Dual-Functional Electrolyte Additive for Lithium–Sulfur Batteries Limits Lithium Dendrite Formation and Increases Sulfur Utilization Rate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Electrolyte
2.2. Cathode Preparation
2.3. Electrochemical Measurements
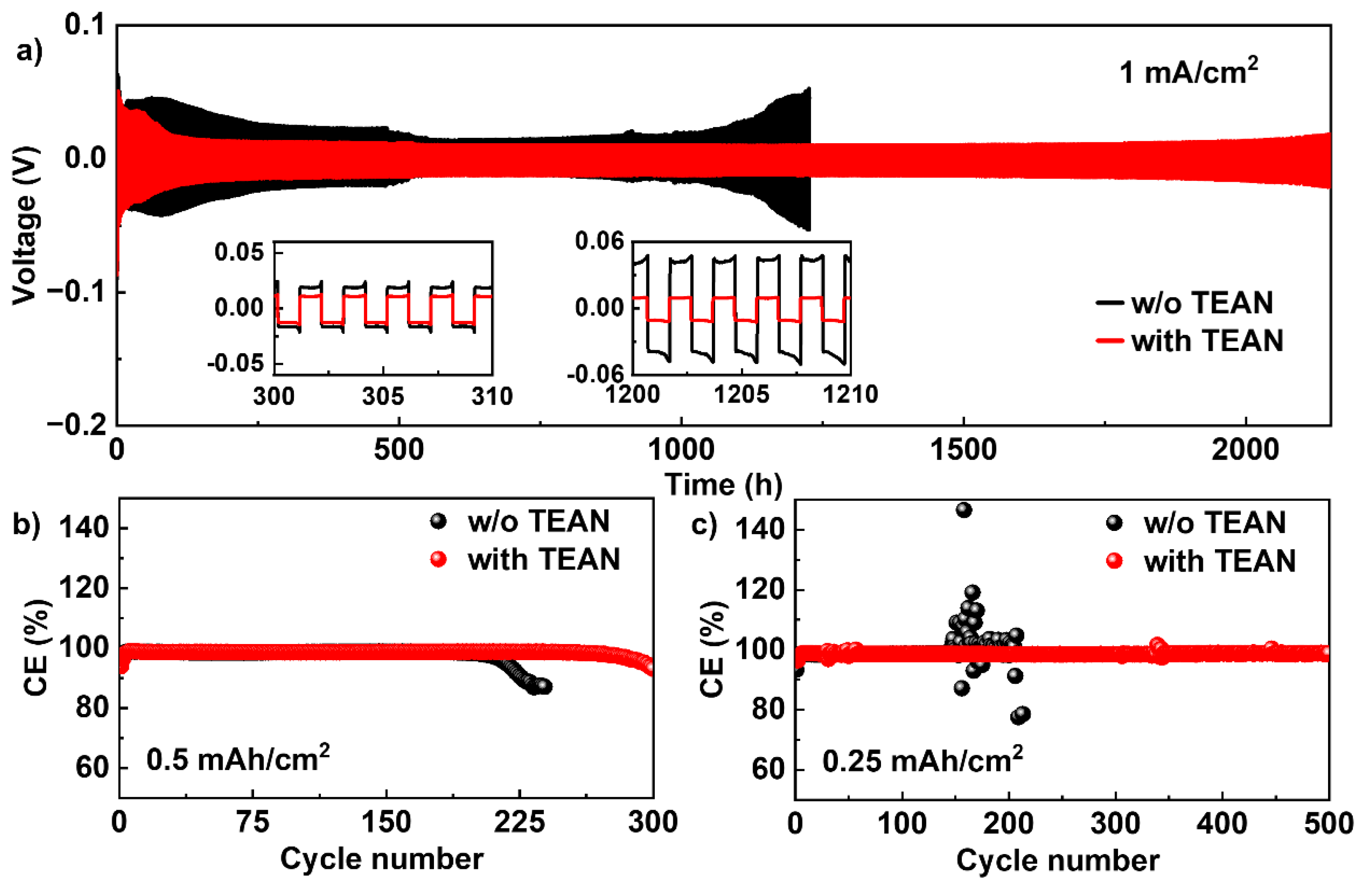
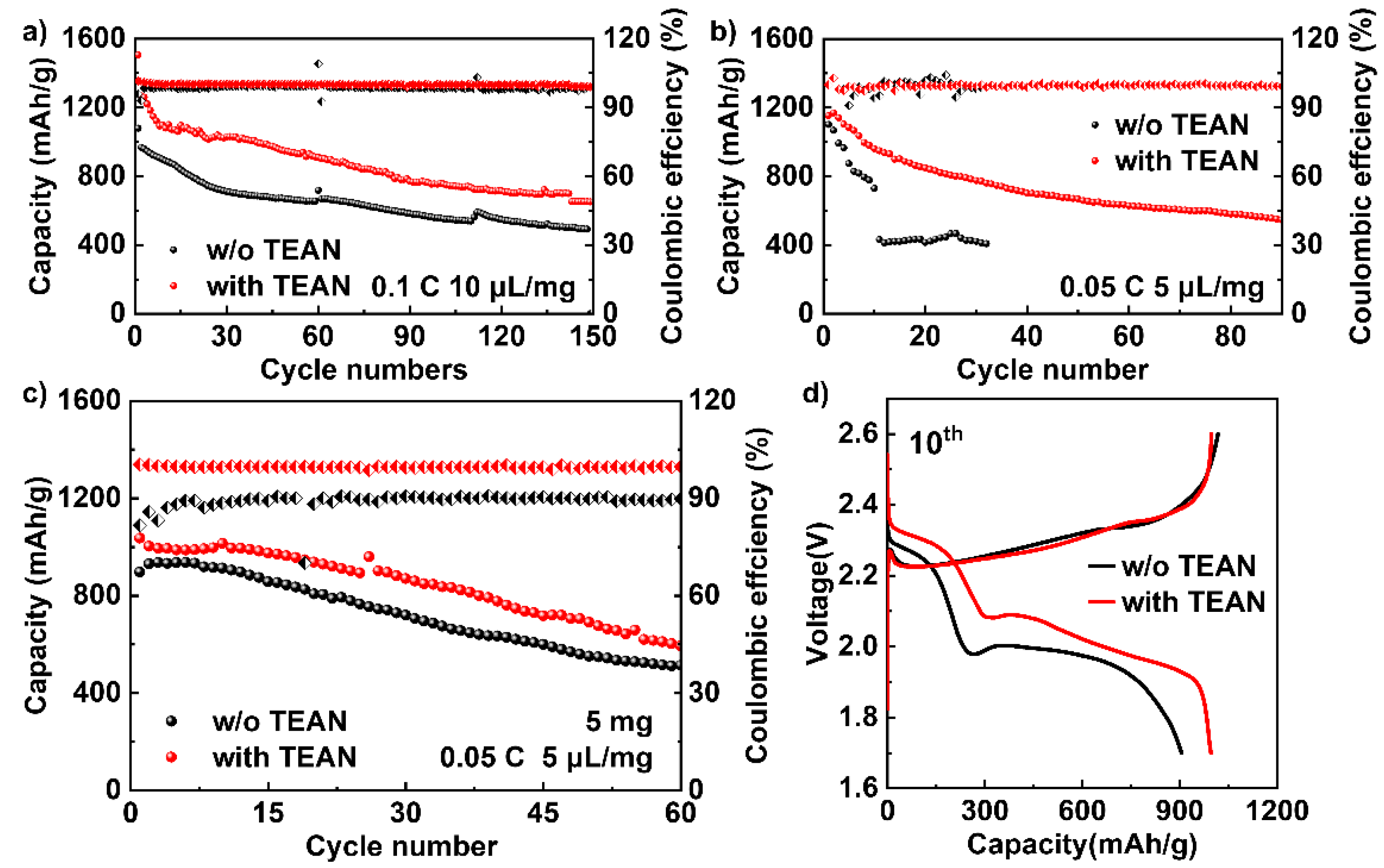
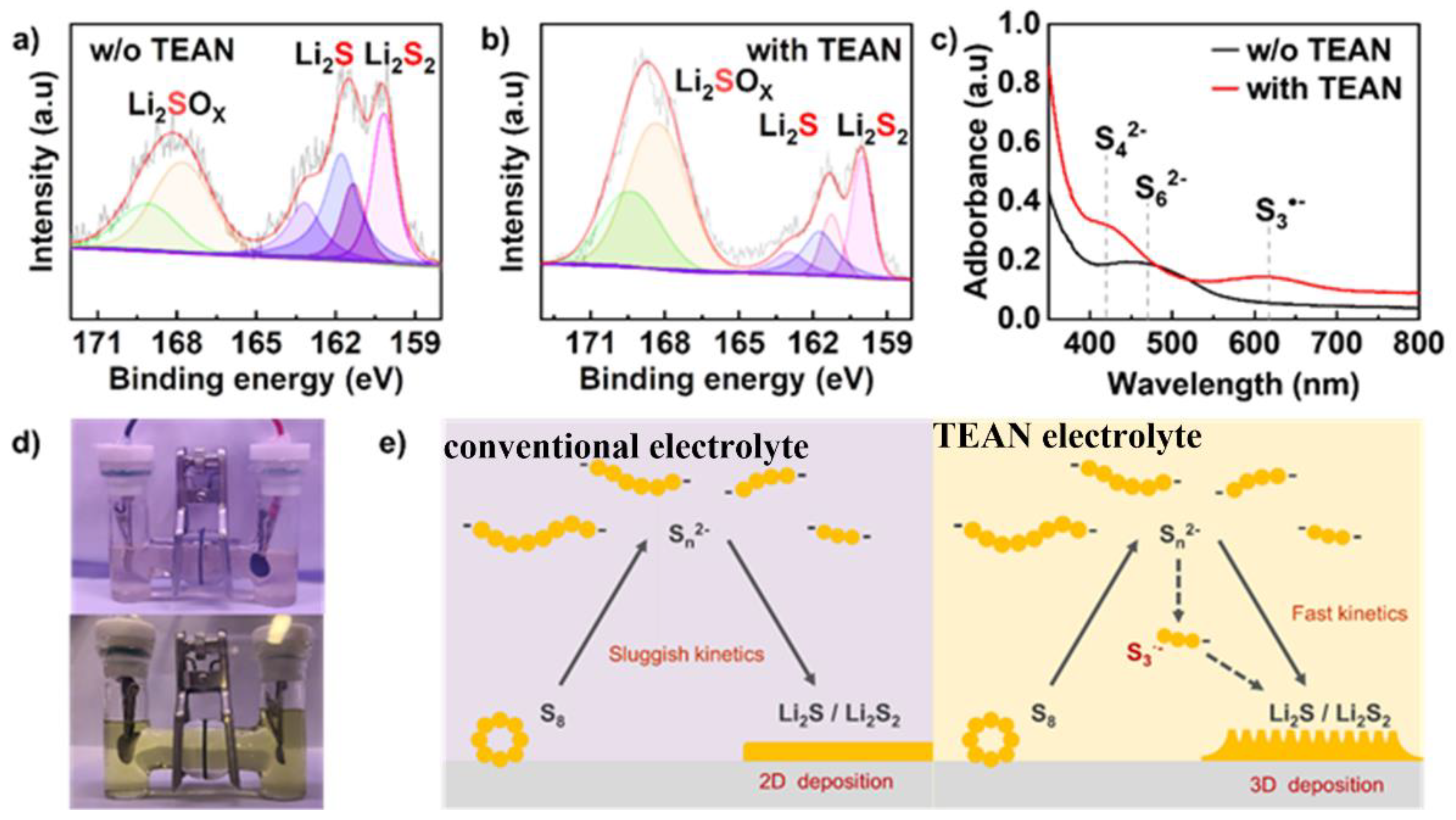
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Evers, S.; Nazar, L.F. New Approaches for High Energy Density Lithium–Sulfur Battery Cathodes. Acc. Chem. Res. 2013, 46, 1135–1143. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium–sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Tian, H.; Su, D.; Zhang, Q.; Wang, G. Advances in Lithium–Sulfur Batteries: From Academic Research to Commercial Viability. Adv. Mater. 2021, 33, 2003666. [Google Scholar] [CrossRef]
- Yu, L.; Ong, S.J.H.; Liu, X.; Mandler, D.; Xu, Z.J. The importance of the dissolution of polysulfides in lithium-sulfur batteries and a perspective on high-energy electrolyte/cathode design. Electrochim. Acta 2021, 392, 139013. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Rosenman, A.; Markevich, E.; Salitra, G.; Aurbach, D.; Garsuch, A.; Chesneau, F.F. Review on Li-Sulfur Battery Systems: An Integral Perspective. Adv. Energy Mater. 2015, 5, 1500212. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Hagen, M.; Fanz, P.; Tübke, J. Cell energy density and electrolyte/sulfur ratio in Li–S cells. J. Power Sources 2014, 264, 30–34. [Google Scholar] [CrossRef]
- Chen, S.; Gao, Y.; Yu, Z.; Gordin, M.L.; Song, J.; Wang, D. High capacity of lithium-sulfur batteries at low electrolyte/sulfur ratio enabled by an organosulfide containing electrolyte. Nano Energy 2017, 31, 418–423. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Y.; Shi, Q.; Wang, Z.; Sun, K.; Wang, H. Electrocatalysis in Lithium Sulfur Batteries under Lean Electrolyte Conditions. Angew. Chem. Int. Ed. 2018, 57, 15549–15552. [Google Scholar] [CrossRef]
- Shin, H.; Baek, M.; Gupta, A.; Char, K.; Manthiram, A.; Choi, J.W. Recent Progress in High Donor Electrolytes for Lithium–Sulfur Batteries. Adv. Energy Mater. 2020, 10, 2001456. [Google Scholar] [CrossRef]
- Chu, H.; Jung, J.; Noh, H.; Yuk, S.; Lee, J.; Lee, J.H.; Baek, J.; Roh, Y.; Kwon, H.; Choi, D.; et al. Unraveling the Dual Functionality of High-Donor-Number Anion in Lean-Electrolyte Lithium-Sulfur Batteries. Adv. Energy Mater. 2020, 10, 2000493. [Google Scholar] [CrossRef]
- Ebadi, M.; Lacey, M.J.; Brandell, D.; Araujo, C.M.G. Density Functional Theory Modeling the Interfacial Chemistry of the LiNO3 Additive for Lithium–Sulfur Batteries by Means of Simulated Photoelectron Spectroscopy. J. Phys. Chem. C 2017, 121, 23324–23332. [Google Scholar] [CrossRef]
- Xu, H.; Han, C.; Li, W.; Li, H.; Qiu, X. Quantification of lithium dendrite and solid electrolyte interphase (SEI) in lithium-ion batteries. J. Power Sources 2022, 529, 231219. [Google Scholar] [CrossRef]
- Fan, X.-Z.; Liu, M.; Zhang, R.; Zhang, Y.; Wang, S.; Nan, H.; Han, Y.; Kong, L. An odyssey of lithium metal anode in liquid lithium–sulfur batteries. Chin. Chem. Lett. 2022, 33, 4421–4427. [Google Scholar] [CrossRef]
- Dai, H.; Xi, K.; Liu, X.; Lai, C.; Zhang, S. Cationic Surfactant-Based Electrolyte Additives for Uniform Lithium Deposition via Lithiophobic Repulsion Mechanisms. J. Am. Chem. Soc. 2018, 140, 17515–17521. [Google Scholar] [CrossRef]
- Jana, A.; Woo, S.I.; Vikrant, K.S.N.; García, R.E. Electrochemomechanics of lithium dendrite growth. Energy Environ. Sci. 2019, 12, 3595–3607. [Google Scholar] [CrossRef]
- He, L.; Sun, Q.; Lu, L.; Adams, S. Understanding and Preventing Dendrite Growth in Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 34320–34331. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Subramani, R.; Huang, Y.-T.; Lee, Y.-L.; Jan, J.-S.; Chiu, C.-C.; Hou, S.-S.; Teng, H. Highly stable interface formation in onsite coagulation dual-salt gel electrolyte for lithium-metal batteries. J. Mater. Chem. A 2021, 9, 5675–5684. [Google Scholar] [CrossRef]
- Hyun, J.-H.; Yi, M.-J.; Jung, H.; Lee, S.-H.; Um, J.H.; Yu, S.-H. Electrochemical behavior and morphological evolution of Li metal anode under high cycling capacity. Energy Storage Mater. 2023, 54, 146–155. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Miao, X.; Sun, R.; Li, J.; Zhang, Z.; Wang, R.; Wang, C.; Li, Z.; Yin, L. Achieve Stable Lithium Metal Anode by Sulfurized-Polyacrylonitrile Modified Separator for High-Performance Lithium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 14264–14273. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, X.; Li, R.; Gao, L.; Luo, J. Dendrite-free Li metal anode by lowering deposition interface energy with Cu99Zn alloy coating. Energy Storage Mater. 2018, 14, 143–148. [Google Scholar] [CrossRef]
- Li, P.; Li, C.; Yang, Y.; Zhang, C.; Wang, R.; Liu, Y.; Wang, Y.; Luo, J.; Dong, X.; Xia, Y. Synergistic Effects of Salt Concentration and Working Temperature towards Dendrite-Free Lithium Deposition. Research 2019, 2019, 7481319. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, X.; Liu, L.; Zhu, X.; Guo, Z.; Guo, W.; Han, Q.; He, J.; Zhao, Y. Uniform coverage of high-loading sulfur on cross-linked carbon nanofibers for high reaction kinetics in Li–S batteries with low electrolyte/sulfur ratio. J. Mater. Chem. A 2022, 10, 1433–1441. [Google Scholar] [CrossRef]
- Wang, L.; Zhen, M.; Hu, Z. Status and prospects of electrocatalysts for lithium-sulfur battery under lean electrolyte and high sulfur loading conditions. Chem. Eng. J. 2023, 452, 139344. [Google Scholar] [CrossRef]
- Luo, C.; Hu, E.; Gaskell, K.J.; Fan, X.; Gao, T.; Cui, C.; Ghose, S.; Yang, X.-Q.; Wang, C. A chemically stabilized sulfur cathode for lean electrolyte lithium sulfur batteries. Proc. Natl. Acad. Sci. USA 2020, 117, 14712–14720. [Google Scholar] [CrossRef]
- Wu, C.C.; Chung, S.H. Material, configuration, and fabrication designs for lean-electrolyte lithium-sulfur cell with a high-loading sulfur cathode. J. Power Sources 2023, 566, 232944. [Google Scholar] [CrossRef]
- Zhang, L.; Ling, M.; Feng, J.; Mai, L.; Liu, G.; Guo, J. The synergetic interaction between LiNO3 and lithium polysulfides for suppressing shuttle effect of lithium-sulfur batteries. Energy Storage Mater. 2018, 11, 24–29. [Google Scholar] [CrossRef]
- Soni, R.; Robinson, J.B.; Shearing, P.R.; Brett, D.J.; Rettie, A.J.; Miller, T.S. Lithium-sulfur battery diagnostics through distribution of relaxation times analysis. Energy Storage Mater. 2022, 51, 97–107. [Google Scholar] [CrossRef]
- Capkova, D.; Knap, V.; Fedorkova, A.S.; Stroe, D.-I. Analysis of 3.4 Ah lithium-sulfur pouch cells by electrochemical impedance spectroscopy. J. Energy Chem. 2022, 72, 318–325. [Google Scholar] [CrossRef]
- Lv, X.; Yang, Q.; Zhang, X.; Song, J.; Guo, W.; Wang, Q. Acceleration of Cathode Interfacial Kinetics by Liquid Organosulfides in Lithium Metal Batteries. Angew. Chem. Int. Ed. 2022, 61, e202213160. [Google Scholar] [CrossRef]
- Zeng, W.; Cheng, M.M.-C.; Ng, S.K.-Y. Effects of transition metal cation additives on the passivation of lithium metal anode in Li–S batteries. Electrochim. Acta 2019, 319, 511–517. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, M.; Wu, X.; Liang, J.; Liu, Y.; Zhang, T.; Zhu, Y.; Qian, Y. Dendrite-tamed deposition kinetics using single-atom Zn sites for Li metal anode. Energy Storage Mater. 2019, 23, 587–593. [Google Scholar] [CrossRef]
- Lovrić, M. Maxima in chronoamperometry of irreversible electrode reactions. J. Electroanal. Chem. 2021, 888, 115187. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Shen, K.; Xu, H.; Wang, W.; Yang, Z.; Zhang, L.; Chen, J.; Huang, Y.; Chen, M.; et al. Stable Lithium Sulfur Battery Based on In Situ Electrocatalytically Formed Li2S on Metallic MoS2-Carbon Cloth Support. Small Methods 2020, 4, 2000353. [Google Scholar] [CrossRef]
- Zhong, N.; Lei, C.; Meng, R.; Li, J.; He, X.; Liang, X. Electrolyte Solvation Chemistry for the Solution of High-Donor-Number Solvent for Stable Li-S Batteries. Small 2022, 18, e2200046. [Google Scholar] [CrossRef]
- Jung, J.; Chu, H.; Kim, I.; Lee, D.H.; Doo, G.; Kwon, H.; Jo, W.; Kim, S.; Cho, H.; Kim, H. Confronting Sulfur Electrode Passivation and Li Metal Electrode Degradation in Lithium-Sulfur Batteries Using Thiocyanate Anion. Adv. Sci. 2023, 10, 2301006. [Google Scholar] [CrossRef]
- Wang, S.; Huang, F.; Li, X.; Li, W.; Chen, Y.; Tang, X.; Jiao, S.; Cao, R. Regulating Li2S Deposition by Ostwald Ripening in Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2022, 14, 4204–4210. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Shin, H.; Char, K.; Choi, J.W. New High Donor Electrolyte for Lithium–Sulfur Batteries. Adv. Mater. 2020, 32, e2005022. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Xiao, Z.; Guo, Z.; Lai, Y.; Zhang, Y.; Zhou, S.; Ding, X.; Nie, H.; Yang, Z. Titanium silicalite as a radical-redox mediator for high-energy-density lithium–sulfur batteries. Nanoscale 2019, 11, 16968–16977. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Lu, Y.-C. Solvent-Dictated Lithium Sulfur Redox Reactions: An Operando UV–vis Spectroscopic Study. J. Phys. Chem. Lett. 2016, 7, 1518–1525. [Google Scholar] [CrossRef]
- Xu, N.; Qian, T.; Liu, X.; Liu, J.; Chen, Y.; Yan, C. Greatly Suppressed Shuttle Effect for Improved Lithium Sulfur Battery Performance through Short Chain Intermediates. Nano Lett. 2017, 17, 538–543. [Google Scholar] [CrossRef]
- Nahian, S.; Jayan, R.; Islam, M. Atomic-Scale Insights into Comparative Mechanisms and Kinetics of Na–S and Li–S Batteries. ACS Catal. 2022, 12, 7664–7676. [Google Scholar] [CrossRef]
- Shao, Q.; Lu, P.; Xu, L.; Guo, D.; Gao, J.; Wu, Z.-S.; Chen, J. Rational design of MoS2 nanosheets decorated on mesoporous hollow carbon spheres as a dual-functional accelerator in sulfur cathode for advanced pouch-type Li–S batteries. J. Energy Chem. 2020, 51, 262–271. [Google Scholar] [CrossRef]
- Gupta, A.; Bhargav, A.; Manthiram, A. Evoking High-Donor-Number-Assisted and Organosulfur-Mediated Conversion in Lithium–Sulfur Batteries. ACS Energy Lett. 2021, 6, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bhargav, A.; Manthiram, A. Highly Solvating Electrolytes for Lithium–Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1803096. [Google Scholar] [CrossRef]
- He, Q.; Gorlin, Y.; Patel, M.U.M.; Gasteiger, H.A.; Lu, Y.-C. Unraveling the Correlation between Solvent Properties and Sulfur Redox Behavior in Lithium-Sulfur Batteries. J. Electrochem. Soc. 2018, 165, A4027–A4033. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wu, H.; Wu, J.; Xiao, Y.; Deng, Y. Dual-Functional Electrolyte Additive for Lithium–Sulfur Batteries Limits Lithium Dendrite Formation and Increases Sulfur Utilization Rate. Batteries 2023, 9, 444. https://doi.org/10.3390/batteries9090444
Liu C, Wu H, Wu J, Xiao Y, Deng Y. Dual-Functional Electrolyte Additive for Lithium–Sulfur Batteries Limits Lithium Dendrite Formation and Increases Sulfur Utilization Rate. Batteries. 2023; 9(9):444. https://doi.org/10.3390/batteries9090444
Chicago/Turabian StyleLiu, Chang, Huiyuan Wu, Jiachun Wu, Yinglin Xiao, and Yonghong Deng. 2023. "Dual-Functional Electrolyte Additive for Lithium–Sulfur Batteries Limits Lithium Dendrite Formation and Increases Sulfur Utilization Rate" Batteries 9, no. 9: 444. https://doi.org/10.3390/batteries9090444
APA StyleLiu, C., Wu, H., Wu, J., Xiao, Y., & Deng, Y. (2023). Dual-Functional Electrolyte Additive for Lithium–Sulfur Batteries Limits Lithium Dendrite Formation and Increases Sulfur Utilization Rate. Batteries, 9(9), 444. https://doi.org/10.3390/batteries9090444






