Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries
Abstract
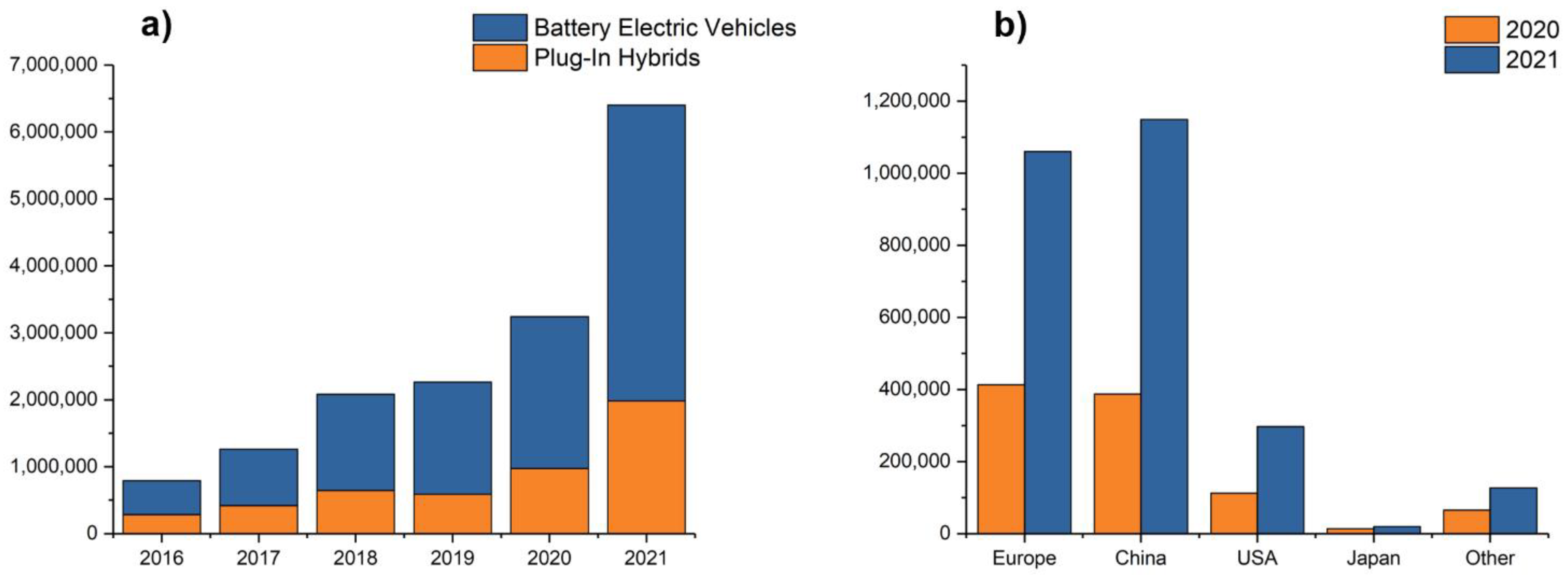
:1. Introduction
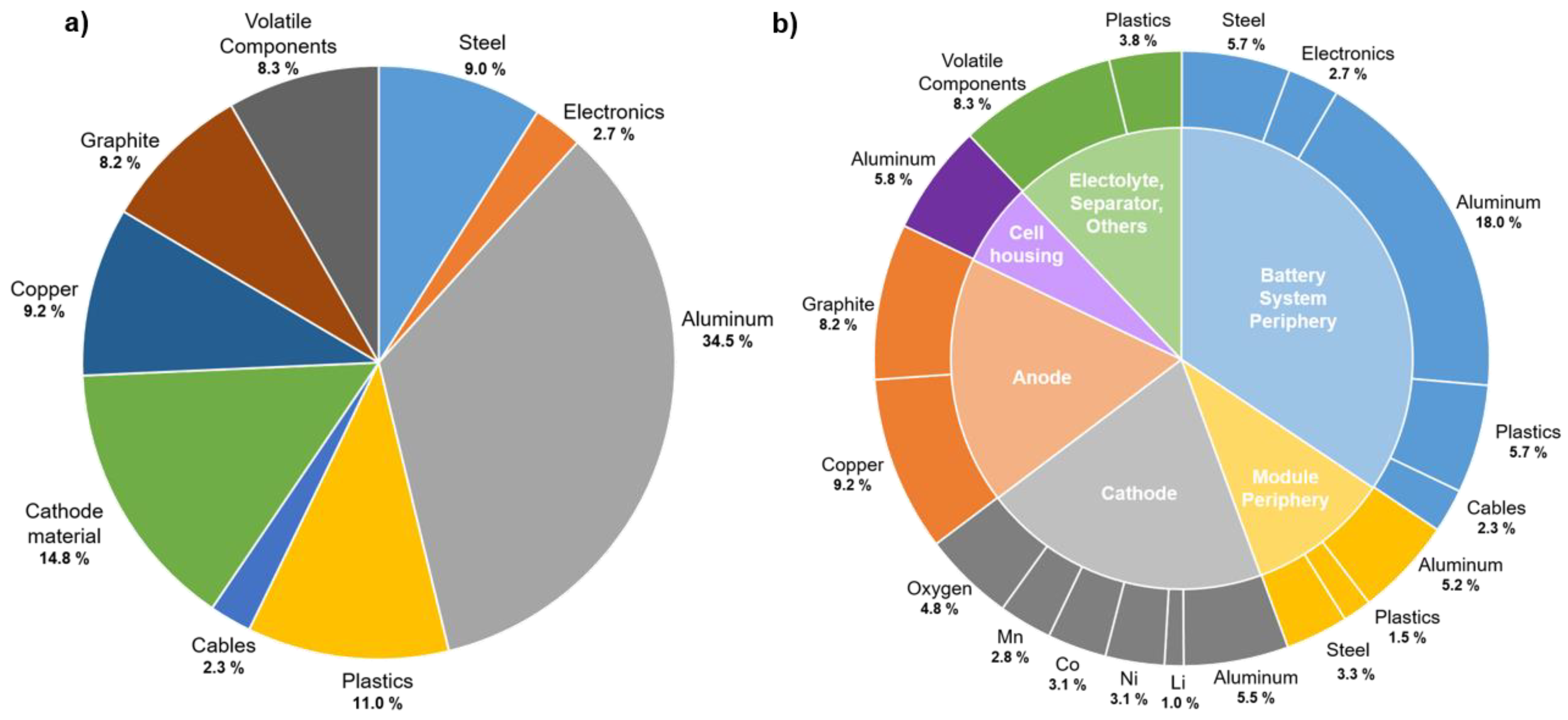
2. Recycling Chains for Lithium-Ion Batteries
2.1. Hydrometallurgical Recycling Routes
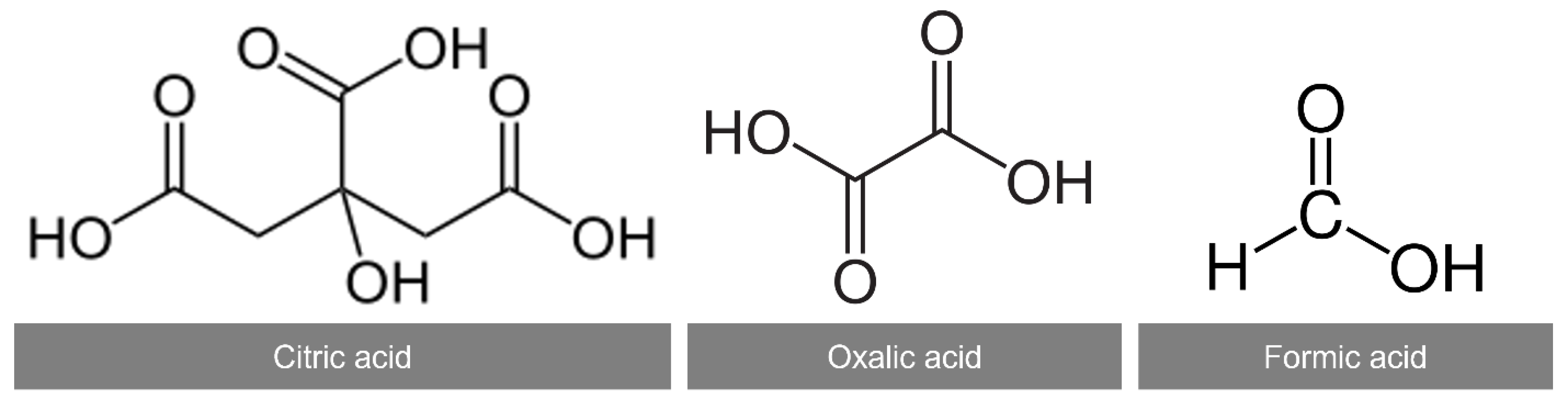
2.2. Effect of Organic Acids in Leaching of Black Mass
3. Materials and Methods
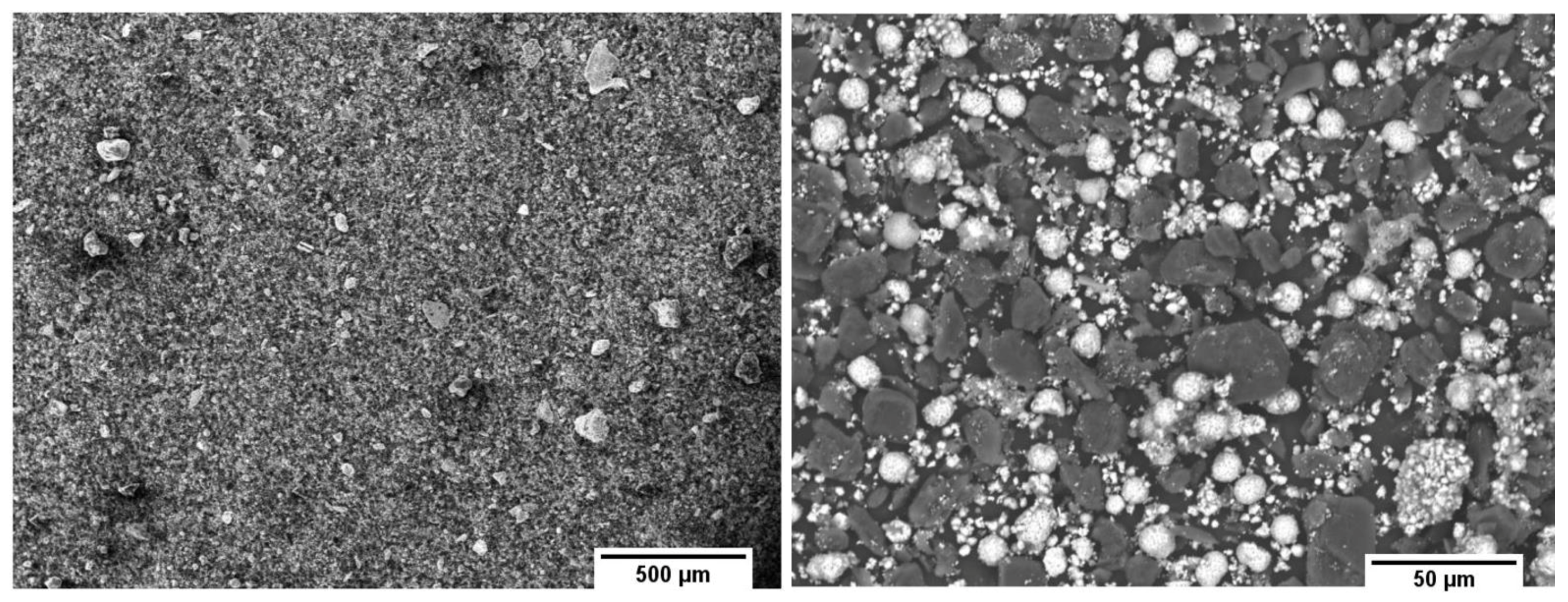
3.1. Sample Preparation and Analysis of the Input Material
3.2. Experimental Procedure

3.3. Analysis of the Obtained Product
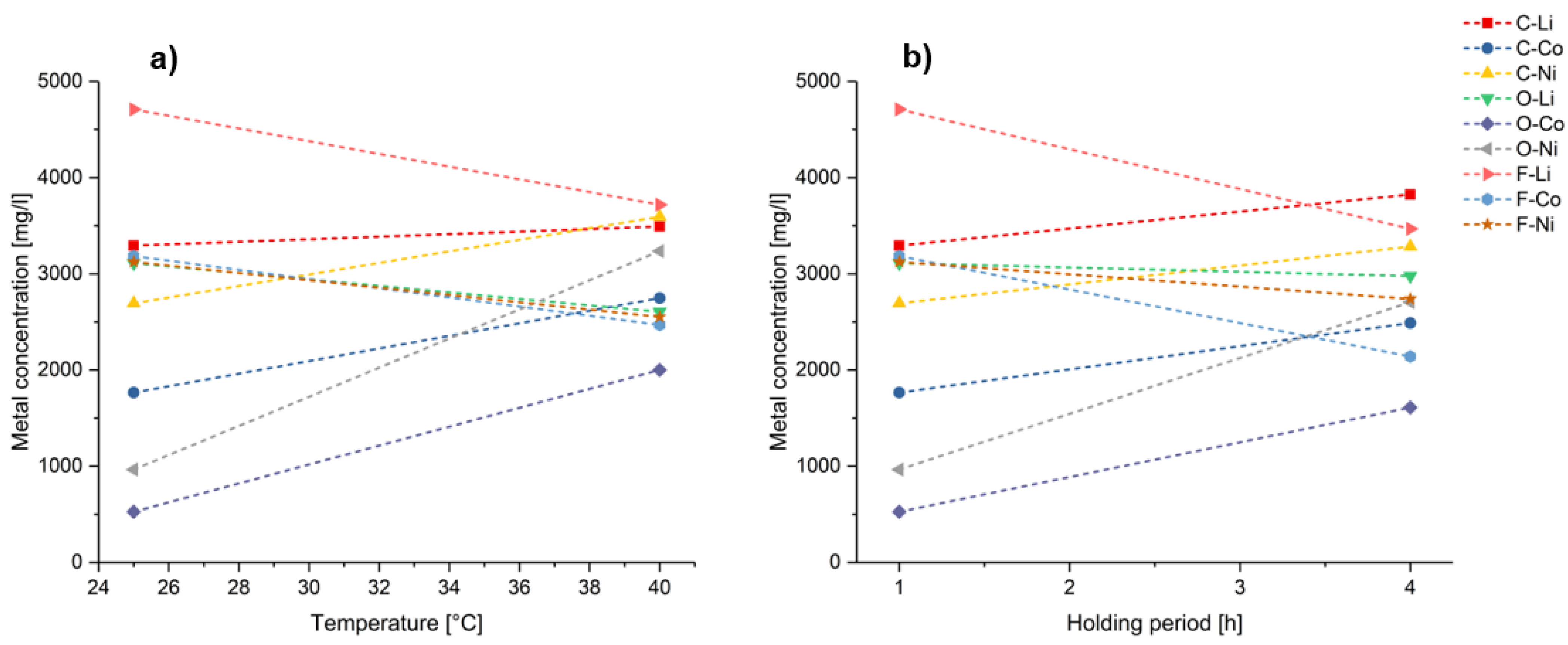
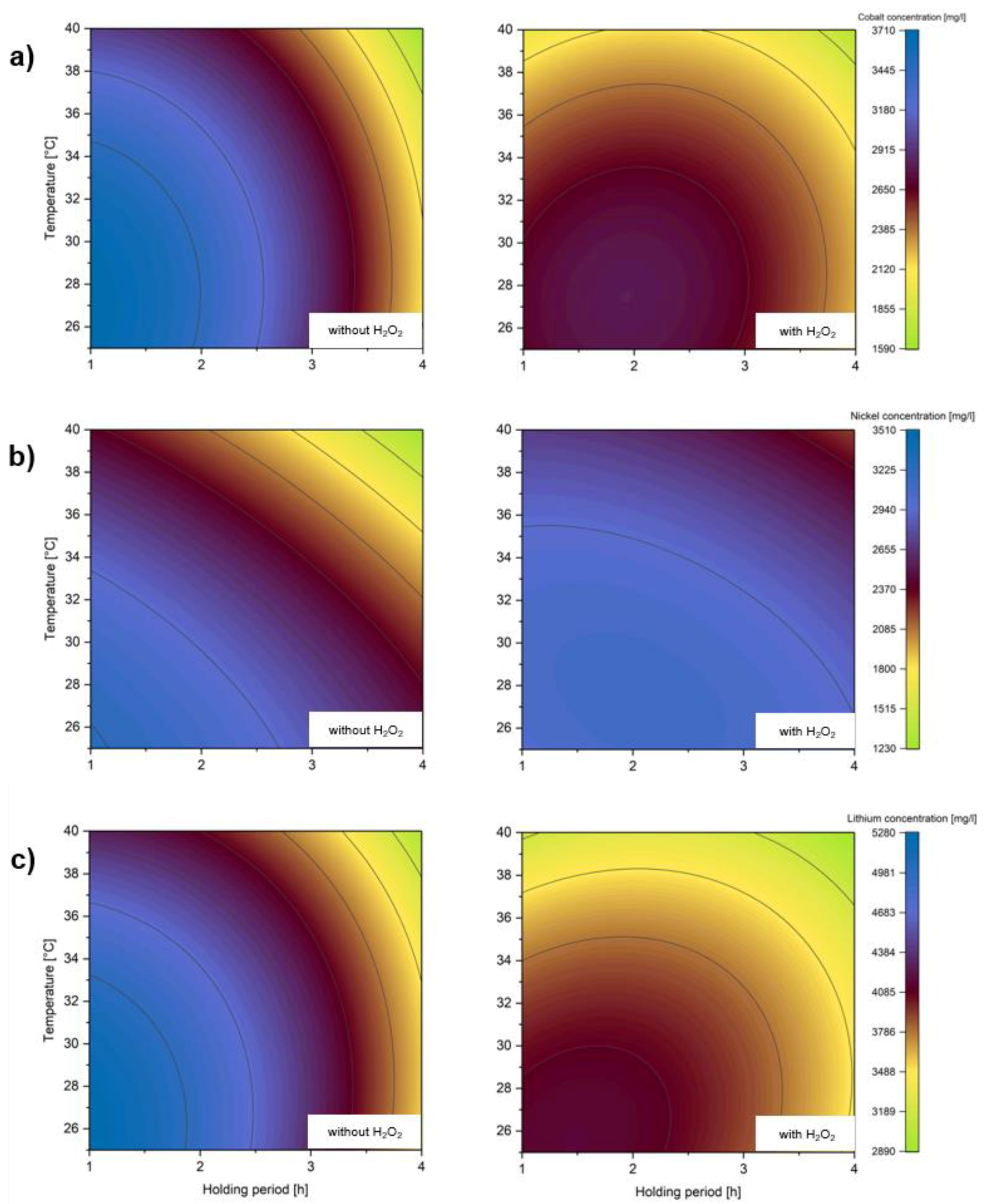
4. Results
5. Discussion
5.1. Influence of Operating Variables
5.2. Statistical Test Evaluation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IEA. Global EV Sales by Scenario 2020–2030. Available online: https://www.iea.org/data-and-statistics/charts/global-ev-sales-by-scenario-2020-2030 (accessed on 24 November 2021).
- Irle, R. Global EV Sales for 2021 H1. Available online: https://www.ev-volumes.com/news/global-ev-sales-for-2021-h1/ (accessed on 24 November 2021).
- Contestabile, M.; Panero, S.; Scrosati, B. A laboratory-scale lithium-ion battery recycling process. J. Power Sources 2001, 92, 65–69. [Google Scholar] [CrossRef]
- Kanamori, T.; Matsuda, M.; Miyake, M. Recovery of rare metal compounds from nickel–metal hydride battery waste and their application to CH4 dry reforming catalyst. J. Hazard. Mater. 2009, 169, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Buchert, M.; Sutter, J. Aktualisierte Ökobilanzen zum Recyclingverfahren LithoRec II für Lithium-Ionen-Batterien; Öko-Institut e.V.: Freiburg, Germany, 2016. [Google Scholar]
- Diekmann, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.-S.; Kwade, A. Ecological Recycling of Lithium-Ion Batteries from Electric Vehicles with Focus on Mechanical Processes. J. Electrochem. Soc. 2017, 164, A6184–A6191. [Google Scholar] [CrossRef]
- Cobalt Institute. Cobalt Mining. Available online: https://www.cobaltinstitute.org/about-cobalt/the-cobalt-value-chain/cobalt-mining/ (accessed on 10 December 2021).
- U.S. Geological Survey. Mineral Commodity Summaries 2021; U.S. Geological Survey: Reston, VA, USA, 2021.
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Lithium Carbonate Recovery from Cathode Scrap of Spent Lithium-Ion Battery: A Closed-Loop Process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qiu, K. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef]
- Li, J.-H.; Li, X.-H.; Zhang, Y.-H.; Hu, Q.-Y.; Wang, Z.-X.; Zhou, Y.-Y.; Fu, F.-M. Study of spent battery material leaching process. Trans. Nonferrous Met. Soc. China 2009, 19, 751–755. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Hu, Q.-Y.; Li, X.-H.; Wang, Z.-X.; Guo, H.-J. Recycle and synthesis of LiCoO2 from incisors bound of Li-ion batteries. Trans. Nonferrous Met. Soc. China 2006, 16, 956–959. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Recovery of valuable metals from cathodic active material of spent lithium ion batteries: Leaching and kinetic aspects. Waste Manag. 2015, 45, 306–313. [Google Scholar] [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Wang, F.; Ge, L.; Zhu, X.; Li, H. Chemical and process mineralogical characterizations of spent lithium-ion batteries: An approach by multi-analytical techniques. Waste Manag. 2014, 34, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Chen, X.; Zhou, T.; Zhang, J.; Xu, B. A sustainable process for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. Res. J. Sustain. Circ. Econ. 2016, 34, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, C.; Diekmann, J.; Stieger, A.; Haselrieder, W.; Kwade, A. Recycling of Lithium-Ion Batteries. Handbook of Clean Energy Systems Online. Available online: https://www.researchgate.net/profile/Christian-Hanisch-2/publication/280644244_Recycling_of_Lithium-Ion_Batteries_from_Electric_Vehicles/links/55c0a82508ae092e9666d0ae/Recycling-of-Lithium-Ion-Batteries-from-Electric-Vehicles.pdf (accessed on 10 December 2021). [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Hu, Q.; Wang, Z.; Zheng, J.; Wu, L.; Zhang, L. Study of extraction and purification of Ni, Co and Mn from spent battery material. Hydrometallurgy 2009, 99, 7–12. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Sources 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef] [Green Version]
- Gerold, E. Entwicklung Eines Hydrometallurgischen Recyclingkonzeptes für Lithium-Ionen-Batterien. Ph.D. Dissertation, Montanuniversität Leoben, Leoben, Austria, 2021. [Google Scholar]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef] [Green Version]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of lithium and cobalt from spent lithium ion batteries (LIBs) using organic acids as leaching reagents: A review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, T. Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media. Waste Manag. Res. J. Sustain. Circ. Econ. 2014, 32, 1083–1093. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Guan, Y.; Fan, E.; Wu, F.; Chen, R. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching. Waste Manag. 2018, 71, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, D.A.; Prados, L.M.Z.; Majuste, D.; Mansur, M.B. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. J. Power Sources 2009, 187, 238–246. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef]
- Swain, B.; Jeong, J.; Lee, J.-C.; Lee, G.-H.; Sohn, J.-S. Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of lithium ion batteries. J. Power Sources 2007, 167, 536–544. [Google Scholar] [CrossRef]
- Yao, X.; Xu, Z.; Yao, Z.; Cheng, W.; Gao, H.; Zhao, Q.; Li, J.; Zhou, A. Oxalate co-precipitation synthesis of LiNi0.6Co0.2Mn0.2O2 for low-cost and high-energy lithium-ion batteries. Mater. Today Commun. 2019, 19, 262–270. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Y.; Xu, S.; Fan, E.; Xue, Q.; Guan, Y.; Wu, F.; Li, L.; Chen, R. Innovative Application of Acid Leaching to Regenerate Li(Ni1/3Co1/3Mn1/3)O2 Cathodes from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 5959–5968. [Google Scholar] [CrossRef]
- de Oliveira Demarco, J.; Cadore, J.S.; de Oliveira, F.D.S.; Tanabe, E.H.; Bertuol, D.A. Recovery of metals from spent lithium-ion batteries using organic acids. Hydrometallurgy 2019, 190, 105169. [Google Scholar] [CrossRef]
- Meshram, P.; Mishra, A.; Abhilash; Sahu, R. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids–A review. Chemosphere 2020, 242, 125291. [Google Scholar] [CrossRef]
- Fanghänel, E. Organikum; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Gerold, E.; Antrekowitsch, H. Potenzialabschätzung von Synergieeffekten zur simultanen Rückgewinnung von Wertmetallen aus komplexen, metallhaltigen Reststoffen. Osterr. Wasser-Und Abfallwirtsch. 2022, 74, 22–31. [Google Scholar] [CrossRef]
- Gerold, E.; Luidold, S.; Antrekowitsch, H. Decomposition of hydrogen peroxide in selected organic acids. In Proceedings of the European Metallurgical Conference, Salzburg, Austria, 27–30 June 2021. [Google Scholar]
- Marques, R.C.; Simões, P.; Pinto, F.S. Tariff regulation in the waste sector: An unavoidable future. Waste Manag. 2018, 78, 292–300. [Google Scholar] [CrossRef] [PubMed]

| Type | Complexation Reaction (M = Co2+, Li+, Mn2+, Ni2+) | Reaction No. |
|---|---|---|
| Citric-metallic complexes [30] | (1) (2) (3) | |
| Oxalic-metallic complexes [30] | (4) (5) | |
| Formic-metallic complexes [10] | (6) |
| Element | Co | Ni | Li | Mn | Cu | Al | Zn | Fe |
|---|---|---|---|---|---|---|---|---|
| [Mass-%] | 6.5 | 22.0 | 5.2 | 7.3 | 4.8 | 3.7 | 0.7 | 0.01 |
| Reducing Agent | Holding Period | Temperature | Leaching Efficiency Co | Leaching Efficiency Ni | Leaching Efficiency Li | ||
|---|---|---|---|---|---|---|---|
| Unit | [-] | [h] | [°C] | [%] | [%] | [%] | |
| C1 | Yes | 1 | 40 | 80.5 | 31.5 | 100.0 | |
| C2 | Yes | 4 | 25 | 70.7 | 27.3 | 100.0 | |
| C3 | No | 1 | 25 | 33.5 | 15.0 | 100.0 | |
| C4 | No | 4 | 40 | 30.5 | 13.7 | 96.2 | |
| C5 | Yes | 1 | 25 | 44.6 | 17.0 | 88.0 | |
| O1 | Yes | 1 | 40 | 36.8 | 17.8 | 66.0 | |
| O2 | Yes | 4 | 25 | 23.4 | 12.2 | 72.8 | |
| O3 | No | 1 | 25 | 24.5 | 12.9 | 79.4 | |
| O4 | No | 4 | 40 | 55.4 | 23.1 | 71.9 | |
| O5 | Yes | 1 | 25 | 20.9 | 8.7 | 83.4 | |
| F1 | Yes | 1 | 40 | 10.3 | 10.7 | 48.5 | |
| F2 | Yes | 4 | 25 | 1.4 | 1.1 | 6.3 | |
| F3 | No | 1 | 25 | 3.7 | 6.0 | 68.7 | |
| F4 | No | 4 | 40 | 2.0 | 3.1 | 59.4 | |
| F5 | Yes | 1 | 25 | 8.3 | 10.1 | 56.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerold, E.; Schinnerl, C.; Antrekowitsch, H. Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries. Recycling 2022, 7, 4. https://doi.org/10.3390/recycling7010004
Gerold E, Schinnerl C, Antrekowitsch H. Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries. Recycling. 2022; 7(1):4. https://doi.org/10.3390/recycling7010004
Chicago/Turabian StyleGerold, Eva, Clemens Schinnerl, and Helmut Antrekowitsch. 2022. "Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries" Recycling 7, no. 1: 4. https://doi.org/10.3390/recycling7010004
APA StyleGerold, E., Schinnerl, C., & Antrekowitsch, H. (2022). Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries. Recycling, 7(1), 4. https://doi.org/10.3390/recycling7010004





