Abstract
Food waste and by-products are intricately linked to sustainable food production, as reducing waste can play a significant role in achieving a more sustainable and efficient food system. Sustainable utilization and recovery of by-products can significantly contribute by creating strategies that can lead to cost savings and increased efficiency across the food supply chain. Worldwide, more than 40% of whey from cheese production is discarded, resulting in the loss of valuable nutrients and potentially polluting the environment. Effective use of whey reduces environmental impact and enhances manufacturing sustainability. Thus, a circular approach to food waste management in the dairy industry supports sustainability goals and creates opportunities for innovation. Whey contains most of the soluble components of milk, including a large number of serum proteins and all the essential amino acids, making it suitable for producing beverages with high nutritional value. This study aims to produce whey-based beverages with different additions to obtain dairy products with high nutritional value. Three different ingredients, sea buckthorn, ginger, and cinnamon, were chosen for their numerous health benefits to the consumer. Six samples were prepared utilizing both unmodified and deproteinized whey in a 75% proportion, with the addition of 25% sea buckthorn juice, 0.75% ginger juice, and 0.2% cinnamon powder. The resultant samples were packaged in 200 mL bottles and maintained at a controlled temperature of 6 °C to ensure optimal preservation. Given the paramount importance of consumer acceptability in novel beverage development, a comprehensive evaluation was conducted to assess the sensory properties of the formulated beverages. In addition, physico-chemical properties and their evolution over 14 days of storage were examined. The sample containing whey, sea buckthorn juice, ginger juice, and cinnamon powder received the highest marks from the tasters. The values of the physico-chemical parameters varied depending on the type of whey used and the storage period. Thus, a pH of approximately 5 and an acidity between 30 and 80 °T were recorded. The average lactose content was 4%, the average protein content was 2.5%, and the total soluble solids content was 11.5 °Brix. The beverages developed in this study represent viable alternatives for diversifying food production through sustainable, environmentally friendly technological variants. By applying circular economy principles, these products contribute to reducing food waste in the dairy industry.
1. Introduction
According to the Food and Agriculture Organization (FAO), 33% of global food production is lost during the supply and consumption processes [1]. Because food waste affects the environment and resources, it is a big concern in the food sector [2]. By adopting practices related to the circular economy, regarding the reduction, reuse, and recycling of food waste [3], companies can transform these by-products into valuable resources [4]. Therefore, a responsible approach to food waste management is essential for promoting sustainable food production and environmental conservation [5,6]. Sustainable utilization and recovery of by-products can significantly contribute by creating strategies [7] that can lead to cost savings and increased efficiency across the food supply chain [8,9].
In the dairy industry, the responsible management of food waste is of particular importance in the context of the circular economy [10]. This industry generates a wide range of waste, with whey from cheese production being a notable example [11]. Recycling and reusing whey in other food products or other industries, as well as turning it into valuable resources, are essential to reduce the impact on the environment and to promote sustainability throughout the production chain [12]. This approach creates opportunities for innovation [13] and industry-wide growth [14]. However, the circular economy encourages the valorization of these materials, transforming them into valuable products with higher economic and nutritional value [15,16]. Whey, for example, is rich in proteins and can be processed into whey protein concentrate or isolate, which is widely used in the food and beverage industry, particularly in sports nutrition. Similarly, lactose, a by-product of whey, can be purified and used in the pharmaceutical industry.
Whey is a by-product resulting from the manufacture of cheeses, as a result of coagulation with rennet, with other enzymes, or through natural fermentation. Its appearance is a greenish-yellowish liquid. Because whey removal raises environmental concerns, whey usage is a beneficial approach that deserves more consideration [12]. Worldwide, more than 40% of whey from cheese production is discarded, leading to the loss of valuable nutrients and potentially polluting the environment [13]. Whey disposal is a complex environmental problem due to the high organic load (biological oxygen demand: 35–45 kg/m3; chemical oxygen demand: 60–80 kg/m3), fat content, salinity, and other suspended solids present in whey [17]. Whey plays an important role in promoting the circular economy, as this by-product of the dairy production process can be valorized and turned into a valuable resource in other food industries [18]. Through its efficient valorization, it is possible to obtain high-quality biosurfactants that can be used in the production of fermented milk. Also, the biosurfactants obtained show a protective effect on the lactobacilli population during storage, contributing to the increased shelf-life of dairy products [19]. Integrating them into food production processes helps reduce the amount of food waste, contributing to sustainability goals and saving resources [20]. Additionally, whey proteins have several qualities that make them invaluable in the development of functional foods [12].
The raw material, type, and technology used to produce cheese influence the chemical composition of the resulting whey [21]. The main component is lactose. The proteins found in the composition are albumin (α-lactalbumin, serum albumin [22]), globulin (β-lactoglobulins, immunoglobulins [22]), and a small proportion of casein [23,24]. Whey protein is also called fast protein because it reaches the muscles quickly [25,26]. The mineral salts (calcium, magnesium, phosphorus [22]) and vitamins in milk are found in whey, increasing its nutritional value [23,24]. Vitamins present in whey are vitamin C (ascorbic acid), vitamin B2 (riboflavin [22]), vitamin B6 (adermin), vitamin B1(thiamine [22]), and vitamin H (p-amino benzoic acid) [27].
By reusing whey, the dairy industry reduces its environmental footprint. Whey, when improperly disposed of, can contribute to water pollution and greenhouse gas emissions. However, when it is processed and used in products such as animal feed [28], biofuels [29,30], or biodegradable plastics [31], the environmental impact is significantly reduced [12]. Furthermore, using whey in these ways helps to reduce the demand for other resources, such as petroleum for plastics or land for animal feed production, thereby conserving natural resources.
In the food industry, whey and its protein concentrate serve as valuable ingredients, prized for their foaming and emulsifying properties [32]. In addition, whey protein provides an excellent way to fortify foods, increasing the nutritional quality of cheese, dairy products, bakery products, etc. [33,34,35].
Whey can be used for ethanol production because it has a high lactose content (74%) and other nutrients necessary for microbial growth (protein 10–12%, minerals 8%, and fat 3%) [36]. Studies have shown that whey possesses buffering capacity due to protein, and deproteinized whey is widely used to avoid protein interference [17]. Deproteinized whey also has a higher lipid content, while untreated whey has a high cell biomass [37]. After deproteinization, whey contains 4.8% lactose, 0.66% ash, 0.46% fat, and 0.40% protein adjusted to pH 6.4 [38].
Sea buckthorn (Hippophae rhamnoides L.), also known in some parts as river sea buckthorn is a very branched and thorny shrub that grows in Romania, starting from the coastal sands and gravels to the mountainous region, sometimes forming rather extensive groves and bushes. Vitamin C content in sea buckthorn is ten times higher than in citrus fruits and double that of rose hips. Over 400–800 mg is present in ripe fruits for every 100 g of fresh juice [39]. The fruit of the sea buckthorn plant is high in bioactive compounds such as flavonoids, steroids, pro-anthocyanidins, triterpenes, tannins, and 5-hydroxytryptamine, as well as nutrients like proteins, amino acids, vitamins, trace elements, and polysaccharides [40]. It has anti-inflammatory, antioxidant, antimicrobial, anticancer, and immune system-stimulating properties due to being rich in active substances [41].
Ginger (Zingiber officinale) is the name given to a herbaceous plant from tropical regions, with an aromatic rhizome rich in essential oils. It is a perennial species that needs a high and constant temperature as well as permanent humidity to develop properly [42]. Numerous phytochemicals, including essential oils and phenolic compounds, as well as carbohydrates, amino acids and proteins, vitamins, minerals, fatty acids, and lipids, are all present in ginger. Ginger also offers a host of other advantageous qualities, such as anticancer properties [43], anti-inflammatory properties [44], immunomodulatory properties [45], antioxidant properties [46], antibacterial properties [47], and antidiabetic properties [48,49].
Cinnamon (Cinnamomum verum) is a spice that is derived from the inner bark of many Cinnamomum tree species. In many different types of food, including sweet and savory dishes, breakfast cereals, snacks, bagels, teas, hot chocolate, and traditional foods, cinnamon is mostly employed as an aromatic spice and flavoring addition. The primary ingredient and essential oil of cinnamon, cinnamaldehyde, as well as a variety of additional ingredients, such as eugenol, are responsible for the flavor and aroma of cinnamon [50,51]. Traditional medicine uses cinnamon bark to treat a variety of conditions, such as respiratory tract infections, diabetes, and digestive issues [52]. However, the medicinal qualities of cinnamon have been researched over time. Studies show that cinnamon possesses antioxidant activities [53], antibacterial properties [54], and antimicrobial properties [55,56].
Whey beverages with fruit additions have been studied a lot lately, because they can offer an alternative to classic soft beverages, and more than that, they can meet consumer expectations for quality and healthy food choices due to the functional and nutritional characteristics that whey and fruit possess [57]. There are whey soft beverages on the market, non-alcoholic beverages made from deproteinized whey (Lactone, Milone), alcoholic beverages made from deproteinized whey (a beer made from whey, wine made from whey), and beverages made from deproteinized whey with added cultures: Saccharomyces cerevisiae (sherry), kefir culture (Lactrone), Str. Lactis, Str. Diacetylactis, Str. Fragellis (diet drink) [58]. These types of whey beverages are known and consumed especially abroad; in Romania, they are known to a smaller group of consumers. Other researchers have studied the development of whey beverages by mixing them with carrot juice and other ingredients (sugar, ginger, etc.) [59]. Beverages based on whey–pineapple [60], whey–grape juice [61], and whey–orange juice [62] can also be formulated.
The purpose of this research is to make soft beverages from whey and deproteinized whey with different additions of fruits and spices. To achieve this purpose, it was decided to add in different amounts of three ingredients that bring high nutritional value, namely sea buckthorn, ginger, and cinnamon. Six samples were prepared from whey and deproteinized whey in a proportion of 75% with the addition of 25% sea buckthorn juice, 0.75% ginger juice, and 0.2% cinnamon powder. The samples obtained were packaged in 200 mL bottles and stored at a temperature of 6 °C. To test the degree of acceptability of the obtained samples, a sensory analysis was carried out during 14 days of storage. The obtained results were statistically processed using a non-numerical method. Additionally, the influence of the additives on physico-chemical properties and their evolution during storage was examined by determining acidity, pH, lactose content, protein titer, and total solids content (TSS).
2. Results and Discussion
2.1. Sensory Analysis
The first step involved calculating the negation of the importance levels of the criteria using Formula (1). The results of these calculations, representing the denial of criteria importance, are presented in Table 1.

Table 1.
Negating the importance level of the criteria.
All the sensory assessments for each person are in Table 2.

Table 2.
The sensory assessment for each person.
2.1.1. Criteria of Aggregation
The aggregation criteria for each alternative were calculated using Formula (2). The approval criteria for each alternative on each day of storage can be seen in Table 3.

Table 3.
The approval criteria for each alternative.
2.1.2. Person Aggregation
The value weights were calculated using Formula (3). The following results were obtained: Q1 = DM; Q2 = NT; Q3 = LM; Q4 = LV; Q5 = LV.
Formula (4) was used to calculate the person aggregation for each alternative. The results can be seen in Table 4.

Table 4.
The person aggregation for each alternative.
According to research in the field [63] of sensory and textural analysis of whey, it has been shown that whey as is has a disagreeable taste and texture. The relatively high lactose–glucose ratio and excessive acidity are factors that influence the development of methods to improve sensory attributes. As can be seen in Table 4, Alternative 5 yielded the most favorable results, indicating that the most appreciated beverage is composed of normal-composition whey, sea buckthorn, ginger, and cinnamon. This formulation consistently scored “Like moderately” on all three days of analysis. Samples with deproteinized whey generally received lower ratings compared to those with normal-composition whey, which implies that the protein content influences the sensory analysis of the samples. The samples with whey and deproteinized whey with sea buckthorn and ginger obtained a similar acceptance rate to those with whey and deproteinized whey with sea buckthorn, referring to the same product category, indicating that the addition of ginger does not influence the tasters’ perception of such products.
The combination of ginger and cinnamon is highly regarded by consumers and has been a prized mixture since medieval France [64]. This can also be seen in the case of the two beverages containing this mixture, which are more appreciated by tasters compared to the others. The positive impact of whey preparations was also highlighted by Królczyk et al., who demonstrated that whey enhances the sensory properties of ice cream by providing a mild, sweet, and milky flavor. An important benefit of these types of preparations is that they are compatible with flavoring additives [65].
In a study by Reis et al., it was concluded that the most popular whey-based drink with added mangaba pulp was the one containing the highest amount of whey (50% of milk and 50% of whey). Thus, it was concluded that the use of whey in such beverages is an excellent alternative because it is accessible and inexpensive and provides mineral and protein intake [66]. Tkachenko et al. demonstrated that by adding 0.2% citric acid, the organoleptic properties of a drink based on cheese whey, marigold flower extract, and a strawberry filler were improved because at that content, the product scored highest for taste and aroma, while a higher amount of citric acid gave an excessive acidity feel [67]. In 2019, Schoina et al. analyzed the influence of Pistacia terebinthus resin on whey-based beverages from a sensory point of view, observing a significant improvement in flavor for samples containing the resin compared to a control. This result underlines the potential for valorizing whey as a by-product through the incorporation of aromatic resins [68]. In another investigation, Larinov et al. found that the organoleptic qualities of their product were significantly impacted when more mint was added to 1 L of whey. As a result, the sample with 1 g of mint and 1 L of whey produced the greatest results because it tasted refreshingly sour and sweet and had a mild scent. The flavor and fragrance of the sample containing 2 g of mint to 1 L of whey were equally nice, but the sample containing 3 g of mint to 1 L of whey had an overpowering stench [69].
2.2. Physico-Chemical Analyses
2.2.1. pH
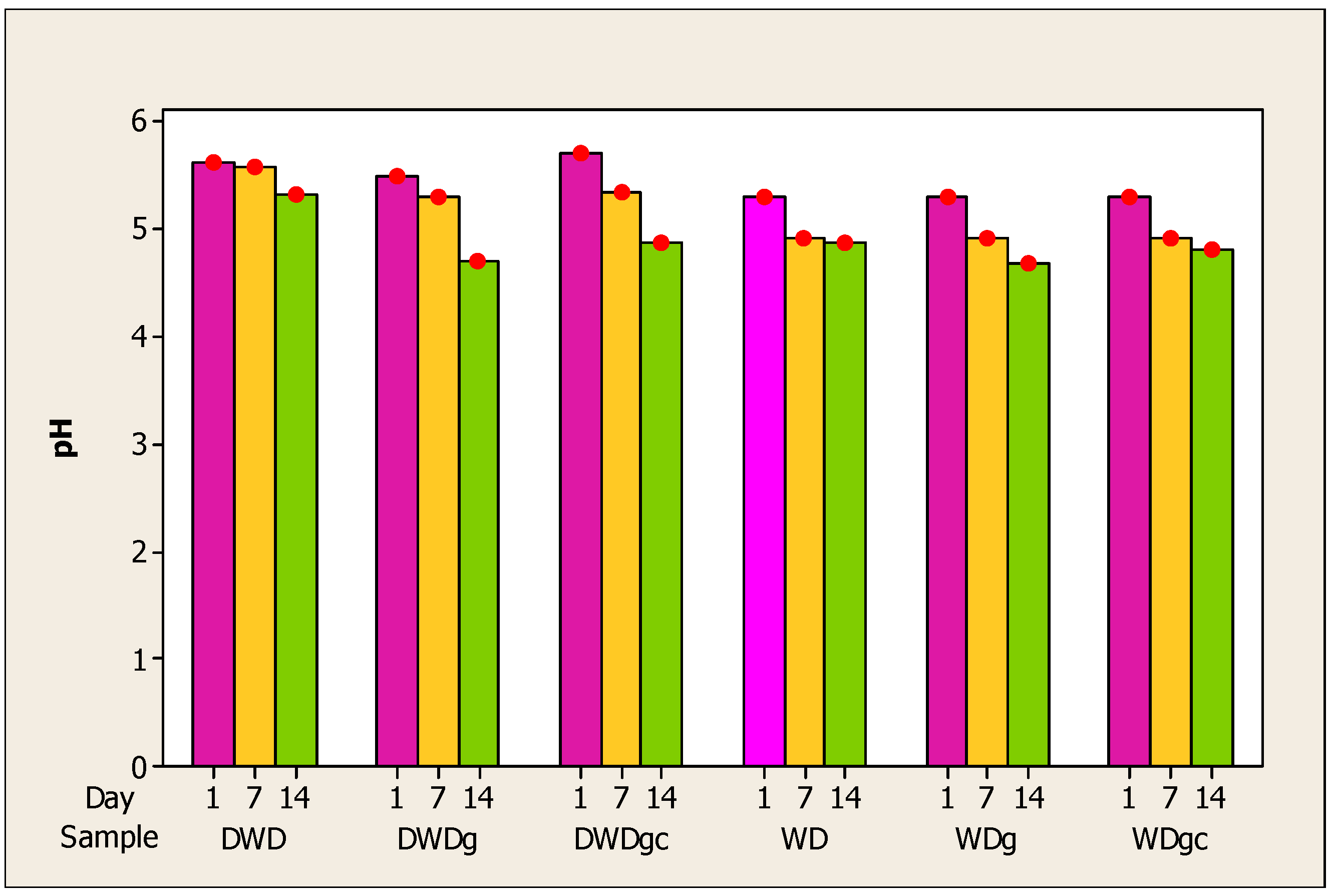
The pH evolution of the six beverages during the storage period is depicted in Figure 1. Monitoring pH and acidity is essential for maintaining viable cells during a fermentation process [66]. A decrease in pH was recorded during the storage period for all samples. The highest value for pH was recorded for the DWD sample followed by the deproteinized whey samples, DWDgc and then WD. Regarding the deproteinized whey drink samples, on the first day of analysis and the seventh, approximately equal values were obtained for the three types of samples. On the fourteenth day, there was a greater decrease for the WDg sample, which obtained the lowest pH value on this day. Thus, it can be concluded that the decrease is statistically significant. One plausible explanation for the observed pH reduction concomitant with increasing acidity is the conversion of lactose to lactic acid [70]. In addition, the acidic nature of the three additions may also contribute to the increase in acidity during storage.
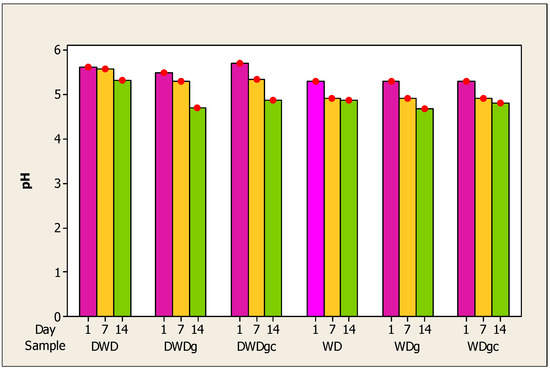
Figure 1.
Evolution of pH in whey samples during 14 days of storage. Note: Red dots = mean (n = 5).
While whey beverages were being stored, other writers also noticed a drop in pH. Mango–whey beverages achieved a decrease in pH with an increasing percentage of mango juice in the drink [57]. Ahmed et al. reported a decrease in pH and an increase in acidity for mango–whey beverages during 25-day storage [71]. Other authors also observed a decrease in pH and an increase in acidity in stored carrot juice-added whey beverages [59]. Research by Sady et al. showed that during the 28 days of storage, the pH of samples of whey and fruit drinks decreased slightly, with the highest values for those with added apple and orange juice [72].
2.2.2. Titratable Acidity
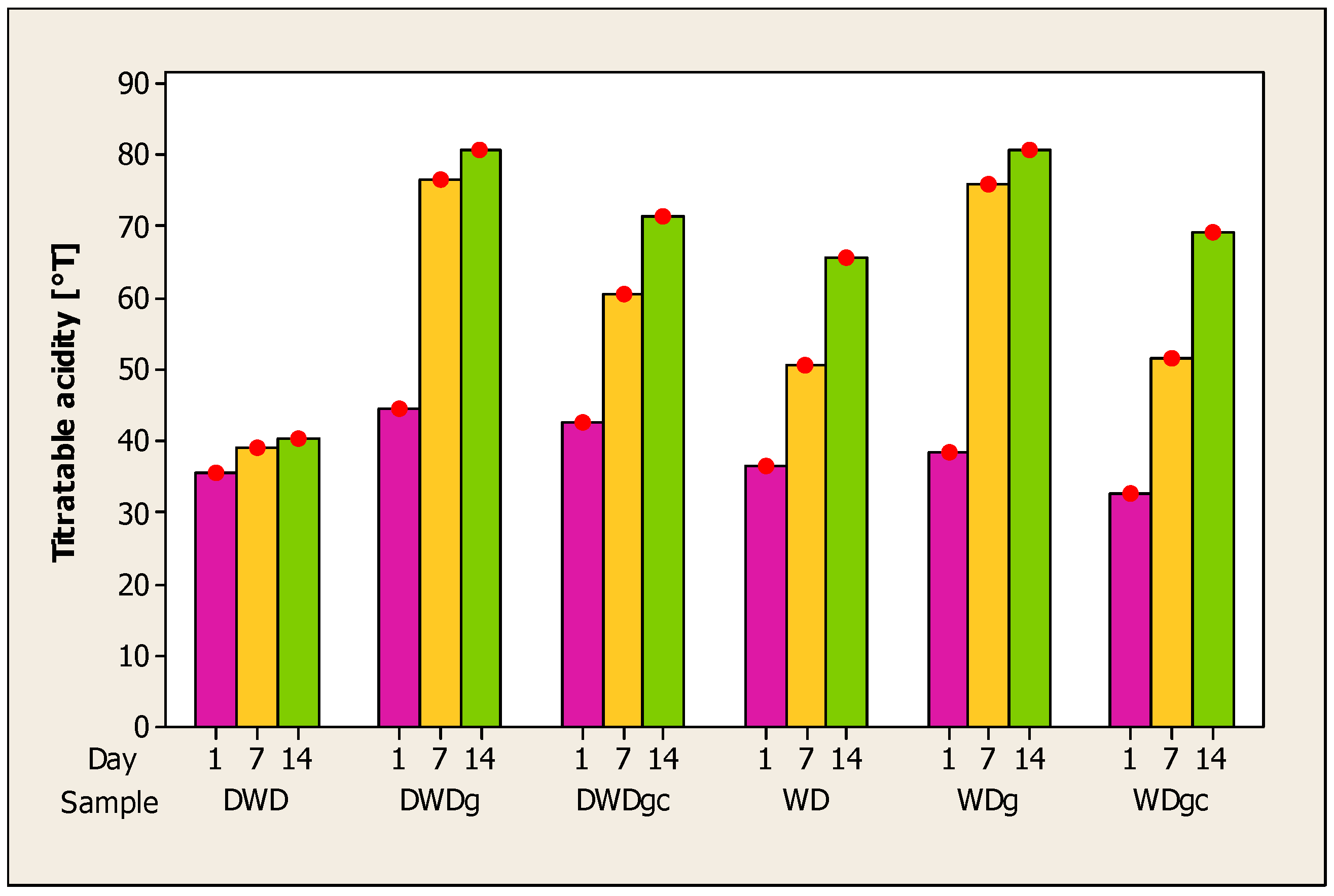
The evolution of acidity in beverages made from whey and deproteinized whey with the addition of sea buckthorn, ginger, and cinnamon is depicted in Figure 2. An increase in the value of acidity can be observed during the storage period of the samples; the results obtained are inversely proportional to those obtained for pH. Acidity determines the flavor of the product and is influenced by the additional ingredients added [72].
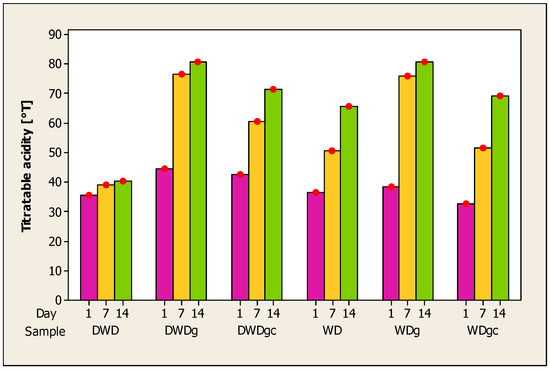
Figure 2.
Evolution of titratable acidity in whey samples during 14 days of storage. Note: Red dots = mean (n = 5).
An increase in the acidity of whey beverages has also been reported by other authors for mango and whey beverages [71], whey and mint beverages [73], and whey and orange juice [62]. An increase in acidity during 60 days of storage of whey drink samples with added fructooligosaccharides was reported by Yasmin et al. [70].
The acidity of whey products is achieved by lactic acid fermentation [74], and the preservation of lactic acid levels can result from the fermentation process’s decrease in lactose [66]. An inversely proportional reduction in pH and an increase in acidity may result from the breakdown of lactose and proteins during storage, which produces amino acids and organic acids [71]. The higher values obtained by deproteinized whey beverages may be because these products have a lower content of protein available to be broken down.
2.2.3. Lactose Content
The influence of the storage period on the lactose content of the whey beverage samples is presented in Table 5. Whey retains almost all the macro- and microelements from milk, particularly lactose and water-soluble vitamins [67]. A consistent decrease in lactose content was observed for all six products during storage, likely due to consumption by live probiotic microorganisms [68]. The lowest values for lactose were obtained for the samples with sea buckthorn and ginger (DWDg and WDg), both with normal-composition whey and with deproteinized whey, on analysis days seven and 14. Because lactose does not greatly contribute to the appropriate sweetness, goods with high whey content may have a high calorie content. This means that additional sugars must be added to reach an acceptable flavor profile [74]. Schoina et al. also validated the decrease in lactose content throughout 30 days of storage in a study on whey beverages containing probiotic cells immobilized on Pistacia terebinthus resin [68].

Table 5.
Evolution of lactose, protein titer, and TSS in whey samples during the storage period.
2.2.4. Protein Titer
Table 5 also illustrates the influence of the storage period on the protein titer of the six whey drink formulations. Whey is a valuable source of high-quality protein [74], minerals, vitamins, and lactose [68,72]. It can be seen that for the WD and DWD samples, the variation in protein titer differs due to the degradation of protein substances, which is more visible in the case of deproteinized whey. These products go through a stagnation period on the 7th and 14th day of storage because their hydrolysis is very slow.
The addition of ginger in these products influences the values obtained for protein titer. It is observed that the DWDg sample shows a lower value (2.86 ± 0.011%) due to the addition of ginger compared to the DWD product (2.93 ± 0.013%). For untreated whey samples, the effect of ginger is evident with WDg at 3.01 ± 0.011% and WD at 3.26 ± 0.010%. The stagnation period on days 7 and 14 of storage is not recorded for ginger samples. Thus, we can conclude that it influences protein hydrolysis.
The lowest protein titers were recorded for cinnamon-containing samples. The sample containing deproteinized whey has 2.72 ± 0.013% protein and the sample with untreated whey has 2.90 ± 0.008% protein. As with the ginger samples, the addition of cinnamon influences protein hydrolysis. This is because between day 7 and day 14 of storage, the protein content does not stagnate as in the DWD and WD samples. Whey proteins promote plasma amino acid concentrations and are readily digested. Numerous whey amino acids [67] function as direct secretors of insulin [70]. The research by Yasmin et al. on whey drink samples containing fructooligosaccharides likewise comes to the conclusion that the protein content decreases after storage [70]. In the study by de Matos Reis et al., it was shown that the 70% milk and 30% whey sample had a higher content than the 50% milk and 50% whey sample, but it was concluded that both variants developed provided an important protein and mineral intake [66].
2.2.5. Total Soluble Solids Content (TSS)
Table 5 illustrates the influence of the storage period on the total soluble solids content. The data reveal that samples with deproteinized whey obtained lower values compared to the samples with untreated whey. As for the addition of ginger, it slightly raised the TSS content, and the addition of cinnamon increased the TSS content even more. Whey has a comparatively high total solids content, which is further increased by the added sugar in whey-based beverages [74].
The samples’ TSS content drops throughout storage. Compared to the specialized literature, the same things were observed by other authors in whey and fruit juice-based beverages [57,59,71]. A slight decrease over time in total soluble solids content during 60 days of storage was evidenced by Yasmin et al. in samples of whey drinks with fructooligosaccharides [70].
3. Materials and Methods
3.1. Materials
The research was carried out in the laboratory of the CCBIA (Research Center for Biotechnology and Food Engineering) research center within the faculty, by using whey purchased from a local cheese producer using organic cow’s milk from Sibiu County. The fresh ginger and powdered cinnamon were purchased from a company specialized in the production and marketing of spices, and the sea buckthorn fruits were from a producer and processor of these fruits, well known on the Romanian market.
3.1.1. Preparation of Sea Buckthorn Juice
The selected fruits were washed using warm water, and the fruit juice was extracted using a cold-pressing juicer. The obtained juice was filtered using filter paper and stored at refrigeration temperature until use.
3.1.2. Preparation of Ginger Extract
Fresh ginger was washed and peeled. Then, using a cold pressing juicer, it was chopped into little pieces to extract the juice. The obtained juice was filtered using filter paper and stored at refrigeration temperature until use.
3.1.3. Preparation of Whey Beverages
The whey beverages were made as follows: the whey was filtered and heated at 50 °C for 5 min, then it was cooled and mixed with the ingredients according to Table 6. The packaging was made in 200 mL glass bottles with metal caps that were properly labeled.

Table 6.
List of ingredients added to whey-based beverages.
A heat treatment method was used to deproteinize whey. The whey was heated to a temperature of 75 °C for 10 min. The precipitated proteins were separated by centrifugation followed by filtration through filter paper. The deproteinized whey was cooled to 25 °C and the rest of the ingredients were added. Properly packaged and labeled bottles were stored at 6 °C for 14 days.
3.2. Methods
3.2.1. Sensory Analysis
A group of five amateur tasters conducted the tasting, which took place on the first, seventh, and fourteenth days after storage. A non-numerical multi-criteria multi-personal agreement method as suggested by Fadhil et al. was employed to conduct the sensory analysis [75].
The evaluated characteristics were the appearance of the drink in natural light, consistency when pouring from one glass to another, the smell, the color, and the taste. Table 7 shows the evaluation scale used, and in Table 8, the level of importance of the criteria based on the scale is illustrated.

Table 7.
Linguistic assessment scale [75].

Table 8.
Criteria importance level [75].
Formula (1) was used to calculate the negation of the importance level of the criteria [75].
- Neg (Wk)—score negation of criteria k;
- k—index;
- q—scale amount.
The process of aggregation of the criteria relied on Formula (2) [75].
- Vij—the score of alternative i by person j;
- Vij (ak)—the score of alternative i by person j on criteria k;
- k—1, 2, …., m.
We determined the score using Formula (3) [75].
Qk = Int [ 1 + (k ∗ (q − 1)/r)]
- Qk—the score k;
- Int—integer;
- R—the number of assessors.
The aggregation process for a person (assessor) was calculated using Formula (4) [75].
- Vi—the total value for alternative i;
- Qj—score j;
- j—1, 2, …, m;
- bj—order from the biggest alternative score i from alternative score j.
3.2.2. Physico-Chemical Analyses
The pH was monitored using the ORION 2 STAR digital pH meter. The pH meter was calibrated and then, it was introduced into 30 mL of homogenized sample and the pH was recorded [76].
Titratable acidity: By titrating 10 mL of sample combined with 10 mL of distilled water titrated with 0.1 n NaOH in the presence of phenolphthalein until the color turned pale pink and remained that way for 30 s, titratable acidity was ascertained [77]. Formula (5) was used to compute the acidity, which is measured in degrees Thörner.
Titratable Acidity [°T] = (V ∗ 10)/m
- V = volume of 0,1 n sodium hydroxide solution, used for titration, in mL;
- 10 = volume of sample taken;
- m = mass of the analyzed sample, in g [77].
Determination of lactose: Following the deproteinization process, the filtrate was polarized, and Formula 6 was used to calculate the proportion of lactose in the examined sample based on the rotation measurement [78].
- a = the angle read at the polarimeter;
- = lactose-specific rotation, equal to +52.53;
- 2 = wavelength of the polarimetric tube, dm [78].
Protein titer: Briefly, 25 cm2 of sample to be analyzed, 0.25 cm2 of phenolphthalein solution, and 1 cm2 of sodium hydroxide solution were mixed using a burette with a division value of 0.05 cm2 until a color identical to that of the comparison solution was obtained. The comparison solution was obtained by mixing 25 cm2 of the sample with 1 cm2 of potassium oxalate solution and 0.5 cm2 of cobalt sulfate solution. A 5 cm2 amount of formic aldehyde was added to the sample to be analyzed and thus neutralized, and after 1 min, it was titrated again with a sodium hydroxide solution until the coloration was identical to that of the comparison solution [78].
The volume of the 0.143 n sodium hydroxide solution, in cm2, used in the second titration represents the protein titer, expressed as a percentage.
Protein titer % = V ∗ f
- V—volume of 0.143 n sodium hydroxide solution, in cm2, used in the second titration;
- f—factor of the 0.143 n sodium hydroxide solution used for titration [78].
Total solids content (TSS) was determined using the refractometer, and the results were expressed in °Brix. A drop of the homogenized sample at a temperature of 20 °C was placed on the prism of a pre-calibrated refractometer and a reading was taken [79].
3.3. Statistical Analysis
Minitab software version 14 was utilized for statistical processing of the data derived from titratable acidity, pH, lactose concentration, protein titer, and TSS measurements. The mean values were compared and the outcomes were evaluated using ANOVA and Duncan’s multiple range test, both used at the 5% level of significance (p < 0.05) [80]. Five measurements were made for each.
4. Conclusions
The dairy industry’s adoption of circular economy principles, particularly through the utilization of whey, represents a significant shift towards sustainability. By recognizing the value of whey and other by-products, the industry has transitioned from a linear, wasteful model to a more sustainable and resource-efficient system. This transformation not only benefits the environment by reducing waste and conserving resources but also offers economic and social advantages, fostering new markets, driving innovation, and contributing to the overall well-being of society. As the dairy industry continues to innovate and expand the use of whey and other by-products, it sets a powerful example of how the circular economy can drive sustainable growth and create a more resilient future for all.
Whey recovery is a beneficial choice because its disposal poses significant environmental problems. A significant proportion of the whey from cheese production is discarded, leading to a loss of valuable nutrients. This study aimed to explore the potential of whey valorization through the development of innovative whey-based beverages with different additions. Six samples were prepared from whey and deproteinized whey in a proportion of 75% with the addition of 25% sea buckthorn juice, 0.75% ginger juice, and 0.2% cinnamon powder.
Sensory evaluation revealed a clear preference for untreated whey beverages, with the WDgc sample (whey with ginger and cinnamon) receiving the highest scores for taste, color, and aroma. The physico-chemical analysis demonstrated an inverse relationship between acidity and pH during storage. One explanation for the pH drop that occurs when acidity increases is the conversion of lactose to lactic acid. Lactose levels decrease over time due to consumption by live probiotic microorganisms. In the WD and DWD samples, the variation in protein titer is different because degradation of protein substances takes place, which is more visible in deproteinized whey. Whey has a comparatively high total solids content. Deproteinized whey samples have a lower TSS content compared to untreated whey samples. As for the addition of ginger, it slightly raised the TSS content, and the addition of cinnamon increased the TSS content even more.
The resulting beverages, thoroughly discussed and analyzed in this study, present viable alternatives for diversifying food production by developing sustainable technological variants, friendly to the environment.
Future research should explore additional whey-based beverage formulations, as they offer a versatile solution for maximizing whey utilization and creating consumer-friendly, nutritious beverages.
Author Contributions
Conceptualization, M.A.T. and C.M.B.; methodology, M.A.T., V.-M.M., and M.A.C.; software, V.-M.M. and M.A.C.; validation, O.T. and C.M.B.; formal analysis, M.A.C.; investigation, V.-M.M.; resources, M.A.T.; data curation, O.T. and V.-M.M.; writing—original draft preparation, C.M.B.; writing—review and editing, V.-M.M. and M.A.C.; visualization, M.A.T. and O.T.; supervision, M.A.T. and C.M.B.; project administration, M.A.T.; funding acquisition, M.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was financed by Lucian Blaga University of Sibiu through the research grant LBUS-IRG-2022-08 (2416).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We sincerely thank the Research Center in Biotechnology and Food Engineering (CCBIA) and the Lucian Blaga University of Sibiu for their support throughout the research period.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al-Obadi, M.; Ayad, H.; Pokharel, S.; Ayari, M. Perspectives on food waste management: Prevention and social innovations. Sustain. Prod. Consum. 2022, 31, 190–208. [Google Scholar] [CrossRef]
- Markina, I.; Somych, N.; Shkilniak, M.; Chykurkova, A.; Lopushynska, O. Managing Resource-Saving Development of Agri-Food Enterprises in the Context of Food Security and Sustainability: Strategic Aspects. Cent. Eur. Manag. J. 2021, 29, 114–135. [Google Scholar] [CrossRef]
- Topleva, S.; Prokopov, T. Integrated business model for sustainability of small and medium-sized enterprises in the food industry: Creating value added through ecodesign. Br. Food J. 2020, 122, 1463–1483. [Google Scholar] [CrossRef]
- Khan, S.; Amin, N.; Ansari, Z.; Majumder, D. Whey: Waste to health and wealth. Int. J. Curr. Microbiol. Appl. Sci. 2015, 2, 245–253. [Google Scholar]
- Zucchella, A.; Previtali, P. Circular business models for sustainable development: A “waste is food” restorative ecosystem. Bus. Strategy Environ. 2019, 28, 274–285. [Google Scholar] [CrossRef]
- Narasimmalu, A.; Ramasamy, R. Food Processing Industry Waste and Circular Economy. IOP Conf. Ser. Mater. Sci. Eng. 2020, 955, 012089. [Google Scholar] [CrossRef]
- Fancello, F.; Zara, G.; Hatami, F.; Scano, E.A.; Mannazzu, I. Unlocking the potential of second cheese whey: A comprehensive review on valorisation strategies. Rev. Environ. Sci. Biotechnol. 2024, 23, 411–441. [Google Scholar] [CrossRef]
- Paraskevopoulou, C.; Vlachos, D. A circular economy perspective for dairy supply chains. In Research on Interdisciplinary Approaches to Decision Making for Sustainable Supply Chains; Publisher Engineering Science Reference: Hershey, PA, USA, 2021; pp. 406–426. [Google Scholar]
- Latif, A.; Cahyandito, M.F.; Utama, G.L. Dynamic System Modeling and Sustainability Strategies for Circular Economy-Based Dairy Cow Waste Management. Sustainability 2023, 15, 3405. [Google Scholar] [CrossRef]
- Rajković, M.; Popović, M.; Milinčić, D.; Zdravković, M. Circular economy in food industry. Zastita Materijala 2020, 61, 229–250. [Google Scholar] [CrossRef]
- Ostojić, S.; Pavlović, M.; Živić, M.; Filipović, Z.; Gorjanović, S.; Hranisavljević, S.; Dojčinović, M. Processing of whey from dairy industry waste. Environ. Chem. Lett. 2005, 3, 29–32. [Google Scholar] [CrossRef]
- Zandona, E.; Blažić, M.; Režek Jambrak, A. Whey utilization: Sustainable uses and environmental approach. Food Technol. Biotechnol. 2021, 59, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Soumati, B.; Atmani, M.; Benabderrahmane, A.; Benjelloun, M. Whey Valorization-Innovative Strategies for Sustainable Development and Value-Added Product Creation. J. Ecol. Eng. 2023, 24, 86–104. [Google Scholar] [CrossRef]
- Uvarova, I.; Atstaja, D.; Grinbergs, U.; Petersons, J.; Gegere-Zetterstroma, A.; Kraze, S. Transition to the circular economy and new circular business models—An in-depth study of whey recycling. IOP Conf. Ser. Earth Environ. Sci. 2020, 578, 012019. [Google Scholar] [CrossRef]
- Adesra, A.; Srivastava, V.K.; Varjani, S. Valorization of Dairy Wastes: Integrative Approaches for Value Added Products. Indian J. Med. Microbiol. 2021, 61, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J. An integrated approach for the valorization of cheese whey. Foods 2021, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.L.; dos Santos, L.F.; Soccol, C.R.; Suguimoto, H.H. Dynamics of ethanol production from deproteinized whey by Kluyveromyces marxianus: An analysis about buffering capacity, thermal and nitrogen tolerance. Braz. Arch. Biol. Technol. 2015, 58, 454–461. [Google Scholar] [CrossRef]
- Tsermoula, P.; Khakimov, B.; Nielsen, J.H.; Engelsen, S. WHEY-The waste-stream that became more valuable than the food product. Trends Food Sci. Technol. 2021, 118, 230–241. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Alexandri, M.; Nascimento, M.F.; Alimpoumpa, D.; Faria, N.T.; Papadaki, A.; Ferreira, F.C.; Kopsahelis, N. Lactobacilli and Moesziomyces Biosurfactants: Toward a Closed-Loop Approach for the Dairy Industry. Fermentation 2022, 8, 517. [Google Scholar] [CrossRef]
- Gregg, J.; Jürgens, J.; Happel, M.; Strom-Andersen, N.; Tanner, A.; Bolwig, S.; Klitkou, A. Valorization of bio-residuals in the food and forestry sectors in support of a circular bioeconomy: A review. J. Clean. Prod. 2020, 267, 122093. [Google Scholar] [CrossRef]
- Toma, C.; Meleghi, E. Tehnologia laptelui și a produselor lactate; Publisher Didactică și Pedagogică: Bucharest, Romania, 1963. [Google Scholar]
- Pescuma, M.; Hébert, E.; Mozzi, F.; de Valdez, G.F. Whey fermentation by thermophilic lactic acid bacteria: Evolution of carbohydrates and protein content. Food Microbiol. 2008, 25, 442–451. [Google Scholar] [CrossRef]
- Bondoc, I. Tehnolohia și controlul calității laptelui și produselor lactate; Publisher Ion Ionescu de la Brad: Iași, Romania, 2013. [Google Scholar]
- Hanreich, L.; Zelther, E. Brânzeturi pentru casă și pentru piață 120 de rețete pentru prelucrarea laptelui; Publisher M.A.S.T.: New York, NY, USA, 2008. [Google Scholar]
- Begum, T.; Islam, Z.; Siddiki, M.; Habib, R.; Rashid, H.U. Preparation of fermented beverage from whey-based watermelon (Citrullus lanatus) juice. Asian J. Dairy Food Res. 2019, 38, 301–306. [Google Scholar] [CrossRef]
- Vivas, Y.; Morales, A.; Otálvaro, Á. Utilization of whey in the development of a refreshing beverage with natural antioxidants. Aliment. Hoy. 2017, 24, 185–199. [Google Scholar]
- Guy, A. 22 de specii de condimente care vă ocrotesc sănătatea; Publisher M.A.S.T.: New York, NY, USA, 2014. [Google Scholar]
- Macwan, S.R.; Dabhi, B.K.; Parmar, S.C.; Aparnathi, K.D. Whey and its utilization. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 134–155. [Google Scholar] [CrossRef]
- Szyba, M.; Mikulik, J. Energy production from biodegradable waste as an example of the circular economy. Energies 2022, 15, 1269. [Google Scholar] [CrossRef]
- Casallas-Ojeda, M.; Torres-Guevara, L.E.; Caicedo-Concha, D.M.; Gómez, M.F. Opportunities for waste to energy in the milk production industry: Perspectives for the circular economy. Sustainability 2021, 13, 12892. [Google Scholar] [CrossRef]
- Caltzontzin-Rabell, V.; Feregrino-Pérez, A.A.; Gutiérrez-Antonio, C. Bio-upcycling of cheese whey: Transforming waste into raw materials for biofuels and animal feed. Heliyon 2024, 10, e32700. [Google Scholar] [CrossRef]
- Ji, T.; Haque, Z.U. Cheddar whey processing and source: I. Effect on composition and functional properties of whey protein concentrates. Int. J. Food Sci. Technol. 2003, 38, 453–461. [Google Scholar] [CrossRef]
- Mistry, V.V.; Metzger, L.E.; Maubois, J.L. Use of Ultrafiltered Sweet Buttermilk in the Manufacture of Reduced Fat Cheddar Cheese. J. Dairy Sci. 1996, 79, 1137–1145. [Google Scholar] [CrossRef]
- Kenny, S.; Wehrle, K.; Auty, M.A.E.; Arendt, E.K. Influence of Sodium Caseinate and Whey Protein on Baking Properties and Rheology of Frozen Dough. Cereal Chem. 2001, 78, 458–463. [Google Scholar] [CrossRef]
- Carunchia Whetstine, M.E.; Croissant, A.E.; Drake, M.A. Characterization of Dried Whey Protein Concentrate and Isolate Flavor. J. Dairy Sci. 2005, 88, 3826–3839. [Google Scholar] [CrossRef]
- Tesfaw, A.; Oner, E.T.; Assefa, F. Evaluating crude whey for bioethanol production using non-Saccharomyces yeast, Kluyveromyces marxianus. SN Appl. Sci. 2021, 3, 42. [Google Scholar] [CrossRef]
- Vyas, S.; Chhabra, M. Assessing oil accumulation in the oleaginous yeast Cystobasidium oligophagum JRC1 using dairy waste cheese whey as a substrate. 3 Biotech 2019, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Kar, T.; Misra, A.K. Therapeutic properties of whey used as fermented drink. Rev. Microbiol. 1999, 30, 163–169. [Google Scholar] [CrossRef][Green Version]
- Hippophae rhamnoides. Available online: https://en.wikipedia.org/wiki/Hippophae_rhamnoides (accessed on 18 February 2024).
- Yang, W.; Laaksonen, O.; Kallio, H.; Yang, B. Proanthocyanidins in sea buckthorn (Hippophaë rhamnoides L.) berries of different origins with special reference to the influence of genetic background and growth location. J. Agric. Food Chem. 2016, 64, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, S.; Guichard, E. The effect of sugars and pectin on flavor release from a fruit pastille model system. Food Chem 2003, 81, 269–273. [Google Scholar] [CrossRef]
- Ginger. Available online: https://en.wikipedia.org/wiki/Ginger (accessed on 24 February 2024).
- Meysam, M.; Rahaie, M.; Ebrahimi, A.; Samiee, F. Four Matrix Metalloproteinase genes involved in murine breast cancer affected by ginger extract. Gene Rep. 2021, 25, 101332. [Google Scholar] [CrossRef]
- Aryaeian, N.; Shahram, F.; Mahmoudi, M.; Tavakoli, H.; Yousefi, B.; Arablou, T.; Jafari-Karegar, S. The effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active Rheumatoid Arthritis. Gene 2019, 698, 179–185. [Google Scholar] [CrossRef]
- Yang, X.; Wei, S.; Lu, X.; Qiao, X.; Simal-Gandara, J.; Capanoglu, E.; Woźniak, Ł.; Zou, L.; Cao, H.; Xiao, J.; et al. A neutral polysaccharide with a triple helix structure from ginger: Characterization and immunomodulatory activity. Food Chem. 2012, 350, 129261. [Google Scholar] [CrossRef]
- Seif, M.; El-Aziz, A.; Sayed, M.; Wang, Z. Zingiber officinale ethanolic extract attenuates oxidative stress, steroidogenic gene expression alterations, and testicular histopathology induced by sodium arsenite in male rats. Environ. Sci. Pollut. Res. 2021, 28, 19783–19798. [Google Scholar] [CrossRef]
- Abdullahi, A.; Khairulmazmi, A.; Yasmeen, S.; Ismail, I.; Norhayu, A.; Sulaiman, M.R.; Ahmed, O.H.; Ismail, M.R. Phytochemical profiling and antimicrobial activity of ginger (Zingiber officinale) essential oils against important phytopathogens. Arabian J. Chem. 2020, 13, 8012–8025. [Google Scholar] [CrossRef]
- El Gayar, M.; Aboromia, M.; Ibrahim, N.; Abdel Hafiz, M. Effects of ginger powder supplementation on glycemic status and lipid profile in newly diagnosed obese patients with type 2 diabetes mellitus. Obes. Med. 2019, 14, 100094. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, Z.; Yan, J.; Xie, J. Nutritional components, phytochemical compositions, biological properties, and potential food applications of ginger (Zingiber officinale): A comprehensive review. J. Food Compos. Anal. 2024, 128, 106057. [Google Scholar] [CrossRef]
- Cinnamon. Available online: https://en.wikipedia.org/wiki/Cinnamon (accessed on 26 February 2024).
- Arangannal, P.; Nithya, S.; Jeevarathan, J.; Rekha, V.; Krishnan, M.; Padmavathy, K. Antibacterial effectiveness of cinnamon chewing gum on Streptococcus Mutans. IJPHRD 2019, 10, 1694–1698. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.A.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Compl. Alternative Med. 2013, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 2006, 94, 520–528. [Google Scholar] [CrossRef]
- Lee, H.; Ahn, Y. Growth-inhibiting effects of Cinnamomum cassia bark-derived materials on human intestinal bacteria. J. Agric. Food Chem. 1998, 46, 8–12. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.; Brooks, J.; Corke, H. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): Activity against foodborne pathogenic bacteria. J. Agric. Food Chem. 2007, 55, 5484–5490. [Google Scholar] [CrossRef]
- Spence, C. Cinnamon: The historic spice, medicinal uses, and flavour chemistry. Int. J. Gastron. Food Sci. 2024, 35, 100858. [Google Scholar] [CrossRef]
- Tanwar, T.; Wagh, R.M.; Malav, O.; Kour, S.; Kumar, P. Preparation of functional beverage from whey-based mango juice. Pharm. Innov. 2022, 11, 4710–4716. [Google Scholar]
- Ahmed, S.G.; Wafaa, H.E.; Gamal, F.M.; Ahmed, F.S. Utilization Whey in Production of Functional Healty Beverage “Whey-mango Beverages”. Am. J. Food Technol. 2013, 8, 133–148. [Google Scholar]
- Naik, B.; Kohli, D.; Walter, N.; Gupta, A.; Mishra, S.; Khan, J.M.; Saris, P.E.J.; Irfan, M.; Rustagi, S.; Kumar, V. Whey-carrot based functional beverage: Development and storage study. J. King Saud Univ. Sci. 2023, 35, 102775. [Google Scholar] [CrossRef]
- Islam, M.; Tabassum, S.; Harun-ur-Rashid, M.; Vegarud, G.; Alam, M.; Islam, M. Development of probiotic beverage using whey and pineapple (Ananas comosus) juice: Sensory and physico-chemical properties and probiotic survivability during in-vitro gastrointestinal digestion. J. Agric. Food Res. 2021, 4, 100144. [Google Scholar] [CrossRef]
- Amaral, G.; Silva, K.; Costa, A.; Alvarenga, V.; Cavalcanti, R.; Esmerino, E.A.; Guimarães, J.T.; Freitas, M.Q.; Sant’Ana, A.S.; Cunha, R.L.; et al. Whey-grape juice drink processed by supercritical carbon dioxide technology: Physical properties and sensory acceptance. LWT 2018, 92, 80–86. [Google Scholar] [CrossRef]
- Oliveira, G.A.R.; Guimaraes, J.T.; Ramos, G.L.P.A.; Esmerino, E.A.; Pimentel, T.C.; Neto, R.P.C.; Tavares, M.I.B.; Sobral, L.A.; Souto, F.; Freitas, M.Q.; et al. Benefits of thermosonication in orange juice whey drink processing. Innov. Food Sci. Emerg. Technol. 2022, 75, 102876. [Google Scholar] [CrossRef]
- Arsic, S.; Bulatovic, M.; Zaric, D.; Kokeza, G.; Subic, J.; Rakin, M. Functional fermented whey carrot beverage—Qualitative, nutritive and techno-economic analysis. Rom. Biotechnol. Lett. 2018, 23, 13496–13504. [Google Scholar]
- Soares, C.; Silveira, A.J.T.; Laurioux, B. Mesados Sentidos & Sentidos da Mesa; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2021; Volume 1, pp. 119–129. [Google Scholar]
- Królczyk, J.B.; Dawidziuk, T.; Janiszewska-Turak, E.; Sołowiej, B. Use of Whey and Whey Preparations in the Food Industry—A Review. Pol. J. Food Nutr. Sci. 2016, 66, 157–165. [Google Scholar] [CrossRef]
- de Matos Reis, S.; Mendes, G.R.L.; Mesquita, B.M.A.C.; Lima, W.J.N.; Pinheiro, C.A.F.D.; Ruas, F.A.O.; Santos, G.L.M.; Brandi, I.V. Development of milk drink with whey fermented and acceptability by children and adolescents. J. Food Sci. Technol. 2021, 58, 2847–2852. [Google Scholar] [CrossRef]
- Tkachenko, N.; Nekrasov, P.; Vikul, S.; Honcharuk, Y. Modelling formulae of strawberry whey drinks of prophylactic application. Food Sci. Technol. 2017, 11, 80–88. [Google Scholar] [CrossRef]
- Schoina, V.; Terpou, A.; Papadaki, A.; Bosnea, L.; Kopsahelis, N.; Kanellaki, M. Enhanced aromatic profile and functionality of cheese whey beverages by incorporation of probiotic cells immobilized on pistacia terebinthus resin. Foods 2020, 9, 13. [Google Scholar] [CrossRef]
- Larionov, G.; Semenov, V.; Lavrentyev, A.; Sherne, V.; Kayukova, O.; Mardaryeva, N.; Ivanova, R. Production of mint whey drink at private and collective farms and agricultural holdings. IOP Conf. Ser. Earth Environ. Sci. 2020, 604, 012042. [Google Scholar] [CrossRef]
- Yasmin, A.; Butt, M.S.; Yasin, M.; Qaisrani, T.B. Compositional analysis of developed whey based fructooligosaccharides supplemented low- calorie drink. J. Food Sci. Technol. 2015, 52, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Sabuz, A.; Mohaldar, A.; Fardows, H.; Inbaraj, B.; Sharma, M.; Rana, M.R.; Sridhar, K. Development of Novel Whey-Mango Based Mixed Beverage: Effect of Storage on Physicochemical, Microbiological, and Sensory Analysis. Foods 2023, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Sady, M.; Najgebauer-Lejko, D.; Domagała, J. The suitability of different probiotic strains for the production of fruit-whey beverages. Acta Sci. Pol. Technol. Aliment. 2017, 16, 421–429. [Google Scholar]
- Divya, D.; Kumari, A. Effect of different temperatures, timings and storage periods on the physico-chemical and nutritional characteristics of whey-guava beverage. WJDFS 2009, 4, 118–122. [Google Scholar]
- Jelen, P.; Currie, R.; Kadis, V.W. Compositional Analysis of Commercial Whey Drinks. J. Dairy Sci. 1987, 70, 892–895. [Google Scholar] [CrossRef]
- Fadhil, R.; Agustina, R.; Hayati, R. Sensory Assessment of Sauerkraut Using a Non-Numeric Approach Based on Multi-Criteria and Multi-Person Aggregation. Bull. Transilv. 2020, 13, 112–118. [Google Scholar] [CrossRef]
- Thermo Scientific Oreon 2 Stars. Available online: https://www.fondriest.com/pdf/thermo_2-star_ph_spec.pdf (accessed on 15 April 2024).
- Association of Official Analytical Chemists. Official Methods of Analysis, 18th ed; Association of Official Analytical Chemists: Rockville, MD, USA, 2015. [Google Scholar]
- Tița, M. Manual de analiză și control calității în industria laptelui; “Lucian Blaga” University Sibiu: Sibiu, Romania, 2002. [Google Scholar]
- Sattar, A.; Durrani, M.; Khan, R.; Hussain, B. Effect of packaging material and fluorescent light on HTST-pasteurized orange drink. Z. Lebensm. Unters. Forch. 1998, 188, 430–433. [Google Scholar] [CrossRef]
- Minitab. Available online: https://www.minitab.com/en-us/ (accessed on 29 April 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).