Molybdenum-Based Electrode Materials Applied in High-Performance Supercapacitors
Abstract
:1. Introduction
2. Mo-Based Electrode Materials for Supercapacitors
2.1. Binary Mo-Based Materials
2.1.1. Molybdenum Oxides

2.1.2. Molybdenum Carbides
2.1.3. Molybdenum Nitrides
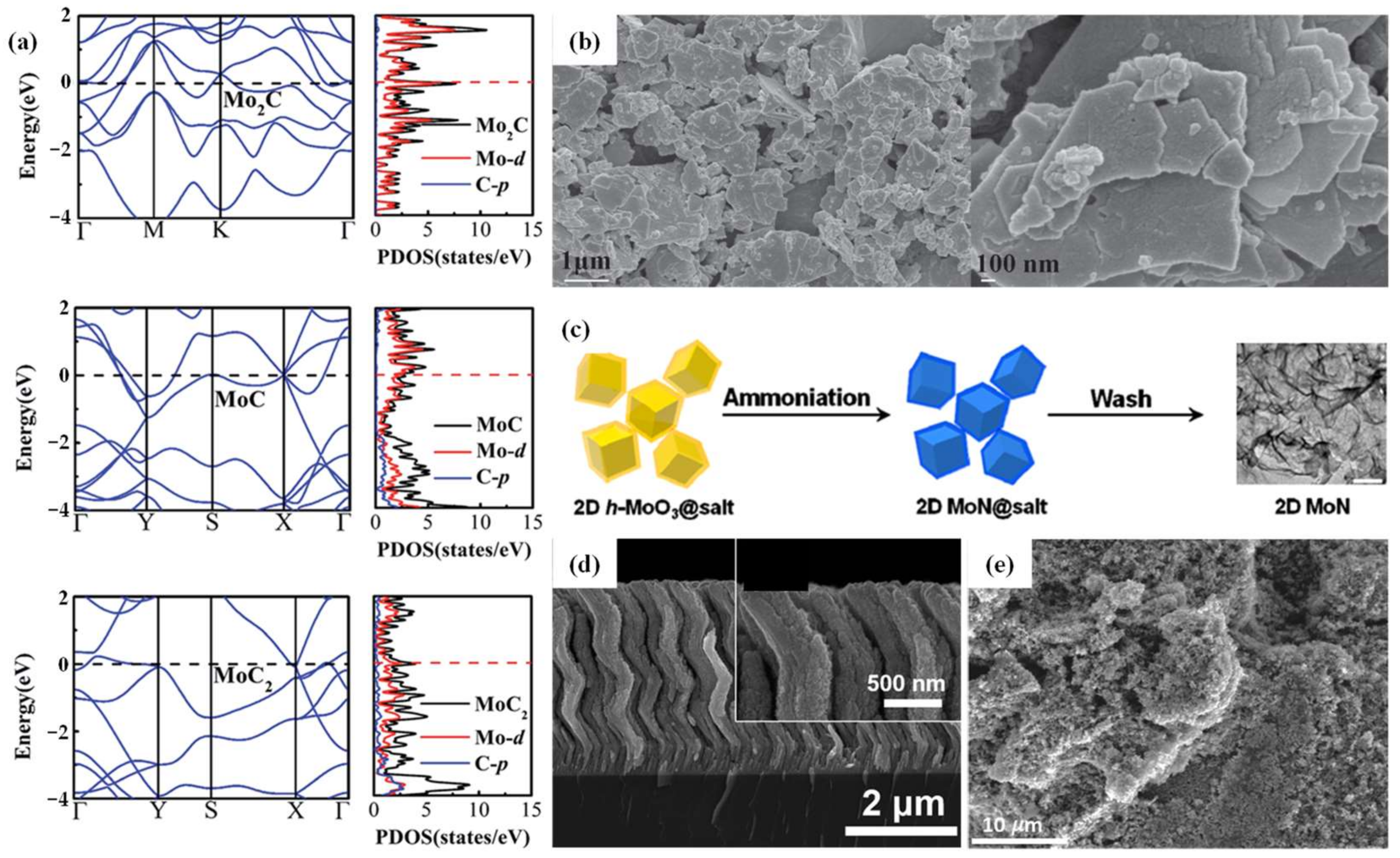
2.1.4. Molybdenum Sulfides
| Electrode Material | Method | Structure | Specific Capacitance | Capacitance Retention | Ref. |
|---|---|---|---|---|---|
| Mo2C | calcination | nanosheets | 88 F g−1 (0.5 A g−1) | 95%, 1200 cycles | [42] |
| MoN | template | nanosheets | 928 F cm−3 (2 mV s−1) | 95%, 25,000 cycles | [45] |
| γ-Mo2N | magnetron sputtering | thin films | 248 mF cm−2 (50 mV s−1) | 95%, 20,000 cycles | [46] |
| γ-Mo2N | calcination | porous | 1500 F g−1 (N/A) | N/A | [47] |
| MoS2 | hydrothermal | nanoflowers | 137 mF cm−2 (10 mA cm−2) | 81.6%, 10,000 cycles | [52] |
| MoS2 | electrodeposition | nanosheets | 416.9 mF cm−2 (1 mA cm−2) | 90.1%, 3000 cycles | [53] |
| 2H-MoS2 | hydrothermal | nanosheets | 379 F g−1 (1 A g−1) | 92%, 3000 cycles | [54] |
| MoS2 | hydrothermal | nanoflowers | 255.65 F g−1 (0.25 A g−1) | 70%, 1000 cycles | [55] |
| MoSe2 | microwave | mesoporous | 257.38 F g−1 (1 A g−1) | 95%, 5000 cycles | [56] |
| 2H-MoSe2 | in situ selenization | nanosheets | 46.22 mA h g−1 (2 A g−1) | 64%, 2000 cycles | [57] |
| MoSe2 | hydrothermal | nanoflowers | 641.5 mA h g−1 (0.1 A g−1) | 70.28%, 5000 cycles | [58] |
2.2. Ternary Mo-Based Materials
2.2.1. Metal Molybdates
2.2.2. Mo-MXenes
| Electrode Material | Method | Structure | Specific Capacitance | Capacitance Retention | Ref. |
|---|---|---|---|---|---|
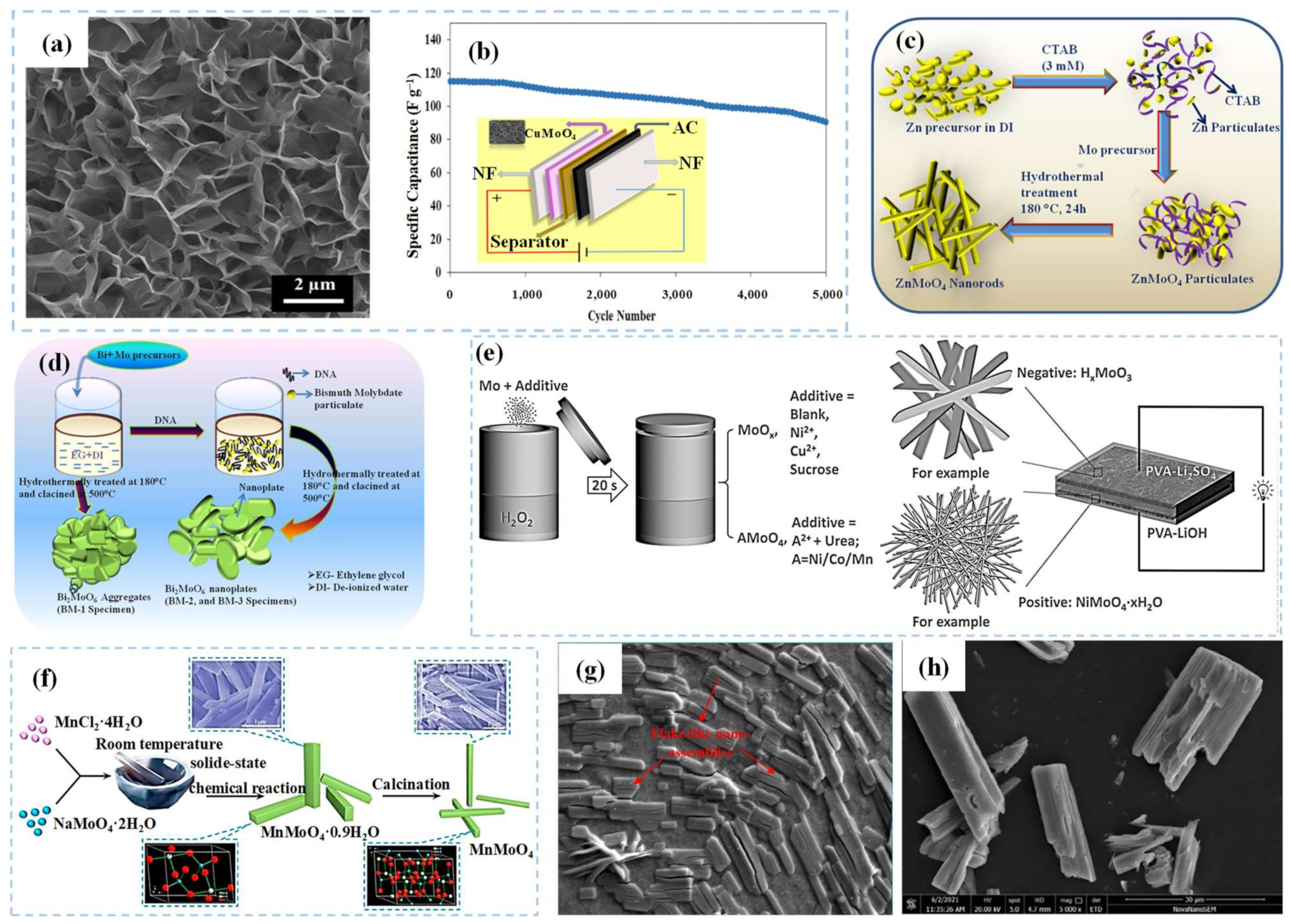
| CuMoO4 | hydrothermal | nanosheets | 2259.55 F g−1 (1 A g−1) | 90.08%, 5000 cycles | [62] |
| ZnMoO4 | template | nanorods | 779 F g−1 (5 mV s−1) | 90%, 3000 cycles | [63] |
| Bi2MoO6 | template | nanoplates | 698 F g−1 (5 mV s−1) | 86%, 3000 cycles | [64] |
| NiMoO4·xH2O | mixture | nanowires | 549 C g−1 (1 A g−1) | 81%, 5000 cycles | [65] |
| MnMoO4 | nitriding | nanorods | 210.2 F g−1 (1 A g−1) | 112.6%, 10,000 cycles | [66] |
| Sn(MoO4)2 | solution method | nanosheets | 109 F g−1 (5 mV s−1) | 70%, 4000 cycles | [67] |
| CoMoO4 | hydrothermal | nanorods | 11.11 F cm−2 (3 mA cm−2) | N/A | [68] |
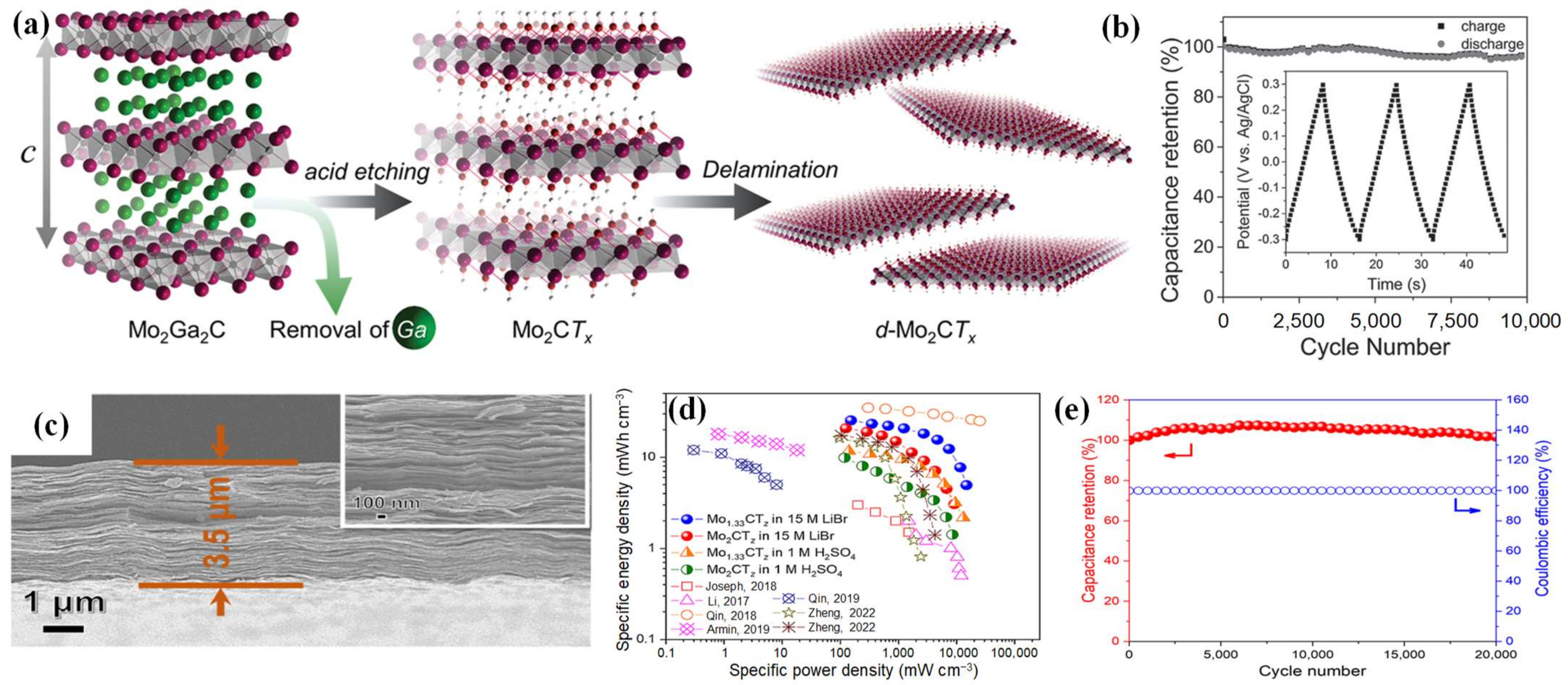
| Mo2CTx | etching | nanosheets | 700 F cm−3 (2 mV s−1) | ~100%, 10,000 cycles | [71] |
| Mo1.33CTz | etching | nanofilms | 127 F cm−3 (2 mV s−1) | 99.4%, 20,000 cycles | [73] |
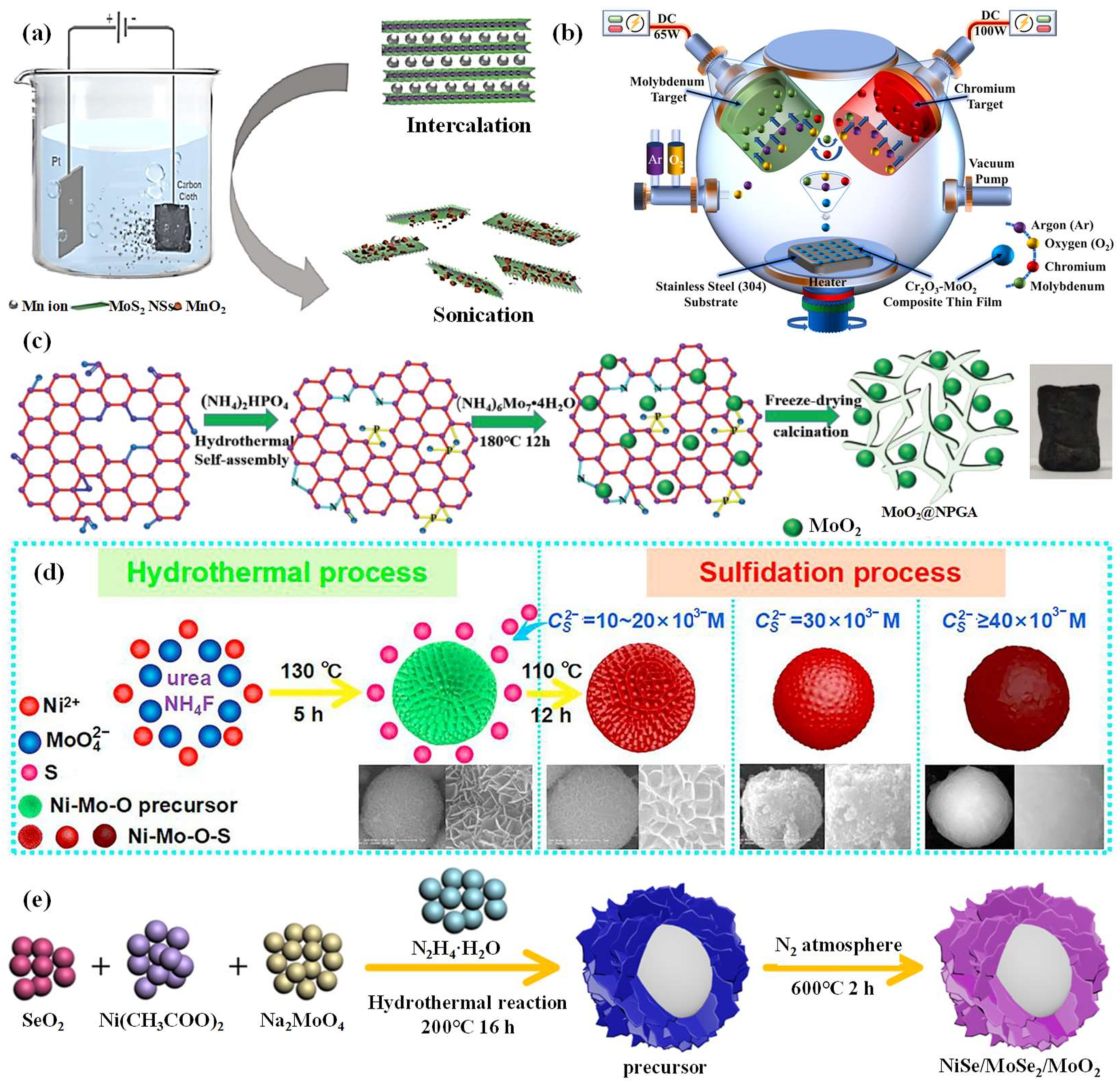
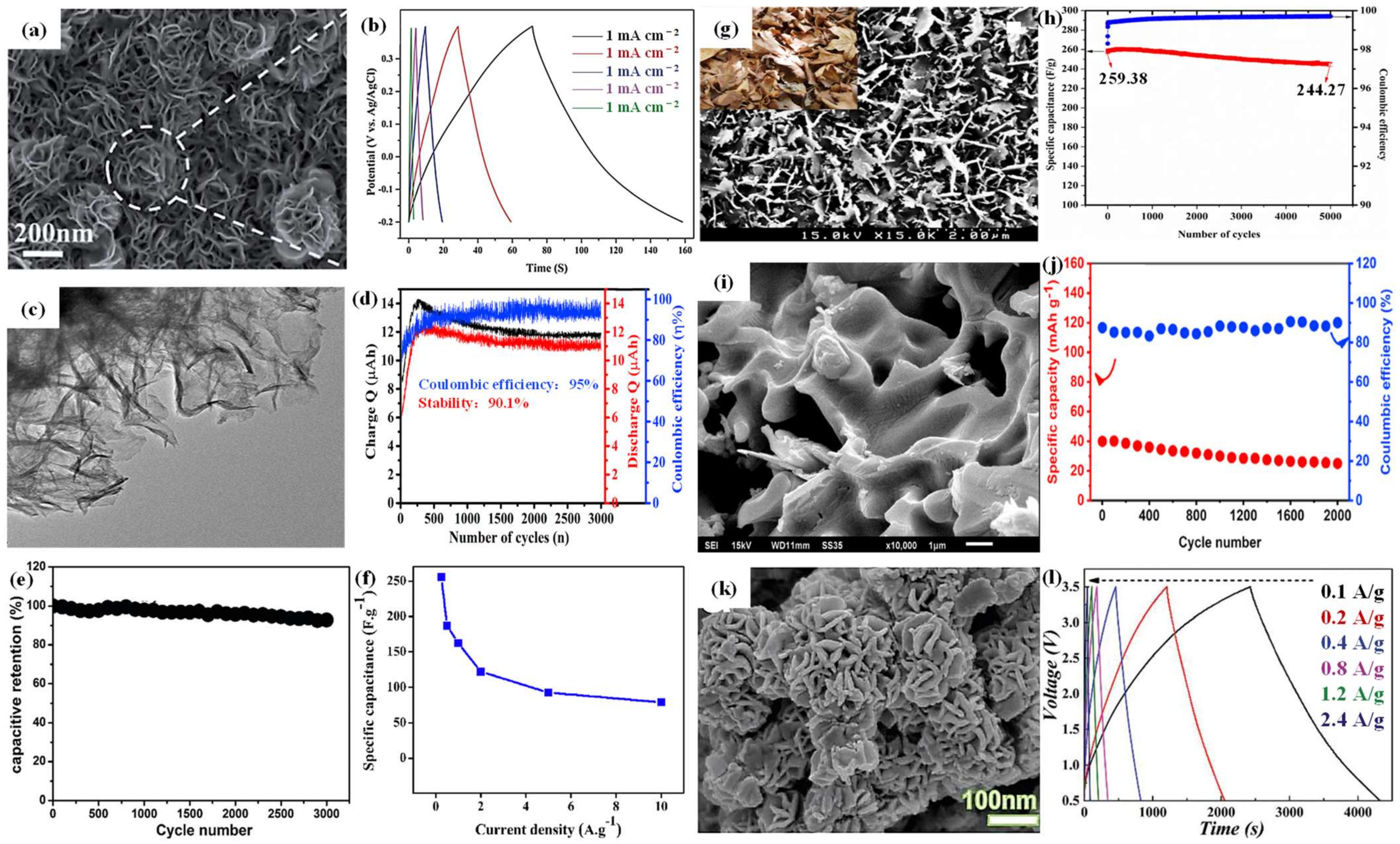
2.3. Nanocomposites of Mo-Based Materials
2.3.1. Nanocomposites of Mo-Based Materials and Metallic Oxides
2.3.2. Nanocomposites of Mo-Based Materials and Carbon
2.3.3. Nanocomposites of Mo-Based Materials and Metallic Sulfides
2.4. Mo-Based MOFs and Mo-Based Materials Deriving from MOFs
3. Conclusions and Outlook
- (1)
- Conductivity and electrochemical stability: The optimized Mo-based electrode materials should possess high conductivity and excellent electrochemical stability to facilitate improved performance and long cycling life.
- (2)
- The excellent electrode materials should present a high specific surface area and a hierarchical porous structure to facilitate fast ion transport.
- (3)
- Cost of mass-industrial manufacture: The cost of mass-industrial manufacture for Mo-based materials is still a challenge, which should be further improved in the application of supercapacitors.
- (4)
- Research on the energy storage mechanism: The energy storage mechanism in supercapacitors remain controversial. Therefore, it is essential to make efforts in investigating the energy storage mechanism.
- (5)
- Application of computational materials science: It is important to resort to computational materials science to design and exploit novel Mo-based electrode materials. In addition, this approach can decrease experimental costs and accelerate experimental processes through a large number of parallel experiments.
- (6)
- There are limited reports on Mo-based MXene materials. It is necessary to etch various MAX phases to develop a series of Mo-based MXenes and explore their application in supercapacitors.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, D.; Liu, Y.; Qin, T.; Gu, H.; Cao, Y.; Shi, H. Overview of the policy instruments for renewable energy development in China. Energies 2022, 15, 6513. [Google Scholar] [CrossRef]
- Pan, Y.; Dong, F. Dynamic evolution and driving factors of new energy development: Fresh evidence from China. Technol. Forecast. Soc. Chang. 2022, 176, 14. [Google Scholar] [CrossRef]
- Guo, L.; Hu, P.; Wei, H. Development of supercapacitor hybrid electric vehicle. J. Energy Storage 2023, 65, 8. [Google Scholar] [CrossRef]
- Lamba, P.; Singh, P.; Singh, P.; Singh, P.; Bharti; Kumar, A.; Gupta, M.; Kumar, Y. Recent advancements in supercapacitors based on different electrode materials: Classifications, synthesis methods and comparative performance. J. Energy Storage 2022, 48, 103871. [Google Scholar] [CrossRef]
- Frackowiak, E.; Abbas, Q.; Béguin, F. Carbon/carbon supercapacitors. J. Energy Chem. 2013, 22, 226–240. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from “supercapacitor” to “battery” behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539–1548. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, X.; Qiao, Z.; Jung, J.; Zhu, Y.; Lu, Y.; Zhang, L.L.; MacDonald, A.H.; Ruoff, R.S. Capacitance of carbon-based electrical double-layer capacitors. Nat. Commun. 2014, 5, 3317. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.F.; Wang, A.L.; Xu, H.; He, X.J.; Tong, Y.X.; Li, G.R. High-performance supercapacitors based on MnO2 tube-in-tube arrays. J. Mater. Chem. A 2015, 3, 16560–16566. [Google Scholar] [CrossRef]
- Sharma, S.; Chand, P. Supercapacitor and electrochemical techniques: A brief review. Results Chem. 2023, 5, 100885. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Wang, D.; Tang, K.; Xiao, J.; Li, X.; Long, M.; Chen, J.; Gao, H.; Chen, W.; Liu, C.; Liu, H. Advances of electrospun Mo-based nanocomposite fibers as anode materials for supercapacitors. Sustain. Mater. Technol. 2021, 29, e00302. [Google Scholar] [CrossRef]
- Xiong, C.; Zhang, Y.; Xu, J.; Dang, W.; Sun, X.; An, M.; Ni, Y.; Mao, J. Kinetics process for structure-engineered integrated gradient porous paper-based supercapacitors with boosted electrochemical performance. Nano Res. 2023, 16, 9471–9479. [Google Scholar] [CrossRef]
- Xiong, C.; Zheng, C.; Jiang, X.; Xiao, X.; Wei, H.; Zhou, Q.; Ni, Y. Recent progress of green biomass based composite materials applied in supercapacitors, sensors, and electrocatalysis. J. Energy Storage 2023, 72, 108633. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, T.; Han, J.; Zhang, Z.; Ni, Y. Recent research progress of paper-based supercapacitors based on cellulose. Energy Environ. Mater. 2023, e12651. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, B.; Brousse, T.; Ramirez Castro, C.; Nicolosi, V.; Grant, P.S. An investigation of nanostructured thin film α-MoO3 based supercapacitor electrodes in an aqueous electrolyte. Electrochim. Acta 2013, 91, 253–260. [Google Scholar] [CrossRef]
- Ma, H.; Liang, J.; Qiu, J.; Jiang, L.; Ma, L.; Sheng, H.; Shao, M.; Wang, Q.; Li, F.; Fu, Y.; et al. A biocompatible supercapacitor diode with enhanced rectification capability toward ion/electron-coupling logic operations. Adv. Mater. 2023, 35, e2301218. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Hussain, S.; Karuppasamy, K.; Santhoshkumar, P.; Kathalingam, A.; Jung, J.; Kim, H.S. Fabrication of asymmetric supercapacitors using molybdenum dichalcogenide nanoarray structures. Int. J. Energy Res. 2022, 46, 18410–18425. [Google Scholar] [CrossRef]
- Yuksel, R.; Coskun, S.; Unalan, H.E. Coaxial silver nanowire network core molybdenum oxide shell supercapacitor electrodes. Electrochim. Acta 2016, 193, 39–44. [Google Scholar] [CrossRef]
- Saji, V.S.; Lee, C.W. Molybdenum, molybdenum oxides, and their electrochemistry. ChemSusChem 2012, 5, 1146–1161. [Google Scholar] [CrossRef]
- Mai, L.Q.; Hu, B.; Chen, W.; Qi, Y.Y.; Lao, C.S.; Yang, R.S.; Dai, Y.; Wang, Z.L. Lithiated MoO3 nanobelts with greatly improved performance for lithium batteries. Adv. Mater. 2007, 19, 3712–3716. [Google Scholar] [CrossRef]
- De Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos Gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-Zadeh, K. Molybdenum oxides—From fundamentals to functionality. Adv. Mater. 2017, 29, 31. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, W.; Zheng, W. The debut and spreading the landscape for excellent vacancies-promoted electrochemical energy storage of nano-architected molybdenum oxides. Mater. Today Energy 2022, 30, 101154. [Google Scholar] [CrossRef]
- Pan, W.; Tian, R.; Jin, H.; Guo, Y.; Zhang, L.; Wu, X.; Zhang, L.; Han, Z.; Liu, G.; Li, J.; et al. Structure, optical, and catalytic properties of novel hexagonal metastable h-MoO3 nano- and microrods synthesized with modified liquid-phase processes. Chem. Mater. 2010, 22, 6202–6208. [Google Scholar] [CrossRef]
- Prakash, N.G.; Dhananjaya, M.; Narayana, A.L.; Shaik, D.P.M.D.; Rosaiah, P.; Hussain, O.M. High performance one dimensional α-MoO3 nanorods for supercapacitor applications. Ceram. Int. 2018, 44, 9967–9975. [Google Scholar] [CrossRef]
- Niu, Y.; Li, X.; Su, H.; Li, J.; Qi, Y. Formation of three dimensional porous h-MoO3 architecture and its application in supercapacitors. Mater. Lett. 2022, 316, 132062. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, Y.; Li, H. MoO3 nanoplates preparation via self-sacrifice C3N4 for supercapacitors in an acid electrolyte. J. Energy Storage 2023, 60, 106657. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, J.; Xia, G.; Liu, Y.; Zheng, Y.; Zhang, W.; Tang, Y.; Pang, W.K.; Guo, Z. Heterostructure manipulation via in situ localized phase transformation for high-rate and highly durable lithium ion storage. ACS Nano 2018, 12, 10430–10438. [Google Scholar] [CrossRef]
- Ma, B.; Hao, W.; Ruan, W.; Yuan, C.; Wang, Q.; Teng, F. Unveiling capacitive behaviors of MoO2 in different electrolytes and flexible MoO2-based asymmetric micro-supercapacitor. J. Energy Storage 2022, 52, 104833. [Google Scholar] [CrossRef]
- Wu, K.; Zhao, J.; Zhang, X.; Zhou, H.; Wu, M. Hierarchical mesoporous MoO2 sphere as highly effective supercapacitor electrode. J. Taiwan Inst. Chem. Eng. 2019, 102, 212–217. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.E.; Cao, J.; Cai, W.; Sui, J. Amorphous/crystalline hybrid MoO2 nanosheets for high-energy lithium-ion capacitors. Chem. Commun. 2017, 53, 10723–10726. [Google Scholar] [CrossRef]
- Luo, Z.; Miao, R.; Huan, T.D.; Mosa, I.M.; Poyraz, A.S.; Zhong, W.; Cloud, J.E.; Kriz, D.A.; Thanneeru, S.; He, J.; et al. Mesoporous MoO3–x material as an mfficient mlectrocatalyst for hydrogen evolution reactions. Adv. Energy Mater. 2016, 6, 11. [Google Scholar] [CrossRef]
- Gurusamy, L.; Karuppasamy, L.; Anandan, S.; Liu, N.; Lee, G.J.; Liu, C.H.; Wu, J.J. Enhanced performance of charge storage supercapattery by dominant oxygen deficiency in crystal defects of 2-D MoO3-x nanoplates. Appl. Surf. Sci. 2021, 541, 148676. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Furuichi, N.; Seki, H.; Yamashita, H. One-pot synthesis of molybdenum oxide nanoparticles encapsulated in hollow silica spheres: An efficient and reusable catalyst for epoxidation of olefins. J. Mater. Chem. A 2017, 5, 18518–18526. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Zhang, Y.; Liu, L.; Wang, W. Surface-ligand protected reduction on plasmonic tuning of one-dimensional MoO3−x nanobelts for solar steam generation. Nano Res. 2020, 13, 3025–3032. [Google Scholar] [CrossRef]
- Wu, Q.L.; Zhao, S.X.; Yu, L.; Zheng, X.X.; Wang, Y.F.; Yu, L.Q.; Nan, C.W.; Cao, G.Z. Oxygen vacancy-enriched MoO3-x nanobelts for asymmetric supercapacitors with excellent room/low temperature performance. J. Mater. Chem. A 2019, 7, 13205–13214. [Google Scholar] [CrossRef]
- Cong, S.; Hadipour, A.; Sugahara, T.; Wei, T.; Jiu, J.; Ranjbar, S.; Hirose, Y.; Karakawa, M.; Nagao, S.; Aernouts, T.; et al. Modifying the valence state of molybdenum in the efficient oxide buffer layer of organic solar cells via a mild hydrogen peroxide treatment. J. Mater. Chem. C 2017, 5, 889–895. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, Y.; Zheng, S.; Zhang, C.; Hu, C.; Liang, B.; Xu, Y.; Huang, G.; Yang, R. Oxygen deficiency and single-crystalline MoO3−x nanobelt as advanced supercapacitor negative electrode and dye adsorbent. Colloids Surf. A 2022, 647, 129064. [Google Scholar] [CrossRef]
- Salkar, A.V.; Naik, A.P.; Peña, G.D.J.G.; Bharath, G.; Haija, M.A.; Banat, F.; Morajkar, P.P. 2D α-MoO3-x truncated microplates and microdisks as electroactive materials for highly efficient asymmetric supercapacitors. J. Energy Storage 2022, 48, 103958. [Google Scholar] [CrossRef]
- Cao, Y.B.; Zhi, S.X.; Qi, H.B.; Zhang, Y.; Qin, C.; Yang, S.P. Evolution behavior of ex-situ NbC and properties of Fe-based laser clad coating. Opt. Laser Technol. 2020, 124, 8. [Google Scholar] [CrossRef]
- Gao, J.; Xu, C.; Tian, X.; Sun, M.; Zhao, J.; Ma, J.Y.; Zhou, H.; Xiao, J.; Wu, M. Design bifunctional vanadium carbide embedded in mesoporous carbon electrode for supercapacitor and dye-sensitized solar cell. Sol. Energy 2020, 206, 848–854. [Google Scholar] [CrossRef]
- Wan, C.; Zhang, R.; Wang, S.; Liu, X. Molten salt electrolytic fabrication of TiC-CDC and its applications for supercapacitor. J. Mater. Sci. Technol. 2017, 33, 788–792. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Q.Q.; Zhang, W.B.; Liu, M.C.; Kong, L.B. Enhanced performance for a high electrical conductive Mo2C electrode based proton ionic liquid electrolytes in supercapacitors. Mater. Res. Express 2018, 5, 75508. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, Z.; Peng, Q.; Zhou, J.; Sun, Z. Novel two-dimensional molybdenum carbides as high capacity anodes for lithium/sodium-ion batteries. J. Mater. Chem. A 2019, 7, 12145–12153. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, C.; Liu, L.; Zhu, M. A review on the synthesis of transition metal nitride nanostructures and their energy related applications. Green Energy Environ. 2023, 8, 406–437. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, H.; Jin, H.; Wu, M.; Fang, Y.; Sun, J.; Hu, Z.; Li, T.; Wu, J.; Huang, L.; et al. Salt-templated synthesis of 2D metallic MoN and other nitrides. ACS Nano 2017, 11, 2180–2186. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, T.; Wang, Q.; Heinz Mayrhofer, P. Nanostructured zig-zag γ-Mo2N thin films produced by glancing angle deposition for flexible symmetrical solid-state supercapacitors. Mater. Des. 2023, 225, 111432. [Google Scholar] [CrossRef]
- Djire, A.; Siegel, J.B.; Ajenifujah, O.; He, L.; Thompson, L.T. Pseudocapacitive storage via micropores in high-surface area molybdenum nitrides. Nano Energy 2018, 51, 122–127. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Luo, J.; Duan, X.; Crittenden, J.; Liu, C.; Zhang, S.; Pei, Y.; Zeng, Y.; Duan, X. Self-Optimization of the Active Site of Molybdenum Disulfide by an Irreversible Phase Transition during Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2017, 56, 7610–7614. [Google Scholar] [CrossRef]
- Li, R.; Liang, S.; Aihemaiti, A.; Li, S.; Zhang, Z. Effectively enhanced piezocatalytic activity in flower-like 2H-MoS2 with tunable S vacancy towards organic pollutant degradation. Appl. Surf. Sci. 2023, 631, 157461. [Google Scholar] [CrossRef]
- Marinov, A.D.; Bravo Priegue, L.; Shah, A.R.; Miller, T.S.; Howard, C.A.; Hinds, G.; Shearing, P.R.; Cullen, P.L.; Brett, D.J.L. Ex situ characterization of 1T/2H MoS2 and their carbon composites for energy applications, a review. ACS Nano 2023, 17, 5163–5186. [Google Scholar] [CrossRef]
- Li, H.; Han, X.; Jiang, S.; Zhang, L.; Ma, W.; Ma, R.; Zhou, Z. Controllable fabrication and structure evolution of hierarchical 1T-MoS2 nanospheres for efficient hydrogen evolution. Green Energy Environ. 2022, 7, 314–323. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Wu, Y.; Wang, Y.; Yang, J.; Wang, Z. Rosette-like MoS2 nanoflowers as highly active and stable electrodes for hydrogen evolution reactions and supercapacitors. RSC Adv. 2019, 9, 13820–13828. [Google Scholar] [CrossRef] [PubMed]
- Teli, A.M.; Beknalkar, S.A.; Mane, S.M.; Bhat, T.S.; Kamble, B.B.; Patil, S.B.; Sadale, S.B.; Shin, J.C. Electrodeposited crumpled MoS2 nanoflakes for asymmetric supercapacitor. Ceram. Int. 2022, 48, 29002–29010. [Google Scholar] [CrossRef]
- Joseph, N.; Muhammed Shafi, P.; Chandra Bose, A. Metallic 1T-MoS2 with defect induced additional active edges for high performance supercapacitor application. N. J. Chem. 2018, 42, 12082–12090. [Google Scholar] [CrossRef]
- Gupta, H.; Chakrabarti, S.; Mothkuri, S.; Padya, B.; Rao, T.N.; Jain, P.K. High performance supercapacitor based on 2D-MoS2 nanostructures. Mater. Today Proc. 2020, 26, 20–24. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Devarayapalli, K.C.; Nagajyothi, P.C.; Shim, J. Microwave synthesized dry leaf-like mesoporous MoSe2 nanostructure as an efficient catalyst for enhanced hydrogen evolution and supercapacitor applications. Microchem. J. 2020, 153, 104446. [Google Scholar] [CrossRef]
- Upadhyay, S.; Pandey, O.P. Synthesis of layered 2H-MoSe2 nanosheets for the high-performance supercapacitor electrode material. J. Alloys Compd. 2021, 857, 157522. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Wang, Y.-K.; Kong, L.-B. A facile strategy for the synthesis of three-dimensional heterostructure self-assembled MoSe2 nanosheets and their application as an anode for high-energy lithium-ion hybrid capacitors. Nanoscale 2019, 11, 7263–7276. [Google Scholar] [CrossRef]
- Sha, R.; Maity, P.C.; Rajaji, U.; Liu, T.-Y.; Bhattacharyya, T.K. Review—MoSe2 nanostructures and related electrodes for advanced supercapacitor developments. J. Electrochem. Soc. 2022, 169, 13503. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, S.; Wang, L.; Tang, H.; Xue, H.; Wang, G.; Pang, H. Fabrication of metal molybdate micro/nanomaterials for electrochemical energy storage. Small 2017, 13, 19. [Google Scholar] [CrossRef]
- Yang, J.; Yao, G.; Sun, S.; Chen, Z.; Yuan, S.; Wu, K.; Fu, X.; Wang, Q.; Cui, W. Structural, magnetic properties of in-plane chemically ordered (Mo2/3R)2AlC (R=Gd, Tb, Dy, Ho, Er and Y) MAX phase and enhanced capacitance of Mo1.33C MXene derivatives. Carbon 2021, 179, 104–110. [Google Scholar] [CrossRef]
- Farahpour, M.; Arvand, M. Single-pot hydrothermal synthesis of copper molybdate nanosheet arrays as electrode materials for high areal-capacitance supercapacitor. J. Energy Storage 2021, 40, 102742. [Google Scholar] [CrossRef]
- Gurusamy, H.; Sivasubramanian, R.; Johnbosco, Y.; Bhagavathiachari, M. Morphology-controlled synthesis of one-dimensional zinc molybdate nanorods for high-performance pseudocapacitor electrode application. Chem. Pap. 2020, 75, 1715–1726. [Google Scholar] [CrossRef]
- Yesuraj, J.; Austin Suthanthiraraj, S.; Padmaraj, O. Synthesis, characterization and electrochemical performance of DNA-templated Bi2MoO6 nanoplates for supercapacitor applications. Mater. Sci. Semicond. Process. 2019, 90, 225–235. [Google Scholar] [CrossRef]
- Qu, G.; Li, T.; Jia, S.; Zheng, H.; Li, L.; Cao, F.; Wang, H.; Ma, W.; Tang, Y.; Wang, J. Rapid and scalable synthesis of Mo-based binary and ternary oxides for electrochemical applications. Adv. Funct. Mater. 2017, 27, 1700928. [Google Scholar] [CrossRef]
- Sheng, R.; Hu, J.; Lu, X.; Jia, W.; Xie, J.; Cao, Y. Solid-state synthesis and superior electrochemical performance of MnMoO4 nanorods for asymmetric supercapacitor. Ceram. Int. 2021, 47, 16316–16323. [Google Scholar] [CrossRef]
- Sakthikumar, K.; Ede, S.R.; Mishra, S.; Kundu, S. Shape-selective synthesis of Sn(MoO4)2 nanomaterials for catalysis and supercapacitor applications. Dalton Trans. 2016, 45, 8897–8915. [Google Scholar] [CrossRef]
- Li, L.; Zhou, J.; Zhang, Y.; Pei, X. Synthesis process optimization and electrochemical properties of CoMoO4 supercapacitor prepared by in situ growth method. J. Mater. Sci. Mater. Electron. 2022, 33, 23851–23866. [Google Scholar] [CrossRef]
- Liang, C.; Meng, Y.; Zhang, Y.; Zhang, H.; Wang, W.; Lu, M.; Wang, G. Insights into the impact of interlayer spacing on MXene-based electrodes for supercapacitors: A review. J. Energy Storage 2023, 65, 20. [Google Scholar] [CrossRef]
- Garg, R.; Agarwal, A.; Agarwal, M. A review on MXene for energy storage application: Effect of interlayer distance. Mater. Res. Express 2020, 7, 21. [Google Scholar] [CrossRef]
- Halim, J.; Kota, S.; Lukatskaya, M.R.; Naguib, M.; Zhao, M.Q.; Moon, E.J.; Pitock, J.; Nanda, J.; May, S.J.; Gogotsi, Y.; et al. Synthesis and characterization of 2D molybdenum carbide (MXene). Adv. Funct. Mater. 2016, 26, 3118–3127. [Google Scholar] [CrossRef]
- Das, M.; Ghosh, S. Theoretical investigation of capacitances in functionalised MXene supercapacitors Mn+1CnO2, M. = Ti, V., Nb, Mo. J. Phys. D Appl. Phys. 2021, 55, 85502. [Google Scholar] [CrossRef]
- Zheng, W.; Halim, J.; Persson, P.O.Å.; Rosen, J.; Barsoum, M.W. Effect of vacancies on the electrochemical behavior of Mo-based MXenes in aqueous supercapacitors. J. Power Sources 2022, 525, 231064. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Mojtabavi, M.; Caffrey, N.M.; Wanunu, M.; Beidaghi, M. Assembling 2D MXenes into highly stable pseudocapacitive electrodes with high power and energy densities. Adv. Mater. 2019, 31, 1806931. [Google Scholar] [CrossRef]
- Li, H.; Hou, Y.; Wang, F.; Lohe, M.R.; Zhuang, X.; Niu, L.; Feng, X. Flexible all-solid-state supercapacitors with high volumetric capacitances boosted by solution processable MXene and electrochemically exfoliated graphene. Adv. Energy Mater. 2017, 7, 1601847. [Google Scholar] [CrossRef]
- Halim, J.; Moon, E.J.; Eklund, P.; Rosen, J.; Barsoum, M.W.; Ouisse, T. Variable range hopping and thermally activated transport in molybdenum-based MXenes. Phys. Rev. B 2018, 98, 104202. [Google Scholar] [CrossRef]
- Zheng, W.; Halim, J.; Rosen, J.; Barsoum, M.W. Aqueous electrolytes, MXene-based supercapacitors and their self-discharge. Adv. Energy Sustain. Res. 2022, 3, 202100147. [Google Scholar] [CrossRef]
- Qin, L.; Tao, Q.; El Ghazaly, A.; Fernandez-Rodriguez, J.; Persson, P.O.Å.; Rosen, J.; Zhang, F. High-Performance ultrathin flexible solid-state supercapacitors based on solution processable Mo1.33C MXene and PEDOT:PSS. Adv. Funct. Mater. 2018, 28, 1703808. [Google Scholar] [CrossRef]
- Qin, L.; Tao, Q.; Liu, X.; Fahlman, M.; Halim, J.; Persson, P.O.Å.; Rosen, J.; Zhang, F. Polymer-MXene composite films formed by MXene-facilitated electrochemical polymerization for flexible solid-state microsupercapacitors. Nano Energy 2019, 60, 734–742. [Google Scholar] [CrossRef]
- Hu, R.; Liao, Y.; Qiao, H.; Li, J.; Wang, K.; Huang, Z.; Qi, X. Electrochemical method integrating exfoliation and in-situ growth to synthesize MoS2 nanosheets/MnO2 heterojunction for performance-enhanced supercapacitor. Ceram. Int. 2022, 48, 23498–23503. [Google Scholar] [CrossRef]
- Sharma, M.; Adalati, R.; Kumar, A.; Chawla, V.; Chandra, R. Single step fabrication of nanostructured Cr2O3-MoO2 composite flexible electrode for top-notch asymmetric supercapacitor. Appl. Surf. Sci. 2021, 555, 149721. [Google Scholar] [CrossRef]
- Li, R.; Ba, X.; Wang, Y.; Zuo, W.; Wang, C.; Li, Y.; Liu, J. Direct growth of Fe3O4-MoO2 hybrid nanofilm anode with enhanced electrochemical performance in neutral aqueous electrolyte. Prog. Nat. Sci. Mater. Int. 2016, 26, 258–263. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, C.; Yan, X.H.; Wang, J.J.; Wang, D.F.; Yuan, X.X.; Cheng, X.N. TiO2 nanoparticles modified MoO3 nanobelts as electrode materials with superior performances for supercapacitors. Energy Technol. 2018, 6, 2367–2373. [Google Scholar] [CrossRef]
- Chen, J.; Nakate, U.T.; Nguyen, Q.T.; Wei, Y.; Park, S. Surface activated Co3O4/MoO3 nanostructured electrodes by air-plasma treatment toward enhanced supercapacitor. Mater. Sci. Eng. B 2022, 285, 115928. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Cai, X.; Song, Y.; Sun, X.; Liu, X.-X. VOx@MoO3 nanorod composite for high-performance supercapacitors. Adv. Funct. Mater. 2018, 28, 1803901. [Google Scholar] [CrossRef]
- Muduli, S.; Pati, S.K.; Swain, S.; Martha, S.K. MoO3@ZnO nanocomposite as an efficient anode material for supercapacitors: A cost effective synthesis approach. Energy Fuels 2021, 35, 16850–16859. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2020, 56, 173–200. [Google Scholar] [CrossRef]
- Si, H.; Sun, L.; Zhang, Y.; Wu, L.; Zhang, Y.; Zhang, Y. Enhanced pseudocapacitive energy storage properties of budding-branch like MoO2@C/CNT nanorods. Dalton Trans. 2020, 49, 1637–1645. [Google Scholar] [CrossRef]
- Tiwari, P.; Jaiswal, J.; Chandra, R. Hierarchal growth of MoS2@CNT heterostructure for all solid state symmetric supercapacitor: Insights into the surface science and storage mechanism. Electrochim. Acta 2019, 324, 134767. [Google Scholar] [CrossRef]
- Tian, Y.; Sarwar, S.; Zheng, Y.; Wang, S.; Guo, Q.; Luo, J.; Zhang, X. Ultrafast microwave manufacturing of MoP/MoO2/carbon nanotube arrays for high-performance supercapacitors. J. Solid State Electrochem. 2020, 24, 809–819. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, G.; Liu, H.; Zhang, W.; Liu, Q.; Zhao, C.; Li, Y.; Wang, Y. Facile construction of MoS2/graphene aerogel composite by depositing MoS2 film with a magnetron sputtering method for high-performance supercapacitors. Solid State Commun. 2022, 351, 114789. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, A.; Yang, B.; Wang, D.; Zhang, Z. Flower-like MoS2 onto nitrogen-doped 3D graphene composite with active material for supercapacitor electrodes. Mater. Lett. 2019, 240, 258–261. [Google Scholar] [CrossRef]
- Chen, J.; Jin, T.; Deng, H.; Huang, J.; Ren, G.; Qian, Y. MoO2 nanoparticles confined in N,P-codoped graphene aerogels with excellent pseudocapacitance performance. Can. J. Chem. 2021, 99, 303–310. [Google Scholar] [CrossRef]
- Qu, G.; Guo, K.; Dong, J.; Huang, H.; Yuan, P.; Wang, Y.; Yuan, H.; Zheng, L.; Zhang, J.-N. Tuning Fe-spin state of FeN4 structure by axial bonds as efficient catalyst in Li-S batteries. Energy Storage Mater. 2023, 55, 490–497. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Peng, K.; Yu, L. One-step hydrothermal synthesis of MoO2/MoS2 nanocomposites as high-performance electrode material for supercapacitors. ACS Appl. Mater. Interfaces 2022, 14, 49909–49918. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, M.; Lu, M.; Guan, X.; Guan, X.; Wang, G.; Jia, B. Facile design and synthesis of nickle-molybdenum oxide/sulfide composites with robust microsphere structure for high-performance supercapacitors. Chem. Eng. J. 2019, 364, 462–474. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, L.; Wei, T.; Liu, X. MoS2/NiS yolk-shell microsphere-based electrodes for overall water splitting and asymmetric supercapacitor. Small 2019, 15, 1803639. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Xu, Q.; Shi, Y.; Tian, Z.; Wang, R.; Zhang, G.; Chen, J.; Wang, Z.; Zheng, W. Controllable synthesis of NiSe/MoSe2/MoO2 3D hierarchical hollow microspheres with enhanced performance for asymmetric supercapacitors. Chem. Eng. J. 2020, 387, 124121. [Google Scholar] [CrossRef]
- Wang, C.; Wu, D.; Qin, Y.; Kong, Y. Nanowired NiMoO4/NiSe2/MoSe2 prepared through in situ selenylation as a high performance supercapacitor electrode. Chem. Commun. 2021, 57, 4019–4022. [Google Scholar] [CrossRef]
- Krishna, B.N.V.; Ankinapalli, O.R.; Reddy, A.R.; Yu, J.S. Facile one-step hydrothermal route to MSe/Mo3Se4 (M: Zn, Mn, and Ni)-based electrode materials for ultralong-life hybrid supercapacitors. J. Mater. Sci. Technol. 2023, 156, 230–240. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Huang, Z. Structure and function tailored metal-organic frameworks for heterogeneous catalysis. Chem. Catal. 2022, 2, 3304–3319. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Dong, C.; Shi, J.; Xu, Y. The synergistic supercapacitive performance of Mo-MOF/PANI and its electrochemical impedance spectroscopy investigation. Mater. Today Commun. 2019, 21, 100711. [Google Scholar] [CrossRef]
- Cai, Z.; Deng, L.; Song, Y.; Li, D.; Hong, L.; Shen, Z. Facile synthesis of hierarchically self-assembled dandelion-like microstructures of bimetallic-MOF as a novel electrode material for high-rate supercapacitors. Mater. Lett. 2020, 281, 128616. [Google Scholar] [CrossRef]
- Li, Q.; Guo, H.; Xue, R.; Wang, M.; Xu, M.; Yang, W.; Zhang, J.; Yang, W. Self-assembled Mo doped Ni-MOF nanosheets based electrode material for high performance battery-supercapacitor hybrid device. Int. J. Hydrogen Energy 2020, 45, 20820–20831. [Google Scholar] [CrossRef]
- Gourji, F.H.; Rajaramanan, T.; Kishore, A.; Heggertveit, M.; Velauthapillai, D. Hierarchical cube-in-cube cobalt-molybdenum phosphide hollow nanoboxes derived from the MOF template strategy for high-performance supercapacitors. ACS Omega 2023, 8, 23446–23456. [Google Scholar] [CrossRef] [PubMed]
- Govindan, R.; Hong, X.J.; Sathishkumar, P.; Cai, Y.P.; Gu, F.L. Construction of metal-organic framework-derived CeO2/C integrated MoS2 hybrid for high-performance asymmetric supercapacitor. Electrochim. Acta 2020, 353, 136502. [Google Scholar] [CrossRef]
- Safartoobi, A.; Mazloom, J.; Ghodsi, F.E. Silver/molybdenum metal-organic framework derived Ag2MoO4 nanoparticles as novel electrode for high-performance supercapacitor. J. Energy Storage 2023, 68, 107818. [Google Scholar] [CrossRef]


| Electrode Material | Method | Structure | Specific Capacitance | Capacitance Retention | Ref. |
|---|---|---|---|---|---|
| α-MoO3 | solution combustion | nanorods | 176 F g−1 (1 mA g−1) | 92%, 1000 cycles | [24] |
| h-MoO3 | hydrothermal | nanorods and nanoparticles | 229.0 F g−1 (0.2 A g−1) | N/A | [25] |
| MoO3 | heat-treating | nanoplates | 994.2 F g−1 (0.5 A g−1) | 84%, 1500 cycles | [26] |
| MoO2 | hydrothermal | nanoparticles | 509.8 F g−1 (0.5 A g−1) | 64.5%, 2500 cycles | [28] |
| MoO2 | hydrothermal | mesoporous | 381.0 F g−1 (0.3 A g−1) | 82.4%, 1000 cycles | [29] |
| MoO2 | hydrothermal | nanosheets | 243 mA h g−1 (0.1 A g−1) | 85%, 4000 cycles | [30] |
| MoO3−x | hydrothermal | nanobelts | 1,220 F g−1 (50 A g−1) | 100%, 38,000 cycles | [35] |
| α-MoO3−x | hydrothermal | nanobelts | 912.5 F g−1 (1 A g−1) | N/A | [37] |
| MoO3−x | liquid phase | microplates and microdisks | 410 F g−1 (20 A g−1) | 90%, 12,000 cycles | [38] |
| Electrode Material | Method | Structure | Specific Capacitance | Capacitance Retention | Ref. |
|---|---|---|---|---|---|
| MoS2/MnO2 | electrochemical exfoliation | heterojunction | 275 F g−1 (2 A g−1) | 89%, 10,000 cycles | [80] |
| Cr2O3-MoO2 | magnetron sputtering | nanosheets | 340.8 F g−1 (2 mA cm−2) | 91.7%, 20,000 cycles | [81] |
| Fe3O4-MoO2 | electrodeposition | nanofilms | 65 mF cm−2 (2 mV s−1) | 230.8%, 1000 cycles | [82] |
| TiO2/MoO3 | hydrothermal | heterojunction | 141 F g−1 (1 A g−1) | 77.5%, 2000 cycles | [83] |
| Co3O4/MoO3 | hydrothermal | nanosheets | 141 F g−1 (1 A g−1) | 91.4%, 1000 cycles | [84] |
| VOx@MoO3 | electrodeposition | nanorods | 1980 mF cm−2 (2 mA cm−2) | 94%, 10,000 cycles | [85] |
| MoO3@ZnO | solid-state impregnation–calcination | nanoparticles and nanorods | 280 F g−1 (1 A g−1) | 98%, 10,000 cycles | [86] |
| Electrode Material | Method | Structure | Specific Capacitance | Capacitance Retention | Ref. |
|---|---|---|---|---|---|
| MoO2@C/CNT | calcination | nanorods | 1667.2 F g−1 (1 A g−1) | 92.8%, 3000 cycles | [88] |
| MoS2/CNT | magnetron sputtering | heterojunction | 337 mF cm−2 (5 mV s−1) | 97.6%, 2500 cycles | [89] |
| MoP/MoO2/CNT | microwave | nanofibers | 447.6 F g−1 (1 A g−1) | 86.5%, 10,000 cycles | [90] |
| MoS2/GA | liquid phase exfoliation | nanofilms | 175 F g−2 (1 A g−1) | 93.5%, 1000 cycles | [91] |
| MoS2/N-3DG | hydrothermal | nanoflowers | 301.2 F g−1 (0.2 A g−1) | 82%, 1000 cycles | [92] |
| MoO2@NPGA | hydrothermal | porous framework | 335 F g−1 (1 A g−1) | 88%, 6000 cycles | [93] |
| Electrode Material | Method | Structure | Specific Capacitance | Capacitance Retention | Ref. |
|---|---|---|---|---|---|
| MoO2/MoS2 | hydrothermal | nanoblocks | 1667.3 F g−1 (1 A g−1) | 94.75%, 5000 cycles | [95] |
| NiMo-O-S | calcination | nanospheres | 2177.5 F g−1 (1 A g−1) | 86.25%, 5000 cycles | [96] |
| MoS2/NiS | hydrothermal | yolk–shell microspheres | 1165 F g−1 (2 A g−1) | ~100%, 10,000 cycles | [97] |
| NiSe/MoSe2/MoO2 | growth-annealing | hierarchical hollow | 1061 F g−1 (2 A g−1) | 93.9%, 10,000 cycles | [98] |
| NiMoO4/NiSe2/MoSe2 | hydrothermal | nanowires | 1020 F g−1 (5 mV s−1) | 86.1%, 5000 cycles | [99] |
| ZnSe/Mo3Se4 | hydrothermal | micro solid spheres | 96 mA h g−1 (1 A g−1) | N/A | [100] |
| MnSe/Mo3Se4 | micro block sheets | 118 mA h g−1 (1 A g−1) | N/A | ||
| NiSe/Mo3Se4 | nanosheet spheres | 252 mA h g−1 (1 A g−1) | 80%, 80,000 cycles |
| Electrode Material | Method | Structure | Specific Capacitance | Capacitance Retention | Ref. |
|---|---|---|---|---|---|
| Mo-MOF/PANI | solution method | nanorod bundles | 110 F g−1 (5 mA g−1) | N/A | [102] |
| BiMo-MOF | electrodeposition | dandelion-like | 864 F g−1 (10 A g−1) | 81.2%, 8000 cycles | [103] |
| Mo-Ni-MOF | hydrothermal | nanosheets | 802 C g−1 (1 A g−1) | 93%, 20,000 cycles | [104] |
| CeO2/C/MoS2 | MOF-derived | nanoparticles | 1325.67 F g−1 (1 A g−1) | 92.8%, 1000 cycles | [106] |
| Ag2MoO4 | MOF-derived | nanoparticles | 1468.7 F g−1 (1 A g−1) | 90%, 5000 cycles | [107] |
| CoMoP-DSHNBs | MOF-derived | hollow nanoboxes | 1204 F g−1 (1 A g−1) | 87%, 20,000 cycles | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, H.; Qu, G. Molybdenum-Based Electrode Materials Applied in High-Performance Supercapacitors. Batteries 2023, 9, 479. https://doi.org/10.3390/batteries9090479
Wang Y, Wang H, Qu G. Molybdenum-Based Electrode Materials Applied in High-Performance Supercapacitors. Batteries. 2023; 9(9):479. https://doi.org/10.3390/batteries9090479
Chicago/Turabian StyleWang, Yu, Hai Wang, and Gan Qu. 2023. "Molybdenum-Based Electrode Materials Applied in High-Performance Supercapacitors" Batteries 9, no. 9: 479. https://doi.org/10.3390/batteries9090479