Recent Advances on Transition Metal Chalcogenide for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Sodium Storage Mechanism
- Intercalation process of the sodium ions:
- 2.
- Transformation reactions (generally transition metal-based chalcogenides, such as Co, Ni, Cu, etc.) occur:
- 3.
- Alloying reactions (often occurring in Bi, Sn-based, and other sulfuric materials with some metal activity):
3. Optimization Strategy for Increasing Sodium Reserves
3.1. Enhanced Structural Stability
3.1.1. Nanostructure Engineering
3.1.2. Defect Engineering
3.1.3. Cladding Engineering
3.2. Enhanced Conductivity of Electrodes
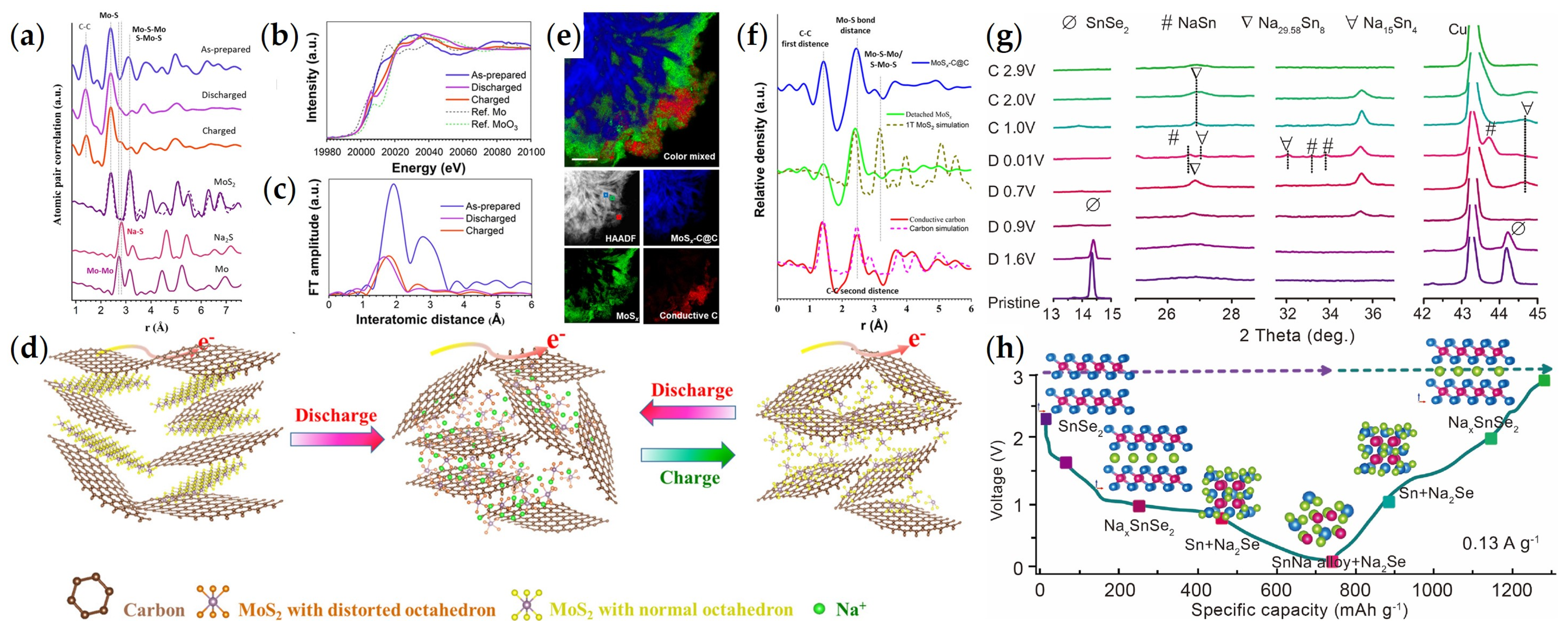
3.2.1. Defective Engineering
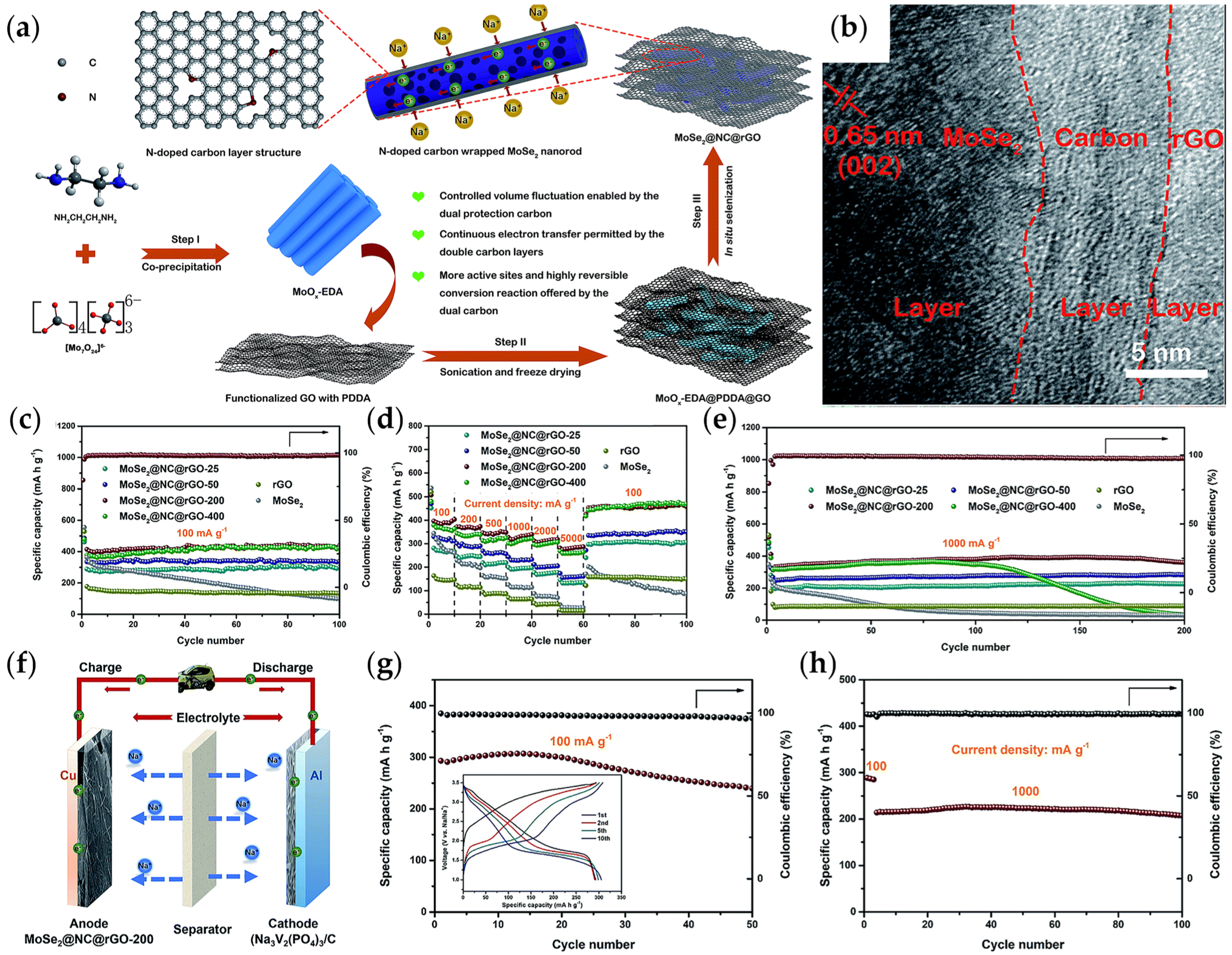
3.2.2. Construction of Heterogeneous Structures
3.2.3. Composite with Carbonaceous Materials
4. Conclusions
- Mitigating the volume expansion and agglomeration of TMX materials during charge/discharge cycles is a crucial issue in improving structural stability. At present, the research mainly focuses on the structural and dimensional design of materials, apparently starting with the construction of small-sized nanomaterials, hollow structures, porous structures, and egg yolk-shell structures; these structures with void spaces are often constructed using MOF and MXene as templates, and the existence of voids can alleviate the volume change. The problem of small-size nanoparticles prone to accumulation and agglomeration can be alleviated by covering the surface of each nanoparticle with carbon layers or carbon networks. The intrinsic aspect is mainly to regulate the crystal structure inside the material, and defects or vacancies are introduced by defect engineering to regulate the electron distribution, which in turn affects the crystal structure.
- Enhancing the conductivity of materials is the key to obtaining high-capacity batteries, and most of the solutions commonly used at present focus on heteroatom doping, introducing vacancy, constructing heterogeneous structures, and composite with carbon matrix materials (such as graphene, MOF precursors, MXene, etc.). Traditional heteroatom doping such as N, S, P, and other atoms can also improve the conductivity of the materials, but such dopants are often more expensive and environmentally unfriendly. The use of cheap and environmentally compatible materials (such as chlorella) may be the future trend of dopant development. Building heterogeneous structures is also a common method to improve the conductivity of TMXs because it can form an internal electric field. The key to this approach is the choice of another material. For example, TMXs combined with a non-polar carbon material will form an unstable heterogeneous structure due to poor electronic coupling but can form a strong and stable electric field with a strongly polar carbon material (such as MXene). The volume energy of TMXs is often poor after forming a heterogeneous structure with conductive carbon, which can be solved by combining with metal elements/metal compounds. In addition, the formation of heterogeneous structures can also play a role in slowing down the agglomeration of intermediate products during the charge–discharge cycle. The development of materials that can form strong and stable heterogeneous structures with TMXs and have high-volume energy may be the trend of future development. Composite TMXs with carbonaceous materials (such as graphene oxide, MOF, MXene, etc.) are also a good choice, especially when using MOF materials, as the carbon matrix and metal ions are derived from the same molecule, and the two can form strong electronic coupling. Therefore, the development of carbon materials with strong electrical conductivity and strong electron coupling is the key to this method.
- The current trend is to combine these approaches to improve structural stability and electrical conductivity and develop simpler, environmentally friendly, and less costly synthetic methods to synthesize TMX composites. In addition, it is necessary to combine more advanced characterization techniques and more theoretical work to fully explore the internal mechanism leading to the structural instability and poor electrical conductivity of active materials and fully understand the sodium storage mechanism and failure mechanism of SIBs. In recent years, full batteries that match TMX-negative electrodes with other positive electrode materials have been developed, but truly commercialized full batteries are few and far between and still require a lot of effort.
| Materials | Synthesis Method | Cycle Performance | Rate Performance | Electrolyte | Voltage Interval | Reference |
|---|---|---|---|---|---|---|
| Fe7Se8 @C@MoSe2 | co-precipitation | 87%/600/1 | 274.5/5 | 1M NaPF6 | / | [129] |
| Willow-leaf-like ZnSe@NC | solvothermal | 242.2/3200/8 | 144.4/10 | 1M NaCF3 SO3 | 0.01–3 | [130] |
| FeSe2 @C microspheres | hydrothermal | 428/1000/1 | / | 1M NaCF3 SO3 | 0.5–2.9 | [131] |
| FeSe2/NC@GE | / | 323/1000/2 | 331/5 | 1M NaClO4 | 1.0–3 | [132] |
| SnSe2/ZnSe@PDA nanobox | co-precipitation | 616/1000/1 | / | 1M NaPF6 | 0.1–3 | [83] |
| FeSe2 @NC microrods | hydrothermal | 401.3/2000/5 | 411/10 | 1M NaCF3 SO3 | 0.4–2.9 | [133] |
| ZnSe⊂N-C@MoSe2/rGO | template engaged | 177.7/5000/10 | 224.4/10 | 1M NaClO4 | 0.01–3 | [134] |
| Mesoporous FeSe2 @C | selenization | 483/100/0.2 | / | 1M NaClO4 | 0–3 | [135] |
| Core/shell FeSe @CNS nanosheet | in situ pyrolysis | 100/10,000/30 | 183.8/30 | 1M NaCF3 SO3 | 0.01–2.8 | [136] |
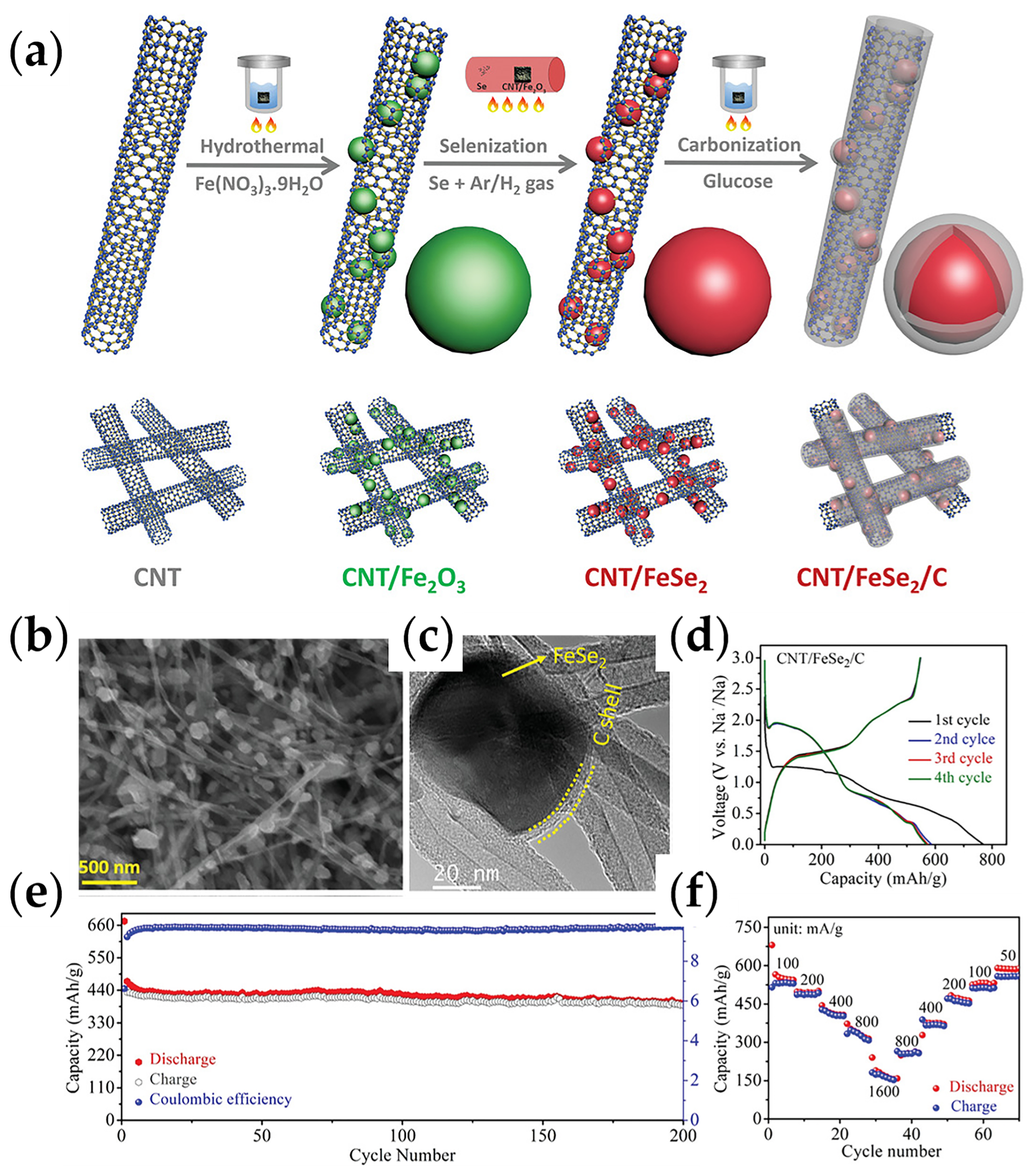
| CNT/FeSe2/C | wet chemistry | 546/100/0.1 | 423/0.5 | 1M NaClO4 | 0.01–3 | [89] |
| Fe7Se8/N-CNF | electrospinning | / | 286.3/20 | 1M NaCF3SO3 | 0.5–2.5 | [78] |
| SnSe2/FeSe2/NC | co-precipitation | 408.1/1200/6 | 345/20 | 1M NaPF6 | / | [137] |
| CoSe/G | self-assembly | 214/600/2 | 290/5 | 1M NaClO4 | 0.01–3 | [92] |
| In2Se3-CoIn2-CoSe2 | / | 205.5/2000/10 | 371.6/20 | 1M NaPF6 | 0.01–2.5 | [138] |
| FeSe2 microspheres | solvothermal | / | 525/20 | 1M NaPF6 | 0.01–3 | [139] |
| FeSe2 @rGO | / | 350/600/5 | / | 1M NaPF6 | 0.01–3 | [140] |
| Co0.85Se@ carbon nanotubes | pyrolysis selenization | 306.4/800/2 | 222.5/5 | 1M NaClO4 | / | [75] |
| Rich-oxygen-doped FeSe2 nanosheets | / | 268/700/1 | 258/3 | 1M NaCF3 SO3 | 0.25–2.5 | [141] |
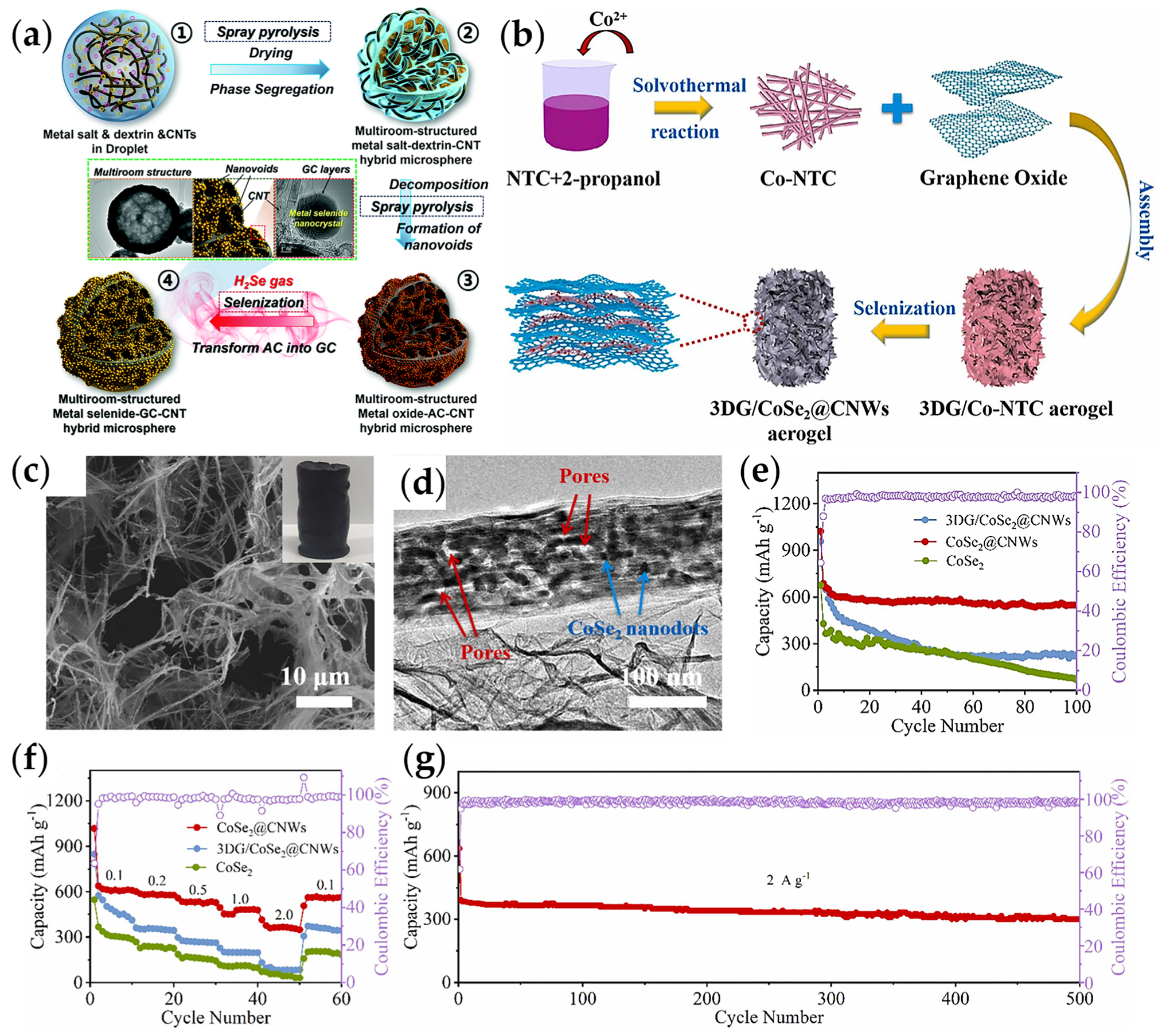
| 3DG/CoSe2 @CNWs | solvothermal | 302/500/2 | / | 1M NaClO4 | 0.01–3 | [64] |
| VSe2 @PPy | selenization | 324.6/2800/4 | 260/10 | 1M NaPF6 | / | [142] |
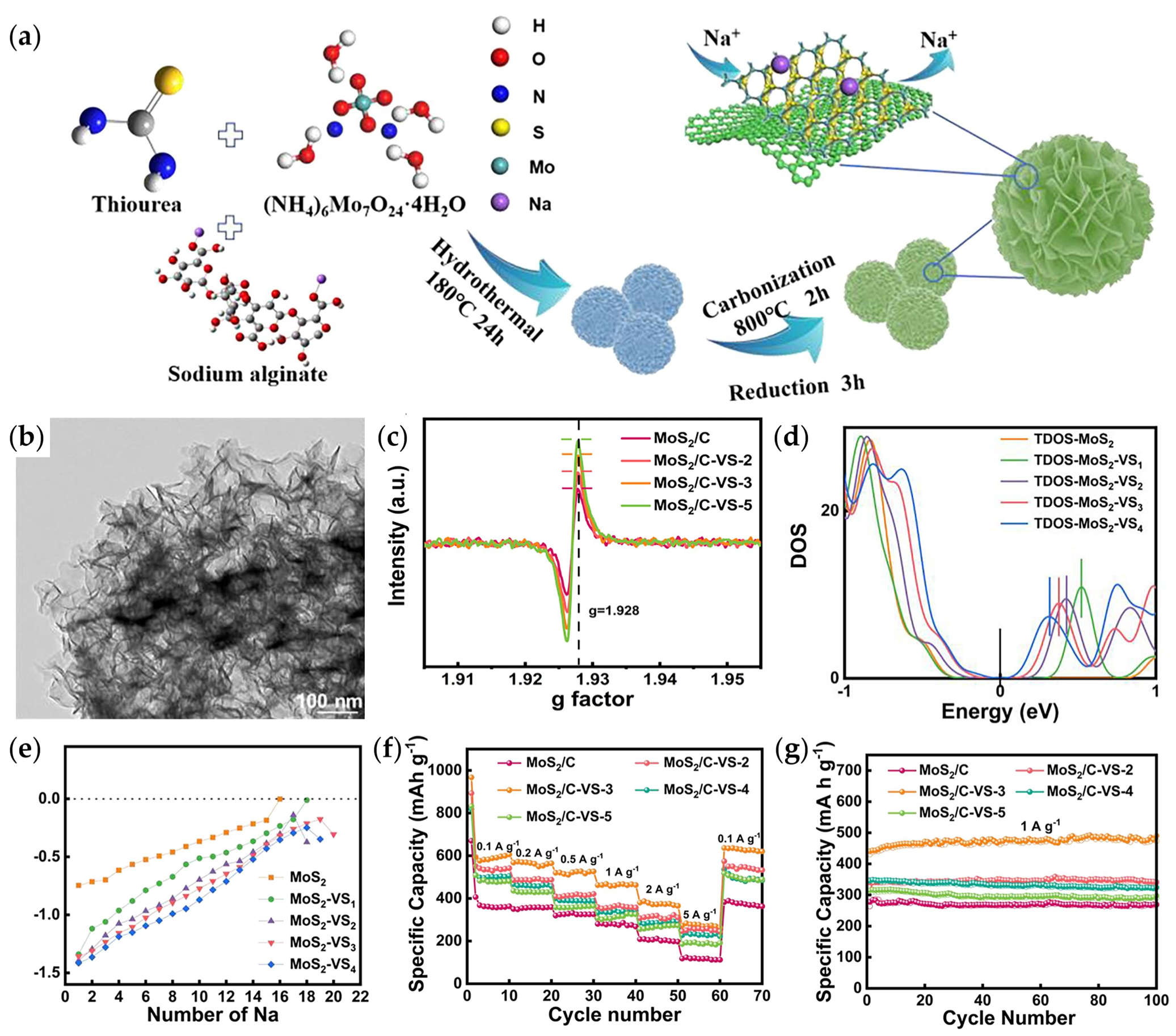
| Bi2S3/MoS2 | solvothermal | 323.4/1200/10 | / | 1M NaCF3SO3 | 0.1–3 | [111] |
| Fe7S8 @HD-C | one-step sulfidation | 480/320/2 | 326/10 | 1M NaPF6 | 0.01–3 | [124] |
| Cu1.81S truss structures | selective reduction | 77.7%/1000/3 | 331/3 | 1M NaPF6 | 0.01–2.6 | [65] |
| SnS2 @C nanobox | metal evaporation | / | 362/5 | 1M NaClO4 | 0.01–2.5 | [118] |
| Hollow CuS | hydrothermal | / | 246.4/5 | 1M NaCF3 SO3 | 0.001–3 | [143] |
| Fe9S10@MoS2 @C | / | 93.4%/1000/2 | 132/50 | 1MNaClO4 | / | [110] |
| Nb2CTx@MoS2 @C | hydrothermal | 403/2000/1 | 260/40 | 1M NaClO4 | 0.01–3 | [58] |
| CuS/FeS2 @NC | two-step pyrolysis | 99.1%/300/5 | 537/5 | 1M NaPF6 | 0.01–2.7 | [144] |
| Hollow MXene@CoS2/NC | carbonization and sulfurization | 620/5000/0.2 | 394/5 | 1M NaCF3 SO3 | 0.25–3 | [128] |
| Lotus-leaf-like FeS @N, S-CNSs | construction | 370/300/5 | / | 1M NaSO3 CF3 | 0.02–2.5 | [145] |
| GeTiS3 | atomic scissors | 209/10,000/32C | 209/32C | 1M NaPF6 | 0.01–2.5 | [146] |
| Ni-Ni3S2 @SC | edge-to-edge | / | 289/2 | 1M NaClO4 | 0.01–3 | [108] |
| Yolk-shell Fe7Se8 @C/N nanoboxes | etching and selenization | Nearly 100%/1000/1 | 316/5 | 1M NaCF3 SO3 | 0.5–2.5 V | [147] |
| Few-layered Ti3C2/Co2Se4 | solvothermal | 379.2/100/0.1 | 289.1/5 | 1M NaClO4 | 0.01–3 | [148] |
| V3Se4/NP CNFs | electrospinning | 340/8000/5 | 240/113,000/10 | 1M NaClO4 | 0.01–3 | [99] |
| Fe3Se4/ZnSe @C | / | 473.8/300/5 | 456.2/5 | 1M NaCF3SO3 | 0.01–3 | [149] |
| Co3Se4 @rGO | selenization | / | 229.3/50 | 1M NaClO4 | 0.01–3 | [150] |
| Cu2PxSe1 -x@C | / | 249.7/1000/20 | / | 1M NaCF3SO3 | 0.01–3.0 | [97] |
| Co0.85Se-Fe7Se8 @rGO | / | 300.8/1000/1 | / | 1M NaPF6 | 0.01–3.0 | [151] |
| Cu2Se@PPy | self-polymerization | 263.5/2000/10 | / | 1M NaCF3 SO3 | / | [152] |
| Branch-leaf CNF@CoSSe@C | electrospinning | 0.01%every cycle/13,000/20 | / | 1M NaCF3 SO3 | 0.01–3.0 | [125] |
| Fe3Se4@SiO2 @C nanorods | situ conformal growth | 272/4200/20 | / | 1M NaCF3 SO3 | / | [153] |
| NiTeSe–NiSe2 nanotubes | hydrothermal | 389.6/1400/10 | 582.5/0.5 | 1M NaPF6 | 0.01–3 | [154] |
| NiSe2@NGCF | template | 406.1/3000/5 | 558.3/200/0.5 | 1M NaClO4 | 0.01–3 | [155] |
| V2C/Fe7S8@C composites | hydrothermal | / | 389.7/5 | 1M NaClO4 | 0.01–3 | [156] |
| SnS/SnS2@SG-K | alkali ion-assisted growth | 372/500/10 | 241/0.05/48 | 1M NaClO4 | 0.01–3 | [157] |
| CuGaSe2@ZnSe-NC | hydrothermal | 276/2000/2 | 595/0.2 | 1M NaClO4 | 0.01–3 | [158] |
| CoSe2/O-C | salt-fixed and thermochemical manners | 346/3500/15 | / | 1M NaCF3 SO3 | 0.01–3 | [159] |
| SnS1.5Se0.5/NS-C | microwave | 670/500/0.2 | 647/10,000/5 | 1M NaPF6 | 0.5–2.8 | [160] |
| NiSe2/CoSe2 nanoparticles | solvothermal co-precipitation | 296.4/1500/10 | 296.4/10 | 1M NaCF3 SO3 | 0.01–3 | [161] |
| SnSe0.5S0.5@ NG | in situ encapsulating | 547/200/0.2 | 387/10 | 1M NaClO4 | 0.01–3 | [162] |
| Ni1/3-xCo1/3-yMn1/3-zSe2/MnSe2 | co-precipitation and high-temperature solid-state route | 400/2000/2 | 400/2 | 1M NaPF6 | 0.3–3 | [163] |
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gu, R.; Ma, Z.; Cheng, T.; Lyu, Y.; Nie, A.; Guo, B. Improved Electrochemical Performances of LiCoO2 at Elevated Voltage and Temperature with an In Situ Formed Spinel Coating Layer. ACS Appl. Mater. Interfaces 2018, 10, 31271–31279. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Rogach, A.L. Anodes and Sodium-Free Cathodes in Sodium Ion Batteries. Adv. Energy Mater. 2020, 10, 2000288. [Google Scholar] [CrossRef]
- U.S. Geological Survey (USGS). Mineral Commodity Summaries 2020; U.S. Geological Survey (USGS): Reston, VA, USA, 2020; p. 204.
- Li, Y.; Feng, X.; Ren, D.; Ouyang, M.; Lu, L.; Han, X. Thermal Runaway Triggered by Plated Lithium on the Anode after Fast Charging. ACS Appl. Mater. Interfaces 2019, 11, 46839–46850. [Google Scholar] [CrossRef]
- Deng, W.-N.; Li, Y.-H.; Xu, D.-F.; Zhou, W.; Xiang, K.-X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Metals 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Feng, L.; Zhang, Q.; Zhang, C.; Ding, Y.; Han, J.; Jiang, S.; He, S. Pore engineering: Structure-capacitance correlations for biomass-derived porous carbon materials. Mater. Des. 2023, 229, 111904. [Google Scholar] [CrossRef]
- Deng, W.; Xu, Y.; Zhang, X.; Li, C.; Liu, Y.; Xiang, K.; Chen, H. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O heterostructure as cathode for high-performance aqueous Zn-ion batteries. J. Alloys Compd. 2022, 903, 163824. [Google Scholar] [CrossRef]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Jiang, S.; Zhang, C.; Ding, Y.; Han, J.; Chen, W.; He, S. High performance supercapacitors based on wood-derived thick carbon electrodes synthesized via green activation process. Inorg. Chem. Front. 2022, 9, 6108–6123. [Google Scholar] [CrossRef]
- Han, B.; Zou, Y.; Zhang, Z.; Yang, X.; Shi, X.; Meng, H.; Wang, H.; Xu, K.; Deng, Y.; Gu, M. Probing the Na metal solid electrolyte interphase via cryo-transmission electron microscopy. Nat. Commun. 2021, 12, 3066. [Google Scholar] [CrossRef]
- Luo, M.; Yu, H.; Hu, F.; Liu, T.; Cheng, X.; Zheng, R.; Bai, Y.; Shui, M.; Shu, J. Metal selenides for high performance sodium ion batteries. Chem. Eng. J. 2020, 380, 122557. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, W.; Zhang, J.; Zhou, D.; Tang, X.; Xu, X.; Li, B.; Liu, H.; Wang, G. Boosting Sodium Storage in Two-Dimensional Phosphorene/Ti3C2Tx MXene Nanoarchitectures with Stable Fluorinated Interphase. ACS Nano 2020, 14, 3651–3659. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, Y.; Li, J.; Wang, G.; Chen, J.; Zhong, G.; Zhan, H.; Wen, Z. Self-Assembling of Conductive Interlayer-Expanded WS2 Nanosheets into 3D Hollow Hierarchical Microflower Bud Hybrids for Fast and Stable Sodium Storage. Adv. Funct. Mater. 2020, 30, 1907677. [Google Scholar] [CrossRef]
- Kang, H.; Liu, Y.; Cao, K.; Zhao, Y.; Jiao, L.; Wang, Y.; Yuan, H. Update on anode materials for Na-ion batteries. J. Mater. Chem. A 2015, 3, 17899–17913. [Google Scholar] [CrossRef]
- Luo, W.; Shen, F.; Bommier, C.; Zhu, H.; Ji, X.; Hu, L. Na-Ion Battery Anodes: Materials and Electrochemistry. Acc. Chem. Res. 2016, 49, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fang, M.; Liu, J.; Yang, D.; Liang, Y.; Zhong, J.; Yuan, Y.-J.; Zhang, Y.; Liu, X.; Zheng, R.; et al. Ultranarrow Bandgap Se-Deficient Bimetallic Selenides for High Performance Alkali Metal-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2205880. [Google Scholar] [CrossRef]
- Ou, X.; Yang, C.; Xiong, X.; Zheng, F.; Pan, Q.; Jin, C.; Liu, M.; Huang, K. A New rGO-Overcoated Sb2Se3 Nanorods Anode for Na+ Battery: In Situ X-Ray Diffraction Study on a Live Sodiation/Desodiation Process. Adv. Funct. Mater. 2017, 27, 1606242. [Google Scholar]
- Zhang, K.; Park, M.; Zhou, L.; Lee, G.-H.; Li, W.; Kang, Y.-M.; Chen, J. Urchin-Like CoSe2 as a High-Performance Anode Material for Sodium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 6728–6735. [Google Scholar] [CrossRef]
- Ge, P.; Hou, H.; Li, S.; Yang, L.; Ji, X. Tailoring Rod-Like FeSe2 Coated with Nitrogen-Doped Carbon for High-Performance Sodium Storage. Adv. Funct. Mater. 2018, 28, 1801765. [Google Scholar] [CrossRef]
- Tang, W.; Xie, D.; Shen, T.; Wang, X.; Wang, D.; Zhang, X.; Xia, X.; Wu, J.; Tu, J. Construction of Nitrogen-Doped Carbon-Coated MoSe2 Microspheres with Enhanced Performance for Lithium Storage. Chem.-Eur. J. 2017, 23, 12924–12929. [Google Scholar] [CrossRef]
- Kwon, H.-T.; Park, C.-M. Electrochemical characteristics of ZnSe and its nanostructured composite for rechargeable Li-ion batteries. J. Power Sources 2014, 251, 319–324. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Zeng, L.; Wei, Q.; Wei, M. In Situ Synthesis of WSe2/CMK-5 Nanocomposite for Rechargeable Lithium-Ion Batteries with a Long-Term Cycling Stability. ACS Sustain. Chem. Eng. 2018, 6, 4688–4694. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.-E.; Yang, Y.; Neale, Z.G.; Massé, R.C.; Cao, J.; Cai, W.; Sui, J.; Cao, G. Reversible and fast Na-ion storage in MoO2/MoSe2 heterostructures for high energy-high power Na-ion capacitors. Energy Storage Mater. 2018, 12, 241–251. [Google Scholar] [CrossRef]
- Ersan, F.; Gökoğlu, G.; Aktürk, E. Adsorption and Diffusion of Lithium on Monolayer Transition Metal Dichalcogenides (MoS2(1–x)Se2x) Alloys. J. Phys. Chem. C 2015, 119, 28648–28653. [Google Scholar] [CrossRef]
- Yang, E.; Ji, H.; Jung, Y. Two-Dimensional Transition Metal Dichalcogenide Monolayers as Promising Sodium Ion Battery Anodes. J. Phys. Chem. C 2015, 119, 26374–26380. [Google Scholar] [CrossRef]
- Xiao, Y.; Lee, S.H.; Sun, Y.-K. The Application of Metal Sulfides in Sodium Ion Batteries. Adv. Energy Mater. 2017, 7, 1601329. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, X.; Song, K.; Zhang, J.; Liu, C.; Mi, L.; Wang, Y.; Chen, W. Se–C bond and reversible SEI in facile synthesized SnSe2⊂3D carbon induced stable anode for sodium-ion batteries. Electrochim. Acta 2020, 337, 135783. [Google Scholar] [CrossRef]
- Yu, S.-H.; Zachman, M.J.; Kang, K.; Gao, H.; Huang, X.; DiSalvo, F.J.; Park, J.; Kourkoutis, L.F.; Abruña, H.D. Atomic-Scale Visualization of Electrochemical Lithiation Processes in Monolayer MoS2 by Cryogenic Electron Microscopy. Adv. Energy Mater. 2019, 9, 1902773. [Google Scholar] [CrossRef]
- Wang, K.; Hua, W.; Li, Z.; Wang, Q.; Kubel, C.; Mu, X. New Insight into Desodiation/Sodiation Mechanism of MoS2: Sodium Insertion in Amorphous Mo-S Clusters. ACS Appl. Mater. Interfaces 2021, 13, 40481–40488. [Google Scholar] [CrossRef]
- Xie, D.; Tang, W.; Wang, Y.; Xia, X.; Zhong, Y.; Zhou, D.; Wang, D.; Wang, X.; Tu, J. Facile fabrication of integrated three-dimensional C-MoSe2/reduced graphene oxide composite with enhanced performance for sodium storage. Nano Res. 2016, 9, 1618–1629. [Google Scholar] [CrossRef]
- Plewa, A.; Kulka, A.; Hanc, E.; Sun, J.; Nowak, M.; Redel, K.; Lu, L.; Molenda, J. Abnormal Phenomena of Multi-Way Sodium Storage in Selenide Electrode. Adv. Funct. Mater. 2021, 31, 2102406. [Google Scholar] [CrossRef]
- Yuan, D.; Dou, Y.; Tian, Y.; Adekoya, D.; Xu, L.; Zhang, S. Robust Pseudocapacitive Sodium Cation Intercalation Induced by Cobalt Vacancies at Atomically Thin Co(1-x) Se2/Graphene Heterostructure for Sodium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2021, 60, 18830–18837. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.M.; Chen, J.H.; Fan, J.J.; Ma, Y.; Radjenovic, P.; Xu, Q.C.; Huang, L.; Passerini, S.; Tian, Z.Q.; Li, J.F. Synthesis and Operando Sodiation Mechanistic Study of Nitrogen-Doped Porous Carbon Coated Bimetallic Sulfide Hollow Nanocubes as Advanced Sodium Ion Battery Anode. Adv. Energy Mater. 2019, 9, 1902312. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, B.; Ou, X.; Wang, C.; Peng, C.; Zhang, J. Synergistical Coupling Interconnected ZnS/SnS2 Nanoboxes with Polypyrrole-Derived N/S Dual-Doped Carbon for Boosting High-Performance Sodium Storage. Small 2019, 15, 1804861. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Yang, J.; Chou, S.-L.; Liu, H.K.; Zhang, W.-X.; Zhao, D.; Dou, S.X. Uniform yolk-shell iron sulfide–carbon nanospheres for superior sodium–iron sulfide batteries. Nat. Commun. 2015, 6, 8689. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahpour, M.; Vafaee, M.; Hosseini, M.R.; Iravani, H. Ab initio study of sodium diffusion and adsorption on boron-doped graphyne as promising anode material in sodium-ion batteries. Phys. Chem. Chem. Phys. 2018, 20, 29889–29895. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, H.; Zou, R.; Mahmood, A.; Liang, Z.; Wang, Q.; Zhang, H.; Gao, S.; Qu, C.; Guo, W.; Guo, S. A Universal Strategy for Hollow Metal Oxide Nanoparticles Encapsulated into B/N Co-Doped Graphitic Nanotubes as High-Performance Lithium-Ion Battery Anodes. Adv. Mater. 2018, 30, 1705441. [Google Scholar] [CrossRef] [PubMed]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef]
- Guo, Q.; Ma, Y.; Chen, T.; Xia, Q.; Yang, M.; Xia, H.; Yu, Y. Cobalt Sulfide Quantum Dot Embedded N/S-Doped Carbon Nanosheets with Superior Reversibility and Rate Capability for Sodium-Ion Batteries. ACS Nano 2017, 11, 12658–12667. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Li, Y.; Zhang, Y.; Dong, Y.; Li, D.; Zhang, J. Construction of uniform SnS2/ZnS heterostructure nanosheets embedded in graphene for advanced lithium-ion batteries. J. Alloys Compd. 2020, 820, 153147. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, P.F.; Cao, M. Core-shell bimetallic carbide nanoparticles confined in a three-dimensional N-doped carbon conductive network for efficient lithium storage. ACS Nano 2014, 8, 7846–7857. [Google Scholar] [CrossRef]
- Lei, C.; Han, F.; Li, D.; Li, W.-C.; Sun, Q.; Zhang, X.-Q.; Lu, A.-H. Dopamine as the coating agent and carbon precursor for the fabrication of N-doped carbon coated Fe3O4 composites as superior lithium ion anodes. Nanoscale 2013, 5, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Chen, B.; Qin, Y.; Yang, S.; Li, C.; Zuo, Y.; Liu, S.; Yang, J. Facile Ultrasonic Synthesis of CoO Quantum Dot/Graphene Nanosheet Composites with High Lithium Storage Capacity. ACS Nano 2012, 6, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Firmiano, E.G.S.; Cordeiro, M.A.L.; Rabelo, A.C.; Dalmaschio, C.J.; Pinheiro, A.N.; Pereira, E.C.; Leite, E.R. Graphene oxide as a highly selective substrate to synthesize a layered MoS2 hybrid electrocatalyst. Chem. Commun. 2012, 48, 7687–7689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Ma, X.; Hou, T.; Guo, K.; Yin, J.; Wang, Z.; Shu, L.; He, M.; Yao, J. Inorganic Salts Induce Thermally Reversible and Anti-Freezing Cellulose Hydrogels. Angew. Chem. Int. Ed. 2019, 58, 7366–7370. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, R.; Liu, M.; Wang, H.; Xu, H.; Guo, Y.; Song, Y.; Fang, F.; Yu, X.; Sun, D. General Synthesis of Dual Carbon-Confined Metal Sulfides Quantum Dots Toward High-Performance Anodes for Sodium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1702046. [Google Scholar] [CrossRef]
- Xia, G.; Zhang, L.; Fang, F.; Sun, D.; Guo, Z.; Liu, H.; Yu, X. General Synthesis of Transition Metal Oxide Ultrafine Nanoparticles Embedded in Hierarchically Porous Carbon Nanofibers as Advanced Electrodes for Lithium Storage. Adv. Funct. Mater. 2016, 26, 6188–6196. [Google Scholar] [CrossRef]
- Yuan, Z.; Dong, L.; Gao, Q.; Huang, Z.; Wang, L.; Wang, G.; Yu, X. SnSb alloy nanoparticles embedded in N-doped porous carbon nanofibers as a high-capacity anode material for lithium-ion batteries. J. Alloys Compd. 2019, 777, 775–783. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, Y.; Gao, P.; Xia, G.; He, S.; Yang, Y.; Pan, H.; Yu, X. Lithium Azides Induced SnS Quantum Dots for Ultra-Fast and Long-Term Sodium Storage. Small 2023, 19, 2302188. [Google Scholar] [CrossRef]
- Jin, T.; Han, Q.; Wang, Y.; Jiao, L. 1D Nanomaterials: Design, Synthesis, and Applications in Sodium–Ion Batteries. Small 2018, 14, 1703086. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Wang, C.; Chen, D.; Zhang, Q.; Jiang, L.; Zhang, C.; Liu, K.; He, S. Capacitive properties of carbon nanofibers derived from blends of cellulose acetate and polyacrylonitrile. New J. Chem. 2023, 47, 13831–13840. [Google Scholar] [CrossRef]
- Xu, X.; Li, F.; Zhang, D.; Liu, Z.; Zuo, S.; Zeng, Z.; Liu, J. Self-Sacrifice Template Construction of Uniform Yolk–Shell ZnS@C for Superior Alkali-Ion Storage. Adv. Sci. 2022, 9, e2200247. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, J.; Wang, Z.; Wang, H.; Zhi, C.; Yu, D.Y.W.; Rogach, A.L. Carbon-Supported Nickel Selenide Hollow Nanowires as Advanced Anode Materials for Sodium-Ion Batteries. Small 2018, 14, 1702669. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhu, J.; Qiu, Y.; Wang, G.; Xu, X.; Ma, S.; Shen, P.K.; Wu, X.L.; Yamauchi, Y. One-dimensional core-shell motif nanowires with chemically-bonded transition metal sulfide-carbon heterostructures for efficient sodium-ion storage. Chem. Sci. 2021, 12, 15054–15060. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Kim, J.H.; Chan Kang, Y. Sodium-ion storage performance of hierarchically structured (Co1/3Fe2/3)Se2 nanofibers with fiber-in-tube nanostructures. J. Mater. Chem. A 2016, 4, 15471–15477. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Jiang, Y.; Wang, J.; Zheng, X.; Han, B.; Xia, K.; Gao, Q.; Cai, Z.; Zhou, C.; et al. Construction of VS2/VOx Heterostructure via Hydrolysis-Oxidation Coupling Reaction with Superior Sodium Storage Properties. Adv. Funct. Mater. 2023, 33, 2212785. [Google Scholar] [CrossRef]
- Wen, X.; Feng, W.; Li, X.; Yang, J.; Du, R.; Wang, P.; Li, H.; Song, L.; Wang, Y.; Cheng, M.; et al. Diatomite-Templated Synthesis of Single-Atom Cobalt-Doped MoS2/Carbon Composites to Boost Sodium Storage. Adv. Mater. 2023, 35, e2211690. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, L.; Li, D.; Cao, J.; Han, W. Carbon-Reinforced Nb2CTx MXene/MoS2 Nanosheets as a Superior Rate and High-Capacity Anode for Sodium-Ion Batteries. ACS Nano 2021, 15, 7439–7450. [Google Scholar] [CrossRef]
- Liu, T.; Ding, J.; Su, Z.; Wei, G. Porous two-dimensional materials for energy applications: Innovations and challenges. Mater. Today Energy 2017, 6, 79–95. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, B.; Feng, L.; Zheng, J.; You, B.; Chen, J.; Zhao, X.; Zhang, C.; Jiang, S.; He, S. Progress in the use of organic potassium salts for the synthesis of porous carbon nanomaterials: Microstructure engineering for advanced supercapacitors. Nanoscale 2022, 14, 8216–8244. [Google Scholar] [CrossRef]
- Li, X.; Han, Z.; Yang, W.; Li, Q.; Li, H.; Xu, J.; Li, H.; Liu, B.; Zhao, H.; Li, S.; et al. 3D Ordered Porous Hybrid of ZnSe/N-doped Carbon with Anomalously High Na+ Mobility and Ultrathin Solid Electrolyte Interphase for Sodium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2106194. [Google Scholar] [CrossRef]
- Ge, P.; Li, S.; Xu, L.; Zou, K.; Gao, X.; Cao, X.; Zou, G.; Hou, H.; Ji, X. Hierarchical Hollow-Microsphere Metal-Selenide@Carbon Composites with Rational Surface Engineering for Advanced Sodium Storage. Adv. Energy Mater. 2019, 9, 1803035. [Google Scholar] [CrossRef]
- Park, G.D.; Kang, Y.C. Multiroom-structured multicomponent metal selenide-graphitic carbon-carbon nanotube hybrid microspheres as efficient anode materials for sodium-ion batteries. Nanoscale 2018, 10, 8125–8132. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Song, Q.; Zheng, K.; Zheng, L.; Zhu, Y.; Chen, Z. CoSe2 nanodots confined in multidimensional porous nanoarchitecture towards efficient sodium ion storage. Nano Energy 2022, 98, 107326. [Google Scholar] [CrossRef]
- Shang, Y.; Li, X.; Huang, S.; Chen, S.; Yang, Z.; Guo, L.; Yang, H.Y. A Selective Reduction Approach to Construct Robust Cu1.81S Truss Structures for High-Performance Sodium Storage. Matter 2020, 2, 428–439. [Google Scholar] [CrossRef]
- Jiang, H.; Huang, L.; Wei, Y.; Wang, B.; Wu, H.; Zhang, Y.; Liu, H.; Dou, S. Bio-Derived Hierarchical Multicore–Shell Fe2N-Nanoparticle-Impregnated N-Doped Carbon Nanofiber Bundles: A Host Material for Lithium-/Potassium-Ion Storage. Nano-Micro Lett. 2019, 11, 56. [Google Scholar] [CrossRef]
- Mai, L.; Sheng, J.; Xu, L.; Tan, S.; Meng, J. One-Dimensional Hetero-Nanostructures for Rechargeable Batteries. Acc. Chem. Res. 2018, 51, 950–959. [Google Scholar] [CrossRef]
- Hasa, I.; Hassoun, J.; Passerini, S. Nanostructured Na-ion and Li-ion anodes for battery application: A comparative overview. Nano Res. 2017, 10, 3942–3969. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Y. 2D material as anode for sodium ion batteries: Recent progress and perspectives. Energy Storage Mater. 2019, 16, 323–343. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, L.; Lou, X.W. Nanostructured Conversion-type Anode Materials for Advanced Lithium-Ion Batteries. Chem 2018, 4, 972–996. [Google Scholar] [CrossRef]
- Li, N.-W.; Yin, Y.-X.; Xin, S.; Li, J.-Y.; Guo, Y.-G. Methods for the Stabilization of Nanostructured Electrode Materials for Advanced Rechargeable Batteries. Small Methods 2017, 1, 1700094. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Chen, Y.; Zhang, H.; Yousaf, M.; Wu, H.; Zou, M.; Cao, A.; Han, R.P.S. Hyperporous Sponge Interconnected by Hierarchical Carbon Nanotubes as a High-Performance Potassium-Ion Battery Anode. Adv. Mater. 2018, 30, 1802074. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cao, Z.; Zhang, J.; Cheng, H.; Liu, G.; Park, G.T.; Cavallo, L.; Wang, L.; Alshareef, H.N.; Sun, Y.K.; et al. Electrolyte-Mediated Stabilization of High-Capacity Micro-Sized Antimony Anodes for Potassium-Ion Batteries. Adv. Mater. 2021, 33, e2005993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, L.; Xie, C.; Wang, D.; Zou, Y.; Chen, R.; Wang, Y.; Jia, C.; Wang, S. Defect Engineering on Electrode Materials for Rechargeable Batteries. Adv. Mater. 2020, 32, 1905923. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, M.; Huang, M.; Xiong, Y.; Yang, X.; Miao, Z.; Yang, Z.; Yu, J. Co0.85Se@carbon nanotubes surface-seeding grown on carbon microplates as superior anode material for sodium ion batteries. Electrochim. Acta 2022, 414, 140167. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Z.; Wang, K.; Gao, M.; Ding, S. Phase boundary-enhanced Ni3N–Co3N@CNT composite materials for lithium-ion batteries. J. Mater. Chem. A 2019, 7, 1779–1784. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Quan, W.; Hong, Y.; Zhang, Z.; Tang, Z.; Li, J. Ti3+-free three-phase Li4Ti5O12/TiO2 for high-rate lithium ion batteries: Capacity and conductivity enhancement by phase boundaries. Nano Energy 2017, 32, 294–301. [Google Scholar] [CrossRef]
- Zhang, D.M.; Jia, J.H.; Yang, C.C.; Jiang, Q. Fe7Se8 nanoparticles anchored on N-doped carbon nanofibers as high-rate anode for sodium-ion batteries. Energy Storage Mater. 2020, 24, 439–449. [Google Scholar] [CrossRef]
- Li, Y.; Qian, J.; Zhang, M.; Wang, S.; Wang, Z.; Li, M.; Bai, Y.; An, Q.; Xu, H.; Wu, F.; et al. Co-Construction of Sulfur Vacancies and Heterojunctions in Tungsten Disulfide to Induce Fast Electronic/Ionic Diffusion Kinetics for Sodium-Ion Batteries. Adv. Mater. 2020, 32, e2005802. [Google Scholar] [CrossRef]
- Su, Q.; Cao, X.; Yu, T.; Kong, X.; Wang, Y.; Chen, J.; Lin, J.; Xie, X.; Liang, S.; Pan, A. Binding MoSe2 with dual protection carbon for high-performance sodium storage. J. Mater. Chem. A 2019, 7, 22871–22878. [Google Scholar] [CrossRef]
- Zhao, X.; Gong, F.; Zhao, Y.; Huang, B.; Qian, D.; Wang, H.-E.; Zhang, W.; Yang, Z. Encapsulating NiS nanocrystal into nitrogen-doped carbon framework for high performance sodium/potassium-ion storage. Chem. Eng. J. 2020, 392, 123675. [Google Scholar] [CrossRef]
- Hou, J.; Zhu, Z.; Li, C.; Zhang, J.; Shen, S.; Yao, Z.; Liu, T.; Li, W.; Xia, X.; Yang, Y. Spatially Confined Synthesis of SnSe Spheres Encapsulated in N, Se Dual-Doped Carbon Networks toward Fast and Durable Sodium Storage. ACS Appl. Mater. Interfaces 2022, 14, 4230–4241. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Han, J.; Zhu, K.; Dong, Z.; Jiao, L. Heterostructure SnSe2/ZnSe@PDA Nanobox for Stable and Highly Efficient Sodium-Ion Storage. Adv. Energy Mater. 2020, 10, 2000741. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, G.; Ni, J.; Li, L. Architecting core-shell nanosheets of MoS2-polypyrrole on carbon cloth as a robust sodium anode. Sustain. Mater. Technol. 2021, 28, e00255. [Google Scholar] [CrossRef]
- Zang, R.; Li, P.; Guo, X.; Man, Z.; Zhang, S.; Wang, C.; Wang, G. Yolk–shell N-doped carbon coated FeS2 nanocages as a high-performance anode for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 14051–14059. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem 2020, 2, 100027. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, Q.; Wang, D.; Pang, Q.; Gao, Y.; Missiul, A.; Nemausat, R.; Sarapulova, A.; Ehrenberg, H.; Wei, Y.; et al. Co9S8@carbon yolk-shell nanocages as a high performance direct conversion anode material for sodium ion batteries. Energy Storage Mater. 2019, 18, 51–58. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, M.; Wang, L.; Huang, Q.; Su, Z.; Xu, P.; Zou, R.; Wang, X.; Zeng, C.; Ba, K. MOFs-Derived Flower-Like Hierarchically Porous Zn-Mn-Se/C Composite for Extraordinary Rate Performance and Durable Anode of Sodium-Ion and Potassium-Ion Batteries. Small 2022, 18, e2203964. [Google Scholar] [CrossRef]
- Yousaf, M.; Wang, Z.; Wang, Y.; Chen, Y.; Ali, U.; Maqbool, M.; Imran, A.; Mahmood, N.; Gao, P.; Han, R.P.S. Core-Shell FeSe2 /C Nanostructures Embedded in a Carbon Framework as a Free Standing Anode for a Sodium Ion Battery. Small 2020, 16, e2002200. [Google Scholar] [CrossRef]
- Kong, Z.; Huang, M.; Liang, Z.; Tu, H.; Zhang, K.; Shao, Y.; Wu, Y.; Hao, X. Phosphorus doping induced the co-construction of sulfur vacancies and heterojunctions in tin disulfide as a durable anode for lithium/sodium-ion batteries. Inorg. Chem. Front. 2022, 9, 902–913. [Google Scholar] [CrossRef]
- Kiciński, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, M.; Wu, F.; Ye, Z.; Zhang, Y.; Wang, Z.; Zhou, Y.; Li, L.; Chen, R. Cobalt Selenide Hollow Polyhedron Encapsulated in Graphene for High-Performance Lithium/Sodium Storage. Small 2021, 17, e2102893. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liang, X.; Ou, X.; Zhang, Q.; Zheng, H.S.; Zheng, F.; Wang, J.H.; Huang, K.; Liu, M. Heterostructured Nanocube-Shaped Binary Sulfide (SnCo)S2 Interlaced with S-Doped Graphene as a High-Performance Anode for Advanced Na+ Batteries. Adv. Funct. Mater. 2019, 29, 1807971. [Google Scholar] [CrossRef]
- Ye, J.; Li, X.; Xia, G.; Gong, G.; Zheng, Z.; Chen, C.; Hu, C. P-doped CoSe2 nanoparticles embedded in 3D honeycomb-like carbon network for long cycle-life Na-ion batteries. J. Mater. Sci. Technol. 2021, 77, 100–107. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Sumboja, A.; Zang, W.; Xie, J.; Gao, D.; Pennycook, S.J.; Liu, Z.; Guan, C.; Wang, J. Integrated Hierarchical Carbon Flake Arrays with Hollow P-Doped CoSe2 Nanoclusters as an Advanced Bifunctional Catalyst for Zn–Air Batteries. Adv. Funct. Mater. 2018, 28, 1804846. [Google Scholar] [CrossRef]
- Wang, L.; Han, Z.; Zhao, Q.; Yao, X.; Zhu, Y.; Ma, X.; Wu, S.; Cao, C. Engineering yolk–shell P-doped NiS2/C spheres via a MOF-template for high-performance sodium-ion batteries. J. Mater. Chem. A 2020, 8, 8612–8619. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Long, X.; Luo, H.-C.; Xu, C.; Wang, G.; Zhao, W. Construction of phosphorus-doping with spontaneously developed selenium vacancies: Inducing superior ion-diffusion kinetics in hollow Cu2Se@C nanospheres for efficient sodium storage. J. Energy Chem. 2022, 77, 227–238. [Google Scholar] [CrossRef]
- Niu, F.; Yang, J.; Wang, N.; Zhang, D.; Fan, W.; Yang, J.; Qian, Y. MoSe2-Covered N,P-Doped Carbon Nanosheets as a Long-Life and High-Rate Anode Material for Sodium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1700522. [Google Scholar] [CrossRef]
- Xu, L.; Guo, W.; Zeng, L.; Xia, X.; Wang, Y.; Xiong, P.; Chen, Q.; Zhang, J.; Wei, M.; Qian, Q. V3Se4 embedded within N/P co-doped carbon fibers for sodium/potassium ion batteries. Chem. Eng. J. 2021, 419, 129607. [Google Scholar] [CrossRef]
- Ma, X.; Diao, L.; Wang, Y.; Zhang, L.; Lu, Y.; Li, D.; Yang, D.; She, X. S-vacancies manipulating enhances Na+ insertion of MoS2 for efficient sodium-ion storage. Chem. Eng. J. 2023, 457, 141116. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Zhang, W.; Zheng, Y.; Lou, X.; Yu, B.; Chen, J.; Chen, Y.; Liu, M.; Wang, J. Heterointerface engineering for enhancing the electrochemical performance of solid oxide cells. Energy Environ. Sci. 2020, 13, 53–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Sun, C.; Lu, Z.; Xue, P.; Tang, B.; Bai, Z.; Dou, S. 3D spongy CoS2 nanoparticles/carbon composite as high-performance anode material for lithium/sodium ion batteries. Chem. Eng. J. 2018, 332, 370–376. [Google Scholar] [CrossRef]
- Lian, Y.; Chen, F.; Kang, H.; Wu, C.; Zhang, M.; Xu, S. Co9S8 nanoparticles scaffolded within carbon-nanoparticles-decorated carbon spheres as anodes for lithium and sodium storage. Appl. Surf. Sci. 2020, 507, 145061. [Google Scholar] [CrossRef]
- Shuang, W.; Huang, H.; Kong, L.; Zhong, M.; Li, A.; Wang, D.; Xu, Y.; Bu, X.-H. Nitrogen-doped carbon shell-confined Ni3S2 composite nanosheets derived from Ni-MOF for high performance sodium-ion battery anodes. Nano Energy 2019, 62, 154–163. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Z.; Liu, X.; Li, C.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Zhu, S.; Hu, W.; et al. Interfacial engineering of Bi2S3/Ti3C2Tx MXene based on work function for rapid photo-excited bacteria-killing. Nat. Commun. 2021, 12, 1224. [Google Scholar] [CrossRef] [PubMed]
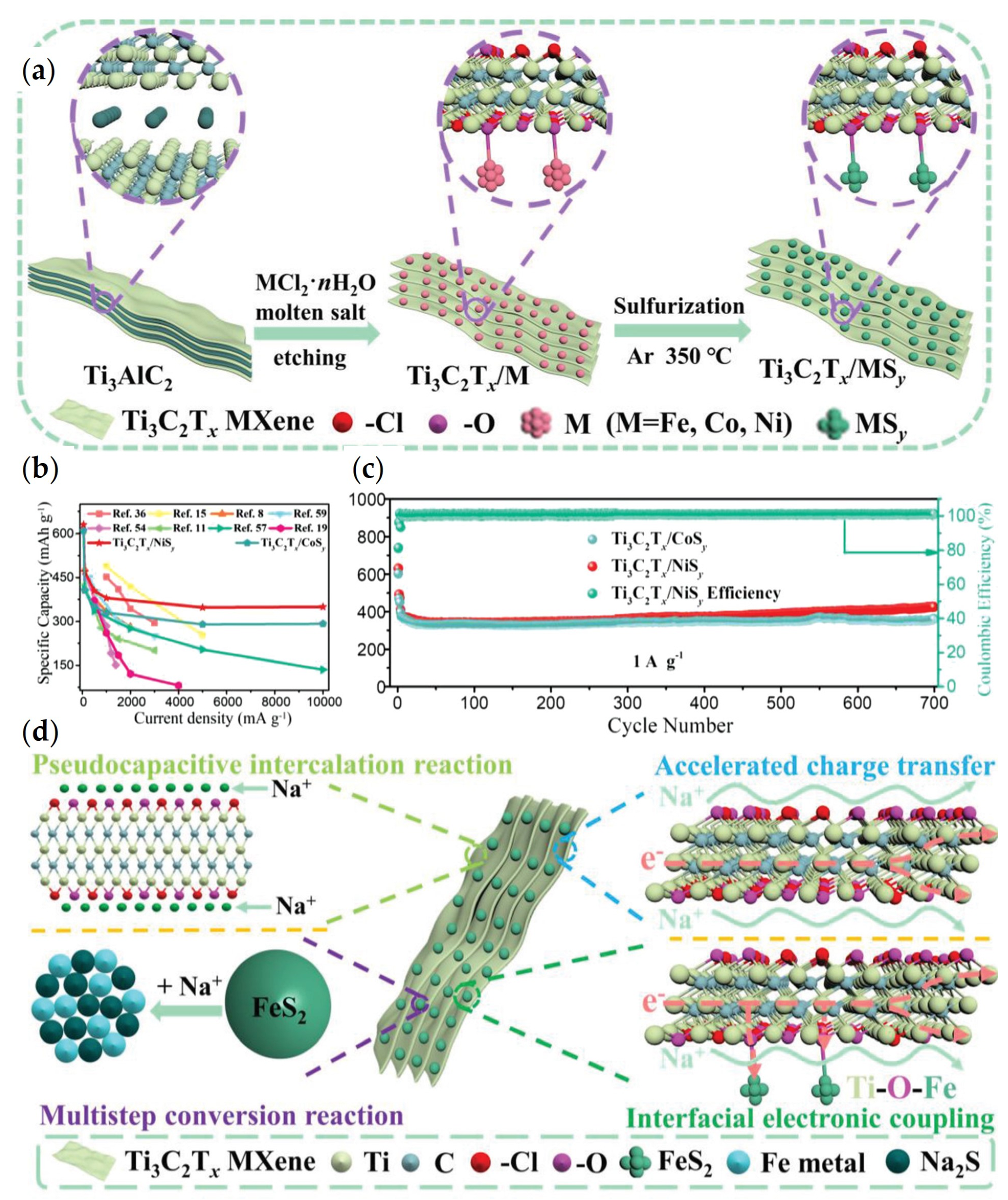
- Huang, P.; Ying, H.; Zhang, S.; Zhang, Z.; Han, W.Q. Molten Salts Etching Route Driven Universal Construction of MXene/Transition Metal Sulfides Heterostructures with Interfacial Electronic Coupling for Superior Sodium Storage. Adv. Energy Mater. 2022, 12, 2202052. [Google Scholar] [CrossRef]
- Sun, Z.; Liang, H.; Wang, H.; Shi, J.; Huang, M.; Chen, J.; Liu, S.; Tian, W.; Cao, H.; Li, Z. Spatially Confined “Edge-to-Edge” Strategy for Achieving Compact Na+/K+ Storage: Constructing Hetero-Ni/Ni3S2 in Densified Carbons. Adv. Funct. Mater. 2022, 32, 2203291. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, W.; Liu, Y.; He, J.; Yang, S.; Liu, M.; Wang, Q.; Guo, Z. Constructing CoO/Co3S4 Heterostructures Embedded in N-doped Carbon Frameworks for High-Performance Sodium-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1901925. [Google Scholar] [CrossRef]
- Zhang, C.; Han, F.; Wang, F.; Liu, Q.; Zhou, D.; Zhang, F.; Xu, S.; Fan, C.; Li, X.; Liu, J. Improving compactness and reaction kinetics of MoS2@C anodes by introducing Fe9S10 core for superior volumetric sodium/potassium storage. Energy Storage Mater. 2020, 24, 208–219. [Google Scholar] [CrossRef]
- Cao, L.; Liang, X.; Ou, X.; Yang, X.; Li, Y.; Yang, C.; Lin, Z.; Liu, M. Heterointerface Engineering of Hierarchical Bi2S3/MoS2 with Self-Generated Rich Phase Boundaries for Superior Sodium Storage Performance. Adv. Funct. Mater. 2020, 30, 1910732. [Google Scholar] [CrossRef]
- Li, H. Interface engineering renders high-rate high-capacity lithium storage in black phosphorous composite anodes with excellent cycling durability. Sci. China Chem. 2020, 63, 1734–1736. [Google Scholar] [CrossRef]
- Chen, W.; Qi, S.; Guan, L.; Liu, C.; Cui, S.; Shen, C.; Mi, L. Pyrite FeS2 microspheres anchoring on reduced graphene oxide aerogel as an enhanced electrode material for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 5332–5341. [Google Scholar] [CrossRef]
- Wang, J.; Yin, H.; Wang, Z.; Gao, J.; Jiang, Q.; Xu, Y.; Chen, Z. High-performance Sn-based anode with robust lignin-derived hard carbon support for sodium-ion batteries. Asia-Pac. J. Chem. Eng. 2022, 17, e2768. [Google Scholar] [CrossRef]
- Yang, L.; Liu, M.; Xiang, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Carbon skeleton confined Sb chalcogenides nanodots for stable sodium storage. Carbon 2022, 197, 341–349. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Yang, Z.; Gu, L.; Chen, Q.; Yu, Y. Sodium-Ion Batteries: Improving the Rate Capability of 3D Interconnected Carbon Nanofibers Thin Film by Boron, Nitrogen Dual-Doping. Adv. Sci. 2017, 4, 1600468. [Google Scholar] [CrossRef]
- Liu, S.; Ren, Z.; Fakudze, S.; Shang, Q.; Chen, J.; Liu, C.; Han, J.; Tian, Z. Structural Evolution of Graphitic Carbon Derived from Ionic Liquids-Dissolved Cellulose and Its Application as Lithium-Ion Battery Anodes. Langmuir 2022, 38, 320–331. [Google Scholar] [CrossRef]
- Sun, Q.; Li, D.; Dai, L.; Liang, Z.; Ci, L. Structural Engineering of SnS2 Encapsulated in Carbon Nanoboxes for High-Performance Sodium/Potassium-Ion Batteries Anodes. Small 2020, 16, e2005023. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal–Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef]
- Ding, Y.-L.; Kopold, P.; Hahn, K.; van Aken, P.A.; Maier, J.; Yu, Y. A Lamellar Hybrid Assembled from Metal Disulfide Nanowall Arrays Anchored on a Carbon Layer: In Situ Hybridization and Improved Sodium Storage. Adv. Mater. 2016, 28, 7774–7782. [Google Scholar] [CrossRef]
- Quan, Y.; Chen, M.; Zhou, W.; Tian, Q.; Chen, J. High-Performance Anti-freezing Flexible Zn-MnO2 Battery Based on Polyacrylamide/Graphene Oxide/Ethylene Glycol Gel Electrolyte. Front. Chem. 2020, 8, 603. [Google Scholar] [CrossRef]
- Zhao, X.; He, D.; You, B. Laser engraving and punching of graphene films as flexible all-solid-state planar micro-supercapacitor electrodes. Mater. Today Sustain. 2022, 17, 100096. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, W.; Yang, Y.; Song, X.; Neale, Z.; Wang, H.-E.; Sui, J.; Cao, G. MoSe2 nanosheets perpendicularly grown on graphene with Mo–C bonding for sodium-ion capacitors. Nano Energy 2018, 47, 224–234. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Zhang, H.; Diemant, T.; Behm, R.J.; Varzi, A.; Passerini, S. Metal–Organic Framework Derived Fe7S8 Nanoparticles Embedded in Heteroatom-Doped Carbon with Lithium and Sodium Storage Capability. Small Methods 2020, 4, 2000637. [Google Scholar] [CrossRef]
- Zhao, W.; Gao, L.; Ma, X.; Yue, L.; Zhao, D.; Li, Z.; Sun, S.; Luo, Y.; Liu, Q.; Asiri, A.M.; et al. An exquisite branch–leaf shaped metal sulfoselenide composite endowing an ultrastable sodium-storage lifespan over 10,000 cycles. J. Mater. Chem. A 2022, 10, 16962–16975. [Google Scholar] [CrossRef]
- Lipatov, A.; Alhabeb, M.; Lukatskaya, M.R.; Boson, A.; Gogotsi, Y.; Sinitskii, A. Effect of Synthesis on Quality, Electronic Properties and Environmental Stability of Individual Monolayer Ti3C2 MXene Flakes. Adv. Electron. Mater. 2016, 2, 1600255. [Google Scholar]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, Q.; Yan, Y.; Li, H.; Zhou, W.; Gu, T.; Shen, X.; Lu, C.; Zhao, Y.; Zhang, Y.; et al. Optimized Co–S bonds energy and confinement effect of hollow MXene@CoS2/NC for enhanced sodium storage kinetics and stability. Chem. Eng. J. 2022, 450, 137922. [Google Scholar] [CrossRef]
- Chen, S.; Huang, S.; Zhang, Y.-F.; Fan, S.; Yan, D.; Shang, Y.; Pam, M.E.; Ge, Q.; Shi, Y.; Yang, H.Y. Constructing stress-release layer on Fe7Se8-based composite for highly stable sodium-storage. Nano Energy 2020, 69, 104389. [Google Scholar] [CrossRef]
- Dong, C.; Wu, L.; He, Y.; Zhou, Y.; Sun, X.; Du, W.; Sun, X.; Xu, L.; Jiang, F. Willow-Leaf-Like ZnSe@N-Doped Carbon Nanoarchitecture as a Stable and High-Performance Anode Material for Sodium-Ion and Potassium-Ion Batteries. Small 2020, 16, e2004580. [Google Scholar] [CrossRef]
- Dong, S.; Su, Q.; Jiao, W.; Ding, S.; Zhang, M.; Du, G.; Xu, B. FeSe2 microspheres coated with carbon layers as anode materials for sodium-ion batteries. J. Alloys Compd. 2020, 842, 155888. [Google Scholar] [CrossRef]
- Jiang, S.; Xiang, M.; Zhang, J.; Chu, S.; Marcelli, A.; Chu, W.; Wu, D.; Qian, B.; Tao, S.; Song, L. Rational design of hierarchical FeSe2 encapsulated with bifunctional carbon cuboids as an advanced anode for sodium-ion batteries. Nanoscale 2020, 12, 22210–22216. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zhang, M.; Zhang, L.; Li, Y.; Li, Y.; Tan, C.; Zheng, F.; Huang, Y.; Wang, H.; Li, Q. FeSe2@C Microrods as a Superior Long-Life and High-Rate Anode for Sodium Ion Batteries. ACS Nano 2020, 14, 17683–17692. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Chu, Y.; Xi, B.; Huang, M.; Chen, W.; Duan, B.; Zhang, C.; Feng, J.; Xiong, S. Sandwich Structures Constructed by ZnSe⊂N-C@MoSe2 Located in Graphene for Efficient Sodium Storage. Adv. Energy Mater. 2020, 10, 2002298. [Google Scholar] [CrossRef]
- Wang, T.; Guo, W.; Wang, G.; Wang, H.; Bai, J.; Wang, B. Highly dispersed FeSe2 nanoparticles in porous carbon nanofibers as advanced anodes for sodium and potassium ion batteries. J. Alloys Compd. 2020, 834, 155265. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, D.; Jia, X.; Zhou, J. Core/shell FeSe/carbon nanosheet-assembled microflowers with ultrahigh coulombic-efficiency and rate performance as nonpresodiate anode for sodium-ion battery. Carbon 2020, 166, 339–349. [Google Scholar] [CrossRef]
- Gao, X.; Kuai, Y.; Xu, Z.; Cao, Y.; Wang, N.; Hirano, S.I.; Nuli, Y.; Wang, J.; Yang, J. SnSe2/FeSe2 Nanocubes Capsulated in Nitrogen-Doped Carbon Realizing Stable Sodium-Ion Storage at Ultrahigh Rate. Small Methods 2021, 5, e2100437. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Li, X.; Zhang, W.; Xiang, Y.; Li, T.; Niu, X.; Chen, J.S.; Yan, Q. Bilateral Interfaces in In2Se3-CoIn2-CoSe2 Heterostructures for High-Rate Reversible Sodium Storage. ACS Nano 2021, 15, 13307–13318. [Google Scholar] [CrossRef]
- Xin, W.; Chen, N.; Wei, Z.; Wang, C.; Chen, G.; Du, F. Self-Assembled FeSe2 Microspheres with High-Rate Capability and Long-Term Stability as Anode Material for Sodium- and Potassium-Ion Batteries. Chemistry 2021, 27, 3745–3752. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Zhong, W.; Xiao, F.; Kashif Aslam, M.; Zhang, X.; Xu, M. Highly Efficient Sodium-Ion Storage Enabled by an rGO-Wrapped FeSe2 Composite. ChemSusChem 2021, 14, 1336–1343. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Zeng, B.; Zhou, D.; Tai, L.; Liu, X.-D.; Lau, W.-M. Rich-oxygen-doped FeSe2 nanosheets with high pseudocapacitance capacity as a highly stable anode for sodium ion battery. Chem. Eng. J. 2022, 428, 132637. [Google Scholar] [CrossRef]
- Yi, Y.; Du, X.; Zhao, Z.; Liu, Y.; Guan, H.; Liu, X.; Pei, X.; Zhang, S.; Li, D. Coupling of Metallic VSe2 and Conductive Polypyrrole for Boosted Sodium-Ion Storage by Reinforced Conductivity Within and Outside. ACS Nano 2022, 16, 7772–7782. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gao, J.; Qin, Z.; Sun, Y.; Tian, R.; Zhang, Q.; Gao, Y. Deactivated-desulfurizer-derived hollow copper sulfide as anode materials for advanced sodium ion batteries. J. Power Sources 2020, 479, 228518. [Google Scholar] [CrossRef]
- Je, J.; Lim, H.; Jung, H.W.; Kim, S.O. Ultrafast and Ultrastable Heteroarchitectured Porous Nanocube Anode Composed of CuS/FeS2 Embedded in Nitrogen-Doped Carbon for Use in Sodium-Ion Batteries. Small 2022, 18, e2105310. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; He, Y.; Sun, H.; Xu, C.; Li, H.; Wang, X.; Zhang, G.; Sun, Z.; Wei, Q.; et al. An integrated strategy based on Schiff base reactions to construct unique two-dimensional nanostructures for intrinsic pseudocapacitive sodium/lithium storage. Chem. Eng. J. 2022, 429, 132339. [Google Scholar] [CrossRef]
- Peng, B.; Lv, Z.; Xu, S.; Pan, J.; Zhao, W.; Dong, C.; Huang, F. Tailoring Ultrafast and High-Capacity Sodium Storage via Binding-Energy-Driven Atomic Scissors. Adv. Mater. 2022, 34, e2200863. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, X.; Gu, Z.; Han, P.; Zhao, B.; Qu, D.; Gao, L.; Liu, Z.; Han, D.; Niu, L. Rationally designed nitrogen-doped yolk-shell Fe7Se8/Carbon nanoboxes with enhanced sodium storage in half/full cells. Carbon 2020, 166, 175–182. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, S.; Ying, H.; Zhang, Z.; Han, W. Few-layered Ti3C2 MXene anchoring bimetallic selenide NiCo2Se4 nanoparticles for superior Sodium-ion batteries. Chem. Eng. J. 2021, 417, 129161. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.L.; Tan, P.; Bao, S.; Zhang, X.; Xu, M. Ultrafast kinetics and high capacity for Stable Sodium Storage enabled by Fe3Se4/ZnSe heterostructure engineering. Composites Part B 2021, 224, 109166. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, S.; Dai, P.; Zeng, Y.; Huang, L.; Liao, H.G.; Sun, S.G. Insights into Electrochemical Processes of Hollow Octahedral Co3Se4@rGO for High-Rate Sodium Ion Storage. ACS Appl. Mater. Interfaces 2022, 14, 37689–37698. [Google Scholar] [CrossRef]
- Wu, H.; Yuan, R.; Li, M.; Liu, L.; Liu, Y.; Song, Q.; Ai, W.; Du, H.; Du, Z.; Wang, K. Co0.85Se–Fe7Se8 nanocuboids embedded in reduced graphene oxides as cycle-stable anodes for sodium-ion batteries. Carbon 2022, 198, 171–178. [Google Scholar] [CrossRef]
- Yue, L.; Wang, D.; Wu, Z.; Zhao, W.; Ren, Y.; Zhang, L.; Zhong, B.; Li, N.; Tang, B.; Liu, Q.; et al. Polyrrole-encapsulated Cu2Se nanosheets in situ grown on Cu mesh for high stability sodium-ion battery anode. Chem. Eng. J. 2022, 433, 134477. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, X.; Yue, L.; Zhang, L.; Luo, Y.; Ren, Y.; Zhao, X.-E.; Li, N.; Tang, B.; Liu, Q.; et al. A gradient hexagonal-prism Fe3Se4@SiO2@C configuration as a highly reversible sodium conversion anode. J. Mater. Chem. A 2022, 10, 4087–4099. [Google Scholar] [CrossRef]
- Wu, H.; Wang, K.; Li, M.; Wang, Y.; Zhu, Z.; Liang, J.; Du, Z.; Ai, W.; He, S.; Yuan, R.; et al. Double-Walled NiTeSe–NiSe2 Nanotubes Anode for Stable and High-Rate Sodium-Ion Batteries. Small 2023, 19, 2300162. [Google Scholar] [CrossRef] [PubMed]
- Gim, H.; Maulana, A.Y.; Choi, J.; Song, J.; Yun, B.; Jeong, Y.; An, N.; Park, M.; Futalan, C.M.; Kim, J. One dimensional pea-shaped NiSe2 nanoparticles encapsulated in N-doped graphitic carbon fibers to boost redox reversibility in sodium-ion batteries. J. Mater. Sci. Technol. 2024, 168, 215–226. [Google Scholar] [CrossRef]
- Xiong, Z.; Shi, H.; Zhang, W.; Yan, J.; Wu, J.; Wang, C.; Wang, D.; Wang, J.; Gu, Y.; Chen, F.-R.; et al. In Situ Growth of Iron Sulfide on Fast Charge Transfer V2C-MXene for Superior Sodium Storage Anodes. Small 2023, 19, 2206767. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, H.; Yang, L.; Lv, R. Ultrahigh rate sodium-ion storage of SnS/SnS2 heterostructures anchored on S-doped reduced graphene oxide by ion-assisted growth. Carbon 2019, 143, 21–29. [Google Scholar] [CrossRef]
- Sun, S.; Zang, J.; Ruan, J.; Fang, F.; Sun, D.; Song, Y.; Wang, F. Two-Dimensional CuGaSe2@ZnSe-NC Heterostructures for Enhanced Sodium Ion Storage. ACS Appl. Energy Mater. 2021, 4, 2761–2768. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, W.; Yuan, S.; Zhang, L.; Yang, Y.; Ge, P.; Ji, X. Designing Rational Interfacial Bonds for Hierarchical Mineral-Type Trogtalite with Double Carbon towards Ultra-Fast Sodium-Ions Storage Properties. Adv. Funct. Mater. 2021, 31, 2100156. [Google Scholar] [CrossRef]
- Zheng, Y.; Wei, S.; Shang, J.; Wang, D.; Lei, C.; Zhao, Y. High-Performance Sodium-Ion Batteries Enabled by 3D Nanoflowers Comprised of Ternary Sn-Based Dichalcogenides Embedded in Nitrogen and Sulfur Dual-Doped Carbon. Small 2023, 19, e2303746. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, J.; Li, H.; Deng, S.; Zuo, Y.; Yan, W.; Zhang, J. Constructing a novel heterostructure of NiSe2/CoSe2 nanoparticles with boosted sodium storage properties for sodium-ion batteries. J. Mater. Chem. A 2022, 10, 16268–16279. [Google Scholar] [CrossRef]
- Hu, X.; Qiu, M.; Liu, Y.; Yuan, J.; Chen, J.; Zhan, H.; Wen, Z. Interface and Structure Engineering of Tin-Based Chalcogenide Anodes for Durable and Fast-Charging Sodium Ion Batteries. Adv. Energy Mater. 2022, 12, 2202318. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Wang, E.; Kuang, Q.; Ming, Y.; Zhong, B.; Wu, Z.; Guo, X. Optimization of NixCo1-x-yMnySe2 composition for efficient sodium storage. Chem. Eng. J. 2023, 456, 140951. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.; Qu, D.; Li, Q.; Sun, Z.; Song, Z.; Guan, H.; Niu, L. Recent Advances on Transition Metal Chalcogenide for Sodium-Ion Batteries. Batteries 2023, 9, 467. https://doi.org/10.3390/batteries9090467
Wei C, Qu D, Li Q, Sun Z, Song Z, Guan H, Niu L. Recent Advances on Transition Metal Chalcogenide for Sodium-Ion Batteries. Batteries. 2023; 9(9):467. https://doi.org/10.3390/batteries9090467
Chicago/Turabian StyleWei, Chunyan, Dongyang Qu, Qiuyu Li, Zhonghui Sun, Zhongqian Song, Hongyu Guan, and Li Niu. 2023. "Recent Advances on Transition Metal Chalcogenide for Sodium-Ion Batteries" Batteries 9, no. 9: 467. https://doi.org/10.3390/batteries9090467






