| Fe3O4@C mesoporous nanoarrays | Self-generated sacrificial template | 2 M KOH | −1.2 to −0.2 V vs. SCE | 292.4 mAh g−1 at 5 mA cm−2 | 90.8% after 5000 cycles at 30 mA cm−2 | [30] |
| Fe/Cu nanocomposites | Cathodic decomposition | 8 M KOH + 0.05M Na2S | −1.3 to 0 V vs. Hg/HgO | ~350 mAh g−1 Fe at 0.05 A g−1 | - | [31] |
| 3D-Fe/Fe2O3@C | Annealing | 1 M KOH | −1.2 to 0 V vs. Hg/HgO | 3.07 mAh cm−2 at 6 mA cm−2 | 80% after 20,000 cycles at 100 mA cm−2 | [63] |
| Fe3O4-NGC (vines-grapes-like) | Hydrothermal synthesis | 6 M KOH | −1.4 to 0 V vs. Ag/AgCl | 308.1 mAh g−1 at 1 A g−1 | - | [65] |
| GE@CNT-Fe-Fe3C/CF | Microwave-assisted synthesis | 1 M KOH | −1.2 to −0.2 V vs. Hg/HgO | 1.74 mAh cm−3 at 1 mA cm−1 | 106.5% after 20,000 cycles at 8 mA cm−2 | [66] |
| Fe3O4@Ni3S2 microspheres | Hydrothermal synthesis | 6 M KOH | −1.0 to −0.4 V vs. Hg/HgO | ~481.2 mAh g−1 at 1.2 A g−1 | 95.1% after 100 cycles at 1.2 A g−1 | [67] |
| Fe3O4@C (micrododecahedral) | Hydrothermal synthesis | 6 M KOH | −1.4 to −0.4 V vs. Hg/HgO | 419 mAh g−1 at 0.6 A g−1 | 40% after 500 cycles at 0.3 A g−1 | [76] |
| α-Fe2O3 nanorods | Hydrothermal synthesis | 1 M KOH | −1.2 to −0.2 V vs. SCE | 308.9 mAh g−1 at 1 A g−1 | 96.1% after 2000 cycles at 5 mV s−1 | [77] |
| FeÌC nanopopcorns | Solid-state synthesis | 3 M KOH | −1.4 to −0.3 V vs. Ag/AgCl | ~480.5 mAh g−1 at ~1 A g−1 | ~95.8% after 4000 cycles at 2 A g−1 | [78] |
| FeOx nanowires | Electrodeposition | 6 M KOH | −1.4 to −0.4 V vs. Hg/HgO | 0.32 mAh cm−2 at 20 mA cm−2 | - | [79] |
| FeOOH nanorods | Electrodeposition | 1 M KOH | −1.2 to −0.4 V vs. Hg/HgO | 184 mAh g−1 at 2 A g−1 | 87.5% after 5000 cycles at 5 A g−1 | [80] |
| rGO/CNTs@α-Fe2O3 | Hydrothermal synthesis | 3 M KOH | −1.2 to 0 V vs. Ag/AgCl | ~486.5 mAh g−1 at 10 mA cm−2 | 93.6% after 5000 cycles at 200 mA cm−2 | [81] |
| C@Fe-based/Bi/FF (flower-like) | Calcination | 6 M KOH | −1.4 to 0 V vs. Hg/HgO | 2.83 mAh cm−2 at 10 mA cm−2 | 92.86% after 25,000 cycles at 10 mA cm−2 | [82] |
| FeP nanowire arrays/CNTF | Hydrothermal synthesis | 1 M KOH | −1.25 to 0 V vs. Ag/AgCl | 0.634 mAh cm−2 at 2 mA cm−2 | 89% after 4000 cycles at 10 mV s−1 | [83] |
| Fe2O3-S@C nanorods | Hydrothermal synthesis and plasma enhanced chemical vapor deposition | 1 M KOH | −1.2 to 0 V vs. Hg/HgO | 224 mAh g−1 at 0.8 mA cm−2 | 92.2% after 100 cycles at 1 mA cm−2 | [84] |
| mc-FeOx/C nanocrystals | Molecular confinement | 1 M KOH | −1.4 to 0 V vs. SCE | 370.2 mAh g−1 at 2 A g−1 | 93.5% after 1000 cycles at 2 A g−1 | [85] |
| Mesostructured carbon/Fe/FeO/Fe3O4 | Solid-state reaction | 4 M KOH + 2% LiOH | −1.2 to 0 V vs. Hg/HgO | 604 mAh g−1 at 1 A g−1 | ~77.3% after 1000 cycles at 1 A g−1 | [86] |
| Fe3O4 mesoporous sheets | Etching | 6 M KOH + 0.5 M LiOH | −1.2 to −0.4 V vs. Hg/HgO | 205.7 mAh g−1 at 0.2 A g−1 | 60.2% after 250 cycles at 0.2 A g−1 | [87] |
| S-Fe2O3/CNTF nanowire arrays | Hydrothermal synthesis and in situ sulfurization | - | −1.4 to 0 V vs. Ag/AgCl | 0.81 mAh cm−2 at 4 mA cm−2 | 92.4% after 5000 cycles at 40 mA cm−2 | [88] |
| Fe3O4 + 5% CuSO4•5H2O | Commercial powders | 6 M KOH | −1.3 to −0.3 V vs. Hg/HgO | 221 mAh g−1 at 0.9 A g−1 | 85.7% after 50 cycles at 0.225 A g−1 | [89] |
| FeCu0.25/15%FeS/5%C (spherical polyhedral) | Autocatalytic Cu and electroless Fe deposition | 6 M KOH + 1 M LiOH | −1.4 to −0.4 V vs. Hg/HgO | ~278 mAh g−1 at 0.6 A g−1 | 85% after 40 cycles at 0.1 A g−1 | [90] |
| Fe@CMFs (nanometal-inlaid fibers) | Colloid-assisted synthesis | 3 M KOH | −1.35 to −0.35 V vs. Ag/AgCl | 348 mAh g−1 at 2 A g−1 | ~80% after 4000 cycles at 2 A g−1 | [91] |
| Fe1-xS@rGO nanosheets | Hydrothermal synthesis | 1 M KOH | −1.2 to −0.2 V vs. SCE | ~270 mAh g−1 at 1 A g−1 | 83.3% after 100 cycles at 1 A g−1 | [92] |
| FeOOH@rGO nanorods | Hydrothermal synthesis | 3 M KOH | −1.0 to 0 V vs. SCE | 180 C g−1 at 1 A g−1 | 66% after - cycles at 10 A g−1 | [93] |
| Core–shell Fe@C nanoparticles | One-step chemical vapor deposition | 3 M KOH | −1.4 to −0.35 V vs. Ag/AgCl | ~405.2 mAh g−1 at 1 A g−1 | ~91.9% after 4000 cycles at 5 A g−1 | [94] |
| S-α-Fe2O3@CNTF (spindle-like) | Hydrothermal synthesis | 3 M KOH | −1.2 to −0.4 V vs. Hg/HgO | 556.7 mAh g−1 at 1.2 A g−1 | 76.8% after 6000 cycles at 20 mA cm−2 | [95] |
| LiFe3PO4/C/FeS spheres | Ball milling | 6 M KOH | −1.2 to −0.4 V vs. Hg/HgO | ~232.9 mAh g−1 at 0.2 C | 75.9% after 300 cycles at 1 C | [96] |
| CuxFe3-xO4 (honeycomb-like) | Co-precipitation method | 8 M KOH + 0.05 M Na2S | −1.2 to −0.8 V vs. Hg/HgO | 230 mAh g−1 at 1 C | - | [97] |
| Mn-Fe2O3 nanoplates | Electrodeposition and electrochemical activation | 1 M KOH | −1.2 to −0.4 V vs. Hg/HgO | 174 mAh g−1 at 8.3 mA cm−2 | ~97% after 10,000 cycles at 20 mV s−1 | [98] |
| Core–shell Fe3O4@C | Polymeric process and magnetic purification | 3 M KOH | −1.35 to −0.4 V vs. Ag/AgCl | ~285 mAh g−1 at ~0.8 A g−1 | - | [99] |
| NiS-Fe3O4 nanoparticles | Co-precipitation method | 6 M KOH + 15 g L−1 + 0.1% Na2S | −1.25 to −0.4 V vs. Hg/HgO | 472.7 mAh g−1 at 1.2 A g−1 | 85.9% after 100 cycles at 0.3 A g−1 | [100] |
| Core–shell Fe3O4@NiS | Sedimentation-oxygenation method | 6 M KOH | −1.4 to −0.4 V vs. Hg/HgO | 346 mAh g−1 at 1.5 C | 85% after 50 cycles at 0.15 C | [101] |
| Core–shell Fe@TCNRs | Gas-phase reactions | 3 M KOH | −1.4 to −0.3 V vs. Ag/AgCl | ~491 mAh g−1 at ~1 A g−1 | ~97.8% after 4000 cycles at 4 A g−1 | [102] |
| Core–bishell Fe-Ni@Fe3O4@C | Pyrolysis | 6 M KOH + 0.35 M LiOH + 0.05 M Na2S | −1.2 to −0.2 V vs. Hg/HgO | 320 mAh g−1 at 1 A g−1 | ~91.7% after 100 cycles at 1 A g−1 | [103] |
| 3D-nanoarrays/Fe-phytate | Electrodeposition and phytic acid treatment | 6 M KOH | −1.3 to −0.3 V vs. Hg/HgO | 223.6 mAh g−1 at 3.85 A g−1 | 114% after 3000 cycles at 76.92 A g−1 | [104] |
| Core–shell 3D hierarchical CC/CF@Fe3O4 (needle-like) | Electrodeposition and hydrothermal growth | 2 M KOH | −1.2 to 0 V vs. Hg/HgO | 207.6 mAh g−1 at 5 mA cm−2 | 83.4% after 1000 cycles at 5 mA cm−2 | [105] |
| FeOx-graphene nanocomposites + 5 wt% Bi2O3 | Solid-state synthesis | 8 M KOH + 1 M LiOH | −1.4 to −0.4 V vs. Hg/HgO | 408.5 mAh g−1 at 1 A g−1 | 90% after 100 cycles at 1 A g−1 | [106] |
| Core–shell Fe/C nanoparticles | Chemical reduction | 8 M KOH + 1 M LiOH | −1.4 to −0.4 V vs. Ag/AgCl | 600 mAh g−1Fe at 0.2 A g−1 | ~66.7% after 40 cycles at 0.2 A g−1 | [107] |
| Fe2O3/MWCNT | Commercial powders | 6 M KOH | −1.2 to −0.3 V vs. Hg/HgO | 350 mAh g−1 at 10 mA cm−2 | - | [108] |
| Core–shell Fe/Cu nanoparticles | Chemical reduction | 8 M KOH + 1 M LiOH | −1.4 to −0.2 V vs. Ag/AgCl | 800 mAh g−1 Fe at 0.2 A g−1 | - | [109] |
| GF/CNTs/Fe2O3 nanocomposites | Hydrothermal synthesis and annealing | 6 M KOH | −1.2 to −0.2 V vs. SCE | 278 mAh g−1 at 1 A g−1 | 96% after 1000 cycles at 10 mV s−1 | [110] |
| Fe3O4/OG (3D nanostructures) | Electrodeposition | 6 M KOH | −1.2 to −0.5 V vs. Hg/HgO | 634 mAh g−1 at 5 A g−1 | ~80% after 2000 cycles at 5 A g−1 | [111] |
| Fe/Fe3O4@CF (rock-like nanocomposites) | Electrodeposition and annealing | 1 M KOH | −1.4 to 0 V vs. Hg/HgO | 163.03 mAh g−1 at 3.43 A g−1 | 91.7% after 6000 cycles at 16 mA cm−2 | [112] |
| Cu-doped Fe3O4 (3D coral-like structure) | Hydrothermal synthesis | 1 M KOH | −1.0 to 0 V vs. Hg/HgO | 117.5 mAh g−1 at 1 A g−1 | 97.6% after 10,000 cycles at 5 A g−1 | [113] |
| Fe3O4/MoS2(5%)/NiS(5%) nanospheres | Chemical co-precipitation | 6 M NaOH + 0.6 M LiOH | −1.25 to −0.4 V vs. Hg/HgO | 639.8 mAh g−1 at 1.2 A g−1 | 84.9% after 100 cycles at 1.2 A g−1 | [114] |
| α-Fe2O3@NC-CTs (3D hollow nanowall arrays) | Seed-assisted hydrothermal synthesis and annealing | 3 M KOH | −1.4 to 0 V vs. Ag/AgCl | 249.9 mAh cm−2 at 5 mA cm−2 | ~81.9% after 5000 cycles at 30 mA cm−2 | [115] |
| Cathode Material | Preparation | Electrolyte | Voltage Window | Specific Capacity | Capacity Retention | Ref. |
|---|---|---|---|---|---|---|
| Ni(OH)2@MWCNTs nanoplates | Hydrothermal synthesis | 1 M KOH | 0 to 0.6 V vs. SCE | 228 mAh g−1 at ~7.46 A g−1 | - | [16] |
| α-Ni(1-x)Fex(OH)2 (layered double hydroxides) | Co-precipitation method | 6 M KOH | 0 to 0.6 V vs. Hg/HgO | 724 mAh g−1 at 0.1 C | 90% after 1000 cycles | [18] |
| Ni0.95Mg0.5(OH)2 | Co-precipitation and hydrothermal treatment | 4 M KOH | 0 to 0.7 V vs. Hg/HgO | 178.4 mAh g−1 at 250 mA g−1 | 88% after 100 cycles | [50] |
| Ni0.95Mn0.5(OH)2 | 181.2 mAh g−1 at 250 mA g−1 | 85% after 100 cycles | ||||
| Ni(OH)2 nanosheets/FGS | Liquid phase epitaxy | 1 M KOH | 0 to 0.5 V vs. SCE | 228.3 mAh g−1 at 1 A g−1 | 86.1% after 5000 cycles at 10 A g−1 | [64] |
| NiCo2O4-CNT-S-PEGm (flower-like microspheres with honeycomb-like ion buffer reservoir) | Thiol-ene click modification and solvothermal method with sintering | 6 M KOH | −0.1 to 0.5 V vs. Ag/AgCl | 195.7 mAh g−1 at 0.5 A g−1 | 84.9% after 2000 cycles at 4 A g−1 | [65] |
| P-NiCo2O4/CC (nanowires array) | Electrolytic deposition and phytic acid treatment | 6 M KOH | −0.2 to 0.8 V vs. Hg/HgO | 247 mAh g−1 at 1 A g−1 | 96.5% after 2000 cycles | [77] |
| NiOx nanoflakes on CC-CF substrate | Chemical deposition with post-annealing | 2 M KOH | −0.2 to 0.6 V vs. Hg/HgO | 39.7 mAh g−1 at 1.68 A g−1 | - | [79] |
| NiO-NiF2/NF nanoparticles | Calcination | 6 M KOH | 0 to 0.7 V vs. Hg/HgO | 1.24 mAh cm−2 at 5 mA cm−2; 0.69 mAh cm−2 at 40 mA cm−2 | 84% after 1000 cycles at 40 mA cm−2 | [82] |
| NiCoP nanosheet arrays/CNTF | Hydrothermal synthesis and phosphating | 1 M KOH | 0 to 0.5 V vs. Ag/AgCl | 0.55 mAh cm−2 at 2 mA cm−2 | 90% after 4000 cycles | [83] |
| Ni-Mn Hydroxide nanosheets/Ni3S2 | Hydrothermal synthesis | 3 M KOH | −0.1 to 0.6 V vs. SCE | 385.17 mAh g−1 at 1 A g−1 | 79% after 5000 cycles | [93] |
| Core–shell CoP Nanowire arrays@Ni(OH)2 nanosheets | Hydrothermal synthesis and phosphating | 3 M KOH | 0 to 0.6 V vs. Ag/AgCl | 0.689 mAh cm−2 at 2 mA cm−2 | 85.4% after 15,000 cycles at 20 mA cm−2 | [95] |
| NiZn-Phytate (on 3D-Cu nanowire network) | In situ sacrifice of Zn via cyclic voltammetry | 6 M KOH | −0.2 to 0.8 V vs. Hg/HgO | 462.02 mAh g−1 at 4.5 A g−1 | 76% after 7000 cycles at 45.45 A g−1 | [104] |
| Ni(OH)2 nanospheres@NiS | Atmospheric reflux in water | 6 M KOH + 0.63 M LiOH | 0 to 0.8 V vs. Hg/HgO | 168.7 mAh g−1 at 5 C | 87.3% after 100 cycles at 5 C; 90% after 100 cycles at 10 C | [165] |
| Anode Material | Cathode Material | Operating Voltage | Specific Capacity | Energy Density | Capacity Retention | Capacity Retention after Mechanical Deformation | Ref. |
|---|---|---|---|---|---|---|---|
| α-Fe2O3@ PPy NRs/ CNTF | CoNiO2@ Ni(OH)2 NWAs/ CNTF | 1.6 V | 70 mAh cm−3 at 1 A cm−3 | 15.47 mWh cm−3 at 228.2 mW cm−3 | 70.6% after 6000 cycles at 5 A cm−3 | 90.1% after 2500 cycles at 1 A cm−3 and 180° bending | [29] |
| 3D-Fe/ Fe2O3@C | NiCo2O4 | 2.0 V | 1.02 mAh cm−2 at 5 mA cm−2 | 15.53 mWh cm−3 at 30.48 W cm−3 | 84.8% after 20,000 cycles at 50 mA cm−2 | 100% after bending at 180° | [63] |
| GE@ CNT-Fe-Fe3C/CF | GE@ NiCoO/CF | 1.8 V | 1.61 mAh cm−3 at 0.3 mA | 1.28 mWh cm−3 at 18.32 mW cm−3 | 94.7% after 20,000 cycles at 5 mA | 100% after bending at 180° | [66] |
| Fe2O3 nano rods | P-NiCo2O4 nanowires | 1.6 V | 134.5 mAh g−1 at 1 A g−1 | 227 Wh kg−1 at 0.59 kW kg−1 | 82.7% after 2600 cycles at 2 A g−1 | 99% after bending | [77] |
| FeOx nano wires | NiOx nanoflakes | 1.0 V | 6.91 mAh cm−3 at 5 mA cm−3 | 7.40 Wh cm−3 at 0.27 W cm−3 | 75% after 1000 cycles at 20 mA cm−2 | 100% after twisting | [79] |
| 3D rGO/ CNTs@ α-Fe2O3 | 3D rGO/ CNTs@ Ni(OH)2 | 2.0 V | 206.4 mAh g−1 at 10 mA cm−2 | 28.1 mWh cm−3 at 10.6 mW cm−3 | 91.3% after 10,000 cycles at 300 mA cm−2 | 84.5% after 2000 cycles at 200 mA cm−3, 60% compression strain | [81] |
| FeP NWAs/ CNTF | NiCoP NSAs/CNTF | 1.6 V | 0.294 mAh cm−2 at 2 mA cm−2 | 235.6 μWh cm−2 | 88% after 4000 cycles at 2 mA cm−2 | 100% after 3000 times bending at 180° | [83] |
| S-Fe2O3 NWAs/ CNTFs | ZNCO@ Ni(OH)2 NWAs | 1.05 V | 0.46 mAh cm−2 at 2 mA cm−2 | 67.32 mWh cm−3 at 591.12 mW cm−3 | 90.2% after 3000 cycles at 10 mA cm−2 | 94.8% after 4000 cycles at 2 mA cm−2 and 90° bending | [88] |
| S-α-Fe2O3/ CNTF | CoP@ Ni(OH)2 NWAs/ CNTF | 1.6 V | 0.203 mAh cm−2 at 3 mA cm−2 | 81 mWh cm−3 at 1200 mW cm−3 | 85.3% after 3000 cycles at 30 mA cm−2 | 92.4% after 3000 cycles at 6 mA cm−2 and 90° bending | [95] |
| Mn-Fe2O3 | Mn-NiO | 2.0 V | 46 mAh cm−3 at 2.5 A cm−3 | 61 mWh cm−3 at 48.4 W cm−3 | 91.5% after 30,000 cycles at 20 mA cm−3 | 96.2% at 10 A cm−3 after 200 times bending at 90° | [98] |
| 3D-NA/Fe-phytate | 3D-NA/ NiZn-phytate | 2.0 V | 225.75 mAh g−1 at 0.46 A g−1 | 185.33 Wh kg−1 at 15.93 kW kg−1 | 86% after 8000 cycles at 20.83 A g−1 | 100% after bending at 180° | [104] |
| CC-CF@ Fe3O4 | CC-CF@NiO | 1.6 V | 88.2 mAh g−1 at 5 mA cm−2 | 94.5 Wh kg−1 at 1.2 kW kg−1 | 80.7% after 2600 cycles | 97% after twisting and bending | [105] |
| GF/CNTs/Fe2O3 | GF/CNTs/ Ni(OH)2 | 1.6 V | 118 mAh g−1 at 0.3 A g−1 | 100.7 Wh kg−1 at 287 Wh kg−1 | 89.1% after 1000 cycles at 1.3 A g−1 | 100% after bending at 60° | [110] |
| Fe/Fe3O4@CF | Ni(OH)2@CF | 1.7 V | 0.466 mAh cm−2 at 10 mA cm−2 | 1.14 mWh cm−3 at 34 mW cm−3 | 86.2% after 6000 cycles at 16 mA cm−2 | 100% after bending at 180° | [112] |
| Cu-doped Fe3O4 | NiCoS4 | 1.6 V | 49.02 mAh g−1 at 1 A g−1 | 45.6 Wh kg−1 at 12 kW kg−1 | 96.8% after 10,000 cycles at 5 A g−1 | - | [113] |
| α-Fe2O3@ NC/CTs | Ni(OH)2@NC/CTs | 2.10 V | 94.8 mAh g−1 at 5 mA cm−2 | 155.4 Wh kg−1 at 1.75 kW kg−1 | 86.1% after 10,000 cycles at 30 mA cm−2 | 100% after bending at 135° | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abarro, J.M.E.; Gavan, J.N.L.; Loresca, D.E.D.; Ortega, M.A.A.; Esparcia, E.A., Jr.; Paraggua, J.A.D.R. A Tale of Nickel-Iron Batteries: Its Resurgence in the Age of Modern Batteries. Batteries 2023, 9, 383. https://doi.org/10.3390/batteries9070383
Abarro JME, Gavan JNL, Loresca DED, Ortega MAA, Esparcia EA Jr., Paraggua JADR. A Tale of Nickel-Iron Batteries: Its Resurgence in the Age of Modern Batteries. Batteries. 2023; 9(7):383. https://doi.org/10.3390/batteries9070383
Chicago/Turabian StyleAbarro, Justine Marie E., Jon Nyner L. Gavan, Daniel Eldrei D. Loresca, Maura Andrea A. Ortega, Eugene A. Esparcia, Jr., and Julie Anne D. R. Paraggua. 2023. "A Tale of Nickel-Iron Batteries: Its Resurgence in the Age of Modern Batteries" Batteries 9, no. 7: 383. https://doi.org/10.3390/batteries9070383
APA StyleAbarro, J. M. E., Gavan, J. N. L., Loresca, D. E. D., Ortega, M. A. A., Esparcia, E. A., Jr., & Paraggua, J. A. D. R. (2023). A Tale of Nickel-Iron Batteries: Its Resurgence in the Age of Modern Batteries. Batteries, 9(7), 383. https://doi.org/10.3390/batteries9070383









