Study of the Mechanical and Electrochemical Performance of Structural Concrete Incorporating Recycled Polyethylene Terephthalate as a Partial Fine Aggregate Replacement
Abstract
:1. Introduction
2. Experimental Section
2.1. Characterization of R-PET
2.2. X-ray Diffraction
2.3. Concrete Mixture Design
2.4. Mechanical Characterization
2.4.1. Compressive Strength
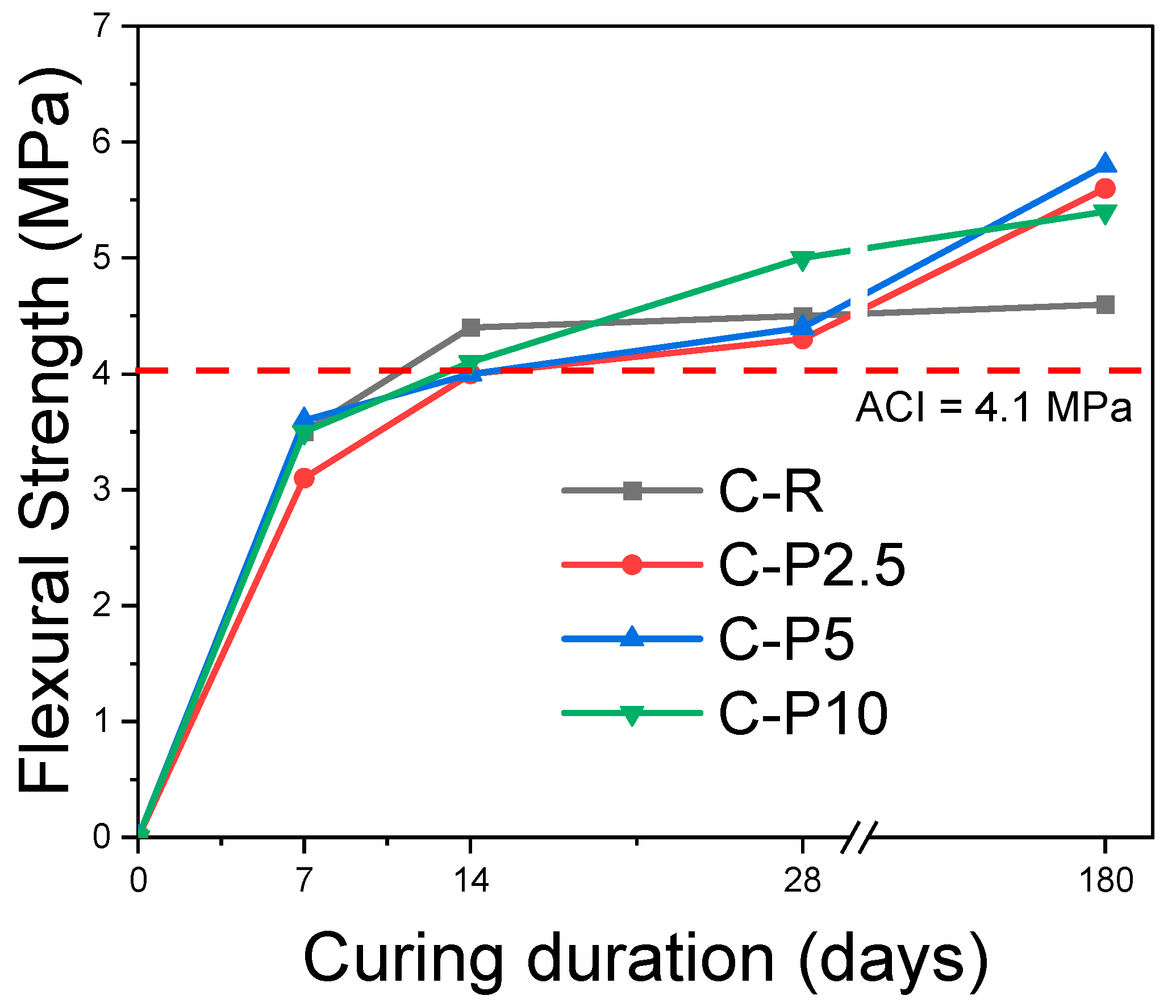
2.4.2. Flexural Strength
2.5. Electrochemical Characterization
3. Results and Analysis
3.1. Characterization of R-PET by X-ray Diffraction
3.2. Tests of Concrete Specimens
3.2.1. Mechanical Characterization
3.2.2. Flexural Strength
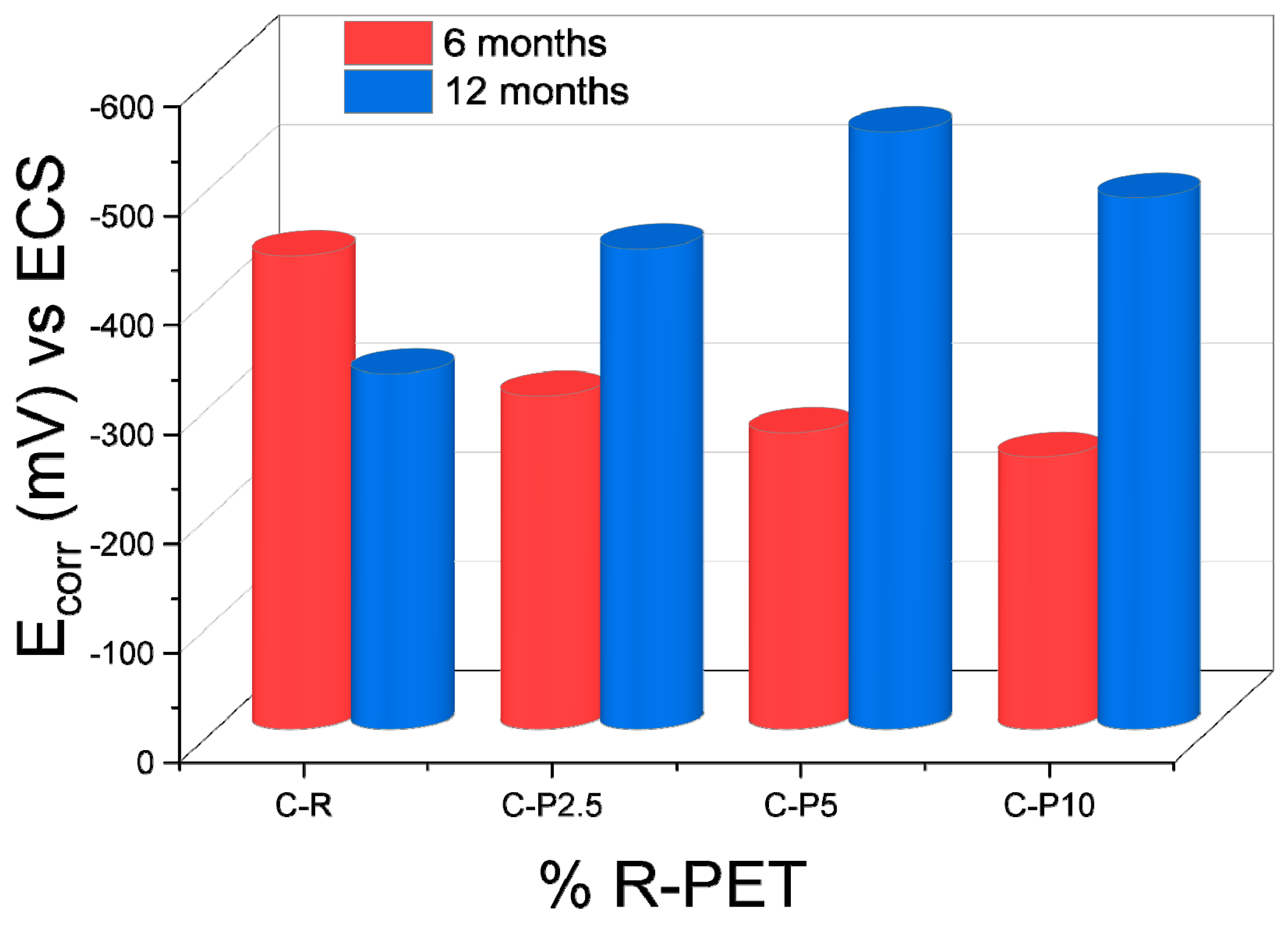
3.2.3. Electrochemical Characterization
Open-Circuit Potential (OCP)
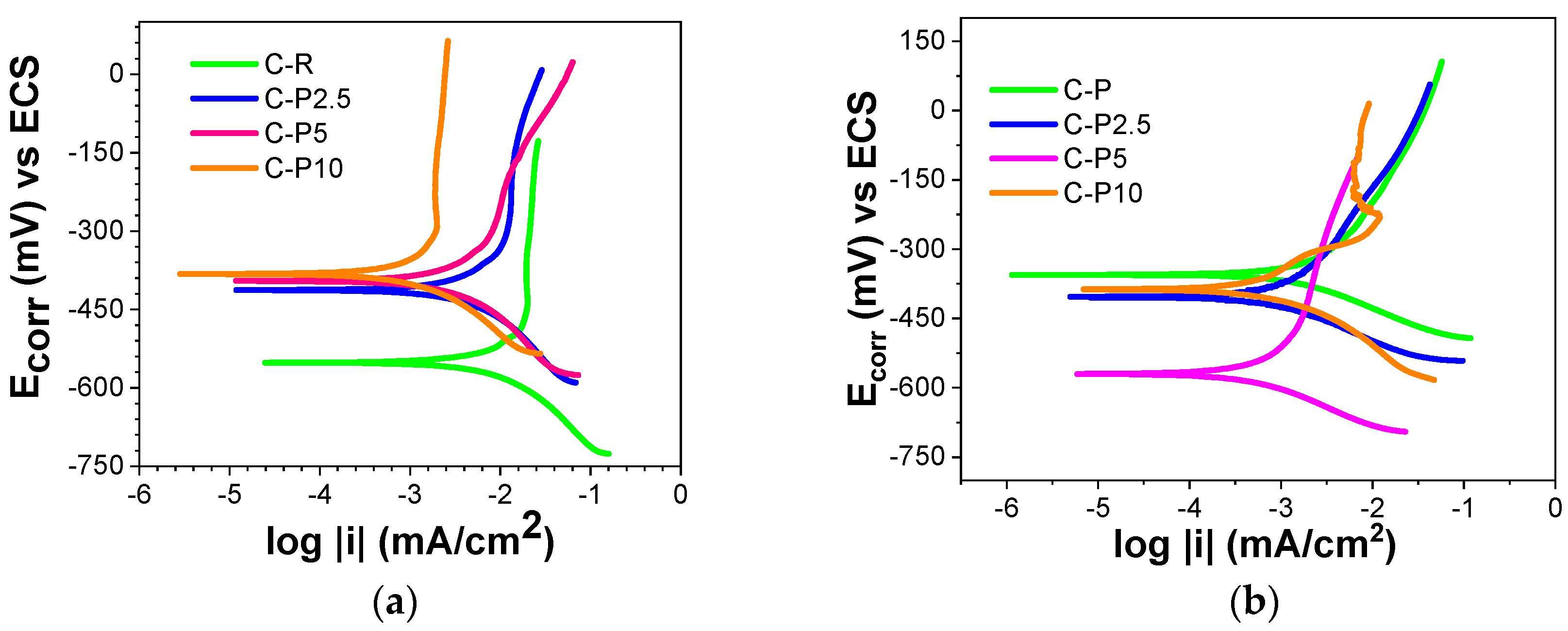
3.2.4. Tafel Extrapolation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gagg, C.R. Cement and concrete as an engineering material: An historic appraisal and case study analysis. Eng. Fail. Anal. 2014, 40, 114–140. [Google Scholar] [CrossRef]
- Mo, K.H.; Thomas, B.S.; Yap, S.P.; Abutaha, F.; Tan, C.G. Viability of agricultural wastes as substitute of natural aggregate in concrete: A review on the durability related properties. J. Clean. Prod. 2020, 275, 123062. [Google Scholar] [CrossRef]
- Alqahtani, F.K.; Zafar, I. Plastic-based sustainable synthetic aggregate in Green Lightweight concrete—A review. Construct. Build. Mater. 2021, 292, 123321. [Google Scholar] [CrossRef]
- Yehia, S.; Helal, K.; Abusharkh, A.; Zaher, A.; Istaitiyeh, H. Strength Durability Evaluation of Recycled Aggregate Concrete. Int. J. Concrete Struct. Mater. 2015, 9, 219–239. [Google Scholar] [CrossRef]
- Ren, Z.; Jiang, M.; Chen, D.; Yu, Y.; Li, F.; Xu, M.; Bringezu, S.; Zhu, B. Stocks and flows of sand, gravel, and crushed stone in China (1978–2018): Evidence of the peaking and structural transformation of supply and demand. Resour. Conserv. Recycl. 2022, 180, 106173. [Google Scholar] [CrossRef]
- Kirthika, S.K.; Singh, S.K.; Chourasia, A. Alternative fine aggregates in production of sustainable concrete—A review. J. Clean. Prod. 2020, 268, 122089. [Google Scholar] [CrossRef]
- Gavriletea, M.D. Environmental impacts of sand exploitation. Analysis of sand market. Sustainability 2017, 9, 1118. [Google Scholar] [CrossRef]
- Timofeeva, Y.R.; Suhacheva, E.Y.; Zakharova, M.K. Soil cover of areas of mining sand and sand-gravel material in the Leningrad region. IOP Conf. Ser. Earth Environ. Sci. 2021, 862, 012064. [Google Scholar] [CrossRef]
- Meador, M.R.; Layher, A.O. Instream sand and gravel mining: Environmental issues and regulatory process in the United States. Fisheries 1998, 23, 6–13. [Google Scholar]
- Bayram, A.; Onsoy, H. Sand and gravel mining impact on the surface water quality: A case study from the city of Tirebolu (Giresun Province, NE Turkey). Environ. Earth Sci. 2015, 73, 1997–2011. [Google Scholar] [CrossRef]
- Padmalal, D.; Maya, K.; Sreebha, S.; Sreeja, R. Environmental effects of river sand mining: A case from the river catchments of Vembanad lake, Southwest coast of India. Environ. Geol. 2008, 54, 879–889. [Google Scholar] [CrossRef]
- Yen, T.P.; Rohasliney, H. Status of water quality subject to sand mining in the Kelantan River, Kelantan. Trop. Life Sci. Res. 2013, 24, 19–34. [Google Scholar]
- Leonhardt, F. Estructuras de Hormigón Armado, 3rd ed.; El Ateneo: Madrid, Spain, 1986. [Google Scholar]
- Ruiz, J.L.R.; Hernández, C.G.C.; López, R.C.; Hernández, R.N. Diseño de un sistema autoconstructivo a base de tapial y bahareque de bajo costo e impacto ambiental para una vivienda. Tópicos Investig. Cienc. Tierra Mater. 2020, 7, 68–78. [Google Scholar] [CrossRef]
- Kore, S.D. Sustainable Utilization of Plastic Waste in Concrete Mixes—A Review. J. Build. Mater. Struct. 2019, 5, 212–217. [Google Scholar] [CrossRef]
- Thorneycroft, J.; Orr, J.; Savoikar, P.; Ball, R.J. Performance of structural concrete with recycled plastic waste as a partial replacement for sand. Constr. Build. Mater 2017, 161, 63–69. [Google Scholar] [CrossRef]
- Naciones Unidas. Available online: https://www.un.org/sustainabledevelopment/es/objetivos-de-desarrollo-sostenible/# (accessed on 19 March 2024).
- Naciones Unidad México. Available online: https://mexico.un.org/es/245562-plan-de-las-naciones-unidas-promete-enormes-reducciones-de-emisiones-en-el-sector-de-la (accessed on 20 March 2024).
- Shi, Y.; Long, G.; Zen, X.; Xie, Y.; Shang, T. Design of binder system of eco-efficient UHPC based on physical packing and chemical effect optimization. Constr. Build. Mater. 2020, 274, 121382. [Google Scholar] [CrossRef]
- Gu, C.; Ye, G.; Sun, W. Ultrahigh performance concrete-properties, applications and perspectives. Sci. China Technol. Sci. 2015, 58, 587–599. [Google Scholar] [CrossRef]
- Zeyad, A.M.; Hakeem, I.Y.; Amin, M.; Tayeh, B.A.; Agwa, I.S. Effect of aggregate and fibre types on ultra-high-performance concrete designed for radiation shielding. J. Build. Eng. 2022, 58, 104960. [Google Scholar] [CrossRef]
- Amin, M.; Hakeem, I.Y.; Zeyad, A.M.; Tayeh, B.A.; Maglad, A.M.; Agwa, I.S. Influence of recycled aggregates and carbon nanofibres on properties of ultra-high- performance concrete under elevated temperatures. Case Stud. Constr. Mater. 2022, 16, e01063. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Subrmani, T.; Pugal, V.K. Experimental study on plastic waste as a coarse aggregate for structural concrete. Int. J. Appl. Innov. Eng. Manag. 2015, 4, 144–152. [Google Scholar]
- Habib, M.Z.; Alom, M.M.; Hoque, M.M. Concrete production using recycled waste plastic as aggregate. J. Civ. Eng. 2017, 45, 11–17. [Google Scholar]
- Patil, B.; Agrawal, P.S.D. An experimental study on partial replacement of coarse aggregate by using recycled plastic aggregate on strength of concrete. Int. J. Eng. Technol. Res. 2018, 8, 2454–4698. [Google Scholar]
- Manjunath, B.T.A. Partial replacement of E-plastic Waste as Coarse-aggregate in Concrete. Procedia Environ. Sci. 2016, 35, 731–739. [Google Scholar] [CrossRef]
- Jaivignesh, B.; Sofi, A. Study on mechanical properties of concrete using plastic waste as an aggregate. IOP Conf. Ser. Earth Environ. Sci. 2017, 80, 012016. [Google Scholar] [CrossRef]
- ASTM C33/C33M-18; Standard Specification for Concrete Aggregates. ASTM: West Conshohocken, PA, USA, 2018.
- ASTM C39/C39M-18; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM-C-293-02; Standard Test Method for Strength of Concrete (Using Simple Beam with Center Point Loading). ASTM International: West Conshohocken, PA, USA, 2004.
- Kirk-Othmer. Enciclopedia of Chemical Technology, 3rd ed.; Wiley: New York, NY, USA, 1978. [Google Scholar]
- Esparza-Juárez, M.E. Evaluación del Efecto de Parámetros de Proceso Sobre las Propiedades de Piezas de PET y PBT Moldeadas por Inyección. Master’s Thesis, Centro de Investigación en Química Aplicada, Saltillo, Mexico, 2005. [Google Scholar]
- Jiří, H.; Petr, S.; Petr, S. Volume relaxation in amorphous and semicrystalline PET. J. Mater. Sci. 2007, 42, 3724–3731. [Google Scholar] [CrossRef]
- Rajabipour, F.; Giannini, E.; Dunant, C.; Ideker, J.H.; Thomas, M.D. Alkali-Silica reaction: Current understanding of the reaction mechanisms and the knowledge gaps. Cem. Concr. Res. 2015, 76, 130–146. [Google Scholar] [CrossRef]
- Thomas, M. The effect of supplementary cementing materials on alkali-silica reaction: A review. Cem. Concr. Res. 2011, 41, 1224–1231. [Google Scholar] [CrossRef]
- Calavera, J. Manual de Ferralla, 3rd ed.; Intemac: Torrejón de Ardoz, Spain, 2003; Chapter 2. [Google Scholar]
- Meseguer, A.G. Hormigón Armado, 15th ed.; Gustavo Gili: Barcelona, Spain, 2010. [Google Scholar]
- Russo, C. Obras Hormigón Armado, 1st ed.; Gustavo Gili: Barcelona, Spain, 1942. [Google Scholar]
- Ismail, Z.Z.; AL-Hashmi, E.A. Use of waste plastic in concrete mixture as aggregate replacement. Waste Manag. 2008, 28, 2041–2047. [Google Scholar] [CrossRef]
- Rai, B.; Rushad, S.T.; Kr, B.; Duggal, S.K. Study of waste plastic mix concrete with plasticizer. ISRN Civil. Eng. 2012, 2012, 469272. [Google Scholar] [CrossRef]
- Mejía, C.E.; Vásquez, C.; Peña, D.Y.; Estupiñán, H. Determinación de la despasivación en varillas de acero de refuerzo en solución poro de agua de mar por medio de técnicas electroquímicas. Prospectiva 2011, 9, 13–20. [Google Scholar]
- Castellote, M.; Andrade, C.; Alonso, C. Accelerated simultaneous determination of the chloride depassivation threshold and of the nonstationary diffusion coefficient values. Corros. Sci. 2002, 44, 2409–2424. [Google Scholar] [CrossRef]
- Alonso, C.; Castellote, M.; Andrade, C. Chloride threshold dependence of pitting potential of reinforcements. Electrochim. Acta 2002, 47, 3469–3481. [Google Scholar] [CrossRef]

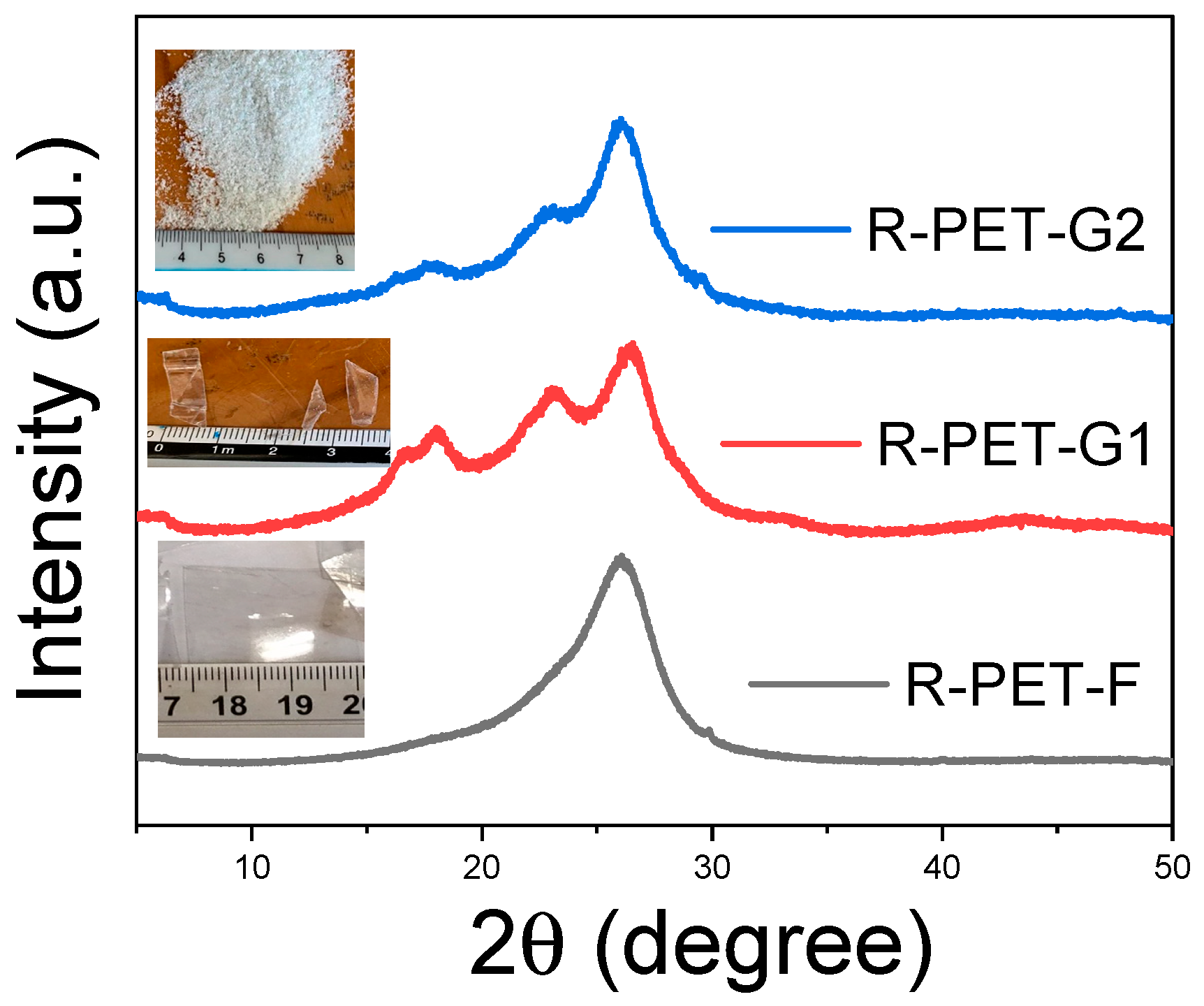


| Miller Index | 2θ Location |
|---|---|
| 011 | 16.43 |
| 010 | 17.54 |
| 111 | 21.56 |
| 110 | 22.85 |
| 103 | 25.28 |
| 100 | 26.12 |
| 111 | 27.77–28.46 |
| 101 | 32.51 |
| Sample | Mixture Component kg/m3 | ||||
|---|---|---|---|---|---|
| Cement | Water | Coarse | Fine | R-PET | |
| C-R | 395 | 180 | 952.8 | 861.3 | 0 |
| C-P2.5 | 395 | 180 | 952.8 | 839.76 | 21.53 |
| C-P5 | 395 | 180 | 952.8 | 818.23 | 43.06 |
| C-P10 | 395 | 180 | 952.8 | 775.17 | 86.13 |
| Sample | Curing Days | |||
|---|---|---|---|---|
| 7 | 14 | 28 | 180 | |
| C-R | 49.4 | 55.4 | 59.8 | 60.8 |
| C-P2.5 | 43 | 52 | 54.9 | 62.1 |
| C-P5 | 38.4 | 51.5 | 60.2 | 72.0 |
| C-P10 | 34.2 | 38.6 | 45.8 | 56.9 |
| Samples | Curing Days | |||
|---|---|---|---|---|
| 7 | 14 | 28 | 180 | |
| C-R | 3.5 | 4.4 | 4.5 | 4.6 |
| C-P2.5 | 3.1 | 4.0 | 4.3 | 5.6 |
| C-P5 | 3.6 | 4.0 | 4.4 | 5.8 |
| C-P10 | 3.5 | 4.1 | 5.0 | 5.4 |
| Sample | 6 Months E (mV) | 12 Months E (mV) |
|---|---|---|
| C-R | −433.2 | −325.3 |
| C-P2.5 | −304.7 | −439.58 |
| C-P5 | −271.2 | −546.65 |
| C-P10 | −248.8 | −486.08 |
| Specimen | Potential (mV) | icorr mA/cm2 | Vcorr mmy | Vcorr mpy |
|---|---|---|---|---|
| 6-month evaluation | ||||
| C-R | −552 | 1.28 × 10−2 | 0.1483 | 5.8400 |
| C-P2.5 | −413 | 3.89 × 10−3 | 0.0450 | 1.7748 |
| C-P5 | −398 | 3.31 × 10−3 | 0.0383 | 1.5101 |
| C-P10 | −382 | 8.91 × 10−4 | 0.0103 | 0.4065 |
| 12-month evaluation | ||||
| C-R | −348 | 3.98 × 10−3 | 0.0461 | 1.8158 |
| C-P2.5 | −506 | 1.9 × 10−3 | 0.0220 | 0.8668 |
| C-P5 | −615 | 7.58 × 10−3 | 0.0087 | 0.3458 |
| C-P10 | −389 | 1.04 × 10−3 | 0.0120 | 0.4745 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espindola-Flores, A.C.; Luna-Jimenez, M.A.; Onofre-Bustamante, E.; Morales-Cepeda, A.B. Study of the Mechanical and Electrochemical Performance of Structural Concrete Incorporating Recycled Polyethylene Terephthalate as a Partial Fine Aggregate Replacement. Recycling 2024, 9, 51. https://doi.org/10.3390/recycling9030051
Espindola-Flores AC, Luna-Jimenez MA, Onofre-Bustamante E, Morales-Cepeda AB. Study of the Mechanical and Electrochemical Performance of Structural Concrete Incorporating Recycled Polyethylene Terephthalate as a Partial Fine Aggregate Replacement. Recycling. 2024; 9(3):51. https://doi.org/10.3390/recycling9030051
Chicago/Turabian StyleEspindola-Flores, Ana Cecilia, Michelle Alejandra Luna-Jimenez, Edgar Onofre-Bustamante, and Ana Beatriz Morales-Cepeda. 2024. "Study of the Mechanical and Electrochemical Performance of Structural Concrete Incorporating Recycled Polyethylene Terephthalate as a Partial Fine Aggregate Replacement" Recycling 9, no. 3: 51. https://doi.org/10.3390/recycling9030051
APA StyleEspindola-Flores, A. C., Luna-Jimenez, M. A., Onofre-Bustamante, E., & Morales-Cepeda, A. B. (2024). Study of the Mechanical and Electrochemical Performance of Structural Concrete Incorporating Recycled Polyethylene Terephthalate as a Partial Fine Aggregate Replacement. Recycling, 9(3), 51. https://doi.org/10.3390/recycling9030051







