Research Progress of Shear Thickening Electrolyte Based on Liquid–Solid Conversion Mechanism
Abstract
1. Introduction
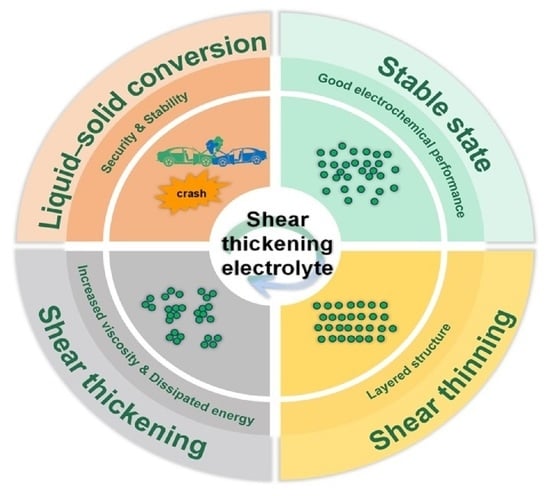
2. Shear Thickening Mechanism
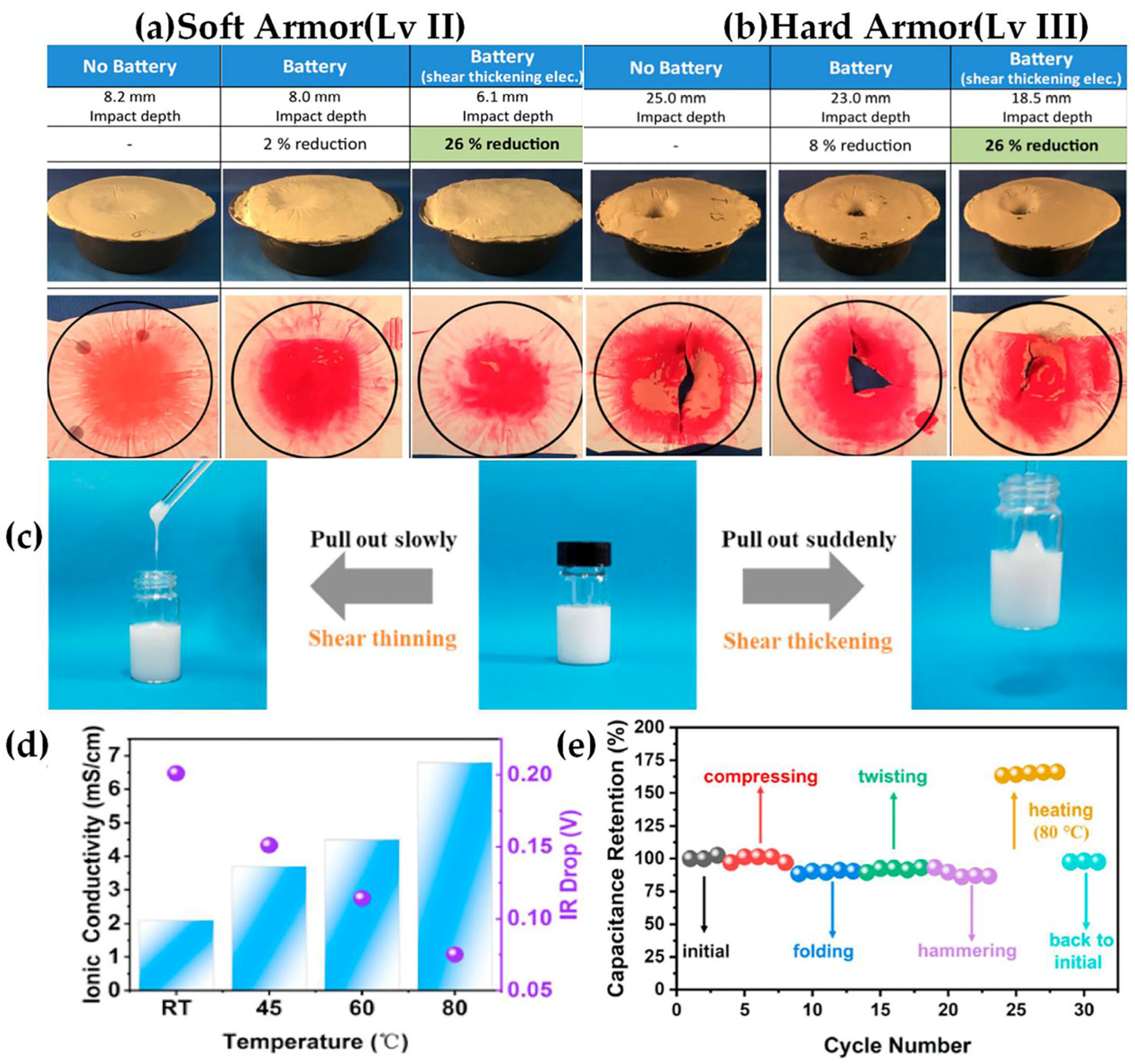
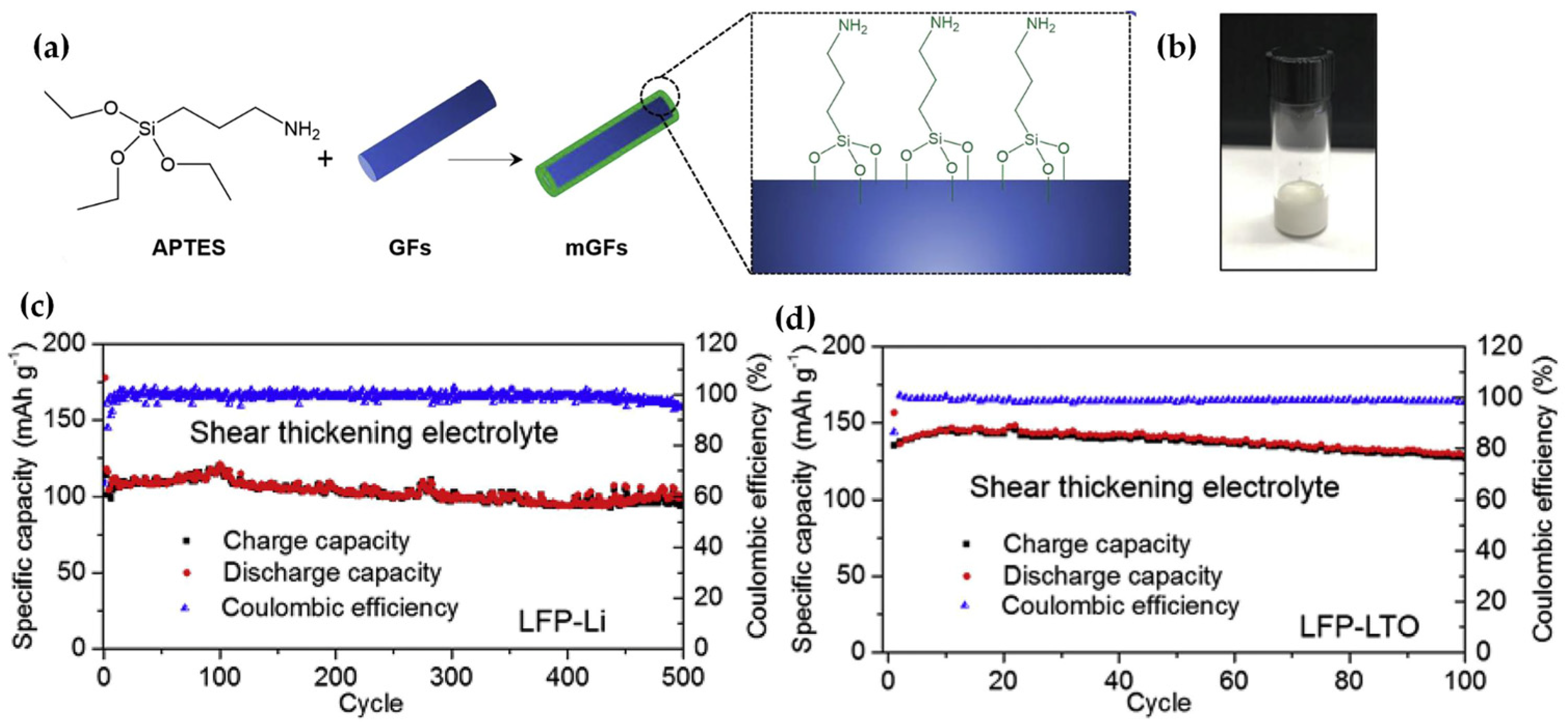
3. Shear Thickening Electrolyte
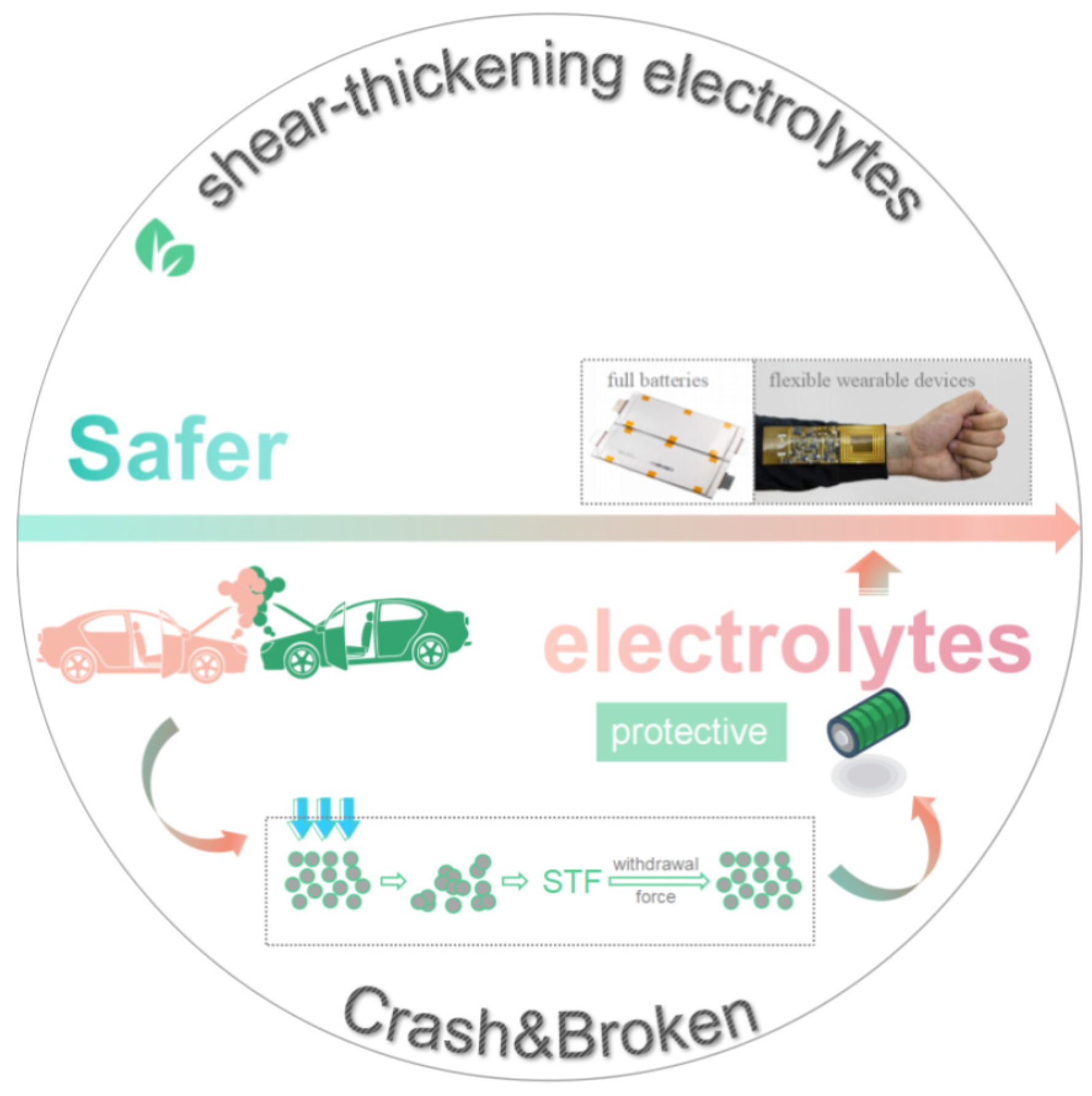
4. Conclusions
5. Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer Electrolytes for Lithium-Ion Batteries: State of the Art and Perspectives. ChemSusChem 2015, 8, 2154–2175. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.G.; Ramesh, R.; Prem Kumar, T. Safety Mechanisms in Lithium-Ion Batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Ming, J.; Cao, Z.; Wu, Y.; Wahyudi, W.; Wang, W.; Guo, X.; Cavallo, L.; Hwang, J.-Y.; Shamim, A.; Li, L.-J.; et al. New Insight on the Role of Electrolyte Additives in Rechargeable Lithium Ion Batteries. ACS Energy Lett. 2019, 4, 2613–2622. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for Lithium-Ion Battery Safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Chen, M.; Huang, Q.; Weng, J.; Wang, Z.; Wang, J. A Review on the Thermal Hazards of the Lithium-Ion Battery and the Corresponding Countermeasures. Appl. Sci. 2019, 9, 2483. [Google Scholar] [CrossRef]
- Arora, S.; Shen, W.; Kapoor, A. Review of Mechanical Design and Strategic Placement Technique of a Robust Battery Pack for Electric Vehicles. Renew. Sustain. Energy Rev. 2016, 60, 1319–1331. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Fan, H.; Wang, H. Flame-Retardant Electrolyte Solution for Dual-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 1363–1370. [Google Scholar] [CrossRef]
- Deng, K.; Zeng, Q.; Wang, D.; Liu, Z.; Wang, G.; Qiu, Z.; Zhang, Y.; Xiao, M.; Meng, Y. Nonflammable Organic Electrolytes for High-Safety Lithium-Ion Batteries. Energy Storage Mater. 2020, 32, 425–447. [Google Scholar] [CrossRef]
- Hyung, Y.E.; Vissers, D.R.; Amine, K. Flame-Retardant Additives for Lithium-Ion Batteries. J. Power Sources 2003, 119, 383–387. [Google Scholar] [CrossRef]
- Xiang, H.; Jin, Q.; Chen, C.H.; Ge, X.; Guo, S.; Sun, J. Dimethyl Methylphosphonate-Based Nonflammable Electrolyte and High Safety Lithium-Ion Batteries. J. Power Sources 2007, 174, 335–341. [Google Scholar] [CrossRef]
- Kizilel, R.; Sabbah, R.; Selman, J.R.; Al-Hallaj, S. An Alternative Cooling System to Enhance the Safety of Li-Ion Battery Packs. J. Power Sources 2009, 194, 1105–1112. [Google Scholar] [CrossRef]
- Kizilel, R.; Lateef, A.; Sabbah, R.; Farid, M.; Selman, J.; Al-Hallaj, S. Passive Control of Temperature Excursion and Uniformity in High-Energy Li-Ion Battery Packs at High Current and Ambient Temperature. J. Power Sources 2008, 183, 370–375. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Howlett, P.C.; Kar, M.; Passerini, S.; Pringle, J.M.; Ohno, H.; Watanabe, M.; Yan, F.; Zheng, W.; et al. Ionic Liquids and Their Solid-State Analogues as Materials for Energy Generation and Storage. Nat. Rev. Mater. 2016, 1, 15005. [Google Scholar] [CrossRef]
- Shu, K.; Wang, C.; Li, W.; Bussell, T.; Ding, J. Electrolytes with Reversible Switch between Liquid and Solid Phases. Curr. Opin. Electrochem. 2020, 21, 297–302. [Google Scholar] [CrossRef]
- Veith, G.M.; Armstrong, B.L.; Wang, H.; Kalnaus, S.; Tenhaeff, W.E.; Patterson, M.L. Shear Thickening Electrolytes for High Impact Resistant Batteries. ACS Energy Lett. 2017, 2, 2084–2088. [Google Scholar] [CrossRef]
- Zhang, S.S. A Review on Electrolyte Additives for Lithium-Ion Batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Haregewoin, A.M.; Wotango, A.S.; Hwang, B.-J. Electrolyte Additives for Lithium Ion Battery Electrodes: Progress and Perspectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
- Barnes, H. Shear-Thickening (“Dilatancy”) in Suspensions of Nonaggregating Solid Particles Dispersed in Newtonian Liquids. J. Rheol. 1989, 33, 329–366. [Google Scholar] [CrossRef]
- Wyart, M.; Cates, M.E. Discontinuous Shear Thickening without Inertia in Dense Non-Brownian Suspensions. Phys. Rev. Lett. 2014, 112, 098302. [Google Scholar] [CrossRef]
- Bag, N.; Bhattacharyya, S. Electroosmotic Flow of a Non-Newtonian Fluid in a Microchannel with Heterogeneous Surface Potential. J. Non-Newton. Fluid Mech. 2018, 259, 48–60. [Google Scholar] [CrossRef]
- Wagner, N.J.; Brady, J.F. Shear Thickening in Colloidal Dispersions. Phys. Today 2009, 62, 27–32. [Google Scholar] [CrossRef]
- Olsson, P.; Teitel, S. Critical Scaling of Shear Viscosity at the Jamming Transition. Phys. Rev. Lett. 2007, 99, 178001. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Cheng, C.-F.; Zhou, L.; Zou, F.; Liang, W.; Wang, M.; Zhu, Y. A Shear Thickening Fluid Based Impact Resistant Electrolyte for Safe Li-Ion Batteries. J. Power Sources 2019, 423, 297–304. [Google Scholar] [CrossRef]
- Ye, Y.; Xiao, H.; Reaves, K.; McCulloch, B.; Mike, J.F.; Lutkenhaus, J.L. Effect of Nanorod Aspect Ratio on Shear Thickening Electrolytes for Safety-Enhanced Batteries. ACS Appl. Nano Mater. 2018, 1, 2774–2784. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Walls, H.J.; Khan, S.A. Rheology of Silica Dispersions in Organic Liquids: New Evidence for Solvation Forces Dictated by Hydrogen Bonding. Langmuir 2000, 16, 7920–7930. [Google Scholar] [CrossRef]
- Li, X.; Cao, H.; Gao, S.; Pan, F.; Weng, L.; Song, S.; Huang, Y. Preparation of Body Armour Material of Kevlar Fabric Treated with Colloidal Silica Nanocomposite. Plast. Rubber Compos. 2008, 37, 223–226. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Gong, X. The Rheology of Shear Thickening Fluid (Stf) and the Dynamic Performance of an Stf-Filled Damper. Smart Mater. Struct. 2008, 17, 035027. [Google Scholar] [CrossRef]
- Gürgen, S.; Kuşhan, M.C.; Li, W. Shear Thickening Fluids in Protective Applications: A Review. Prog. Polym. Sci. 2017, 75, 48–72. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zhou, J.; Wu, J.; Liu, S.; Sang, M.; Liu, B.; Pan, Y.; Gong, X. Multi-Functional Stf-Based Yarn for Human Protection and Wearable Systems. Chem. Eng. J. 2023, 453, 139869. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Pan, Y.; Zhou, J.; Zhang, J.; Liu, S.; Fan, Z.; Deng, H.; Hu, Y.; Gong, X. An Impact-Resistant and Flame-Retardant Cnts/Stf/Kevlar Composite with Conductive Property for Safe Wearable Design. Compos. Part A Appl. Sci. Manuf. 2023, 168, 107489. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, R.; Zhao, Y.; Liu, H.; Zhang, D.; Shi, X.; Zhang, B.; Liu, C.; Shen, C. Conductive Mxene/Cotton Fabric Based Pressure Sensor with Both High Sensitivity and Wide Sensing Range for Human Motion Detection and E-Skin. Chem. Eng. J. 2021, 420, 127720. [Google Scholar] [CrossRef]
- Zarei, M.; Aalaie, J. Application of Shear Thickening Fluids in Material Development. J. Mater. Res. Technol. 2020, 9, 10411–10433. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Gong, X. Study on Magnetorheological Shear Thickening Fluid. Smart Mater. Struct. 2008, 17, 015051. [Google Scholar] [CrossRef]
- Williams, T.H.; Day, J.; Pickard, S. Surgical and Medical Garments and Materials Incorporating Shear Thickening Fluids. U.S. Patent 12/440,086, 27 April 2009. [Google Scholar]
- Liu, B.; Shelley, M.; Zhang, J. Focused Force Transmission through an Aqueous Suspension of Granules. Phys. Rev. Lett. 2010, 105, 188301. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Sang, M.; Shu, Q.; Zhang, J.; Xuan, S.; Gong, X. A Safeguarding and High Temperature Tolerant Organogel Electrolyte for Flexible Solid-State Supercapacitors. J. Power Sources 2021, 505, 230083. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Sheng, W.; Wang, F.; Zhang, J.; Zhu, F.; Zhuang, X.; Jordan, R.; Schmidt, O.G.; Feng, X. Thermoswitchable on-Chip Microsupercapacitors: One Potential Self-Protection Solution for Electronic Devices. Energy Environ. Sci. 2018, 11, 1717–1722. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, W.; Huang, Y.; Zhi, C. Proton-Insertion-Enhanced Pseudocapacitance Based on the Assembly Structure of Tungsten Oxide. ACS Appl. Mater. Interfaces 2014, 6, 18901–18910. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Jiang, Y.; Su, Q.; Zheng, J. Facile Surface Modification of Silica Nanoparticles with a Combination of Noncovalent and Covalent Methods for Composites Application. Compos. Sci. Technol. 2014, 104, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Su, Q.; Zheng, J. Use of Unmodified Sio2 as Nanofiller to Improve Mechanical Properties of Polymer-Based Nanocomposites. Compos. Sci. Technol. 2013, 89, 52–60. [Google Scholar] [CrossRef]
- Tripathi, A.M.; Su, W.-N.; Hwang, B.J. In Situ Analytical Techniques for Battery Interface Analysis. Chem. Soc. Rev. 2018, 47, 736–851. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of Inorganic Solid-State Electrolytes for Batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Isaac, J.A.; Devaux, D.; Bouchet, R. Dense Inorganic Electrolyte Particles as a Lever to Promote Composite Electrolyte Conductivity. Nat. Mater. 2022, 21, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Yi, Q.; Wang, X.; Camacho, R.A.P.; Kungl, H.; Eichel, R.A.; Lu, L.; Zhang, H. Ion Conduction in Composite Polymer Electrolytes: Potential Electrolytes for Sodium-Ion Batteries. ChemSusChem 2023, 16, e202202152. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, J.; Tang, Z.; Wang, H.; Lian, H.; Zhang, J.; Cao, C.-n. Physicochemical Properties of Poly (Ethylene Oxide)-Based Composite Polymer Electrolytes with a Silane-Modified Mesoporous Silica Sba-15. Electrochim. Acta 2009, 54, 3490–3494. [Google Scholar] [CrossRef]
- Chen-Yang, Y.; Wang, Y.; Chen, Y.; Li, Y.; Chen, H.; Chiu, H. Influence of Silica Aerogel on the Properties of Polyethylene Oxide-Based Nanocomposite Polymer Electrolytes for Lithium Battery. J. Power Sources 2008, 182, 340–348. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.; Persi, L.; Scrosati, B. Nanocomposite Polymer Electrolytes for Lithium Batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Jayanthi, S.; Shenbagavalli, S.; Muthuvinayagam, M.; Sundaresan, B. Effect of Nano Tio2 on the Thransport, Structural and Thermal Properties of Pema-Nai Solid Polymer Electrolytes for Energy Storage Devices. Mater. Sci. Eng. B 2022, 285, 115942. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Rychagov, A.Y.; Sosenkin, V.E.; Baskakov, S.A.; Kabachkov, E.N.; Shulga, Y.M. Supercapacitor Properties of Rgo-Tio2 Nanocomposite in Two-Component Acidic Electrolyte. Materials 2022, 15, 7856. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Karlinsey, R.L.; Ritter, K.; Joo, C.G.; Stein, B.; Zwanziger, J.W. Design of Organic–Inorganic Solid Polymer Electrolytes: Synthesis, Structure, and Properties. J. Mater. Chem. 2004, 14, 1812–1820. [Google Scholar] [CrossRef]
- Kumar, B.; Fellner, J. Polymer–Ceramic Composite Protonic Conductors. J. Power Sources 2003, 123, 132–136. [Google Scholar] [CrossRef]
- Charradi, K.; Ahmed, Z.; Aranda, P.; Chtourou, R. Silica/Montmorillonite Nanoarchitectures and Layered Double Hydroxide-Speek Based Composite Membranes for Fuel Cells Applications. Appl. Clay Sci. 2019, 174, 77–85. [Google Scholar] [CrossRef]
- Ding, J.; Tian, T.; Meng, Q.; Guo, Z.; Li, W.; Zhang, P.; Ciacchi, F.T.; Huang, J.; Yang, W. Smart Multifunctional Fluids for Lithium Ion Batteries: Enhanced Rate Performance and Intrinsic Mechanical Protection. Sci. Rep. 2013, 3, 2485. [Google Scholar] [CrossRef]
- Xiaoxia, C.; Kai, L.; Baoguo, W. Research on High-Safety Electrolytes and Their Application in Lithium-Ion Batteries. Energy Storage Sci. Technol. 2020, 9, 583–592. [Google Scholar] [CrossRef]
- Yu, O.; Wenhui, H.; Kai, L. Research Progress of Smart Safety Electrolytes in Lithium-Ion Batteries. Energy Storage Sci. Technol. 2022, 11, 1772. [Google Scholar] [CrossRef]
- Heo, Y.; Larson, R.G. The Scaling of Zero-Shear Viscosities of Semidilute Polymer Solutions with Concentration. J. Rheol. 2005, 49, 1117–1128. [Google Scholar] [CrossRef]
- Srivastava, A.; Majumdar, A.; Butola, B.S. Improving the Impact Resistance of Textile Structures by Using Shear Thickening Fluids: A Review. Crit. Rev. Solid State Mater. Sci. 2012, 37, 115–129. [Google Scholar] [CrossRef]
- Dong, H.; Hu, X.; He, G. A Shear-Thickening Colloidal Electrolyte for Aqueous Zinc-Ion Batteries with Resistance on Impact. Nanoscale 2022, 14, 14544–14551. [Google Scholar] [CrossRef]
- Hamaker, H.C. The London—Van Der Waals Attraction between Spherical Particles. Physica 1937, 4, 1058–1072. [Google Scholar] [CrossRef]
- Mewis, J.; Biebaut, G. Shear Thickening in Steady and Superposition Flows Effect of Particle Interaction Forces. J. Rheol. 2001, 45, 799–813. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Liang, S.; Lu, B.; Zhou, J. Ph-Buffer Contained Electrolyte for Self-Adjusted Cathode-Free Zn–Mno2 Batteries with Coexistence of Dual Mechanisms. Small Struct. 2021, 2, 2100119. [Google Scholar] [CrossRef]
- Yufit, V.; Tariq, F.; Eastwood, D.S.; Biton, M.; Wu, B.; Lee, P.D.; Brandon, N.P. Operando Visualization and Multi-Scale Tomography Studies of Dendrite Formation and Dissolution in Zinc Batteries. Joule 2019, 3, 485–502. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Seol, S.-K.; Lo, T.-N.; Liu, C.-J.; Wang, C.-L.; Lin, C.-S.; Hwu, Y.; Chen, C.; Chang, L.-W.; Je, J.; et al. Hydrogen Bubbles and the Growth Morphology of Ramified Zinc by Electrodeposition. J. Electrochem. Soc. 2008, 155, D400. [Google Scholar] [CrossRef]
- Yi, J.; Guo, S.; He, P.; Zhou, H. Status and Prospects of Polymer Electrolytes for Solid-State Li–O2 (Air) Batteries. Energy Environ. Sci. 2017, 10, 860–884. [Google Scholar] [CrossRef]
- Zheng, G.; Yan, T.; Hong, Y.; Zhang, X.; Wu, J.; Liang, Z.; Cui, Z.; Du, L.; Song, H. A Non-Newtonian Fluid Quasi-Solid Electrolyte Designed for Long Life and High Safety Li-O(2) Batteries. Nat. Commun. 2023, 14, 2268. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Liang, X.; Liu, B.; Deng, H. Research Progress of Shear Thickening Electrolyte Based on Liquid–Solid Conversion Mechanism. Batteries 2023, 9, 384. https://doi.org/10.3390/batteries9070384
Huang Q, Liang X, Liu B, Deng H. Research Progress of Shear Thickening Electrolyte Based on Liquid–Solid Conversion Mechanism. Batteries. 2023; 9(7):384. https://doi.org/10.3390/batteries9070384
Chicago/Turabian StyleHuang, Qianqian, Xin Liang, Bing Liu, and Huaxia Deng. 2023. "Research Progress of Shear Thickening Electrolyte Based on Liquid–Solid Conversion Mechanism" Batteries 9, no. 7: 384. https://doi.org/10.3390/batteries9070384
APA StyleHuang, Q., Liang, X., Liu, B., & Deng, H. (2023). Research Progress of Shear Thickening Electrolyte Based on Liquid–Solid Conversion Mechanism. Batteries, 9(7), 384. https://doi.org/10.3390/batteries9070384