Crosslinked Hyperbranched Polyglycerol-Based Polymer Electrolytes for Lithium Metal Batteries
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Methods
2.3. Polymer Electrolyte Preparation
2.3.1. Partially Methylated and Methacrylated Polyglycerols
2.3.2. Fully Methylated Polyglycerol
2.3.3. Preparation of Polymer Electrolyte Mixtures
2.3.4. Liquid Electrolyte
2.3.5. Electrode Coating via Photopolymerization
2.3.6. Preparation of DMA Specimens
3. Results and Discussion
3.1. Preparation of SPEs
3.2. Structural and Thermal Analysis of SPEs
3.3. Mechanical Properties
3.4. Electrochemical Properties
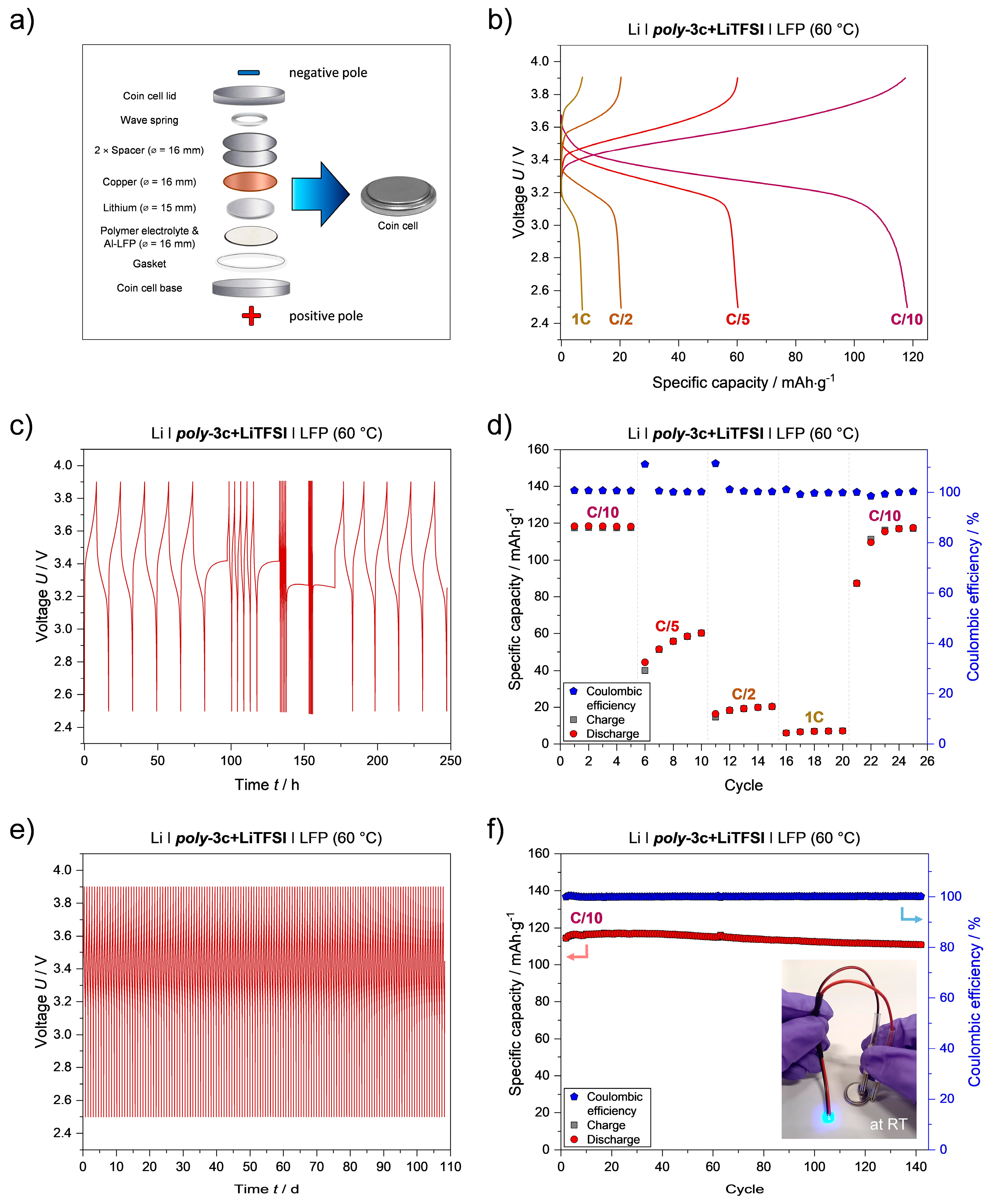
3.5. Cell Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Korthauer, R. Handbuch Lithium-Ionen-Batterien, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-30653-2. [Google Scholar]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Wagner, R.; Preschitschek, N.; Passerini, S.; Leker, J.; Winter, M. Current research trends and prospects among the various materials and designs used in lithium-based batteries. J. Appl. Electrochem. 2013, 43, 481–496. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Jiao, S.; Zheng, J.; Li, Q.; Li, X.; Engelhard, M.H.; Cao, R.; Zhang, J.-G.; Xu, W. Behavior of Lithium Metal Anodes under Various Capacity Utilization and High Current Density in Lithium Metal Batteries. Joule 2018, 2, 110–124. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, S.; Huang, Z.; Wei, Z.; Shen, L.; Gu, H.; Xu, X.; Yao, X. High conductivity polymer electrolyte with comb-like structure via a solvent-free UV-cured method for large-area ambient all-solid-sate lithium batteries. J. Mater. 2019, 5, 195–203. [Google Scholar] [CrossRef]
- Zhang, H.; Armand, M. History of Solid Polymer Electrolyte-Based Solid-State Lithium Metal Batteries: A Personal Account. Isr. J. Chem. 2021, 61, 94–100. [Google Scholar] [CrossRef]
- Barteau, K.P.; Wolffs, M.; Lynd, N.A.; Fredrickson, G.H.; Kramer, E.J.; Hawker, C.J. Allyl Glycidyl Ether-Based Polymer Electrolytes for Room Temperature Lithium Batteries. Macromolecules 2013, 46, 8988–8994. [Google Scholar] [CrossRef]
- Lee, S.-I.; Schömer, M.; Peng, H.; Page, K.A.; Wilms, D.; Frey, H.; Soles, C.L.; Yoon, D.Y. Correlations between Ion Conductivity and Polymer Dynamics in Hyperbranched Poly(ethylene oxide) Electrolytes for Lithium-Ion Batteries. Chem. Mater. 2011, 23, 2685–2688. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li + -conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Li, J.; Cai, Y.; Wu, H.; Yu, Z.; Yan, X.; Zhang, Q.; Gao, T.Z.; Liu, K.; Jia, X.; Bao, Z. Polymers in Lithium-Ion and Lithium Metal Batteries. Adv. Energy Mater. 2021, 11, 2003239. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.-Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Boaretto, N.; Garbayo, I.; Valiyaveettil-SobhanRaj, S.; Quintela, A.; Li, C.; Casas-Cabanas, M.; Aguesse, F. Lithium solid-state batteries: State-of-the-art and challenges for materials, interfaces and processing. J. Power Source 2021, 502, 229919. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Klein, R.; Wurm, F.R. Aliphatic Polyethers: Classical Polymers for the 21st Century. Macromol. Rapid Commun. 2015, 36, 1147–1165. [Google Scholar] [CrossRef]
- Borodin, O.; Smith, G.D. Mechanism of Ion Transport in Amorphous Poly(ethylene oxide)/LiTFSI from Molecular Dynamics Simulations. Macromolecules 2006, 39, 1620–1629. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Fan, L.-Z.; Maier, J. Composite effects in poly(ethylene oxide)–succinonitrile based all-solid electrolytes. Electrochem. Commun. 2006, 8, 1753–1756. [Google Scholar] [CrossRef]
- Jayathilaka, P.; Dissanayake, M.; Albinsson, I.; Mellander, B.-E. Effect of nano-porous Al2O3 on thermal, dielectric and transport properties of the (PEO)9LiTFSI polymer electrolyte system. Electrochim. Acta 2002, 47, 3257–3268. [Google Scholar] [CrossRef]
- Peters, F.; Langer, F.; Hillen, N.; Koschek, K.; Bardenhagen, I.; Schwenzel, J.; Busse, M. Correlation of Mechanical and Electrical Behavior of Polyethylene Oxide-Based Solid Electrolytes for All-Solid State Lithium-Ion Batteries. Batteries 2019, 5, 26. [Google Scholar] [CrossRef]
- Croce, F.; Persi, L.; Scrosati, B.; Serraino-Fiory, F.; Plichta, E.; Hendrickson, M. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim. Acta 2001, 46, 2457–2461. [Google Scholar] [CrossRef]
- Zhang, N.; He, J.; Han, W.; Wang, Y. Composite solid electrolyte PEO/SN/LiAlO2 for a solid-state lithium battery. J Mater. Sci. 2019, 54, 9603–9612. [Google Scholar] [CrossRef]
- Marzantowicz, M.; Dygas, J.R.; Krok, F.; Florjańczyk, Z.; Zygadło-Monikowska, E.; Lapienis, G. Ionic conductivity of electrolytes based on star-branched poly(ethylene oxide) with high concentration of lithium salts. Solid State Ion. 2011, 192, 137–142. [Google Scholar] [CrossRef]
- Nishimoto, A.; Watanabe, M.; Ikeda, Y.; Kohjiya, S. High ionic conductivity of new polymer electrolytes based on high molecular weight polyether comb polymers. Electrochim. Acta 1998, 43, 1177–1184. [Google Scholar] [CrossRef]
- Watanabe, M.; Endo, T.; Nishimoto, A.; Miura, K.; Yanagida, M. High ionic conductivity and electrode interface properties of polymer electrolytes based on high molecular weight branched polyether. J. Power Source 1999, 81–82, 786–789. [Google Scholar] [CrossRef]
- Nishimoto, A.; Agehara, K.; Furuya, N.; Watanabe, T.; Watanabe, M. High Ionic Conductivity of Polyether-Based Network Polymer Electrolytes with Hyperbranched Side Chains. Macromolecules 1999, 32, 1541–1548. [Google Scholar] [CrossRef]
- Watanabe, M.; Hirakimoto, T.; Mutoh, S.; Nishimoto, A. Polymer electrolytes derived from dendritic polyether macromonomers. Solid State Ion. 2002, 148, 399–404. [Google Scholar] [CrossRef]
- Kim, S.-C.; Oh, T.H.; Kim, D.W.; Lee, C.; Kang, Y. Ion-conducting hyperbranched PEG electrolytes derived from poly(glycidol). Macromol. Res. 2009, 17, 141–143. [Google Scholar] [CrossRef]
- Itoh, T. Effect of branching in base polymer on ionic conductivity in hyperbranched polymer electrolytes. Solid State Ion. 2002, 150, 337–345. [Google Scholar] [CrossRef]
- Gao, C.; Yan, D. Hyperbranched polymers: From synthesis to applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Hawker, C.J.; Chu, F.; Pomery, P.J.; Hill, D.J.T. Hyperbranched Poly(ethylene glycol)s: A New Class of Ion-Conducting Materials. Macromolecules 1996, 29, 3831–3838. [Google Scholar] [CrossRef]
- Xinling, W.; Jianjun, C.; Ling, H.; Xiaozhen, T. Synthesis and ionic conductivity of hyperbranched poly(glycidol). J. Polym. Sci. B Polym. Phys. 2001, 39, 2225–2230. [Google Scholar] [CrossRef]
- Xiaoying, S.; Xiaohui, Y.; Yunhang, L.; Xinling, W. Synthesis and characterization of a multiarm star polymer. J. Polym. Sci. A Polym. Chem. 2004, 42, 2356–2364. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Shao, J.; Liu, Y.; Wang, X. Ionic conductivity of multiarm star polymer/LiClO4 electrolytes. J. Polym. Sci. B Polym. Phys. 2004, 42, 4195–4198. [Google Scholar] [CrossRef]
- Sato, T. Ion-Conductive Polymer Electrolyte Composition of Polyglycidol. U.S. Patent 6,469,107 B1, 22 October 2002. [Google Scholar]
- Sato, T. Polymeric Compound, Polymer for Polyelectrolyte, and Composition for Ionically Conductive Polyelectrolyte. U.S. Patent 6,472,106 B1, 29 October 2002. [Google Scholar]
- Neumann, N.; Boskamp, L.; Hartwig, A.; Koschek, K. Synthesis and characterization of hyperbranched polyglycerols with various degree of methylation employing phase-transfer conditions. Polymer 2021, 229, 124002. [Google Scholar] [CrossRef]
- Itoh, T.; Gotoh, S.; Horii, S.; Hashimoto, S.; Uno, T.; Kubo, M.; Fujinami, T.; Yamamoto, O. Polymer electrolytes based on hyperbranched polymer with cross-linkable groups at the terminals. J. Power Source 2005, 146, 371–375. [Google Scholar] [CrossRef]
- Khurana, R.; Schaefer, J.L.; Archer, L.A.; Coates, G.W. Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: A new approach for practical lithium-metal polymer batteries. J. Am. Chem. Soc. 2014, 136, 7395–7402. [Google Scholar] [CrossRef]
- Fu, G.; Kyu, T. Effect of Side-Chain Branching on Enhancement of Ionic Conductivity and Capacity Retention of a Solid Copolymer Electrolyte Membrane. Langmuir 2017, 33, 13973–13981. [Google Scholar] [CrossRef] [PubMed]
- Neumann, N.; Thinius, S.; Abels, G.; Hartwig, A.; Koschek, K.; Boskamp, L. Multifunctional hyperbranched prepolymers with tailored degree of methylation and methacrylation. Polymer 2023, 276, 125886. [Google Scholar] [CrossRef]
- Evans, J.; Vincent, C.A.; Bruce, P.G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar] [CrossRef]
- Scheller, J.; Brenner, T.; Ott, M.; Fladung, T.; Baur, P.J. Release properties of plasma polymeric coated polymer films and adhesive strength of transferred polyurethane coatings to fiber-reinforced thermosets. Adv. Manuf. Polym. Compos. Sci. 2022, 8, 11–21. [Google Scholar] [CrossRef]
- Sunder, A.; Mülhaupt, R.; Haag, R.; Frey, H. Hyperbranched Polyether Polyols: A Modular Approach to Complex Polymer Architectures. Adv. Mater. 2000, 12, 235–239. [Google Scholar] [CrossRef]
- Sunder, A.; Hanselmann, R.; Frey, H.; Mülhaupt, R. Controlled Synthesis of Hyperbranched Polyglycerols by Ring-Opening Multibranching Polymerization. Macromolecules 1999, 32, 4240–4246. [Google Scholar] [CrossRef]
- Frey, H.; Haag, R. Dendritic polyglycerol: A new versatile biocompatible material. Rev. Mol. Biotechnol. 2002, 90, 257–267. [Google Scholar] [CrossRef]
- Wilms, D.; Stiriba, S.-E.; Frey, H. Hyperbranched polyglycerols: From the controlled synthesis of biocompatible polyether polyols to multipurpose applications. Acc. Chem. Res. 2010, 43, 129–141. [Google Scholar] [CrossRef]
- Imholt, L.; Dörr, T.S.; Zhang, P.; Ibing, L.; Cekic-Laskovic, I.; Winter, M.; Brunklaus, G. Grafted polyrotaxanes as highly conductive electrolytes for lithium metal batteries. J. Power Source 2019, 409, 148–158. [Google Scholar] [CrossRef]
- Bardenhagen, I.; Soto, M.; Langer, F.; Koschek, K.; Schwenzel, J. Solid electrolyte based on 2-adamantanone for all-solid-state lithium-ion batteries. Ionics 2022, 28, 3615–3621. [Google Scholar] [CrossRef]
- Wen, S.J.; Richardson, T.J.; Ghantous, D.I.; Striebel, K.A.; Ross, P.N.; Cairns, E.J. FTIR characterization of PEO + LiN(CF3SO2)2 electrolytes. J. Electroanal. Chem. 1996, 408, 113–118. [Google Scholar] [CrossRef]
- Echeverri, M.; Kim, N.; Kyu, T. Ionic Conductivity in Relation to Ternary Phase Diagram of Poly(ethylene oxide), Succinonitrile, and Lithium Bis(trifluoromethane)sulfonimide Blends. Macromolecules 2012, 45, 6068–6077. [Google Scholar] [CrossRef]
- Fox, T.G.; Loshaek, S. Influence of molecular weight and degree of crosslinking on the specific volume and glass temperature of polymers. J. Polym. Sci. 1955, 15, 371–390. [Google Scholar] [CrossRef]
- Everaers, R.; Kremer, K. Test of the Foundations of Classical Rubber Elasticity. Macromolecules 1995, 28, 7291–7294. [Google Scholar] [CrossRef]
- Barai, P.; Higa, K.; Srinivasan, V. Lithium dendrite growth mechanisms in polymer electrolytes and prevention strategies. Phys. Chem. Chem. Phys. 2017, 19, 20493–20505. [Google Scholar] [CrossRef] [PubMed]
- Monroe, C.; Newman, J. The Impact of Elastic Deformation on Deposition Kinetics at Lithium/Polymer Interfaces. Electrochim. Acta 2005, 152, A396. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung e. V. Kunststoffe—Bestimmung Dynamisch-Mechanischer Eigenschaften—Teil 1: Allgemeine Grundlagen; Beuth Verlag GmbH: Berlin, Germany, 2019. [Google Scholar]
- Elias, H.-G. Physikalische Strukturen und Eigenschaften, 6th ed.; Wiley-VCH: Weinheim, Germany, 2009; ISBN 3-527-29960-2. [Google Scholar]
- Bouchet, R.; Lascaud, S.; Rosso, M. An EIS Study of the Anode Li/PEO-LiTFSI of a Li Polymer Battery. Macromolecules 2003, 150, A1385. [Google Scholar] [CrossRef]
- Vadhva, P.; Hu, J.; Johnson, M.J.; Stocker, R.; Braglia, M.; Brett, D.J.L.; Rettie, A.J.E. Electrochemical Impedance Spectroscopy for All-Solid-State Batteries: Theory, Methods and Future Outlook. ChemElectroChem 2021, 8, 1930–1947. [Google Scholar] [CrossRef]
- Saeed, M.A.M.; Abdullah, O.G. Effect of High Ammonium Salt Concentration and Temperature on the Structure, Morphology, and Ionic Conductivity of Proton-Conductor Solid Polymer Electrolytes Based PVA. Membranes 2020, 10, 262. [Google Scholar] [CrossRef]
- Siekierski, M.; Bukat, M.; Ciosek, M.; Piszcz, M.; Mroczkowska-Szerszeń, M. Transference Number Determination in Poor-Dissociated Low Dielectric Constant Lithium and Protonic Electrolytes. Polymers 2021, 13, 895. [Google Scholar] [CrossRef]
- Tułodziecki, M.; Tarascon, J.-M.; Taberna, P.-L.; Guéry, C. Catalytic reduction of TFSI-containing ionic liquid in the presence of lithium cations. Electrochem. Commun. 2017, 77, 128–132. [Google Scholar] [CrossRef]
- Shin, W.; Manthiram, A. A Facile Potential Hold Method for Fostering an Inorganic Solid-Electrolyte Interphase for Anode-Free Lithium-Metal Batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202115909. [Google Scholar] [CrossRef] [PubMed]
- Theuerkauf, D.; Swan, L. Characteristics of Open Circuit Voltage Relaxation in Lithium-Ion Batteries for the Purpose of State of Charge and State of Health Analysis. Batteries 2022, 8, 77. [Google Scholar] [CrossRef]
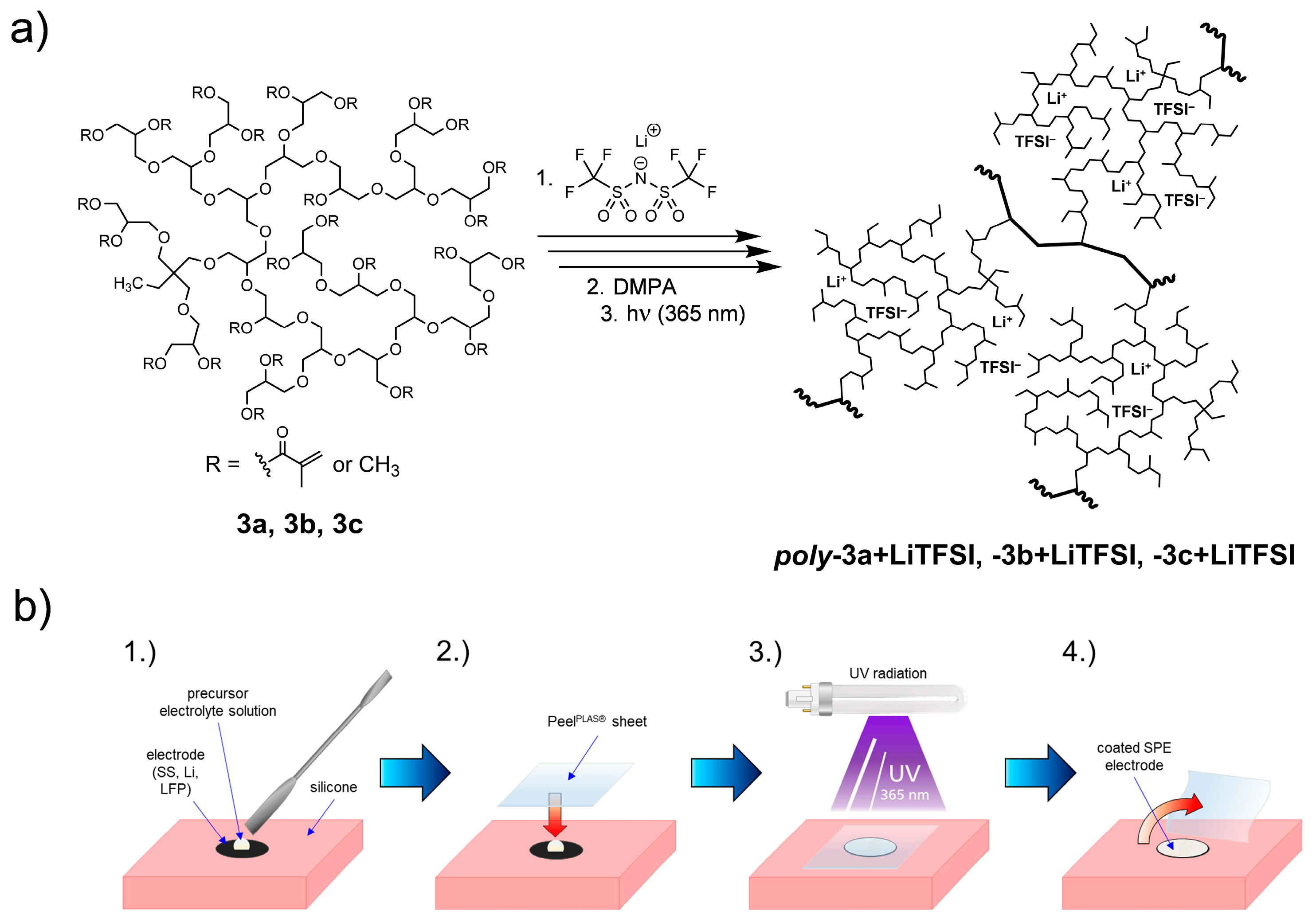
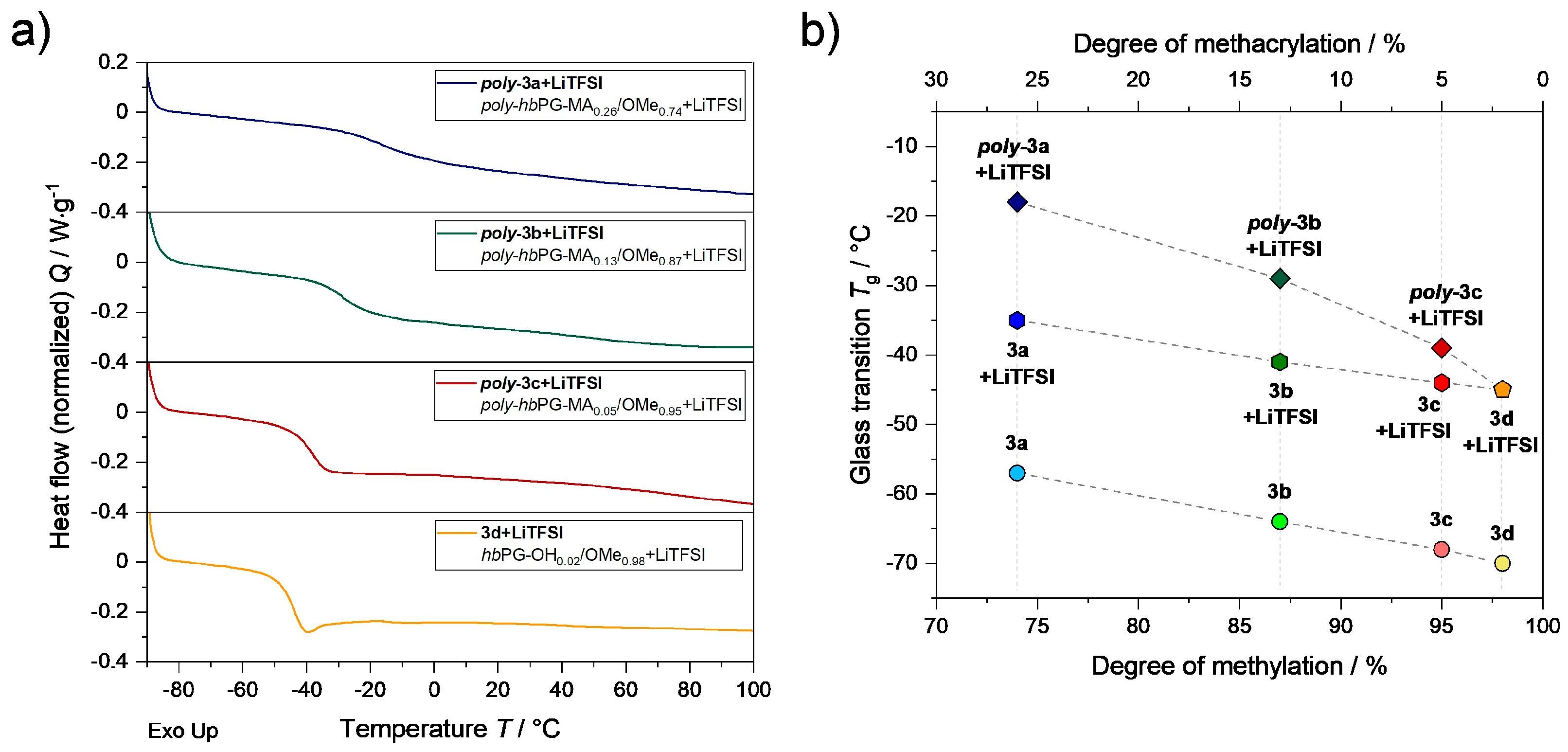
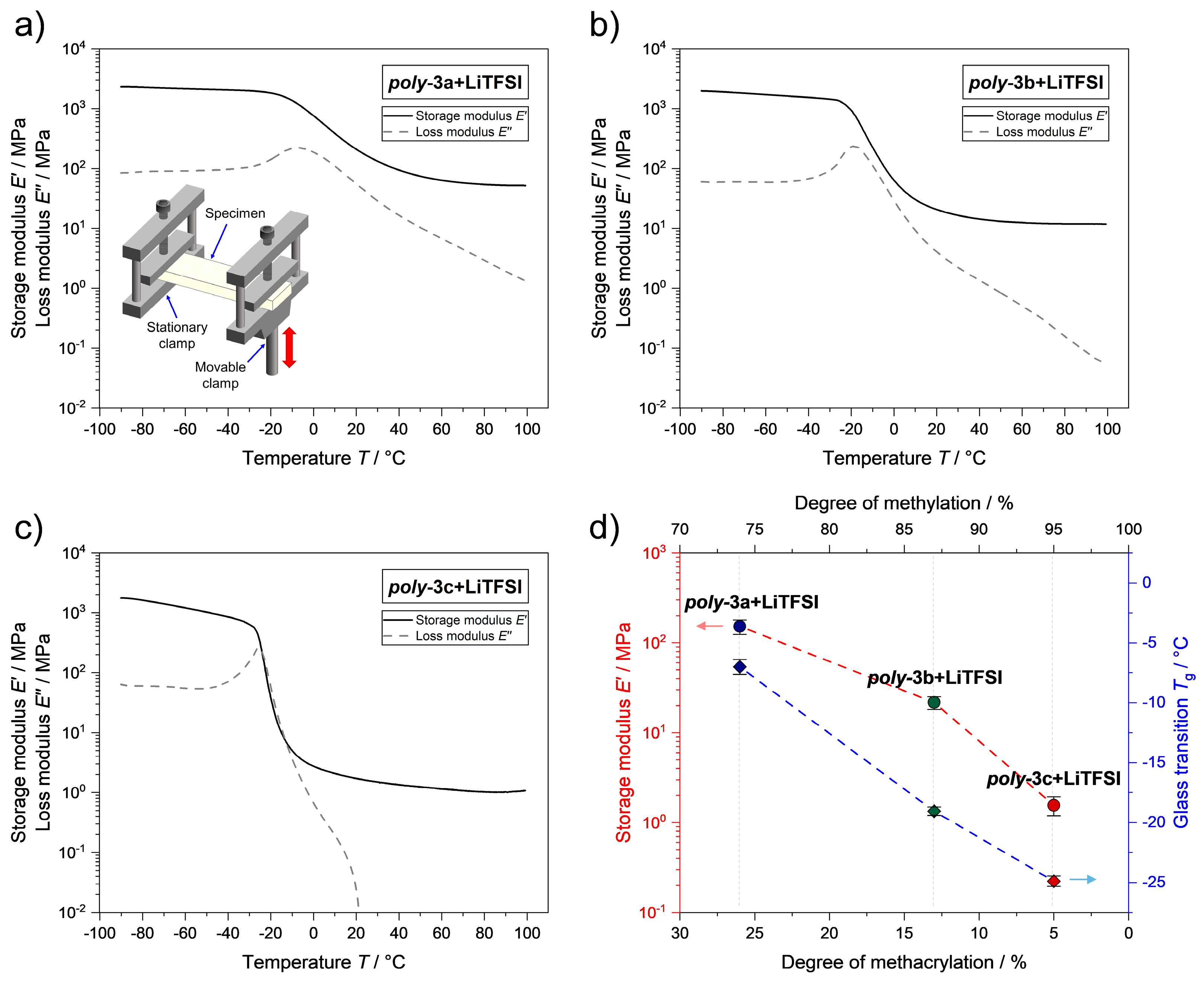
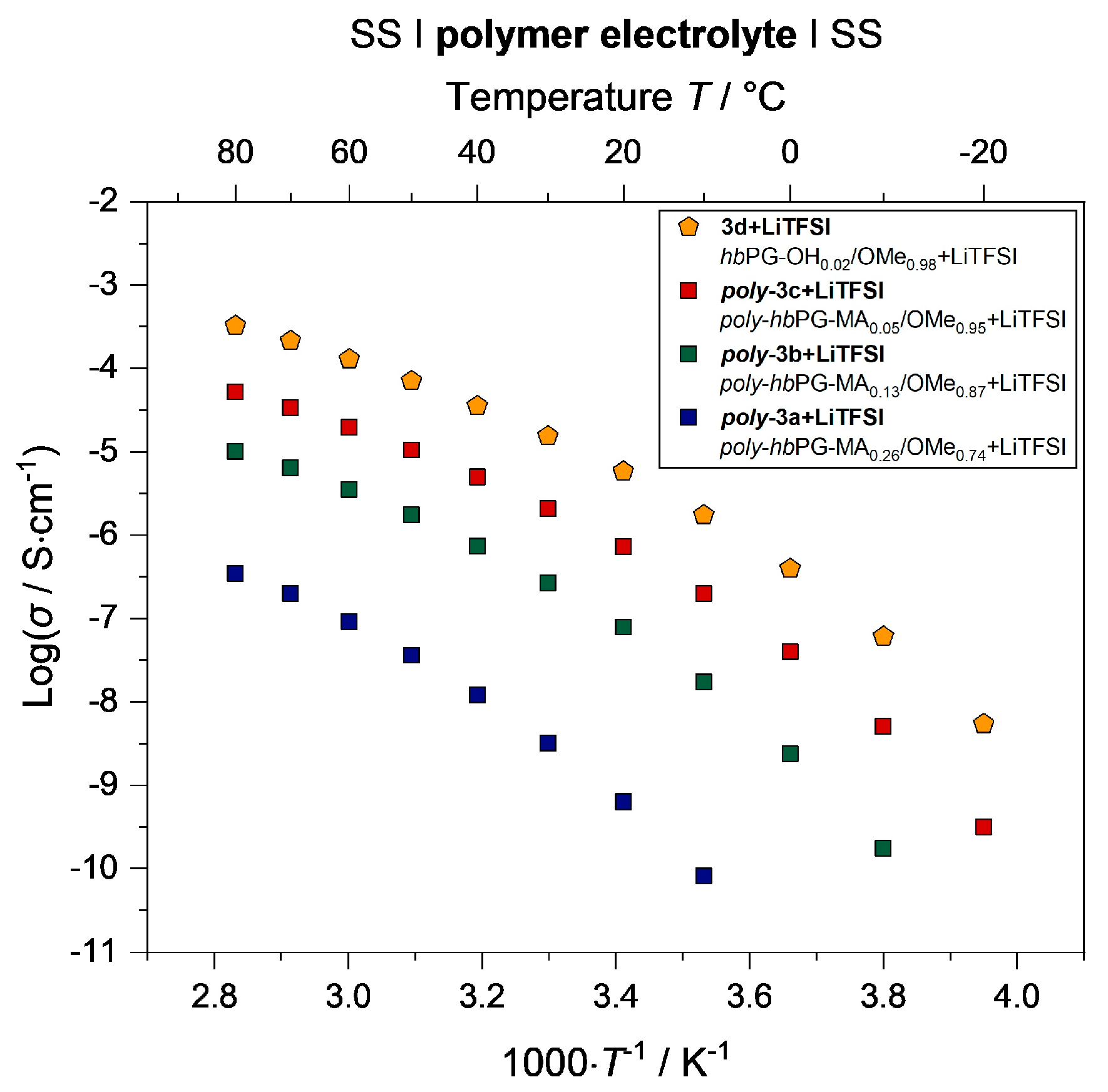

| No. | Name a | DM a /% | DS a /% | Tg DSC b /°C | Tg DMA c /°C | E′ d /MPa | Ionic Conductivity e /S⋅cm−1 (30 °C) /S⋅cm−1 (60 °C) | tLi+ e | |
|---|---|---|---|---|---|---|---|---|---|
| poly-3a+LiTFSI | poly-hbPG-MA0.26/OMe0.74+LiTFSI | 74 | 26 | −18 | −7 | 152 ± 28 | 3.2 × 10−9 | 9.1 × 10−8 | 0.19 |
| poly-3b+LiTFSI | poly-hbPG-MA0.13/OMe0.87+LiTFSI | 87 | 13 | −28 | −19 | 22 ± 4 | 2.7 × 10−7 | 3.5 × 10−6 | 0.08 |
| poly-3c+LiTFSI | poly-hbPG-MA0.05/OMe0.95+LiTFSI | 95 | 5 | −39 | −25 | 1.6 ± 0.4 | 2.1 × 10−6 | 2.0 × 10−5 | 0.07 |
| 3d+LiTFSI | hbPG-OH0.02/OMe0.98+LiTFSI | 98 | 0 | −45 | - | - | 1.5 × 10−5 | 1.3 × 10−4 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann, N.; Abels, G.; Koschek, K.; Boskamp, L. Crosslinked Hyperbranched Polyglycerol-Based Polymer Electrolytes for Lithium Metal Batteries. Batteries 2023, 9, 431. https://doi.org/10.3390/batteries9090431
Neumann N, Abels G, Koschek K, Boskamp L. Crosslinked Hyperbranched Polyglycerol-Based Polymer Electrolytes for Lithium Metal Batteries. Batteries. 2023; 9(9):431. https://doi.org/10.3390/batteries9090431
Chicago/Turabian StyleNeumann, Niklas, Gideon Abels, Katharina Koschek, and Laura Boskamp. 2023. "Crosslinked Hyperbranched Polyglycerol-Based Polymer Electrolytes for Lithium Metal Batteries" Batteries 9, no. 9: 431. https://doi.org/10.3390/batteries9090431
APA StyleNeumann, N., Abels, G., Koschek, K., & Boskamp, L. (2023). Crosslinked Hyperbranched Polyglycerol-Based Polymer Electrolytes for Lithium Metal Batteries. Batteries, 9(9), 431. https://doi.org/10.3390/batteries9090431








