Geopolymerization of Recycled Glass Waste: A Sustainable Solution for a Lightweight and Fire-Resistant Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Recycled Waste Glass
2.1.1. ED-XRF Results
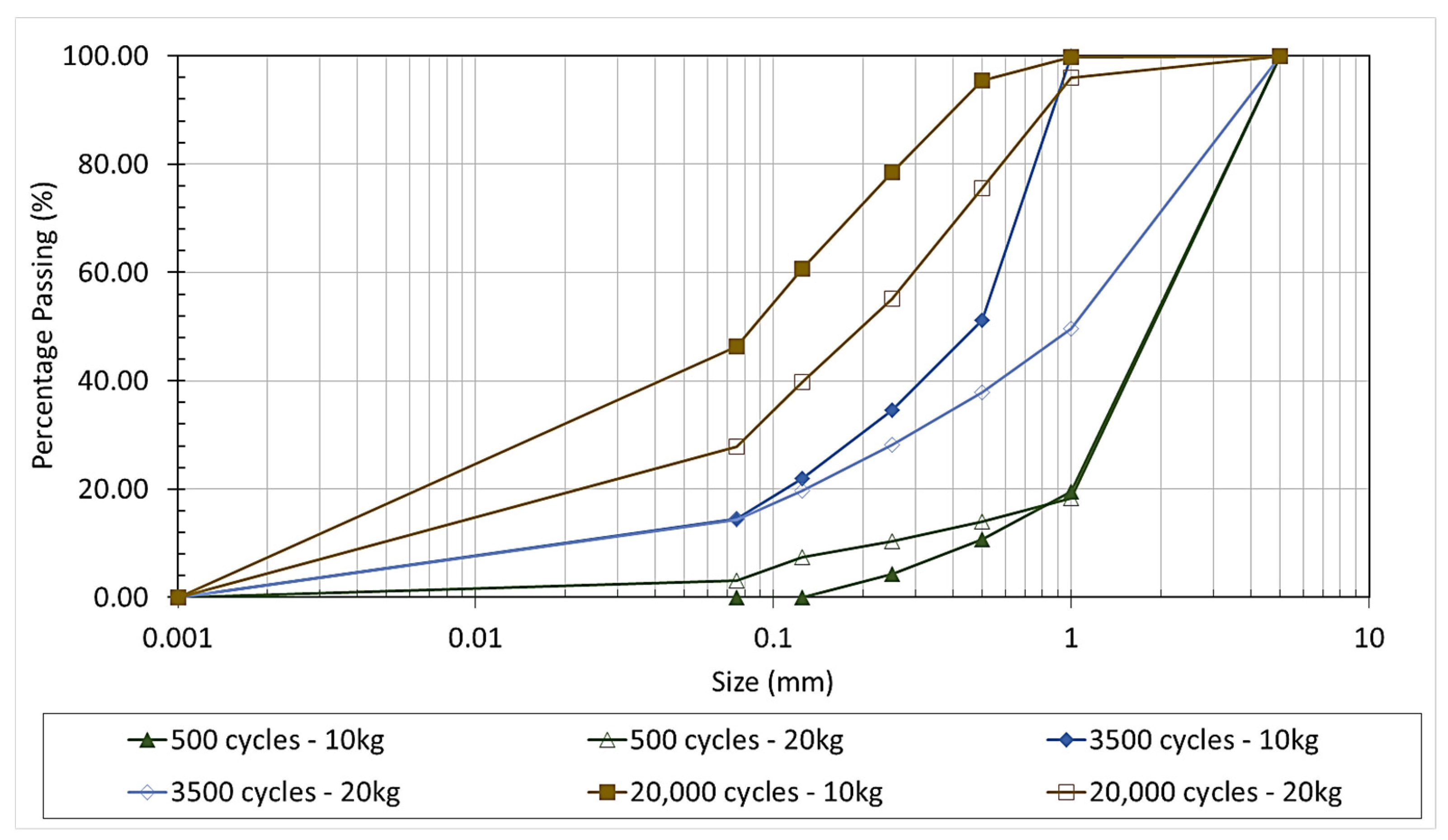
2.1.2. Los Angeles and Micro Deval Grinding
2.1.3. Density, Water Absorption and Fineness
2.1.4. Rest of Materials
2.2. Mixture Design, Properties and Testing
2.2.1. Mixture Design
2.2.2. Mixing Procedure
2.2.3. Hardened Properties
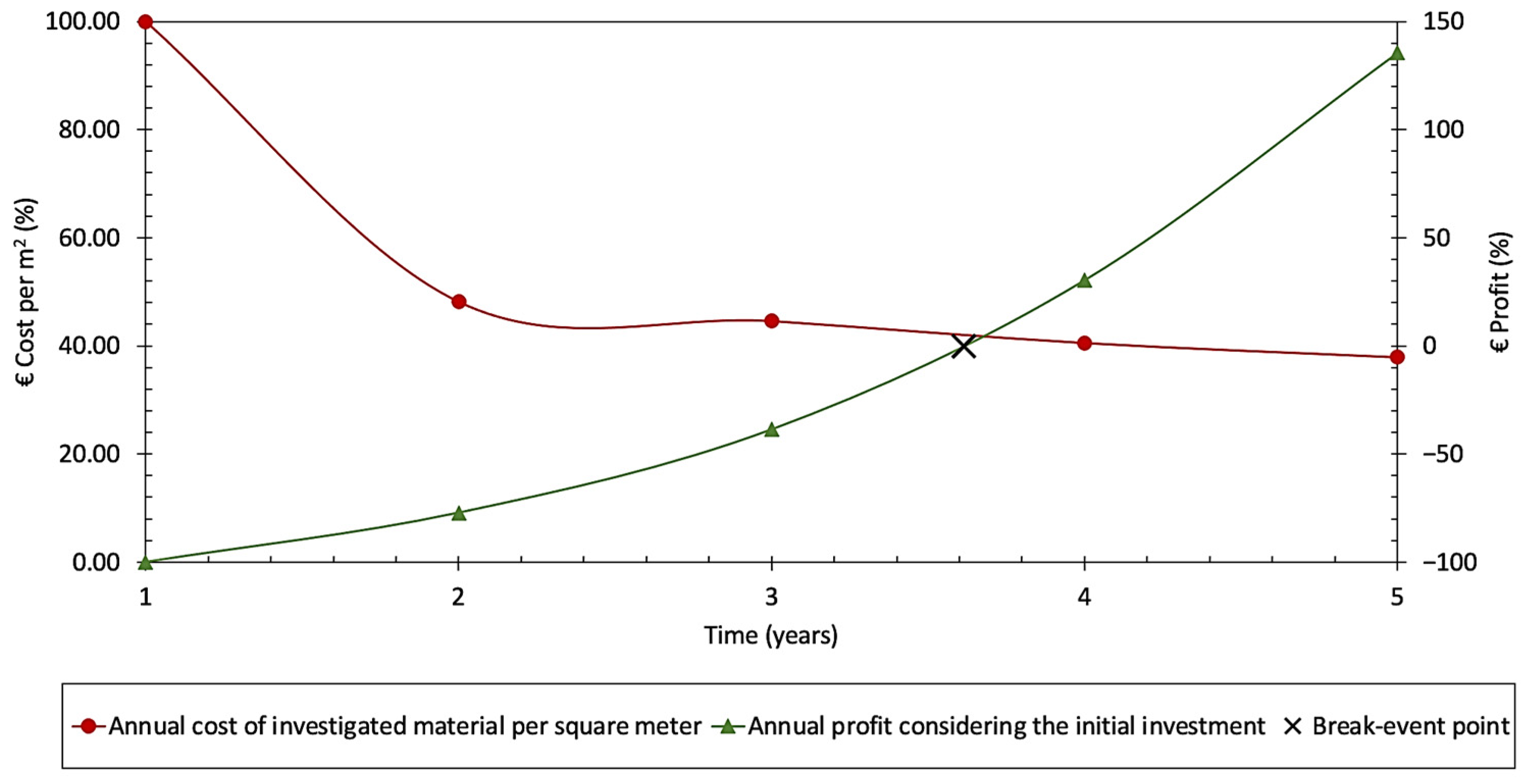
2.3. Techno-Economic Analysis
3. Results and Discussion
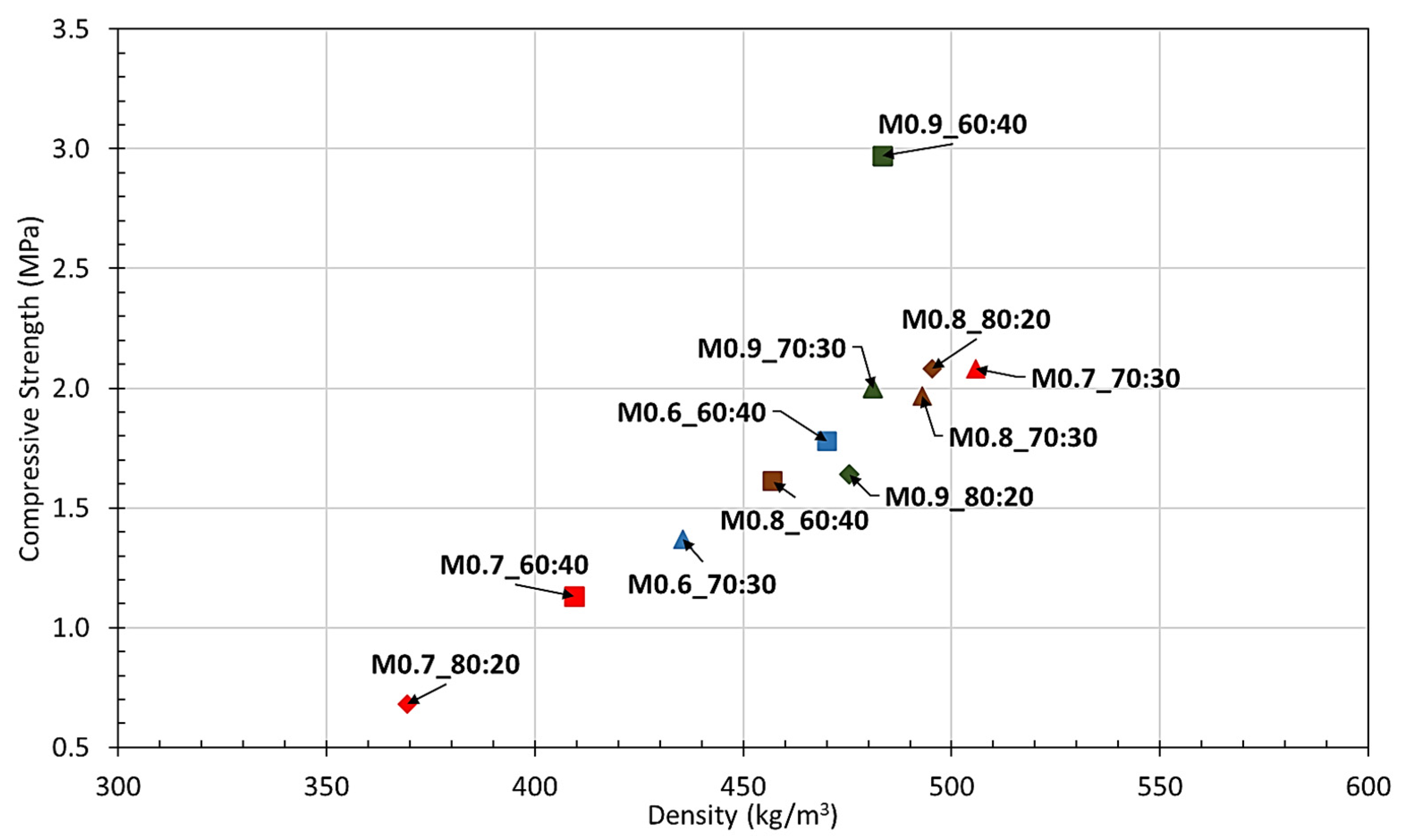
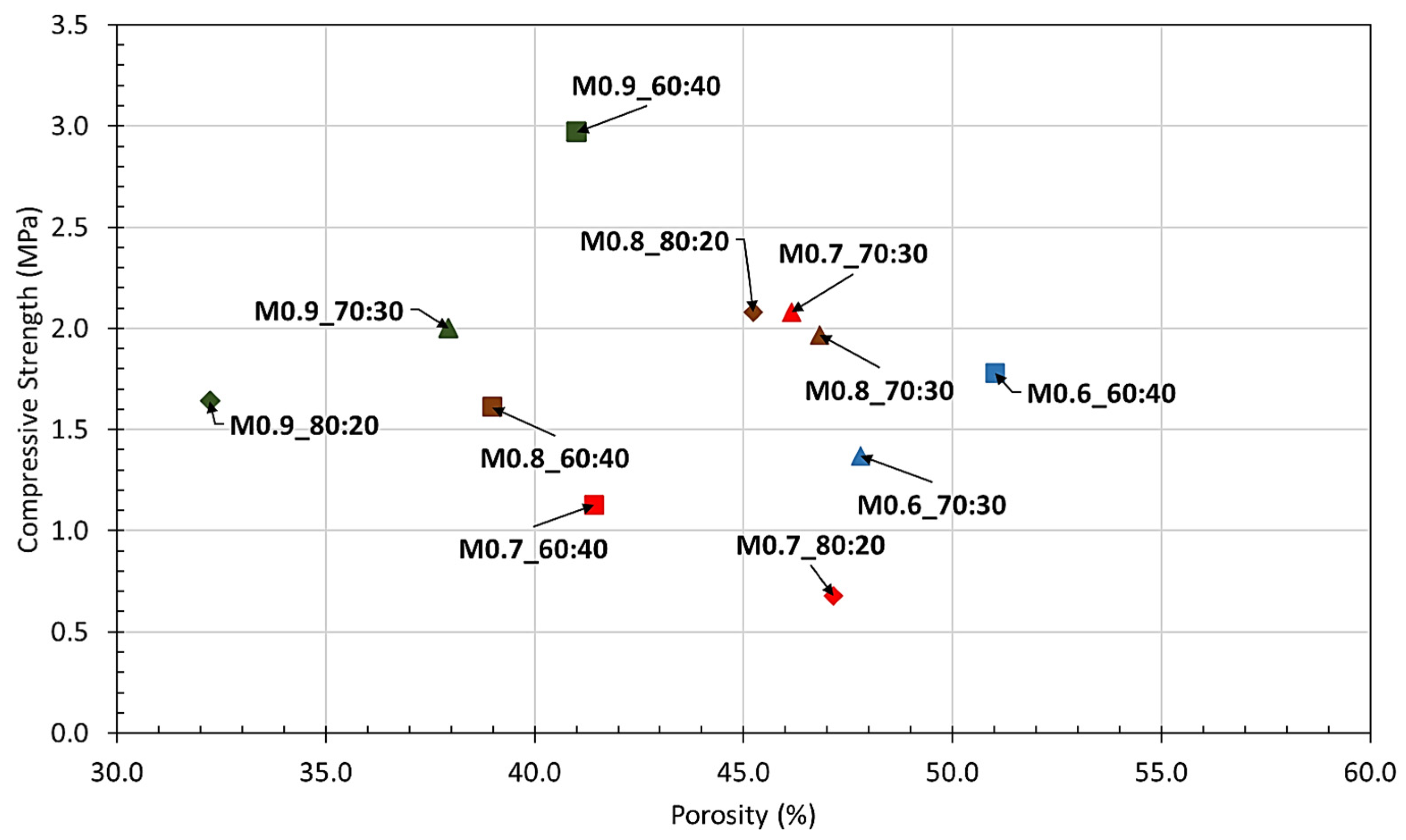
3.1. Hardened Properties
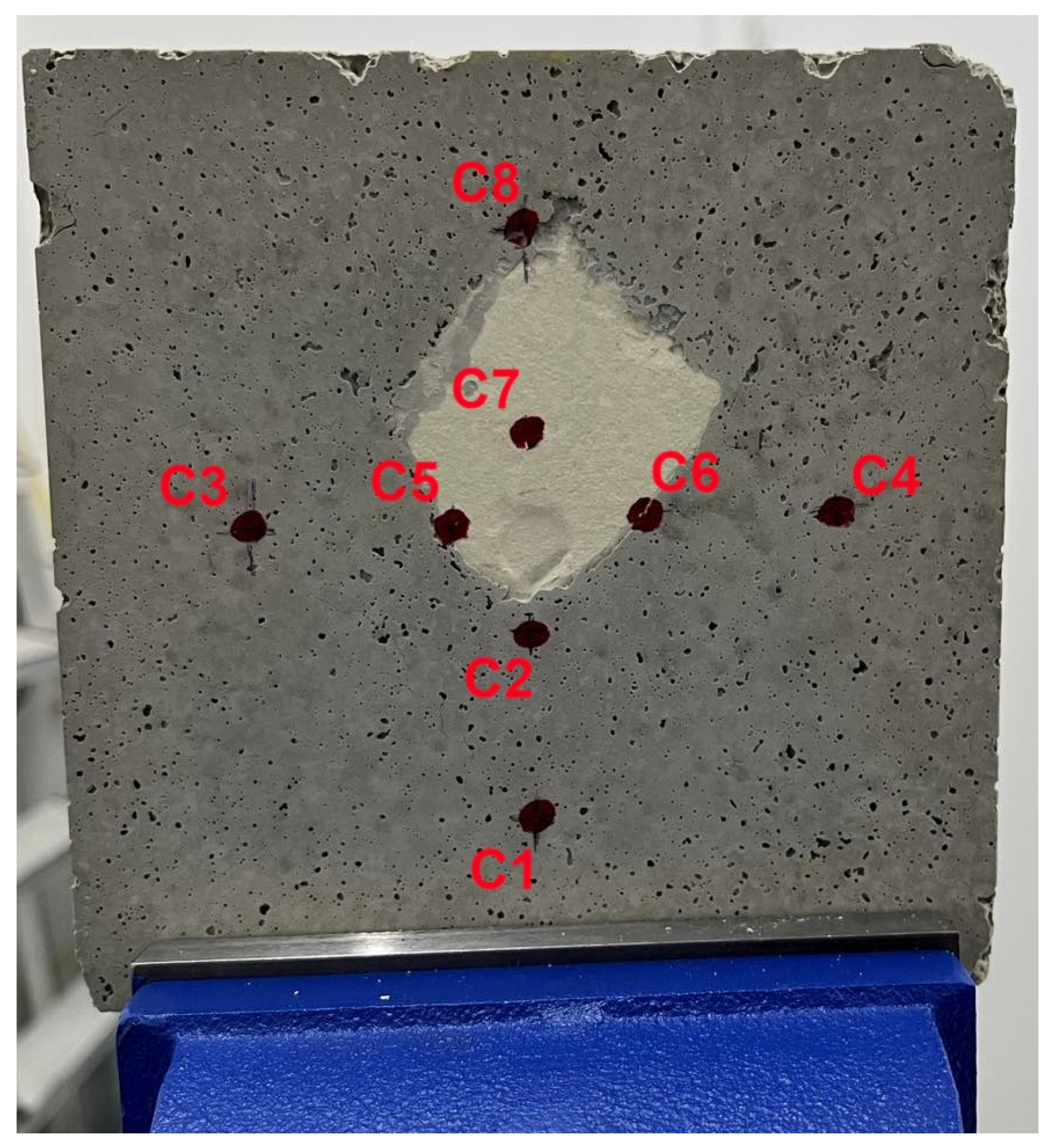
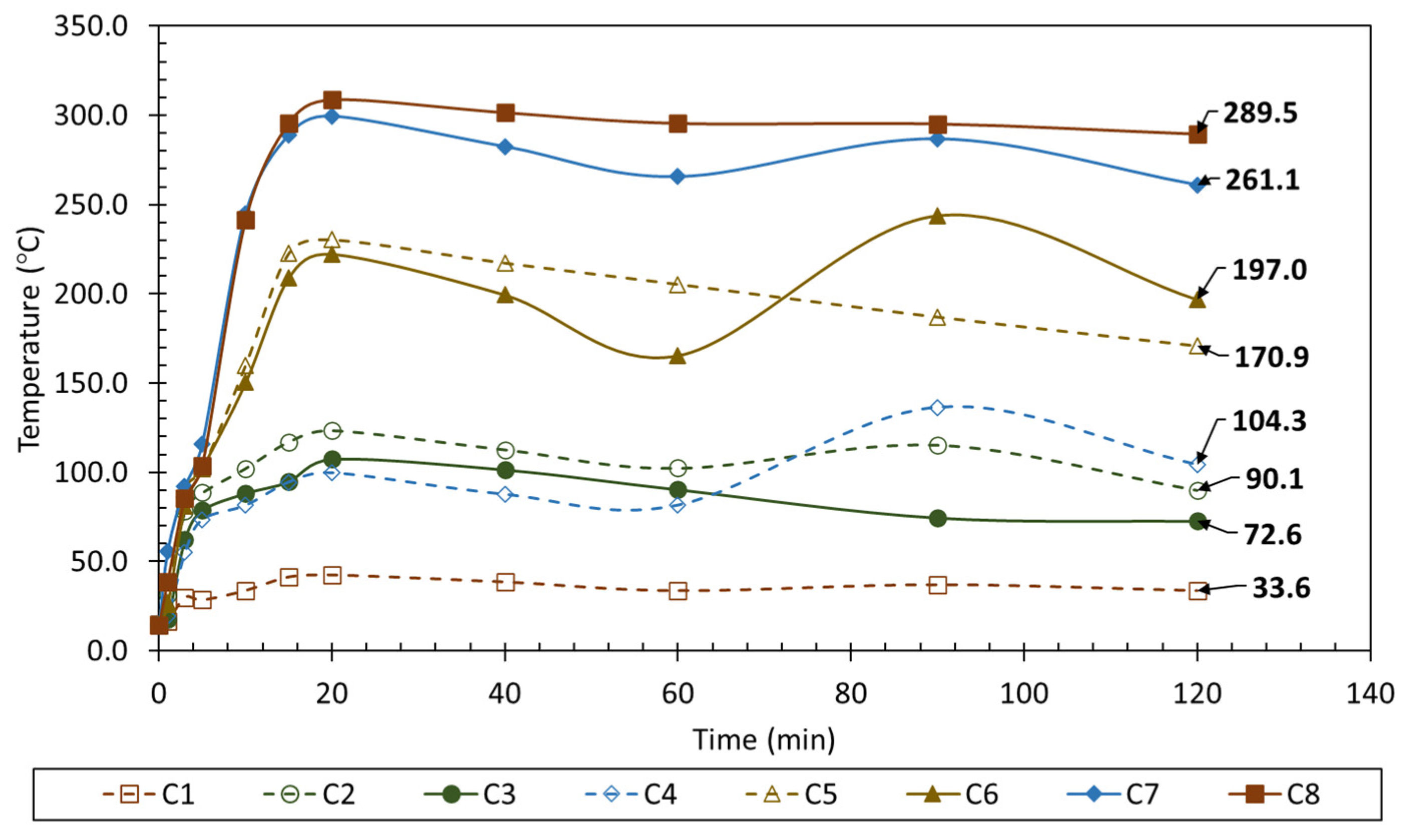
3.2. Fire Testing
3.3. Technoeconomic Analysis
4. Conclusions
- ED-XRF characterization of the recycled waste glass showed a silicate-rich material with an average SiO2 quantity of 76.8%; nevertheless, the absence of Al2O3 in the oxide composition necessitated the obligatory incorporation of aluminum powder to initiate the geopolymerization.
- An optimum mechanical treatment for the adequate grinding of waste glass to achieve the required surface reactivity involved the initial treatment with a Los Angeles apparatus for 20 kg of materials at 20,000 cycles, followed by grinding in a Micro Deval machine at 5 kg per round at 10,000 rpm.
- All formulations yielded a density range from 350 to 550 kg/m3, compressive strengths between 0.5 and 3.0 MPa and flexural strengths exceeding 0.2 MPa, defining the results as promising indicators in the geopolymerization of recycled glass powder.
- The experimental results showed the significance of obtaining an optimum balance between the aluminum powder and solution ratio quantities in strength and physical characteristics. For application of the geopolymerized product as a door core, mixture M0.70_70:30 was defined as the suitable and cost-efficient formulation; however, for utilization as an exterior façade, the requirement for the minimum possible void matrix specimen was crucial. Therefore, porosity was the predominant parameter for selecting M0.9_70:30 as the optimum mixture.
- The thermal performance of M0.70_70:30 was evaluated as satisfactory during a 2 h fire exposure utilizing a blowtorch with a maximum temperature of 1850 °C since the highest temperatures were recorded at the center of the specimen at 308.7 °C and 299.5 °C twenty minutes after experiment initiation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Environmental Protection Agency. Glass: Material-Specific Data. 2017. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/glass-material-specific-data (accessed on 28 March 2022).
- Meena, A.; Singh, R. Comparative Study of Waste Glass Powder as Pozzolanic Material in Concrete. Ph.D. Thesis, Department of Civil and Environmental Engineering, National Institute of Technology, Rourkela, India, 2012. [Google Scholar]
- Guo, P.; Meng, W.; Nassif, H.; Gou, H.; Bao, Y. New perspectives on recycling waste glass in manufacturing concrete for sustainable civil infrastructure. Constr. Build. Mater. 2020, 257, 119579. [Google Scholar] [CrossRef]
- Siddika, A.; Hajimohammadi, A.; Sahajwalla, V. Powder sintering and gel casting methods in making glass foam using waste glass: A review on parameters, performance, and challenges. Ceram. Int. 2022, 48, 1494–1511. [Google Scholar] [CrossRef]
- Cheng, M.; Chen, M.; Wu, S.; Yang, T.; Zhang, J.; Zhao, Y. Effect of waste glass aggregate on performance of asphalt micro-surfacing. Constr. Build. Mater. 2021, 307, 125133. [Google Scholar] [CrossRef]
- Paul, D.; Suresh, M.; Pal, M. Utilization of fly ash and glass powder as fillers in steel slag asphalt mixtures. Case Stud. Constr. Mater. 2021, 15, e00672. [Google Scholar] [CrossRef]
- You, L.; Jin, D.; Guo, S.; Wang, J.; Dai, Q.; You, Z. Leaching evaluation and performance assessments of asphalt mixtures with recycled cathode ray tube glass: A preliminary study. J. Clean. Prod. 2021, 279, 123716. [Google Scholar] [CrossRef]
- Lam, C.S.; Poon, C.S.; Chan, D. Enhancing the performance of pre-cast concrete blocks by incorporating waste glass—ASR consideration. Cem. Concr. Compos. 2007, 29, 616–625. [Google Scholar] [CrossRef]
- Su, N.; Chen, J. Engineering properties of asphalt concrete made with recycled glass. Resour. Conserv. Recycl. 2002, 35, 259–274. [Google Scholar] [CrossRef]
- Ming, N.C.; Putra Jaya, R.; Awang, H.; Siaw Ing, N.L.; Mohd Hasan, M.R.; Al-Saffar, Z.H. Performance of glass powder as bitumen modifier in hot mix asphalt. Phys. Chem. Earth Parts A/B/C 2022, 128, 103263. [Google Scholar] [CrossRef]
- Lin, J.; Guo, Z.; Hong, B.; Xu, J.; Fan, Z.; Lu, G.; Wang, D.; Oeser, M. Using recycled waste glass fiber reinforced polymer (GFRP) as filler to improve the performance of asphalt mastics. J. Clean. Prod. 2022, 336, 130357. [Google Scholar] [CrossRef]
- Kadhim, M.A.; Al-Busaltan, S.; Nemaa, Z.K.; Abo Almaali, Y.; Saghafi, B.; Al-Kafaji, M.; Al-Yasari, R. Evaluating the Cracking Performance Indices of Half-Warm Mix Asphalt Comprising Waste Glass. Int. J. Pavement Res. Technol. 2022, 15, 1262–1276. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Sata, V.; Chindaprasirt, P. 19—Use of construction and demolition waste (CDW) for alkali-activated or geopolymer concrete. In Advances in Construction and Demolition Waste Recycling; Pacheco-Torgal, F., Ding, Y., Colangelo, F., Tuladhar, R., Koutamanis, A., Eds.; Woodhead Publishing: Thorston, UK, 2020; pp. 385–403. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, H.-Y.; Chen, B.-T.; Peng, Y.-C. Study on the engineering properties and prediction models of an alkali-activated mortar material containing recycled waste glass. Constr. Build. Mater. 2017, 132, 130–141. [Google Scholar] [CrossRef]
- Wang, W.-C.; Chen, B.-T.; Wang, H.-Y.; Chou, H.-C. A study of the engineering properties of alkali-activated waste glass material (AAWGM). Constr. Build. Mater. 2016, 112, 962–969. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Seabra, M.P.; Labrincha, J.A. Waste glass from end-of-life fluorescent lamps as raw material in geopolymers. Waste Manag. 2016, 52, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Ozer, I.; Soyer-Uzun, S. Relations between the structural characteristics and compressive strength in metakaolin based geopolymers with different molar Si/Al ratios. Ceram. Int. 2015, 41, 10192–10198. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Pimraksa, K.; Chindaprasirt, P.; Rungchet, A.; Sagoe-Crentsil, K.; Sato, T. Lightweight geopolymer made of highly porous siliceous materials with various Na2O/Al2O3 and SiO2/Al2O3 ratios. Mater. Sci. Eng. A 2011, 528, 6616–6623. [Google Scholar] [CrossRef]
- Puertas, F.; Torres-Carrasco, M. Use of glass waste as an activator in the preparation of alkali-activated slag. Mechanical strength and paste characterization. Cem. Concr. Res. 2014, 57, 95–104. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.-W.; Nicolaides, D.; Luhar, I.; Panias, D.; Sakkas, K. Valorisation of glass waste for development of Geopolymer composites—Mechanical properties and rheological characteristics: A review. Constr. Build. Mater. 2019, 220, 547–564. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.-W.; Nicolaides, D.; Luhar, I.; Panias, D.; Sakkas, K. Valorisation of glass wastes for the development of geopolymer composites—Durability, thermal and microstructural properties: A review. Constr. Build. Mater. 2019, 222, 673–687. [Google Scholar] [CrossRef]
- Tahwia, A.M.; Ellatief, M.A.; Heneigel, A.M.; Elrahman, M.A. Characteristics of eco-friendly ultra-high-performance geopolymer concrete incorporating waste materials. Ceram. Int. 2022, 48, 19662–19674. [Google Scholar] [CrossRef]
- Mahesh, Y.; Lalitha, G. Durablilty propeties of geopolymer concrete partial replacement of fine aggaregate with waste crushed glass. Mater. Today Proc. 2022, 51, 2466–2470. [Google Scholar] [CrossRef]
- Geopolymer Cement a Review 2013, ResearchGate. (n.d.). Available online: https://www.researchgate.net/publication/306946529_Geopolymer_Cement_a_review_2013 (accessed on 29 March 2022).
- Hlaváček, P.; Šmilauer, V.; Škvára, F.; Kopecký, L.; Šulc, R. Inorganic foams made from alkali-activated fly ash: Mechanical, chemical and physical properties. J. Eur. Ceram. Soc. 2015, 35, 703–709. [Google Scholar] [CrossRef]
- Hertz, K.D. Limits of spalling of fire-exposed concrete. Fire Saf. J. 2003, 38, 103–116. [Google Scholar] [CrossRef]
- Sidhu, P.S. Transformation of Trace Element-Substituted Maghemite to Hematite. Clays Clay Miner. 1988, 36, 31–38. [Google Scholar] [CrossRef]
- Przepiera, A.; Przepiera, K.; Wisniewski, M.; Dabrowski, W. Investigations of the thermal transformations of precipitated mixed transition metal hydroxides. J. Therm. Anal. 1993, 40, 1131–1138. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Kealley, C.S.; van Riessen, A. Thermally Induced Microstructural Changes in Fly Ash Geopolymers: Experimental Results and Proposed Model. J. Am. Ceram. Soc. 2015, 98, 929–939. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kelly, S.; Yao, Z. Effect of fire exposure on cracking, spalling and residual strength of fly ash geopolymer concrete. Mater. Design. 2014, 63, 584–592. [Google Scholar] [CrossRef]
- Zhao, R.; Sanjayan, J.G. Geopolymer and Portland cement concretes in simulated fire. Mag. Concr. Res. 2011, 63, 163–173. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, W.; Shi, D. Effect of elevated temperature on the properties of geopolymer synthesized from calcined ore-dressing tailing of bauxite and ground-granulated blast furnace slag. Constr. Build. Mater. 2014, 69, 41–48. [Google Scholar] [CrossRef]
- Zulkifly, K.; Yong, H.C.; Abdullah, M.M.A.B.; Ming, L.Y.; Panias, D.; Sakkas, K. Review of Geopolymer Behaviour in Thermal Environment; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 209, p. 012085. [Google Scholar] [CrossRef]
- Gok, S.G.; Sengul, O. Mechanical properties of alkali-activated slag based SIFCON incorporating waste steel fibers and waste glass. Constr. Build. Mater. 2023, 408, 133697. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F.; de Carvalho, J.M.F.; Ribeiro, J.C.L.; de Souza, C.M.M. Application of mixture design of experiments to the development of alkali-activated composites based on chamotte and waste glass. Constr. Build. Mater. 2023, 379, 131139. [Google Scholar] [CrossRef]
- Rios, L.M.H.; Triviño, A.F.H.; Villaquirán-Caicedo, M.A.; de Gutiérrez, R.M. Effect of the use of waste glass (as precursor, and alkali activator) in the manufacture of geopolymer rendering mortars and architectural tiles. Constr. Build. Mater. 2023, 363, 129760. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, C.; Shi, Q.; Tan, X.; Meng, W. Recycling waste glass aggregate in concrete: Mitigation of alkali-silica reaction (ASR) by carbonation curing. J. Clean. Prod. 2022, 370, 133545. [Google Scholar] [CrossRef]
- Zhao, J.; Li, S. Performance study and environmental evaluation of alkali-activated materials based on waste photovoltaic glass. J. Clean. Prod. 2022, 379 Pt 1, 134576. [Google Scholar] [CrossRef]
- Zhao, J.; Li, S. Alkali-activated binder with waste photovoltaic glass powder and blast furnace slag as precursors: Performance study, shrinkage—reducing technology and mechanism analysis. J. Non-Cryst. Solids 2023, 609, 122263. [Google Scholar] [CrossRef]
- Bianco, I.; Tomos, B.A.D.; Vinai, R. Analysis of the environmental impacts of alkali-activated concrete produced with waste glass-derived silicate activator—A LCA study. J. Clean. Prod. 2021, 316, 128383. [Google Scholar] [CrossRef]
- Mazzi, A.; Sciarrone, M.; Bernardo, E. Environmental performance of glass foam as insulation material from waste glass with the alkali activation process. Heliyon 2023, 8, e19001. [Google Scholar] [CrossRef] [PubMed]
- Europe Passive Fire Protection Coating Market Scope, Revenue, Value, & Industry Trends. Europe Passive Fire Protection Coating Market Scope, Revenue, Value, & Industry Trends. Available online: https://www.databridgemarketresearch.com/reports/europe-passive-fire-protection-coating-market (accessed on 5 October 2023).
- CYS EN 1097-2:2020; Tests for Mechanical and Physical Properties of Aggregates—Part 2. Methods for the Determination of Resistance to Fragmentation. CEN (European Committee for Standardization): Brussels, Belgium, 2020.
- CYS EN 1097-1:2021; Tests for Mechanical and Physical Properties of Aggregates Part 1. Determination of the Resistance to Wear (Micro-Deval). CEN (European Committee for Standardization): Brussels, Belgium, 2021.
- CYS EN 1097-6:2022; Tests for Mechanical and Physical Properties of Aggregates—Part 6: Determination of Particle Density and Water Absorption. CEN (European Committee for Standardization): Brussels, Belgium, 2022.
- CYS EN 196-6:2018; Methods of Testing Cement—Part 6: Determination of Fineness. CEN (European Committee for Standardization): Brussels, Belgium, 2018.
- CYS EN 196-1:2016; Methods of Testing Cement—Part 1: Determination of Strength. CEN (European Committee for Standardization): Brussels, Belgium, 2016.
- Kanellopoulos, A.; Petrou, M.F.; Ioannou, I. Durability performance of self-compacting concrete. Constr. Build. Mater. 2012, 37, 320–325. [Google Scholar] [CrossRef]




| Sample | WG1 | WG2 | WG3 | WG4 | WG5 | |
|---|---|---|---|---|---|---|
| Oxide | ||||||
| SiO2 (%) | 77.46 | 76.20 | 77.46 | 77.04 | 75.99 | |
| CaO (%) | 10.26 | 10.19 | 10.24 | 9.92 | 9.93 | |
| Na2O (%) | 8.71 | 8.21 | 7.58 | 9.23 | 9.16 | |
| Al2O3 (%) | - | - | - | - | - | |
| MgO (%) | 4.15 | 4.52 | 2.16 | 4.39 | 4.48 | |
| K2O (%) | 0.16 | 0.25 | 0.17 | 0.18 | 0.22 | |
| Fe2O3 (%) | 0.27 | 0.64 | 0.40 | 0.25 | 0.23 | |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |
| Sample No. | Passing Percentage 0.075 μm (%) | Passing Percentage 0.063 μm (%) |
|---|---|---|
| Sample 1 | 97.96 | 89.34 |
| Sample 2 | 99.22 | 93.20 |
| Sample 3 | 99.53 | 94.60 |
| Sample 4 | 100.00 | 89.76 |
| Sample 5 | 100.00 | 99.33 |
| Sample 6 | 100.00 | 92.00 |
| Constituent | Nomenclature | Particle Size (μm) | pH at 20 °C | Specific Gravity (g/cm3) |
|---|---|---|---|---|
| Aluminum Powder | Al | <44 | - | 2.70 |
| Hostapur OSB | Alpha olefin sulfate, sodium salt | <63 | 10.0–11.0 | 0.30 |
| Sodium Silicate | Na2SiO3 | - | 11.4 | 1.26–1.46 |
| Sodium Hydroxide | NaOH | - | 14.0 | 2.04 |
| Nomenclature | Glass (% by wt. Solids) | Aluminum Powder (% by wt. Solids) | Hostapur OBS (% by wt. Solids) | Sodium Silicate/Sodium Hydroxide (Na2SiO3:NaOH) Ratio (% by wt. Liquids) | S/L Ratio | M NaOH |
|---|---|---|---|---|---|---|
| M0.6_60:40 | 99.32 | 0.60 | 0.08 | 60:40 | 2.86 | 7 |
| M0.6_70:30 | 99.32 | 0.60 | 0.08 | 70:30 | 2.86 | 7 |
| M0.6_80:20 | 99.32 | 0.60 | 0.08 | 80:20 | 2.86 | 7 |
| M0.7_60:40 | 99.10 | 0.70 | 0.10 | 60:40 | 2.87 | 7 |
| M0.7_70:30 | 99.10 | 0.70 | 0.10 | 70:30 | 2.87 | 7 |
| M0.7_80:20 | 99.10 | 0.70 | 0.10 | 80:20 | 2.87 | 7 |
| M0.8_60:40 | 99.09 | 0.80 | 0.11 | 60:40 | 2.87 | 7 |
| M0.8_70:30 | 99.09 | 0.80 | 0.11 | 70:30 | 2.87 | 7 |
| M0.8_80:20 | 99.09 | 0.80 | 0.11 | 80:20 | 2.87 | 7 |
| M0.9_60:40 | 98.97 | 0.90 | 0.13 | 60:20 | 2.88 | 7 |
| M0.9_70:30 | 98.97 | 0.90 | 0.13 | 70:30 | 2.88 | 7 |
| M0.9_80:20 | 98.97 | 0.90 | 0.13 | 80:20 | 2.88 | 7 |
| Hardened Properties Test | Standard | Age of Testing (Days) | Specimens | Dimensions (mm × mm × mm) |
|---|---|---|---|---|
| Compressive Strength | EN 196-1:2016 [49] | 7 | 3 cubes | 40.0 × 40.0 × 40.0 |
| Flexural Strength | EN 196-1:2016 [49] | 7 | 3 prisms | 40.0 × 41.0 × 160.0 |
| Open Porosity | Reference [50] | 7 | 3 cubes | 40.0 × 40.0 × 40.0 |
| Fire Testing | - | 7 | 1 board | 150.0 × 150.0 × 30.0 |
| Fixed Costs | Variable Costs | Studied Parameters |
|---|---|---|
| Web Host Fees | Cost of Goods Sold | Turnover |
| Accounting and Legal Fees | Overhead | Total Costs |
| Depreciation | Maintenance | Initial Investment |
| Insurance | Cost per m2 | |
| Manufacturing | EBIT (Earnings before interest and taxes) | |
| Payroll | TAX | |
| Rent | NIAT (Net income after tax) | |
| Supplies | Break-event point | |
| Taxes (Real Estate, etc.) | Payback Period | |
| Utilities | ||
| Labor | ||
| Other Startup Costs |
| Nomenclature | Temperature (°C) | Humidity (%) | Density (kg/m3) | Compressive Strength (MPa) | Flexural Strength (MPa) | Porosity (%) |
|---|---|---|---|---|---|---|
| M0.6_60:40 | 23.3 | 57 | 470 | 1.8 | 0.6 | 51.0 |
| M0.6_70:30 | 23.6 | 56 | 435 | 1.4 | 0.5 | 47.8 |
| M0.6_80:20 | 23.8 | 57 | N/A 1 | N/A 1 | N/A 1 | N/A 1 |
| M0.7_60:40 | 23.8 | 60 | 409 | 1.1 | 0.6 | 41.4 |
| M0.7_70:30 | 23.8 | 60 | 506 | 2.1 | 0.9 | 46.1 |
| M0.7_80:20 | 23.5 | 58 | 369 | 0.7 | 0.5 | 47.2 |
| M0.8_60:40 | 23.8 | 57 | 457 | 1.6 | 0.7 | 39.0 |
| M0.8_70:30 | 23.3 | 56 | 493 | 2.0 | 0.8 | 46.8 |
| M0.8_80:20 | 23.8 | 57 | 495 | 2.1 | 0.8 | 45.2 |
| M0.9_60:40 | 23.2 | 57 | 483 | 3.0 | 0.5 | 41.0 |
| M0.9_70:30 | 23.5 | 56 | 481 | 2.0 | 0.7 | 37.9 |
| M0.9_80:20 | 23.5 | 56 | 476 | 1.6 | 0.3 | 32.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valanides, M.; Aivaliotis, K.; Oikonomopoulou, K.; Fikardos, A.; Savva, P.; Sakkas, K.; Nicolaides, D. Geopolymerization of Recycled Glass Waste: A Sustainable Solution for a Lightweight and Fire-Resistant Material. Recycling 2024, 9, 16. https://doi.org/10.3390/recycling9010016
Valanides M, Aivaliotis K, Oikonomopoulou K, Fikardos A, Savva P, Sakkas K, Nicolaides D. Geopolymerization of Recycled Glass Waste: A Sustainable Solution for a Lightweight and Fire-Resistant Material. Recycling. 2024; 9(1):16. https://doi.org/10.3390/recycling9010016
Chicago/Turabian StyleValanides, Marios, Konstantinos Aivaliotis, Konstantina Oikonomopoulou, Alexandros Fikardos, Pericles Savva, Konstantinos Sakkas, and Demetris Nicolaides. 2024. "Geopolymerization of Recycled Glass Waste: A Sustainable Solution for a Lightweight and Fire-Resistant Material" Recycling 9, no. 1: 16. https://doi.org/10.3390/recycling9010016






