Potential of Alternative Organic Binders in Briquetting and Enhancing Residue Recycling in the Steel Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Selection and Analysis
2.2. Recipe Design
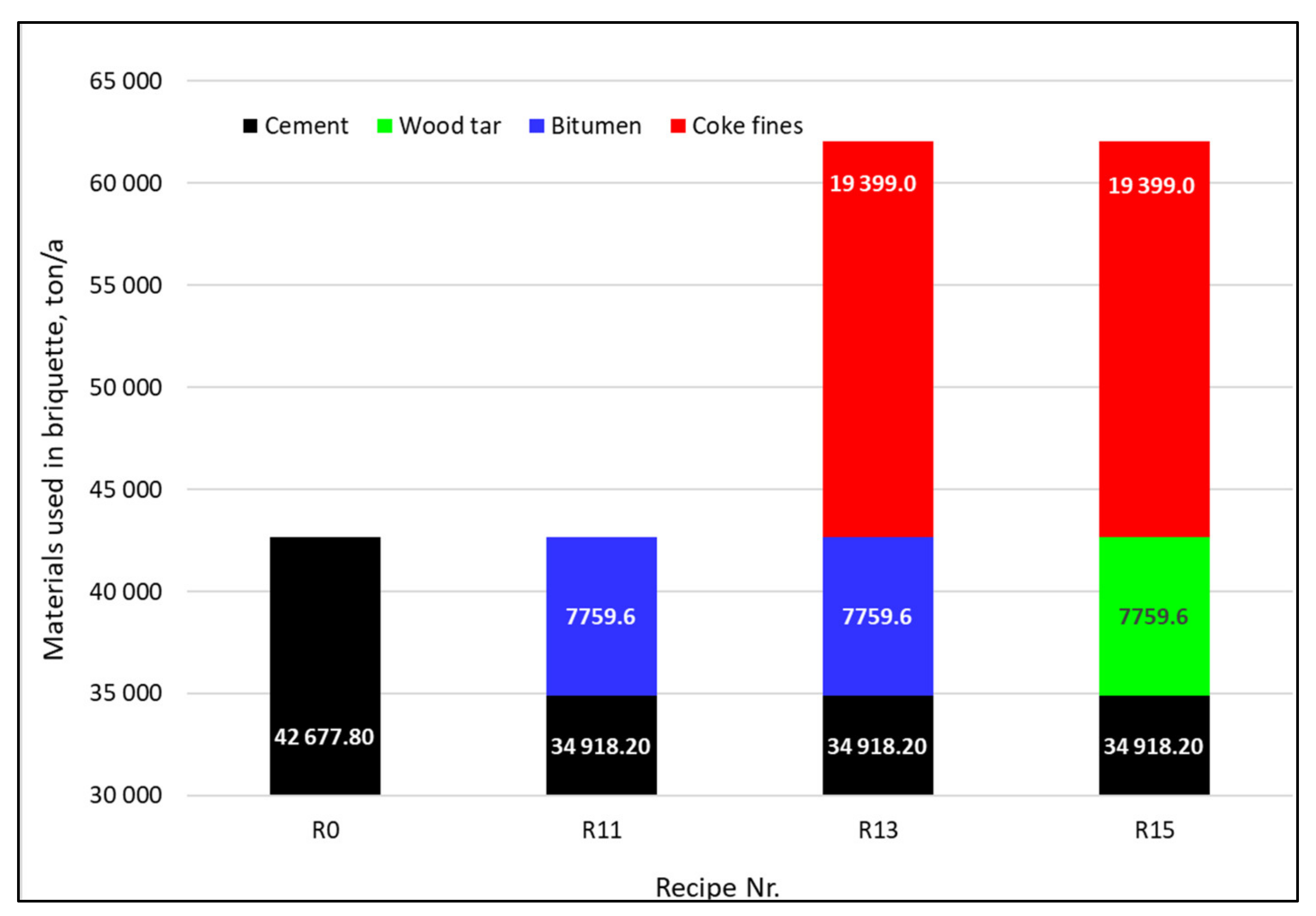
2.2.1. Design of Recipes R0–R3
2.2.2. Design of Recipes R4–R8
2.2.3. Design of Recipes R9–R15
2.3. Technical Scale Briquetting
| Stage No. | Recipe No. | Pre-Mix | BF Dust | Coke Fines | Cement | Molasses/Lime | Keracoal | Bitumen | CMC | Wood Tar | Total. wt.% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage 1 | R0 | 79 | 10 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 100 |
| R1 | 79 | 10 | 0 | 7 | 2.7/1.3 | 0 | 0 | 0 | 0 | 100 | |
| R2 | 79 | 10 | 0 | 7 | 0 | 4 | 0 | 0 | 0 | 100 | |
| R3 | 79 | 10 | 0 | 7 | 0 | 0 | 4 | 0 | 0 | 100 | |
| Stage 2 | R4 | 74 | 10 | 5 | 11 | 0 | 0 | 0 | 0 | 0 | 100 |
| R5 | 74 | 10 | 5 | 7 | 2.7/1.3 | 0 | 0 | 0 | 0 | 100 | |
| R6 | 74 | 10 | 5 | 7 | 0 | 4 | 0 | 0 | 0 | 100 | |
| R7 | 74 | 10 | 5 | 7 | 0 | 0 | 4 | 0 | 0 | 100 | |
| R8 | 77 | 10 | 5 | 7 | 0 | 0 | 0 | 1 | 0 | 100 | |
| Stage 3 | R9 | 81 | 10 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 100 |
| R10 | 79 | 10 | 0 | 9 | 1.3/0.7 | 0 | 0 | 0 | 0 | 100 | |
| R11 | 79 | 10 | 0 | 9 | 0 | 0 | 2 | 0 | 0 | 100 | |
| R12 | 74 | 10 | 5 | 9 | 1.3/0.7 | 0 | 0 | 0 | 0 | 100 | |
| R13 | 74 | 10 | 5 | 9 | 0 | 0 | 2 | 0 | 0 | 100 | |
| R14 | 79 | 10 | 0 | 9 | 0 | 0 | 0 | 0 | 2 | 100 | |
| R15 | 74 | 10 | 5 | 9 | 0 | 0 | 0 | 0 | 2 | 100 |
3. Results and Discussion
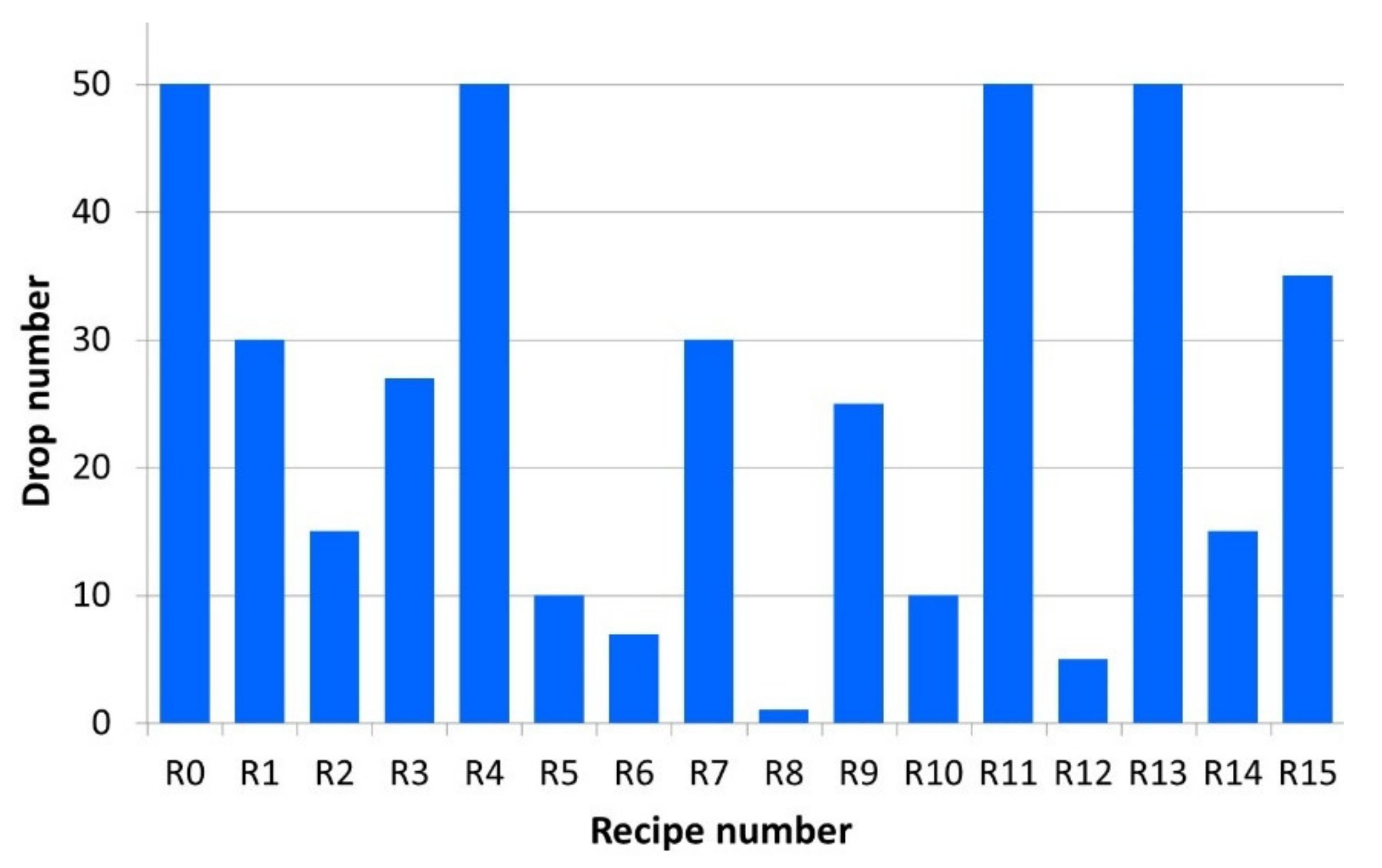
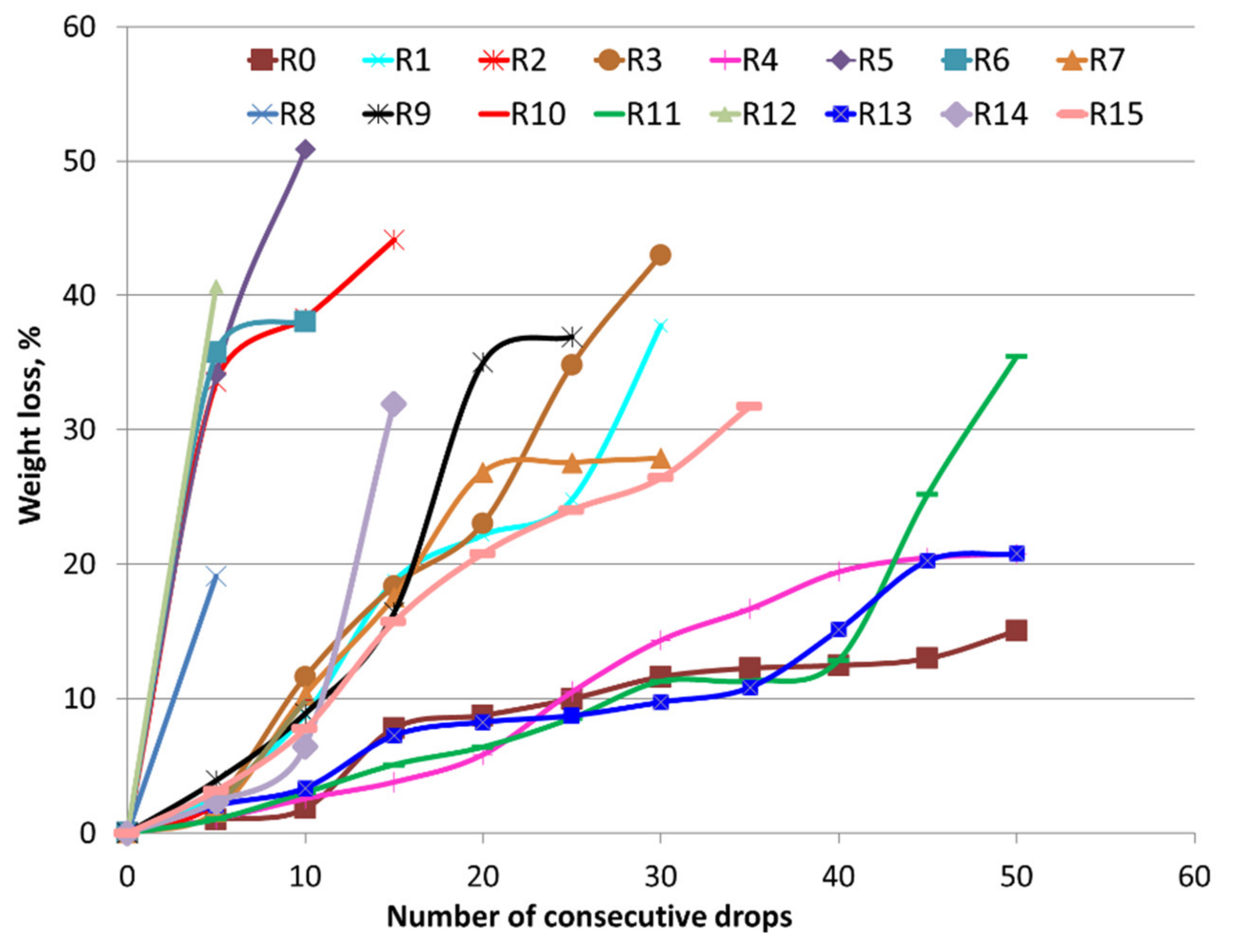
3.1. Mechanical Strength
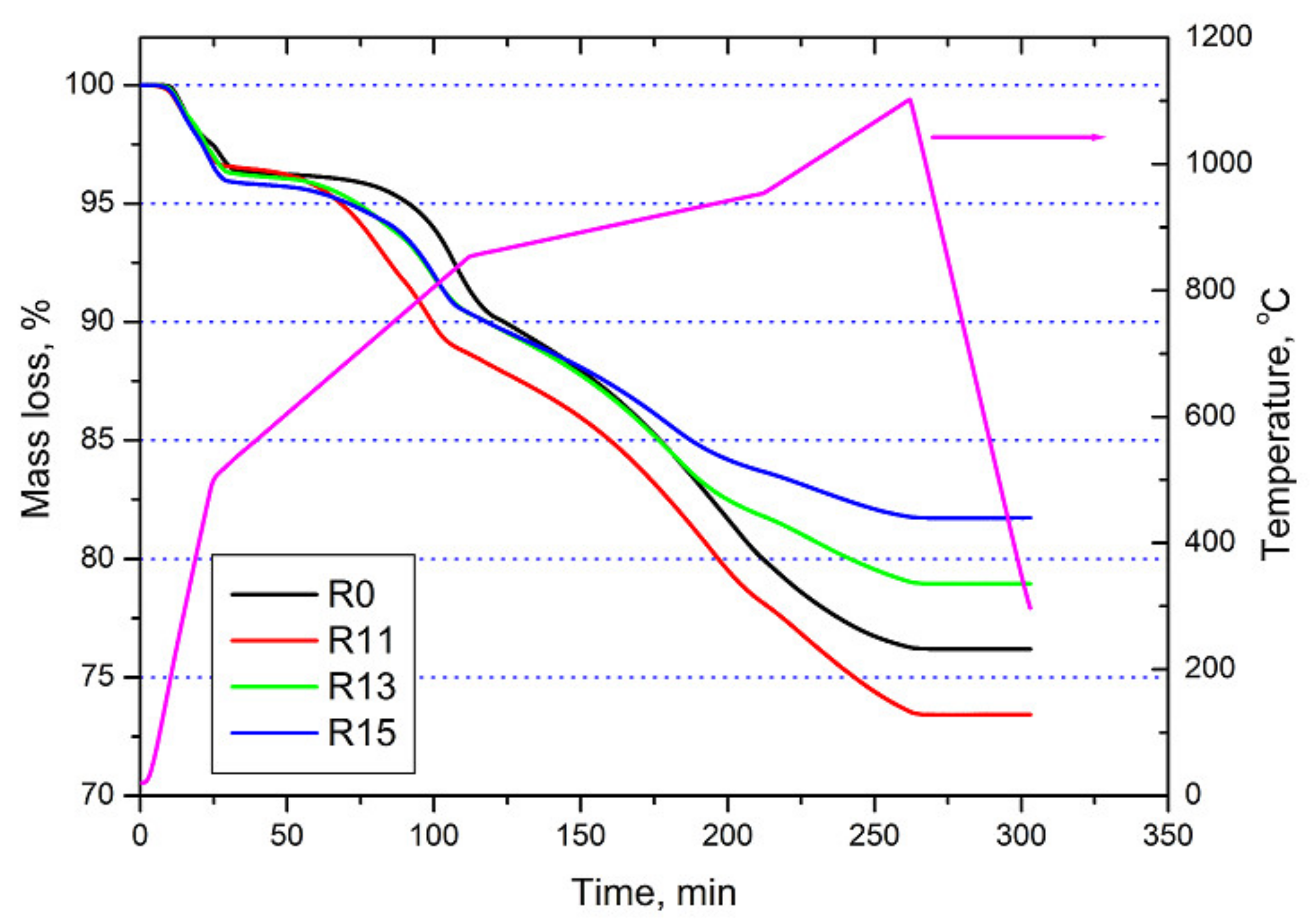
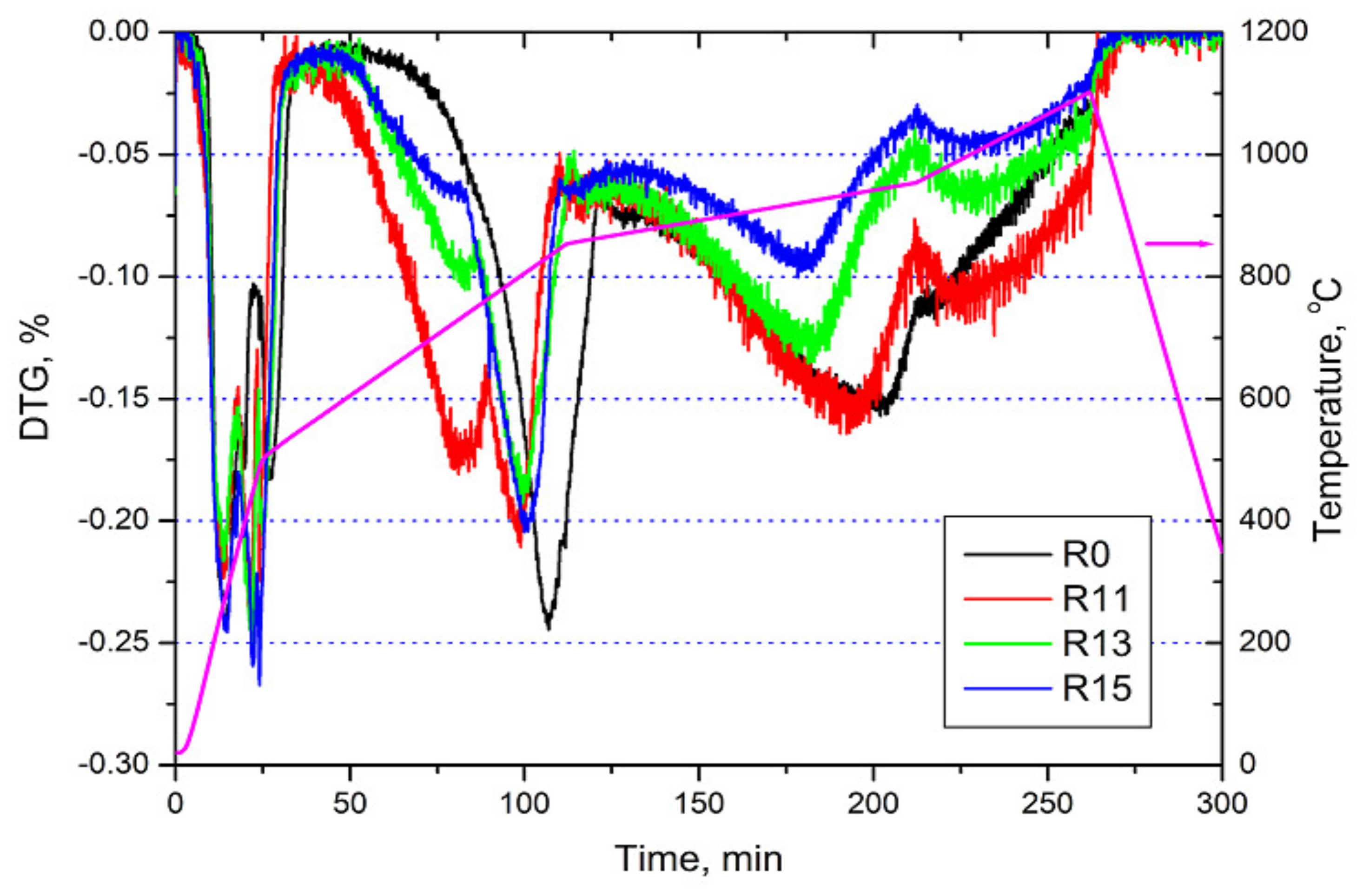
3.2. Reduction Behaviour
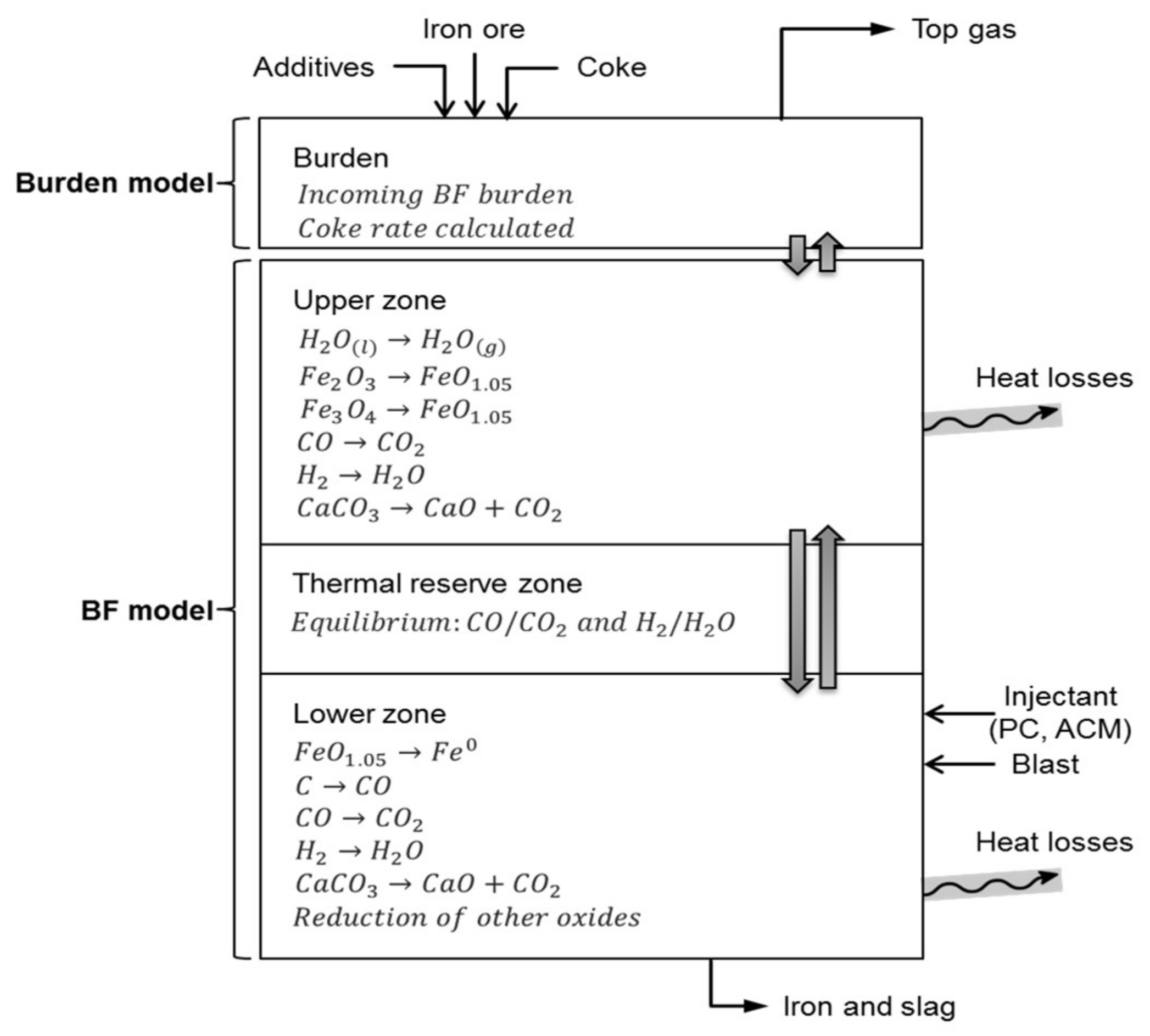
3.3. Heat and Mass Balance Calculations (MASMOD)
- The production rate of hot metal (tHM/h) was kept constant;
- RAFT (Raceway adiabatic flame temperature) was kept constant while TRZT (thermal reserve zone temperature) and top gas temperature were varied;
- Shaft efficiency was assumed to be constant as the reference;
- Slag basicity was kept the same by adjusting the amount of limestone charged to BF, while the slag rate varied;
- PCI (pulverized coal injection) was kept constant, while coke rate was allowed to vary;
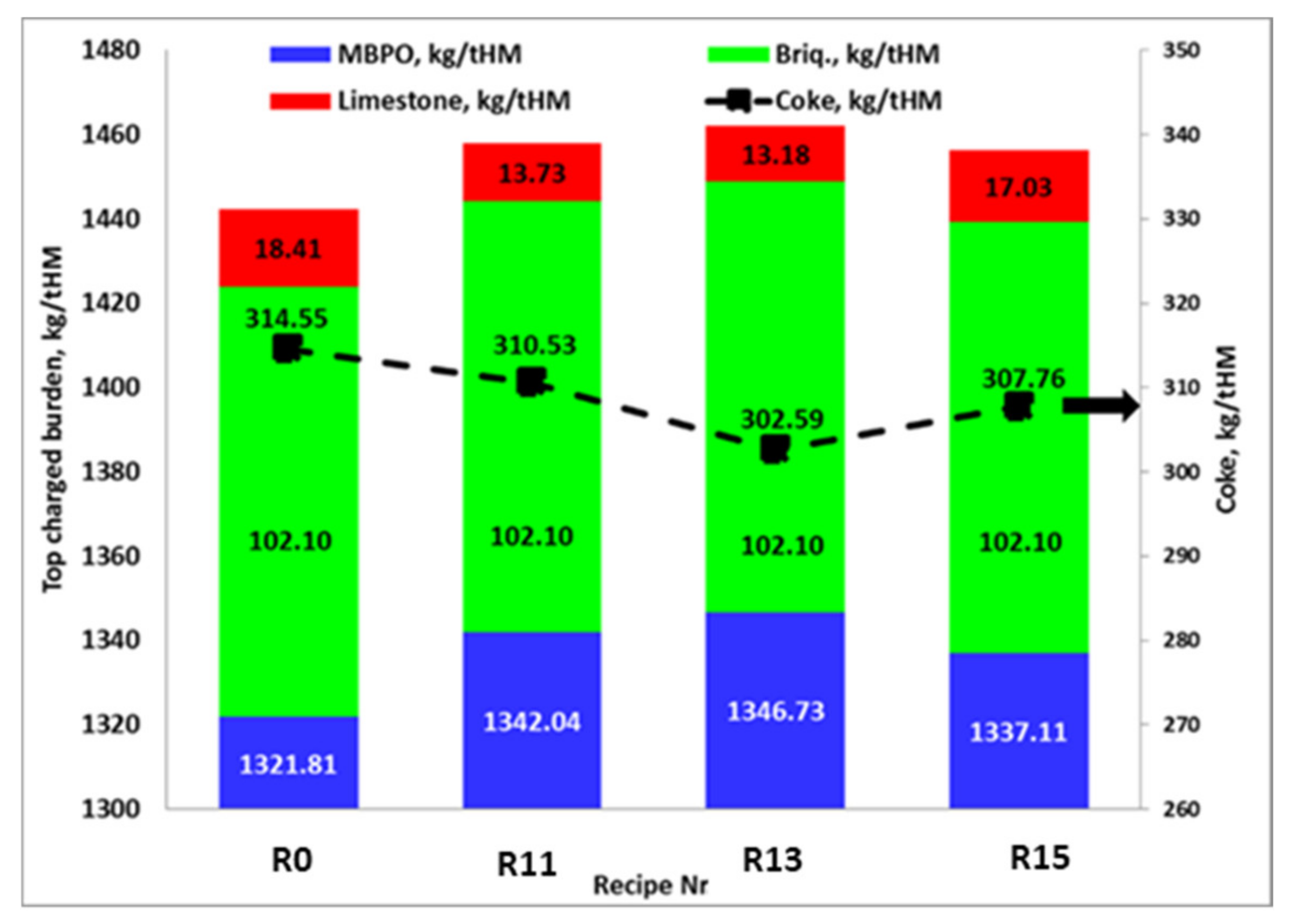
- Briquette rate was kept constant at 102.1 kg/tHM, while the pellets rate was allowed to vary to keep the production rate constant;
- The generated dust and sludge were kept constant at 15 kg/tHM of dust and 5.0 kg/tHM for sludge.
4. Conclusions
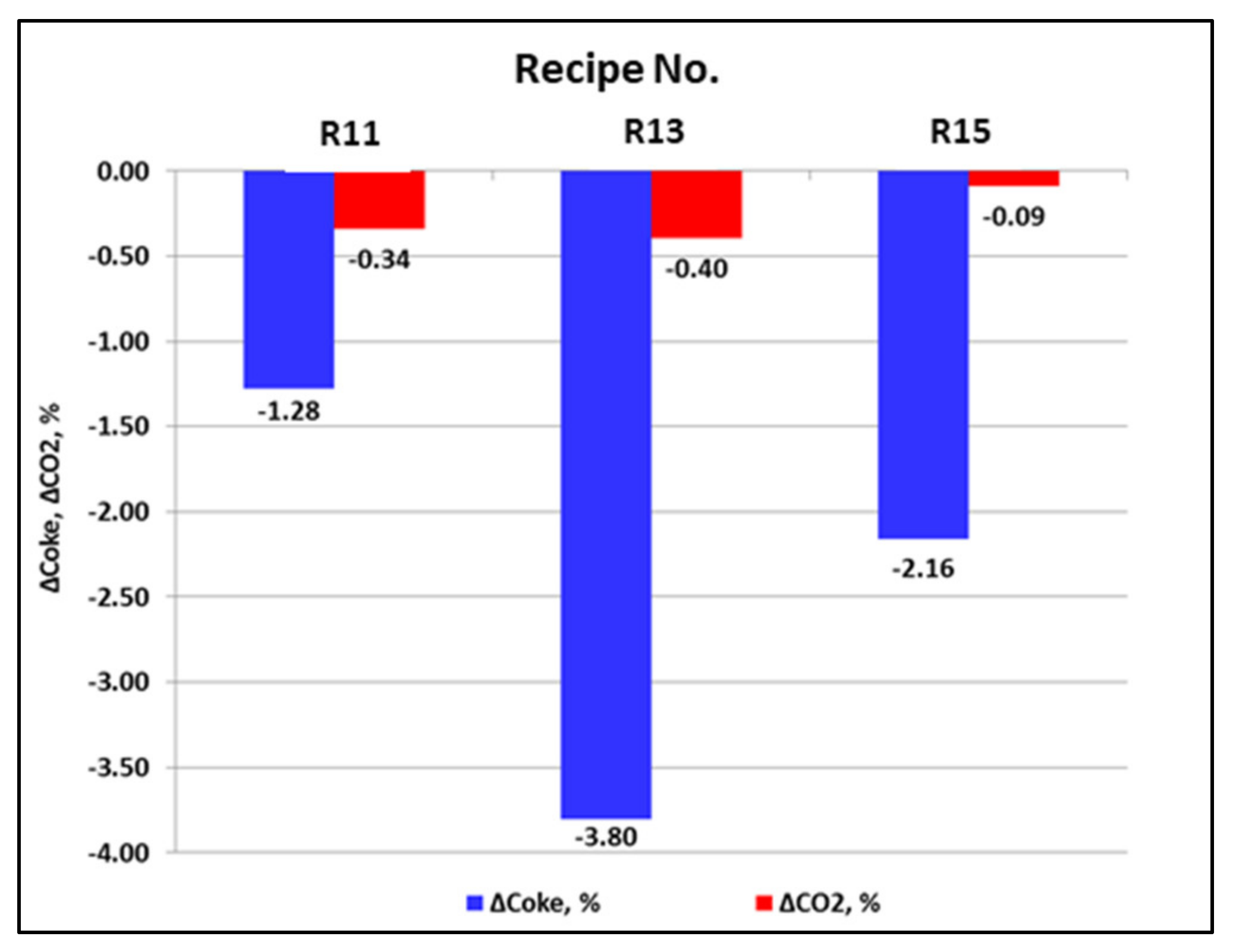
- Among all tested organic binders, bitumen showed cement had equal efficiency with 2 wt.% addition to the briquettes, which replaced 18.2 wt.% of the cement. The other types of organic binders deteriorated the mechanical strength of the briquettes, even at 2 wt.% addition.
- The briquettes with bitumen showed a higher reduction rate compared to the standard briquettes.
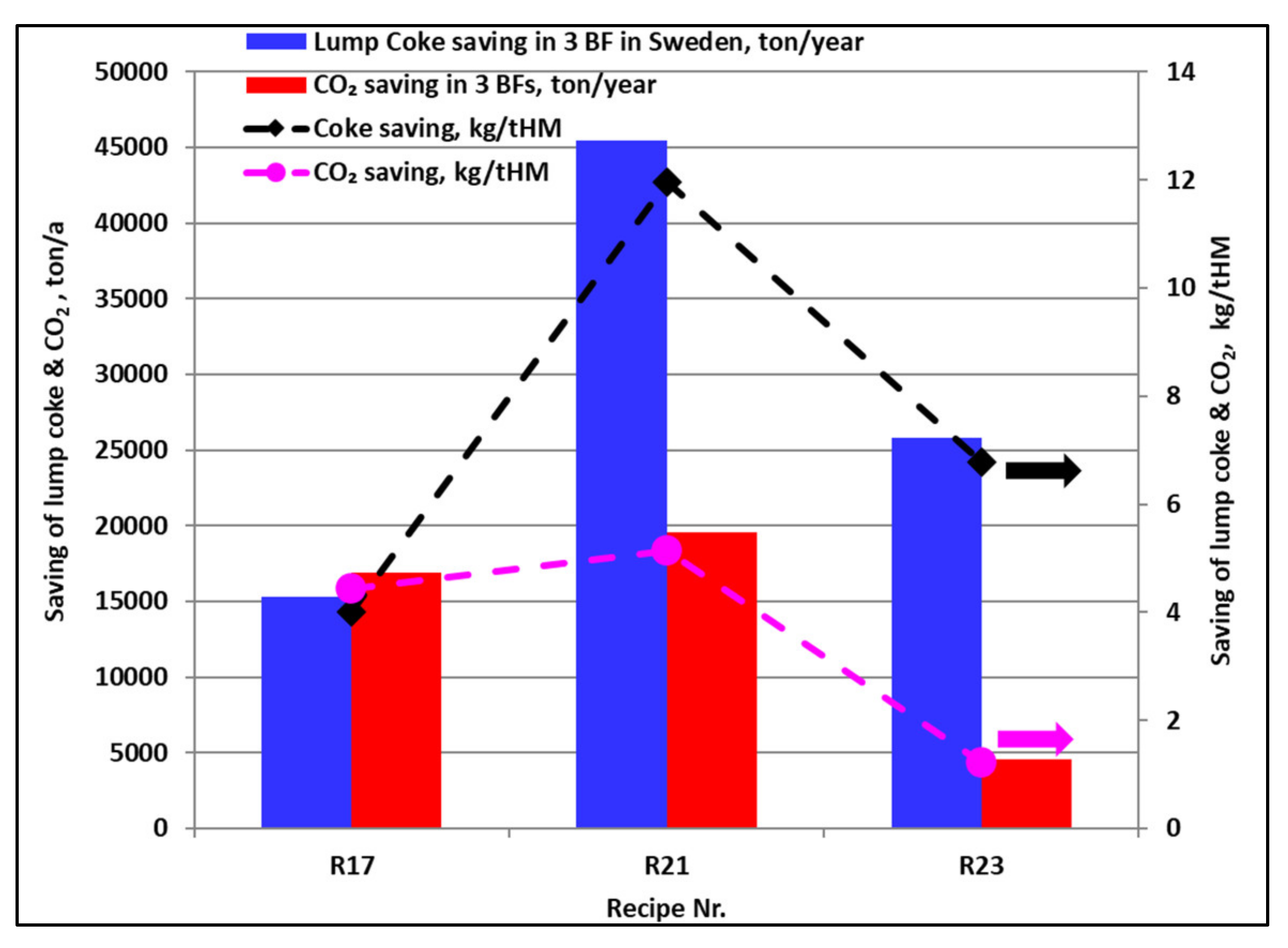
- The developed briquettes have the potential for annual savings of 15,000–45,000 tons of coke and 4500–19,500 tons of CO2 emission from the three BFs in Sweden at a full production rate.
- Approximately 19,000 tons of coke fines can be annually recycled in three BFs in the form of the developed briquettes and this can contribute to savings equal to the amount of lump coke.
- The annual consumption of limestone at the three BFs can be decreased by 5000–20,000 tons, which contributes to further a reduction in CO2 emissions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fact Sheet-Steel Industry By-Products. Available online: https://www.worldsteel.org/en/dam/jcr:1b916a6d-06fd-4e84-b35d-c1d911d18df4/Fact_By-products_2016.pdf (accessed on 18 January 2018).
- Schwelberger, J.; Wimmer, G.; Brunner, C.; Fleischander, A. Innovative solutions for recycling of by-products. In Proceedings of the 46th Steelmaking, Rio de Janeiro, Brazil, 17–21 August 2015. [Google Scholar]
- Mousa, E.A.; Ahmed, H.M.; Wang, C. Novel approach towards biomass lignin utilization in ironmaking blast furnace. ISIJ Int. 2017, 57, 1788–1796. [Google Scholar] [CrossRef]
- Wang, C.; Larsson, M.; Lövgren, J.; Nilsson, L.; Mellin, P.; Yang, W.; Salman, H.; Hultgren, A. Injection of solid biomass products into the blast furnace and its potential effects on an integrated steel plant. Energy Procedia 2014, 61, 2184–2187. [Google Scholar] [CrossRef]
- Pach, M.; Zanzi, R.; Björnbom, E. Torrefied biomass a substitute for wood and charcoal. In Proceedings of the 6th Asia-Pacific International Symposium on Combustion and Energy Utilization, Kuala Lumpur, Malaysia, 20–22 May 2002. [Google Scholar]
- Ribeiro, J.M.C.; Godina, R.; de Oliveira Matias, J.C.; Nunes, L.J.R. Future perspectives of biomass torrefaction: Review of the current state-of-the art and research development. Sustainability 2018, 10, 2323. [Google Scholar] [CrossRef]
- Gudenau, H.W.; Senk, D.; Wang, S.; De Melo Martins, K.; Stephany, C. Research in the reduction of iron ore agglomerates including coal and C-containing dust. ISIJ Int. 2005, 45, 603–608. [Google Scholar] [CrossRef][Green Version]
- Ueda, S.; Yanagiya, K.; Watanabe, K.; Murakami, T.; Inoue, R.; Ariyama, T. Improvement of reactivity of carbon iron ore composite with biomass char for blast furnace. ISIJ Int. 2009, 49, 1505–1512. [Google Scholar] [CrossRef]
- Kundvist, K.; Brämming, M.; Riesbeck, J.; Wedholm, A. New methods for waste minimization in an integrated steel site. Chem. Eng. Trans. 2015, 45, 739–744. [Google Scholar]
- Robinson, R.; Sundqvist, L. Recycling of by-product pellets as burden in the blast furnace process: A lab and pilot scale investigation. Steel Res. 2004, 75, 99–105. [Google Scholar] [CrossRef]
- Sundqvist, L.; Jonsson, K.O.; Lampinen, H.O.; Eriksson, L.E. Recycling of in-plant fines as cold bonded agglomerates. In Committee on Raw Materials-Seminar Proceeding; International Iron and Steel Institute (IISI): Brussels, Belgium, 1999; pp. 44–60. [Google Scholar]
- Singh, M.; Björkman, B. Swelling behaviour of cement-bonded briquettes-proposed model. ISIJ Int. 2004, 44, 482–491. [Google Scholar] [CrossRef]
- Singh, M. Studies on the Cement-Bonded Briquettes of Iron and Steel Plant By-Products as Burden Material for Blast Furnaces. Ph.D. Thesis, Department of Chemical and Metallurgical Engineering, Division of Process Metallurgy, Luleå University of Technology, Luleå, Sweden, 2003. [Google Scholar]
- Tanaka, Y.; Ueno, T.; Okumura, K.; Hayashi, S. Reaction behavior of coal rich composite iron ore hot briquettes under load at high temperatures until 1400 °C. ISIJ Int. 2011, 51, 1240–1246. [Google Scholar] [CrossRef]
- Kasai, A.; Matsui, Y. Lowering thermal reserve zone temperature in blast furnace by adjoining carbonaceous materials and iron ore. ISIJ Int. 2004, 44, 2073–2078. [Google Scholar] [CrossRef]
- Ujisawa, Y.; Nakano, K.; Matsukura, Y.; Sunahara, K.; Komatsu, S.; Yamamoto, T. Subjects for achievement of blast furnace operation with low reducing agent rate. ISIJ Int. 2005, 45, 1379–1385. [Google Scholar] [CrossRef]
- Mousa, E.A.; Lundgren, M.; Ökvist, L.S.; From, L.-E.; Robles, A.; Hällsten, S.; Sundelin, B.; Friberg, H.; El-Tawil, A. Reduced carbon consumption and CO2 emission at the blast furnace by use of briquette containing torrefied sawdust. J. Sustain. Metall. 2019, 5, 391–401. [Google Scholar] [CrossRef]
- Mousa, E.A.; Ahmed, H.M. Utilization of biomass as an alternative fuel in iron and steel making, In Iron Ore Textbook, Mineralogy, Processing and Environmental Sustainability, 2nd ed.; Woodhead Publishing Series in Metals and Surface Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 665–690. [Google Scholar]
- Mousa, E.; Kazemi, M.; Larsson, M.; Karlsson, G.; Persson, E. Potential for developing biocarbon briquettes for foundry industry. Appl. Sci. 2019, 9, 5288. [Google Scholar] [CrossRef]
- Rist, A.; Bonnivard, G. Rduction d’un lit d’oxydes de fer par un gaz. Rev. Métallurgie 1963, 60, 23–37. [Google Scholar] [CrossRef]
- Geerdes, M.; Chaigneau, R.; Lingiardi, O. Modern Blast Furnace Ironmaking: An Introduction; IOS Press: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Ahmed, H.; Viswanathan, N.N.; Bjorkman, B. Isothermal reduction kinetics of self-reducing mixtures. Ironmak. Steelmak. 2017, 44, 66–75. [Google Scholar] [CrossRef]
- Hooey, P.L.; Bodén, A.; Wang, C.; Grip, C.-E.; Jansson, P. Design and application of a spread sheet-based model of the blast furnace factory. ISIJ Int. 2010, 50, 924–930. [Google Scholar] [CrossRef]




| Pre-Mix. | BF Dust | Coke Fines | Cement | |
|---|---|---|---|---|
| wt.%, Dry Basis | ||||
| Fe | 46.41 | 23.80 | 0.35 | 2.46 |
| CaO | 22.85 | 14.74 | 0.05 | 65.00 |
| SiO2 | 5.16 | 6.84 | 7.19 | 20.10 |
| MnO | 1.31 | 0.44 | 0 | 0.04 |
| P2O5 | 0.15 | 0.08 | 0.03 | 0.10 |
| Al2O3 | 2.18 | 2.21 | 3.12 | 3.47 |
| MgO | 2.55 | 1.65 | 0.07 | 2.11 |
| Na2O | 0.06 | 0.06 | 0.05 | 0.17 |
| K2O | 0.02 | 0.03 | 0.16 | 0.93 |
| V2O5 | 1.2 | 0.39 | 0.01 | 0.02 |
| TiO2 | 0.85 | 0.37 | 0.18 | 0.21 |
| Cr2O3 | 0.12 | 0.04 | 0 | 0.03 |
| GLF | 2.9 | -39.0 | - | - |
| C | 5.9 | 33.53 | 87.86 | 0.70 |
| S | 0.73 | 0.63 | 0.56 | 1.37 |
| Moisture | 11.13 | 0.30 | 9.98 | 0.00 |
| Material | Molasses | Hydrated Lime | Keracoal | Bitumen | CMC | Wood Tar |
|---|---|---|---|---|---|---|
| Moisture, wt.% | 3.64 | 4.82 | 2.53 | 9.97 | 6.94 | 2.26 |
| Sample | Peak Temperature | Expected Corresponding Reaction |
|---|---|---|
| All samples | Below 600 °C | Water evaporation and release of volatiles |
| R11, 13 and 15 | ~750 °C | Hematite—wüstite |
| R0 | ~805 °C | Hematite—wüstite |
| R13 and R15 | ~920 °C | Wüstite—metallic iron |
| R11 | ~940 °C | Wüstite—metallic iron |
| R0 | ~950 °C | Wüstite—metallic iron |
| Sample | C/O Molar Ratio | Minimum Mass Loss | Maximum Mass Loss | Actual Mass Loss |
|---|---|---|---|---|
| R0 | 0.6 | 29.9 | 38.0 * | 23.8 |
| R11 | 1.1 | 21.9 | 27.8 | 26.6 |
| R13 | 1.8 | 20.1 | 25.6 | 21.1 |
| R15 | 1.2 | 23.9 | 30.4 | 18.3 |
| Recipe No | Step | Equation of Linear Regression | R2 | Activation Energy, kJ/mol |
|---|---|---|---|---|
| R0 | Magnetite–wüstite | y = −18182x + 9.3913 | 0.97 | 151 |
| Wüstite–metallic iron | y = −31276x + 19.717 | 0.97 | 260 | |
| R11 | Magnetite–wüstite | y = −15707x + 7.1346 | 0.97 | 130 |
| Wüstite–metallic iron | y = −21954x + 12.718 | 0.96 | 182 | |
| R13 | Magnetite–wüstite | y = −12066x + 2.8546 | 0.96 | 100 |
| Wüstite–metallic iron | y = −25308x + 15.343 | 0.97 | 210 | |
| R15 | Magnetite–wüstite | y = −9908.9x + 0.4331 | 0.90 | 82 |
| Wüstite–metallic iron | y = −23845x + 13.949 | 0.98 | 198 |
| Parameter | Unit | Values in Blast Furnace | Limits |
|---|---|---|---|
| Feed and Production Rate | |||
| Coke | kg/tHM | 314.5 | Variable |
| PCI | kg/tHM | 143.0 | Kept constant |
| Limestone | kg/tHM | 18.4 | Varies to adjust the slag basicity |
| Pellets | kg/tHM | 1321.8 | Varies to keep the production constant |
| LD slag | kg/tHM | 45.1 | Kept constant |
| Injected dust | kg/tHM | 6.1 | Kept constant |
| Briquettes | kg/tHM | 102.1 | Kept constant |
| Production rate | tHM/h | 275.3 | Kept constant |
| Operating conditions | |||
| Eta CO | % | 54.9 | Varies |
| Eta H2 | % | 35.5 | Constant as reference |
| Top gas temperature | °C | 131.7 | Varies |
| Shaft efficiency | % | 92.7 | Kept constant |
| Blast temperature | °C | 1076 | Kept constant |
| TRZT | °C | 850 | Varies |
| O2 in the blast | % | 4.17 | Kept constant |
| RAFT | °C | 2161 | Kept constant |
| Heat losses | MJ/tHM | 353.1 | Kept constant |
| Heat loss distribution | % | 55.2 | Kept constant |
| Component, wt.% | Recipe No. | |||
|---|---|---|---|---|
| R0 | R11 | R13 | R15 | |
| CaO | 17.94 | 21.46 | 21.40 | 18.88 |
| MgO | 1.69 | 2.06 | 1.96 | 2.00 |
| SiO2 | 5.66 | 6.74 | 6.91 | 6.16 |
| Al2O3 | 1.69 | 1.91 | 2.13 | 1.99 |
| TiO2 | 0.43 | 0.51 | 0.49 | 0.47 |
| V2O5 | 0.63 | 0.74 | 0.62 | 0.63 |
| Na2O | 0.06 | 0.07 | 0.07 | 0.07 |
| K2O | 0.09 | 0.13 | 0.13 | 0.12 |
| S | 0.56 | 0.79 | 0.88 | 0.64 |
| P | 0.04 | 0.05 | 0.04 | 0.04 |
| Mn | 0.72 | 0.73 | 0.627 | 0.658 |
| Fe | 50.71 | 37.06 | 34.09 | 40.49 |
| C | 9.21 | 12.65 | 19.60 | 15.20 |
| Zn | 0.07 | 0.07 | 0.07 | 0.07 |
| Cr | 0.05 | 0.07 | 0.05 | 0.05 |
| Moisture | 3.69 | 2.55 | 3.00 | 2.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousa, E.; Ahmed, H.; Söderström, D. Potential of Alternative Organic Binders in Briquetting and Enhancing Residue Recycling in the Steel Industry. Recycling 2022, 7, 21. https://doi.org/10.3390/recycling7020021
Mousa E, Ahmed H, Söderström D. Potential of Alternative Organic Binders in Briquetting and Enhancing Residue Recycling in the Steel Industry. Recycling. 2022; 7(2):21. https://doi.org/10.3390/recycling7020021
Chicago/Turabian StyleMousa, Elsayed, Hesham Ahmed, and Daniel Söderström. 2022. "Potential of Alternative Organic Binders in Briquetting and Enhancing Residue Recycling in the Steel Industry" Recycling 7, no. 2: 21. https://doi.org/10.3390/recycling7020021
APA StyleMousa, E., Ahmed, H., & Söderström, D. (2022). Potential of Alternative Organic Binders in Briquetting and Enhancing Residue Recycling in the Steel Industry. Recycling, 7(2), 21. https://doi.org/10.3390/recycling7020021







