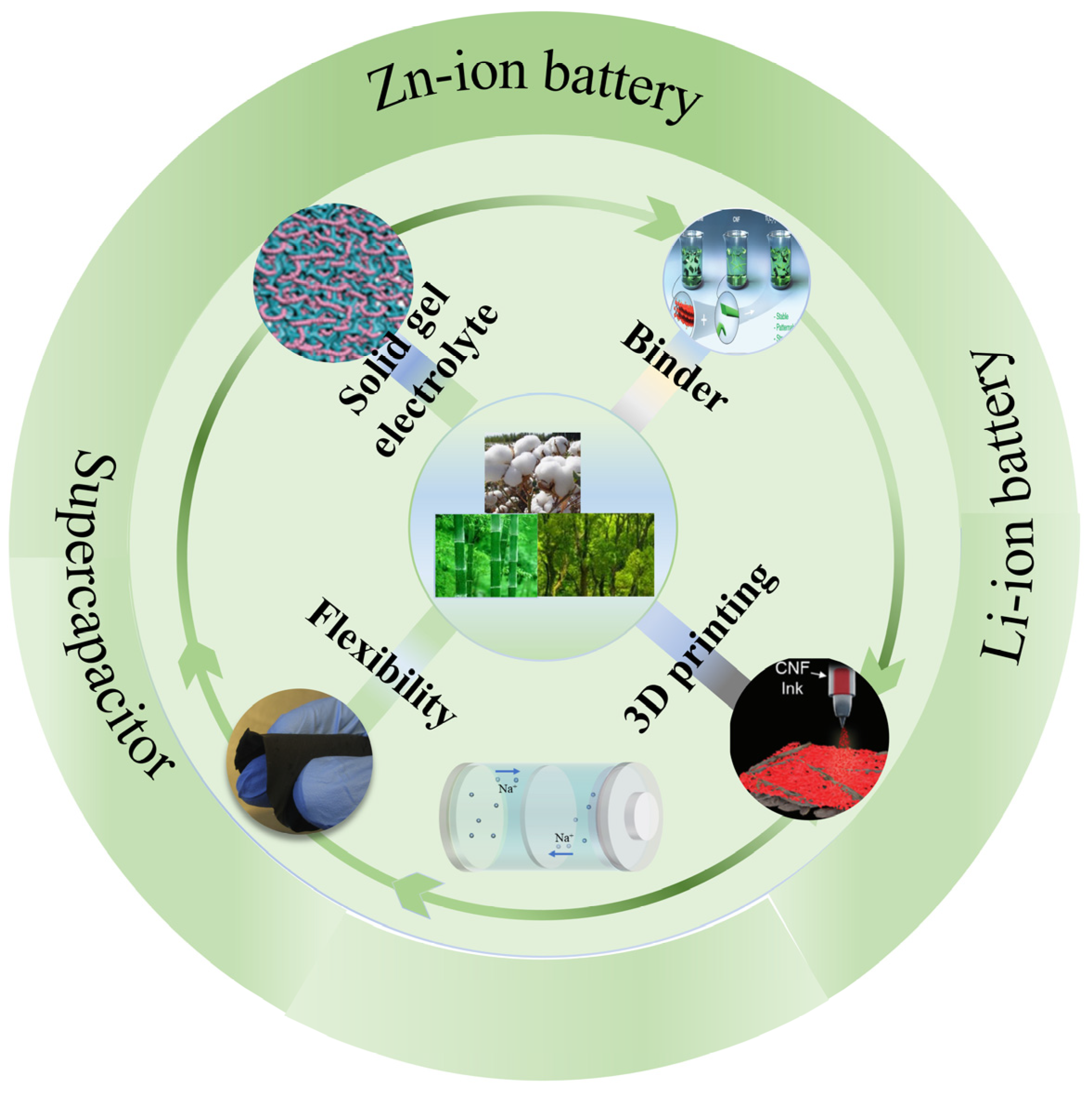
The Application of Cellulose Nanofibrils in Energy Systems
Abstract
:1. Introduction
2. Preparation and Properties of Nanocellulose
2.1. The Fabrication of CNFs

2.2. Production Methods of Biomass-Derived Nanofibers
| Type | Source | Morphology | Representative SEM/TEM Image | Surface Chemical Group | Fabrication Method | Reference |
|---|---|---|---|---|---|---|
| CNF | Wood | Thread-like aggregates |  | Hydroxyl carboxylate | TEMPO-mediated oxidation and stirring | [40] |
| CNC | Cotton | Rod-like particles |  | Hydroxyl | Sulfuric and hydrolysis | [41] |
| BC | Coconut milk | Thread-like aggregates forming hydrogels |  | Hydroxyl | Secreted by Acetobacter xylinum FF-88 | [42] |
3. CNFs as Binders in Energy Storage and Conversion
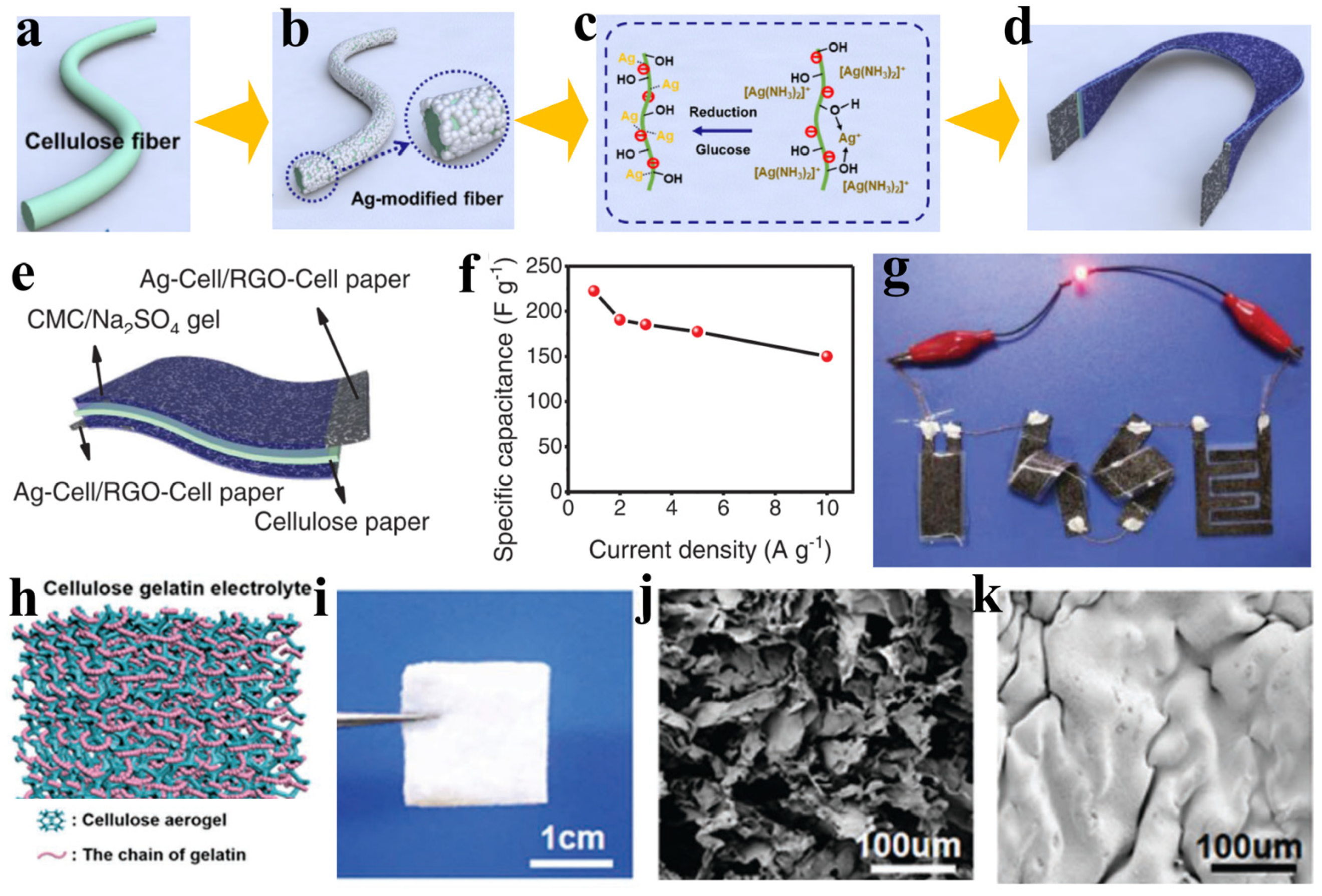
4. The Application of CNFs in Gel Electrolyte
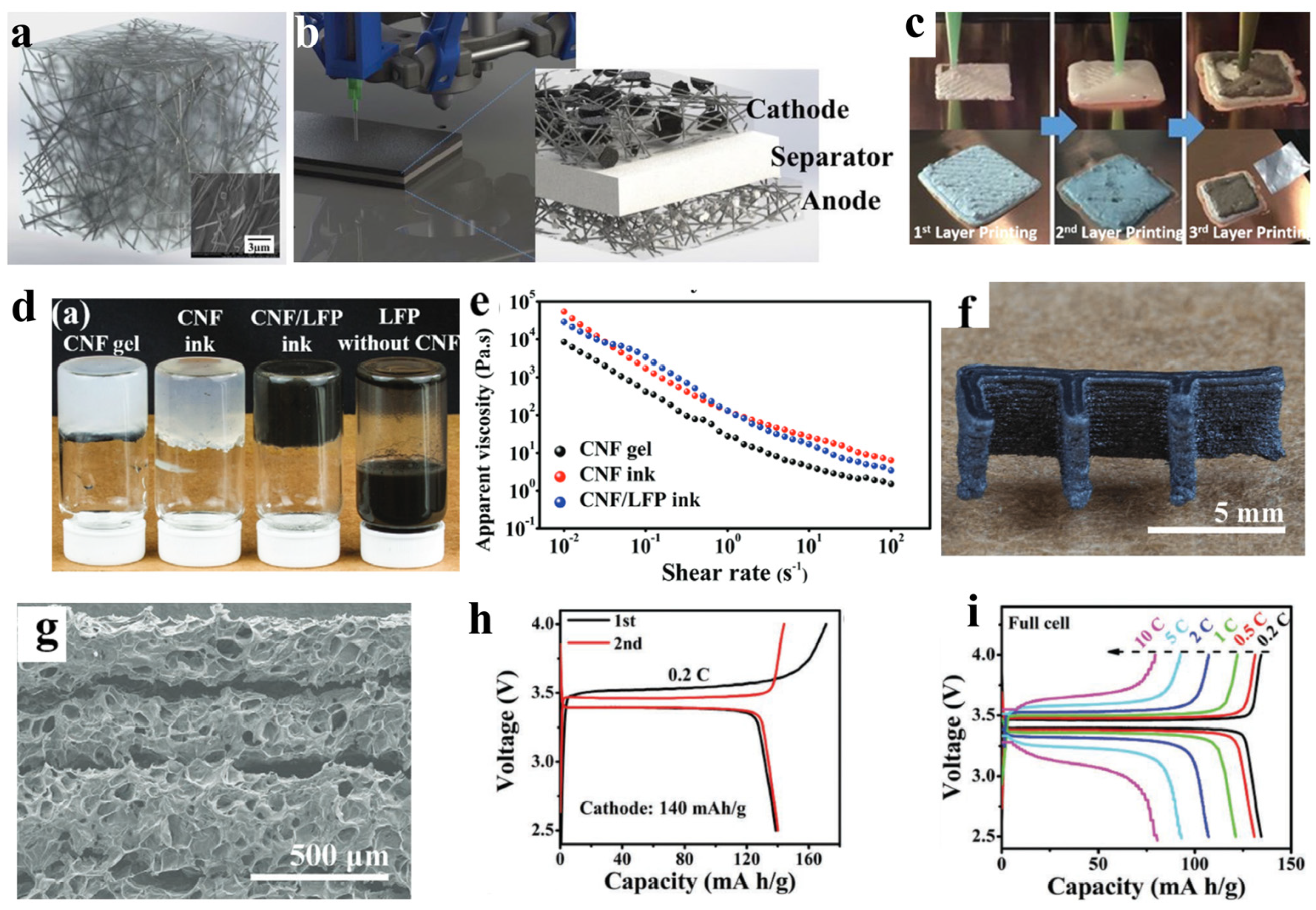
5. The Application of CNFs in 3D Printing
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omer, A.M. Energy, environment and sustainable development. Renew. Sustain. Energy Rev. 2008, 12, 2265–2300. [Google Scholar] [CrossRef]
- Ou, Z.; An, Z.; Ma, Z.; Li, N.; Han, Y.; Yang, G.; Jiang, Q.; Chen, Q.; Chu, W.; Wang, S.; et al. 3D Porous Graphene-like Carbons Encaged Single-Atom-Based Pt for Ultralow Loading and High-Performance Fuel Cells. ACS Catal. 2023, 13, 1856–1862. [Google Scholar] [CrossRef]
- Tang, K.; Hu, H.; Xiong, Y.; Chen, L.; Zhang, J.; Yuan, C.; Wu, M. Hydrophobization Engineering of the Air-Cathode Catalyst for Improved Oxygen Diffusion towards Efficient Zinc-Air Batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202671. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, Y.; Wang, J.; Li, J.; Yu, L.; Zhou, F.; Li, J.; Shen, X.; Wang, X.; Wang, S.; et al. Biomass-Derived Fe2N@NCNTs from Bioaccumulation as an Efficient Electrocatalyst for Oxygen Reduction and Zn-Air Battery. ACS Sustain. Chem. Eng. 2022, 10, 9105–9112. [Google Scholar] [CrossRef]
- Yu, L.; Li, W.; Wei, C.; Yang, Q.; Shao, Y.; Sun, J. 3D Printing of NiCoP/Ti3C2 MXene Architectures for Energy Storage Devices with High Areal and Volumetric Energy Density. Nanomicro Lett. 2020, 12, 143. [Google Scholar] [CrossRef]
- Lawson, S.; Alwakwak, A.A.; Rownaghi, A.A.; Rezaei, F. Gel-Print-Grow: A New Way of 3D Printing Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 56108–56117. [Google Scholar] [CrossRef]
- Jin, H.; Li, J.; Yuan, Y.; Wang, J.; Lu, J.; Wang, S. Recent Progress in Biomass-Derived Electrode Materials for High Volumetric Performance Supercapacitors. Adv. Energy Mater. 2018, 8, 1801007. [Google Scholar] [CrossRef]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Menon, M.P.; Selvakumar, R.; Sureshkumar, P.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Lee, Y.H.; Kim, S.W.; Seo, J.Y.; Lee, S.Y.; Nyholm, L. Why Cellulose-Based Electrochemical Energy Storage Devices? Adv. Mater. 2021, 33, e2000892. [Google Scholar] [CrossRef]
- Calle-Gil, R.; Castillo-Martínez, E.; Carretero-González, J. Cellulose Nanocrystals in Sustainable Energy Systems. Adv. Sustain. Syst. 2022, 6, 2100395. [Google Scholar] [CrossRef]
- Sheng, J.; Tong, S.; He, Z.; Yang, R. Recent developments of cellulose materials for lithium-ion battery separators. Cellulose 2017, 24, 4103–4122. [Google Scholar] [CrossRef]
- Mayilswamy, N.; Kandasubramanian, B. Green composites prepared from soy protein, polylactic acid (PLA), starch, cellulose, chitin: A review. Emergent Mater. 2022, 5, 727–753. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Yi, Z.; Zhang, L.; Zhang, S.; Hu, X. Co-pyrolysis of coke bottle wastes with cellulose, lignin and sawdust: Impacts of the mixed feedstock on char properties. Renew. Energy 2022, 181, 1126–1139. [Google Scholar] [CrossRef]
- Abe, K.; Yano, H. Comparison of the characteristics of cellulose microfibril aggregates isolated from fiber and parenchyma cells of Moso bamboo (Phyllostachys pubescens). Cellulose 2009, 17, 271–277. [Google Scholar] [CrossRef]
- Paakko, M.; Ankerfors, M.; Kosonen, H.; Nykanen, A.; Ahola, S.; Osterberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Okita, Y.; Saito, T.; Isogai, A. Entire surface oxidation of various cellulose microfibrils by TEMPO-mediated oxidation. Biomacromolecules 2010, 11, 1696–1700. [Google Scholar] [CrossRef]
- Cao, D.X.; Xing, Y.J.; Tantratian, K.; Wang, X.; Ma, Y.; Mukhopadhyay, A.; Cheng, Z.; Zhang, Q.G.; Jiao, Y.; Chen, L.; et al. 3D Printed High-Performance Lithium Metal Microbatteries Enabled by Nanocellulose. Adv. Mater. 2019, 31, 1807313. [Google Scholar] [CrossRef]
- Pennells, J.; Godwin, I.D.; Amiralian, N.; Martin, D.J. Trends in the production of cellulose nanofibers from non-wood sources. Cellulose 2019, 27, 575–593. [Google Scholar] [CrossRef]
- Hassan, M.; Berglund, L.; Hassan, E.; Abou-Zeid, R.; Oksman, K. Effect of xylanase pretreatment of rice straw unbleached soda and neutral sulfite pulps on isolation of nanofibers and their properties. Cellulose 2018, 25, 2939–2953. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, W.; Zhang, Y.; Ben, H.; Han, G.; Ragauskas, A.J. Isolation and characterization of cellulosic fibers from kenaf bast using steam explosion and Fenton oxidation treatment. Cellulose 2018, 25, 4979–4992. [Google Scholar] [CrossRef]
- Zhao, G.; Du, J.; Chen, W.; Pan, M.; Chen, D. Preparation and thermostability of cellulose nanocrystals and nanofibrils from two sources of biomass: Rice straw and poplar wood. Cellulose 2019, 26, 8625–8643. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, S.; Liu, Y.; Lin, J. Isolation of hierarchical cellulose building blocks from natural flax fibers as a separation membrane barrier. Int. J. Biol. Macromol. 2020, 155, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Petroudy, S.R.D.; Chabot, B.; Loranger, E.; Naebe, M.; Shojaeiarani, J.; Gharehkhani, S.; Ahvazi, B.; Hu, J.; Thomas, S. Recent Advances in Cellulose Nanofibers Preparation through Energy-Efficient Approaches: A Review. Energies 2021, 14, 6792. [Google Scholar] [CrossRef]
- Baati, R.; Mabrouk, A.B.; Magnin, A.; Boufi, S. CNFs from twin screw extrusion and high pressure homogenization: A comparative study. Carbohydr. Polym. 2018, 195, 321–328. [Google Scholar] [CrossRef]
- Hongrattanavichit, I.; Aht-Ong, D. Nanofibrillation and characterization of sugarcane bagasse agro-waste using water-based steam explosion and high-pressure homogenization. J. Clean. Prod. 2020, 277, 123471. [Google Scholar] [CrossRef]
- Arantes, V.; Dias, I.K.R.; Berto, G.L.; Pereira, B.; Marotti, B.S.; Nogueira, C.F.O. The current status of the enzyme-mediated isolation and functionalization of nanocelluloses: Production, properties, techno-economics, and opportunities. Cellulose 2020, 27, 10571–10630. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Campano, C.; Balea, A.; Tarres, Q.; Delgado-Aguilar, M.; Mutje, P.; Blanco, A.; Negro, C. Critical comparison of the properties of cellulose nanofibers produced from softwood and hardwood through enzymatic, chemical and mechanical processes. Int. J. Biol. Macromol. 2022, 205, 220–230. [Google Scholar] [CrossRef]
- Yamato, K.; Yoshida, Y.; Kumamoto, Y.; Isogai, A. Surface modification of TEMPO-oxidized cellulose nanofibers, and properties of their acrylate and epoxy resin composite films. Cellulose 2021, 29, 2839–2853. [Google Scholar] [CrossRef]
- Koochi, H.; Intyre, J.M.; Viitanen, L.; Puisto, A.; Maleki-Jirsaraei, N.; Alava, M. Local time-dependent microstructure of aging TEMPO nanofibrillated cellulose gel. Cellulose 2022, 30, 61–74. [Google Scholar] [CrossRef]
- Isogai, A.; Kato, Y. Preparation of polyuronic acid from cellulose by TEMPO-mediated oxidation. Cellulose 1998, 5, 153–164. [Google Scholar] [CrossRef]
- Delgado-Aguilar, M.; Tarrés, Q.; Puig, J.; Boufi, S.; Blanco, Á.; Mutjé, P. Enzymatic Refining and Cellulose Nanofiber Addition in Papermaking Processes from Recycled and Deinked Slurries. BioResources 2015, 10, 5730–5743. [Google Scholar] [CrossRef] [Green Version]
- Alila, S.; Besbes, I.; Vilar, M.R.; Mutjé, P.; Boufi, S. Non-woody plants as raw materials for production of microfibrillated cellulose (MFC): A comparative study. Ind. Crops Prod. 2013, 41, 250–259. [Google Scholar] [CrossRef]
- Tarrés, Q.; Boufi, S.; Mutjé, P.; Delgado-Aguilar, M. Enzymatically hydrolyzed and TEMPO-oxidized cellulose nanofibers for the production of nanopapers: Morphological, optical, thermal and mechanical properties. Cellulose 2017, 24, 3943–3954. [Google Scholar] [CrossRef]
- Patterson, G.; Hsieh, Y.L. Tunable dialdehyde/dicarboxylate nanocelluloses by stoichiometrically optimized sequential periodate-chlorite oxidation for tough and wet shape recoverable aerogels. Nanoscale Adv. 2020, 2, 5623–5634. [Google Scholar] [CrossRef]
- Sun, X.; He, Q.; Yang, Y. Preparation of Dicarboxyl Cellulose Nanocrystals from Agricultural Wastes by Sequential Periodate-Chlorite Oxidation. J. Renew. Mater. 2020, 8, 447–460. [Google Scholar] [CrossRef]
- Butchosa, N.; Zhou, Q. Water redispersible cellulose nanofibrils adsorbed with carboxymethyl cellulose. Cellulose 2014, 21, 4349–4358. [Google Scholar] [CrossRef]
- Azrak, S.M.E.A.; Gohl, J.A.; Moon, R.J.; Schueneman, G.T.; Davis, C.S.; Youngblood, J.P. Controlled Dispersion and Setting of Cellulose Nanofibril—Carboxymethyl Cellulose Pastes. Cellulose 2021, 28, 9149–9168. [Google Scholar] [CrossRef]
- Wagberg, L.; Decher, G.; Norgren, M.; Lindstrom, T.; Ankerfors, M.; Axnas, K. The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 2008, 24, 784–795. [Google Scholar] [CrossRef]
- Chen, W.; Li, Q.; Wang, Y.; Yi, X.; Zeng, J.; Yu, H.; Liu, Y.; Li, J. Comparative study of aerogels obtained from differently prepared nanocellulose fibers. ChemSusChem 2014, 7, 154–161. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Handa, K.; Nakatsubo, F.; Yano, H. Surface modification of bacterial cellulose nanofibers for property enhancement of optically transparent composites: Dependence on acetyl-group DS. Biomacromolecules 2007, 8, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
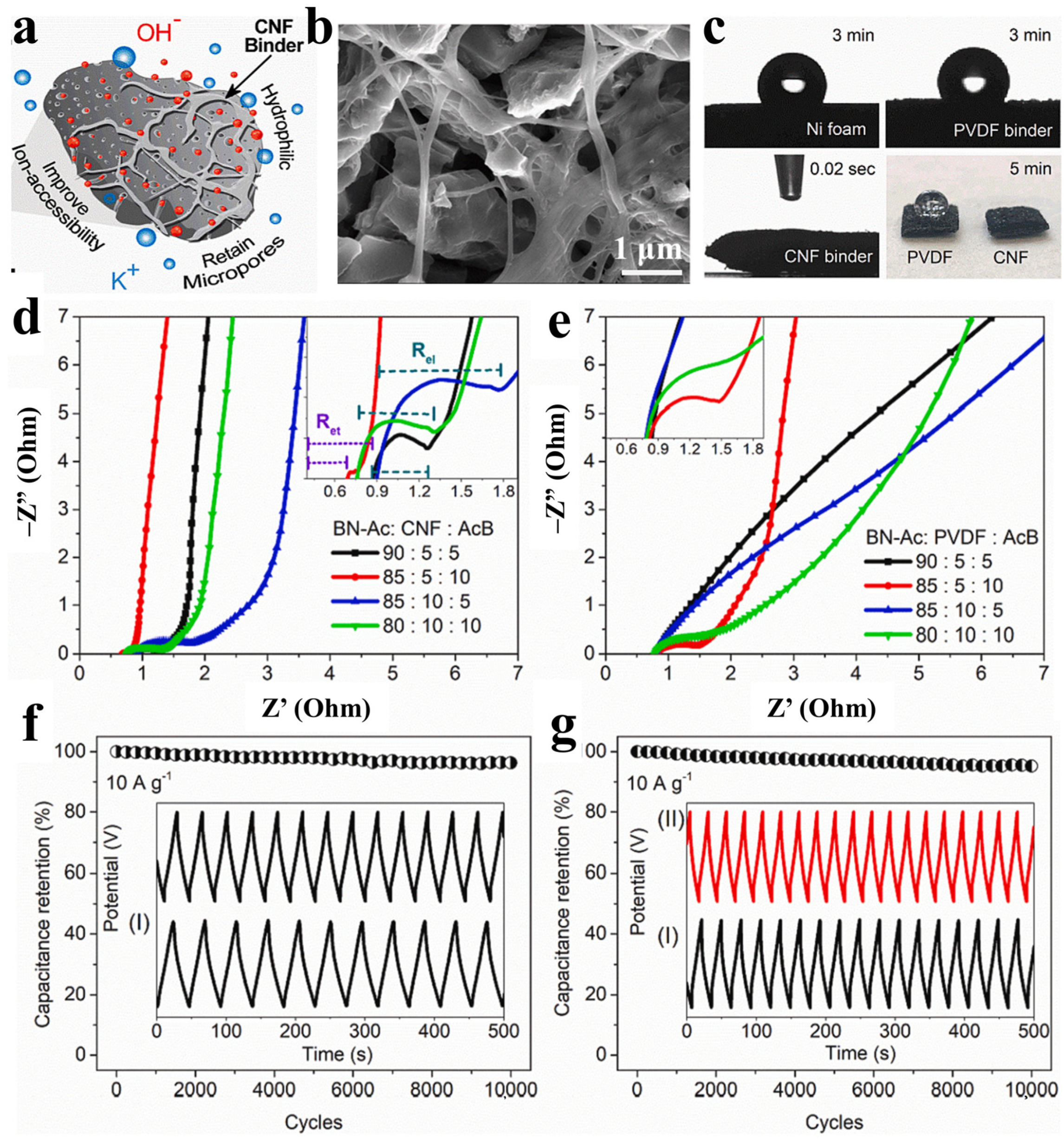
- Zhong, X.; Han, J.; Chen, L.; Liu, W.; Jiao, F.; Zhu, H.; Qin, W. Binding mechanisms of PVDF in lithium ion batteries. Appl. Surf. Sci. 2021, 553, 149564. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Yang, B.; Guo, D.; Lin, P.; Zhou, L.; Li, J.; Fang, G.; Wang, J.; Jin, H.; Chen, X.A.; Wang, S. Hydroxylated Multi-Walled Carbon Nanotubes Covalently Modified with Tris(hydroxypropyl) Phosphine as a Functional Interlayer for Advanced Lithium-Sulfur Batteries. Angew. Chem. 2022, 61, e202204327. [Google Scholar]
- Darjazi, H.; Staffolani, A.S.; Sbrascini, L.; Bottoni, L.; Tossici, R.; Nobili, F. Sustainable Anodes for Lithium- and Sodium-Ion Batteries Based on Coffee Ground-Derived Hard Carbon and Green Binders. Energies 2020, 13, 6216. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, S.; Paik, U.; Choi, Y.-M. Aqueous processing of natural graphite particulates for lithium-ion battery anodes and their electrochemical performance. J. Power Sources 2005, 147, 249–255. [Google Scholar] [CrossRef]
- Kwon, T.W.; Choi, J.W.; Coskun, A. The emerging era of supramolecular polymeric binders in silicon anodes. Chem. Soc. Rev. 2018, 47, 2145–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
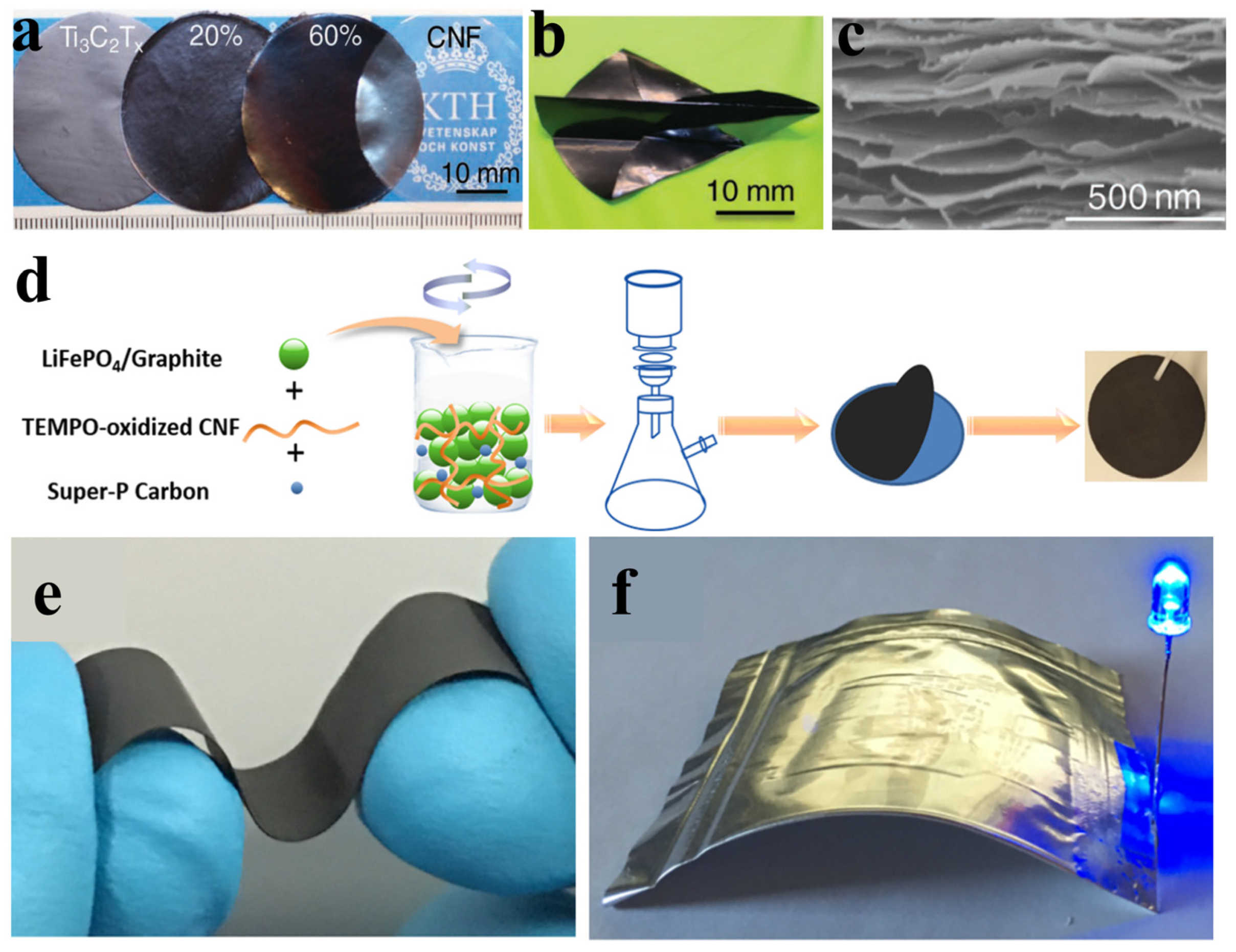
- Tian, W.; VahidMohammadi, A.; Reid, M.S.; Wang, Z.; Ouyang, L.; Erlandsson, J.; Pettersson, T.; Wagberg, L.; Beidaghi, M.; Hamedi, M.M. Multifunctional Nanocomposites with High Strength and Capacitance Using 2D MXene and 1D Nanocellulose. Adv. Mater. 2019, 31, e1902977. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Behm, M.; Leijonmarck, S.; Lindbergh, G.; Cornell, A. Flexible Paper Electrodes for Li-Ion Batteries Using Low Amount of TEMPO-Oxidized Cellulose Nanofibrils as Binder. ACS Appl. Mater. Interfaces 2016, 8, 18097–18106. [Google Scholar] [CrossRef]
- Lu, H.; Guccini, V.; Kim, H.; Salazar-Alvarez, G.; Lindbergh, G.; Cornell, A. Effects of Different Manufacturing Processes on TEMPO-Oxidized Carboxylated Cellulose Nanofiber Performance as Binder for Flexible Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 37712–37720. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Q.; Liu, S.; Long, J.; Wu, Z.; Zhang, W.; Pang, W.K.; Sencadas, V.; Song, R.; Song, W.; et al. Synergy of binders and electrolytes in enabling microsized alloy anodes for high performance potassium-ion batteries. Nano Energy 2020, 77, 105118. [Google Scholar] [CrossRef]
- Rajeevan, S.; John, S.; George, S.C. Polyvinylidene fluoride: A multifunctional polymer in supercapacitor applications. J. Power Sources 2021, 504, 230037. [Google Scholar] [CrossRef]
- Aslan, M.; Weingarth, D.; Jäckel, N.; Atchison, J.S.; Grobelsek, I.; Presser, V. Polyvinylpyrrolidone as binder for castable supercapacitor electrodes with high electrochemical performance in organic electrolytes. J. Power Sources 2014, 266, 374–383. [Google Scholar] [CrossRef]
- Daraghmeh, A.; Hussain, S.; Servera, L.; Xuriguera, E.; Cornet, A.; Cirera, A. Impact of binder concentration and pressure on performance of symmetric CNFs based supercapacitors. Electrochim. Acta 2017, 245, 531–538. [Google Scholar] [CrossRef]
- Lacey, M.J.; Jeschull, F.; Edström, K.; Brandell, D. Porosity Blocking in Highly Porous Carbon Black by PVdF Binder and Its Implications for the Li–S System. J. Phys. Chem. C 2014, 118, 25890–25898. [Google Scholar] [CrossRef]
- Rabuni, M.F.; Sulaiman, N.M.N.; Aroua, M.K.; Hashim, N.A. Effects of Alkaline Environments at Mild Conditions on the Stability of PVDF Membrane: An Experimental Study. Ind. Eng. Chem. Res. 2013, 52, 15874–15882. [Google Scholar] [CrossRef]
- Mian, M.M.; Kamana, I.M.L.; An, X.; Abbas, S.C.; Ahommed, M.S.; He, Z.; Ni, Y. Cellulose nanofibers as effective binders for activated biochar-derived high-performance supercapacitors. Carbohydr. Polym. 2023, 301, 120353. [Google Scholar] [CrossRef]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Yang, Y.Q.; Li, M.X.; Wang, F.X.; Chang, Z.; Wu, Y.P.; Liu, X. A composite membrane based on a biocompatible cellulose as a host of gel polymer electrolyte for lithium ion batteries. J. Power Sources 2014, 270, 53–58. [Google Scholar] [CrossRef]
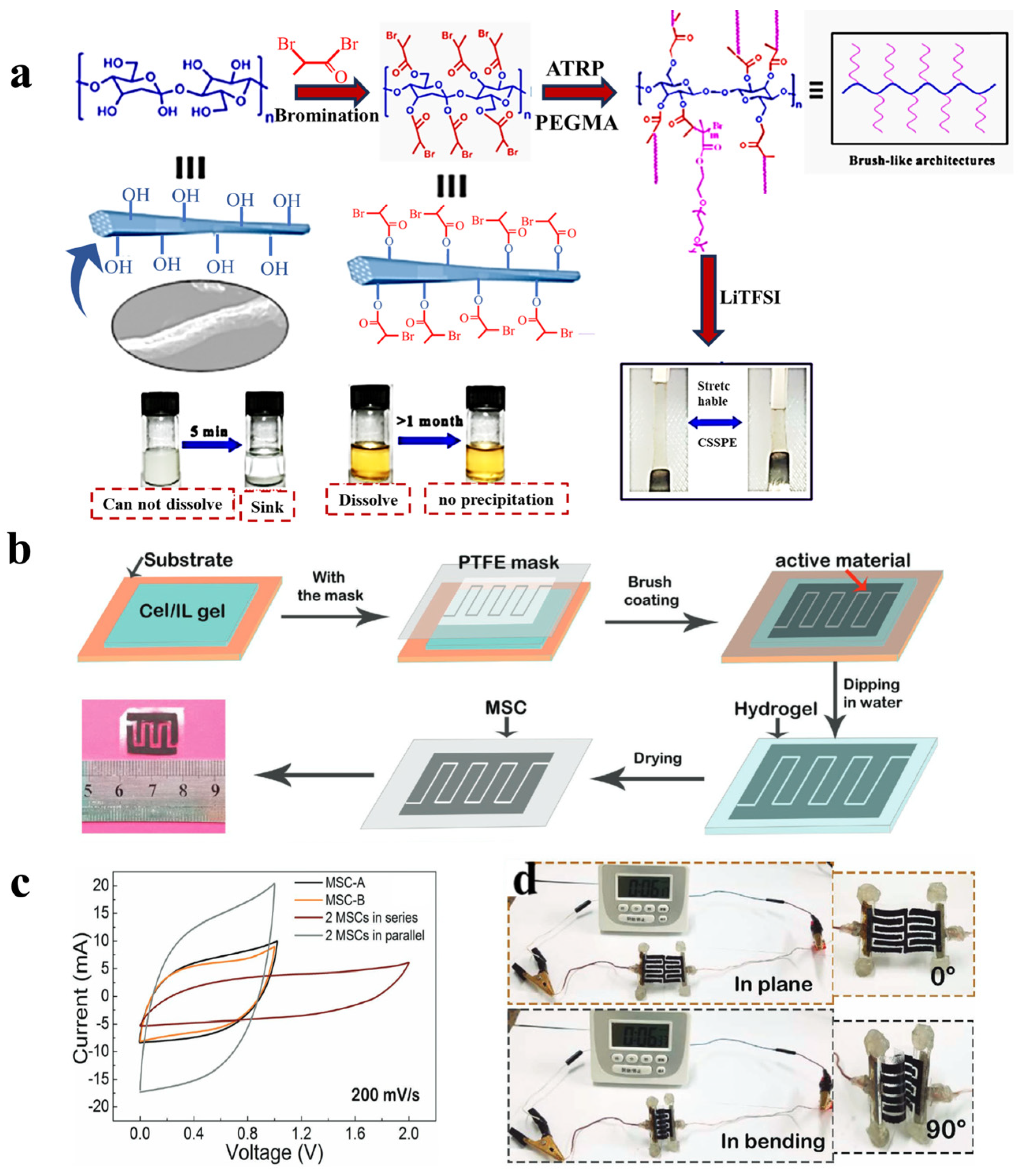
- Wang, S.; Zhang, L.; Zeng, Q.; Liu, X.; Lai, W.-Y.; Zhang, L. Cellulose Microcrystals with Brush-Like Architectures as Flexible All-Solid-State Polymer Electrolyte for Lithium-Ion Battery. ACS Sustain. Chem. Eng. 2020, 8, 3200–3207. [Google Scholar] [CrossRef]
- Qu, S.; Liu, J.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Dynamic stretching–electroplating metal-coated textile for a flexible and stretchable zinc–air battery. Carbon Energy 2022, 4, 867–877. [Google Scholar] [CrossRef]
- Hui, X.; Zhang, P.; Li, J.; Zhao, D.; Li, Z.; Zhang, Z.; Wang, C.; Wang, R.; Yin, L. In Situ Integrating Highly Ionic Conductive LDH-Array@PVA Gel Electrolyte and MXene/Zn Anode for Dendrite-Free High-Performance Flexible Zn–Air Batteries. Adv. Energy Mater. 2022, 12, 2201393. [Google Scholar] [CrossRef]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Kadriadi; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C.; Li, Q.; Pan, Z.; Sun, J.; Zhou, Z.; He, B.; Man, P.; Xie, L.; Kang, L.; et al. Flexible and High-Voltage Coaxial-Fiber Aqueous Rechargeable Zinc-Ion Battery. Nano Lett. 2019, 19, 4035–4042. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, Y.; Chen, R.; Wang, X. High performance fully paper-based all-solid-state supercapacitor fabricated by a papermaking process with silver nanoparticles and reduced graphene oxide-modified pulp fibers. EcoMat 2021, 3, e12076. [Google Scholar] [CrossRef]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, R.; Xu, R.; Li, Y.; Tian, W.; Gao, M.; Wang, M.; Li, D.; Liang, X.; Xie, L.; et al. Super-Assembled Hierarchical Cellulose Aerogel-Gelatin Solid Electrolyte for Implantable and Biodegradable Zinc Ion Battery. Adv. Funct. Mater. 2022, 32, 2111406. [Google Scholar] [CrossRef]
- Wu, M.L.; Zhang, Y.; Xu, L.; Yang, C.P.; Hong, M.; Cui, M.J.; Clifford, B.C.; He, S.M.; Jing, S.H.; Yao, Y.; et al. A sustainable chitosan-zinc electrolyte for high-rate zinc-metal batteries. Matter 2022, 5, 3402–3416. [Google Scholar] [CrossRef]
- Xu, L.; Meng, T.T.; Zheng, X.Y.; Li, T.Y.; Brozena, A.H.; Mao, Y.M.; Zhang, Q.; Clifford, B.C.; Rao, J.C.; Hu, L.B. Nanocellulose-Carboxymethylcellulose Electrolyte for Stable, High-Rate Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2302098. [Google Scholar] [CrossRef]
- Yu, L.; Fan, Z.; Shao, Y.; Tian, Z.; Sun, J.; Liu, Z. Versatile N-Doped MXene Ink for Printed Electrochemical Energy Storage Application. Adv. Energy Mater. 2019, 9, 1901839. [Google Scholar] [CrossRef]
- Wei, C.; Tian, M.; Fan, Z.; Yu, L.; Song, Y.; Yang, X.; Shi, Z.; Wang, M.; Yang, R.; Sun, J. Concurrent realization of dendrite-free anode and high-loading cathode via 3D printed N-Ti3C2 MXene framework toward advanced Li–S full batteries. Energy Storage Mater. 2021, 41, 141–151. [Google Scholar] [CrossRef]
- Wady, P.; Wasilewski, A.; Brock, L.; Edge, R.; Baidak, A.; McBride, C.; Leay, L.; Griffiths, A.; Vallés, C. Effect of ionising radiation on the mechanical and structural properties of 3D printed plastics. Addit. Manuf. 2020, 31, 100907. [Google Scholar] [CrossRef]
- Aeby, X.; Poulin, A.; Siqueira, G.; Hausmann, M.K.; Nystrom, G. Fully 3D Printed and Disposable Paper Supercapacitors. Adv. Mater. 2021, 33, e2101328. [Google Scholar] [CrossRef]
- Seoane-Viano, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef]
- Tan, H.W.; An, J.; Chua, C.K.; Tran, T. Metallic Nanoparticle Inks for 3D Printing of Electronics. Adv. Electron. Mater. 2019, 5, 1800831. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Han, W.; Lin, H.; Li, R.; Zhu, J.; Huang, W. 3D Printed Flexible Strain Sensors: From Printing to Devices and Signals. Adv. Mater. 2021, 33, e2004782. [Google Scholar] [CrossRef]
- Qiu, L.; Shao, Z.; Wang, D.; Wang, W.; Wang, F.; Wang, J. Enhanced electrochemical properties of LiFePO4 (LFP) cathode using the carboxymethyl cellulose lithium (CMC-Li) as novel binder in lithium-ion battery. Carbohydr. Polym. 2014, 111, 588–591. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, T.; Kim, W.S. Conductive Cellulose Composites with Low Percolation Threshold for 3D Printed Electronics. Sci. Rep. 2017, 7, 3246. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Li, M.C.; Liu, C.; Wu, Q.; Mei, C. 3D Printed Ti3C2Tx MXene/Cellulose Nanofiber Architectures for Solid-State Supercapacitors: Ink Rheology, 3D Printability, and Electrochemical Performance. Adv. Funct. Mater. 2022, 32, 2109593. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Tian, D.; Chen, L.; Zhuang, B.; Feng, H.; Li, Q.; Yu, L.; Ling, Y. The Application of Cellulose Nanofibrils in Energy Systems. Batteries 2023, 9, 399. https://doi.org/10.3390/batteries9080399
Li R, Tian D, Chen L, Zhuang B, Feng H, Li Q, Yu L, Ling Y. The Application of Cellulose Nanofibrils in Energy Systems. Batteries. 2023; 9(8):399. https://doi.org/10.3390/batteries9080399
Chicago/Turabian StyleLi, Ruoyu, Dong Tian, Lei Chen, Bocheng Zhuang, Hui Feng, Qiang Li, Lianghao Yu, and Yihan Ling. 2023. "The Application of Cellulose Nanofibrils in Energy Systems" Batteries 9, no. 8: 399. https://doi.org/10.3390/batteries9080399
APA StyleLi, R., Tian, D., Chen, L., Zhuang, B., Feng, H., Li, Q., Yu, L., & Ling, Y. (2023). The Application of Cellulose Nanofibrils in Energy Systems. Batteries, 9(8), 399. https://doi.org/10.3390/batteries9080399









