Abstract
Currently, less attention is paid to zinc–carbon batteries, although they are still widely used and are among the major types of batteries collected and recycled. The recycling technologies currently in use do not allow the complete recovery of resources, are not self-sufficient and require additional financing. Therefore, this paper aims to study the possibility of complete recycling of waste zinc–carbon batteries and to suggest the practical use of the final products generated in the recycling process. The possibility of complex processing of spent zinc–carbon batteries using mechanical separation and processing of the battery’s components (steel case, zinc electrode, graphite electrode, polypropylene and paper insulators) is justified. The separation of spent electrolytes from other components of batteries with hydrochloric acid was studied. It was shown that the extraction of Zn2+ and NH4+ cations takes place following the addition of an equivalent amount of Na3PO4 solution and water-insoluble NH4ZnPO4 salt sedimentation. Waste agglomerate (mixture of MnO2, MnO(OH), and graphite) was regenerated to its initial composition (MnO2, graphite) at a temperature of 300–325 °C; manganese (III) hydroxide was oxidized to manganese (IV) dioxide. Thermal destruction of polypropylene and paper insulators with additional introduction of polyethylene into the primary mixture produced pyrolysis liquid, pyrocarbon and pyrolysis gas as products. The practical use of the products obtained and compliance with the environmental requirements of the suggested method of waste batteries recycling were shown.
1. Introduction
A tremendous amount of portable batteries is used worldwide, including both primary (zinc–carbon, alkaline, zinc–air, lithium, etc.) and rechargeable (nickel–cadmium, nickel–metal hydride, lithium-ion, etc.). Primary zinc–carbon batteries are among the most common due to their cheapness, resource availability, simple manufacturing technology, ease of use and acceptable electrical parameters.
The lack of appropriate waste battery management in many countries results in environmental pollution and loss of resources. Studies on waste battery composition [1,2] and assessment of their environmental impact [3,4] show a high resource potential and the importance of waste battery recycling for sustainable development. At the same time, a circular economy for batteries can only be achieved if spent batteries can be collected comprehensively [5]. In addition, the analysis of waste battery management systems [6] demonstrates many unused options for waste battery recycling. Although separate collection systems for portable batteries have been installed years ago in many countries, high amounts of batteries still do not enter the collection systems and a lot of batteries are misplaced into non-battery-specific collection systems [7].
While spent lithium-ion batteries are of the highest interest for a circular economy [8], less attention is paid to zinc–carbon batteries, although they still are widely used and are among the major types of batteries collected and recycled [9]. Sobianowaska-Turek et al. [10] reported mercury content in some zinc–carbon batteries exceeding the value permissible by the EU Directive. Waste zinc–carbon batteries are also valuable due to the resources that can be recovered, like metals. In addition, this type of battery is known to be used to synthesize graphene [11], a new material for many applications, or upcycle waste battery electrodes to high-value LiMn2O4 cathodes and carbon anodes for lithium-ion battery application [12].
Pyrometallurgy and hydrometallurgy are known to be used for waste battery recycling. While pyrometallurgy is primarily used for the mixture of batteries in order to provide the highest disposal rate, hydrometallurgy is mainly applied when metal recovery is of the highest priority.
Most studies on waste portable battery recycling using the hydrometallurgical method are focused on mechanical grinding (first stage) [13] followed by leaching [14,15], electrolysis [16], or solvent extraction [17]. Each of the methods has its advantages and disadvantages and needs technical improvement. Metals (zinc and manganese) are mainly recovered as sulphates or oxides (after the treatment by acid). While zinc is easily leached, the use of a reductant is needed to fully solubilize Mn from spent batteries during the leaching process [18]. Some techniques [16,19] use a high temperature at some stages, making these methods energy-demanding. Although acid leaching remains the primary step, alkaline [20] or other types [21,22] of leaching are also used.
Summarizing the existing methods of waste batteries processing, it should be noted that portable battery recycling usually begins with mechanical grinding. Components of the batteries (metal case, zinc and graphite electrodes, agglomerate and other components) are mixed and then separated using multi-stage physical, chemical and physic-chemical operations. This makes the recycling technology more complicate and expensive. In addition, complete recycling is hardly possible. Therefore, it requires the improvement of existing technologies.
It should be noted that the recycling technologies currently in use are focused on metal recycling (and thus generating waste), do not allow the complete recovery of resources, are not self-sufficient and require additional financing (for example, through the system of extended producer responsibility). Along with circular economy requirements, this leads to the need for effective zero-waste technology for waste battery recycling. From this perspective, the goal of this research was to study the possibility of complete recycling of waste zinc–carbon batteries and to suggest the practical use of the final products generated in the recycling process.
2. Results and Discussion
Manual dismantling of the waste batteries was carried out (the average composition of waste batteries used in this research can be found in Table 1). This allows for the possibility of recovering the resources compared to methods traditionally used for battery recycling where many resources are lost (metal recovery is prioritized).

Table 1.
Average composition of waste zinc–carbon batteries.
This kind of dismantling, while reducing the loss of resources, requires less operations and energy consumption (traditional battery recycling includes mechanical grinding followed by sieving, magnetic or dry separation, precipitation, filtration, evaporation, drying, absorption, etc.). At the industrial scale, the automatization of battery dismantling can be applied.
While improving the technology of waste zinc–carbon battery recycling, the authors have defined the quantitative dependence of recovered components on the residual voltage of the battery. When comparing different batteries, the least deviation in the weight was found for the components that are inert to battery discharge (metal case, graphite electrode, polymer and paper insulators), while the weight of the components providing battery operation is the most scattered (zinc residues, agglomerates, electrolytes).
2.1. Electrolyte Recycling
In most cases, electrolytes of zinc–carbon batteries consist of aqueous concentrated solutions of ammonium and zinc chlorides.
Kang et al. [23] analyzed the recycling of waste zinc batteries with the recovery of ammonium chloride electrolytes. The water solubility of NH4Cl and ZnCl2 was 37.2 g/100 g (20 °C) and 432 g/100 g (25 °C), respectively. This allows for effective recovery of these salts with a minimum amount of water. However, after battery discharge, a solid mass of electrolyte is generated. It includes a water-soluble zinc complex salt [Zn(NH3)2]Cl2, which can be washed out with hydrochloric acid according to the following reaction:
[Zn(NH3)2]Cl2 + 2HCl → ZnCl2 + 2NH4Cl
In this research, zinc was recovered from the electrolyte by washing out its salts with a 1% solution of hydrochloric acid according to the reaction (1). The conditions for salts recovery are listed in Table 2. The battery components (see Table 2) were placed in separate vessels and processed using a certain amount of hydrochloric acid for 10–30 min, which was followed by filtering and washing using distilled water and a filter.

Table 2.
Conditions for NH4Cl and ZnCl2 recovery from the battery’s components.
The concentrations of zinc and ammonium in the washing solution were 4.5 × 10−2 mol/L and 5.0 × 10−3 mol/L, respectively. It was found that when an equivalent amount of PO43− anion was added, NH4Cl and ZnCl2 salts formed ammonium zinc orthophosphate, which hardly dissolved in water and was easily separated by filtration [19]. By adding sodium phosphate to the solution, the double salt of orthophosphoric acid is generated, which is shown below:
ZnCl2 + NH4Cl + Na3PO4 → ZnNH4PO4↓ + 3NaCl
For this purpose, 200 mL of washing solution containing 0.001 moles of NH4Cl and 0.009 moles of ZnCl2 was mixed with 0.43 g (0.008 moles) of NH4Cl and 1.27 g (0.009 moles) of Na3PO4 (in the form of saturated water solutions) to obtain the equivalent amount of salts. The resulting white precipitate was stirred for 60 min. After coagulation and precipitation, it was filtered on a Schott filter and dried. The amount of ZnNH4PO4 salt obtained was 1.70 g (96% yield by weight). The purity of the recovered ZnNH4PO4 was estimated at 98–99% (based on average metal content in zinc–carbon batteries). The temperature of melting with decomposition was over 200 °C.
It should be noted that double salts, such as NH4MePO4, containing phosphorus, nitrogen and trace elements (Me = Zn, Co, Fe, Cu, Mg) are known as effective microfertilizers for cereal crops [24] and biologically active compounds for some food products [25].
2.2. Agglomerate Recycling
The black powdered material (or agglomerate) is a mixture of manganese (IV) oxide MnO2, manganese (III) hydroxide Mn(O)OH (some properties of manganese oxides are shown in Table 3), and graphite.

Table 3.
Properties of manganese oxides [26,27,28].
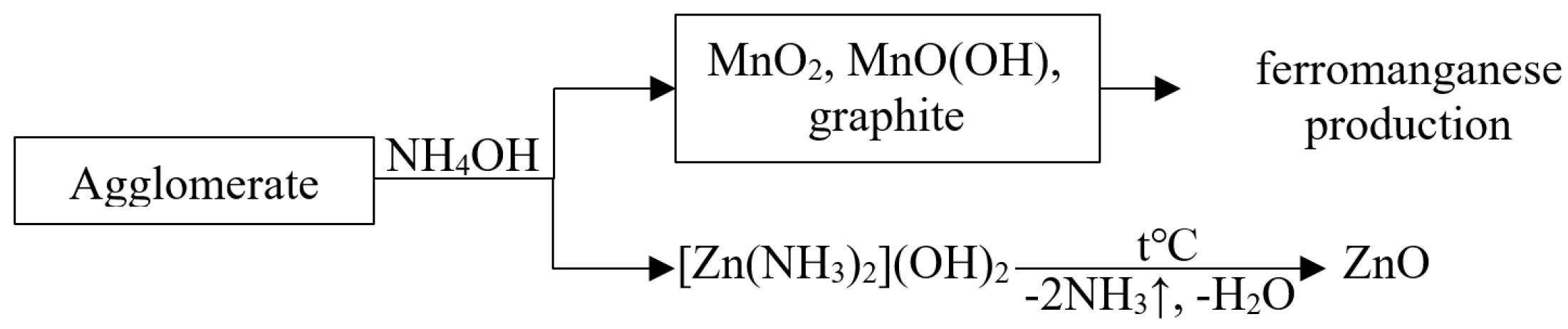
Usually, waste agglomerate is processed by ammonia leaching according to the following scheme (Figure 1):
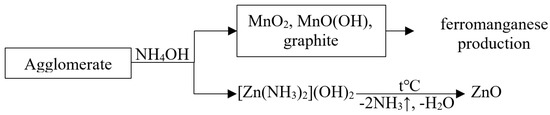
Figure 1.
Scheme of agglomerate recycling.
Waste agglomerate can also be used in the production of ferromanganese. In addition, a regenerated agglomerate consisting of natural pyrolusite can be effectively used as a sorbent for radioactive water treatment from long-lived radionuclides cesium-137 and strontium-90 [29].
In this study, we regenerated waste agglomerate to its original state by moderate calcination (300–325 °C), as shown below:
4MnO(OH) + O2 → 4MnO2 + 2H2O
The redox duality of manganese oxides (III, IV) ensures MnO2 formation. However, at a higher temperature (over 530 °C), Mn2O3 oxide formation is possible. Therefore, the temperature of reaction (3) must be controlled. The recycling procedure included the thermo-oxidation of Mn3+ to Mn4+. For the oxidation, 10 g of agglomerate (residues of electrolyte were washed out before; see previous section) was placed in the muffle furnace (temperature 300–325 °C) for 1 h. After cooling down, grinding and sieving, the regenerated mixture consisting of MnO2 oxide and graphite was suitable for reuse as raw material in electrotechnical or other industries. The yield of the final product was 9.8 g (98.0% by weight).
2.3. Polymer and Paper Insulator Recycling
Previously, other researchers [30,31] used the pyrolysis processing of waste electronic and electrical equipment to produce pyrolysis liquid (can be used as a component for fuel manufacture after additional technological operations, or directly for stove fuel production without further processing), pyrocarbon, and a gas mixture. The efficiency of this technology is obvious [32]. However, its low practical implementation is mostly related to logistical difficulties because of the low weight of the recycled mixture (only 5–7% of battery weight; see Table 1).
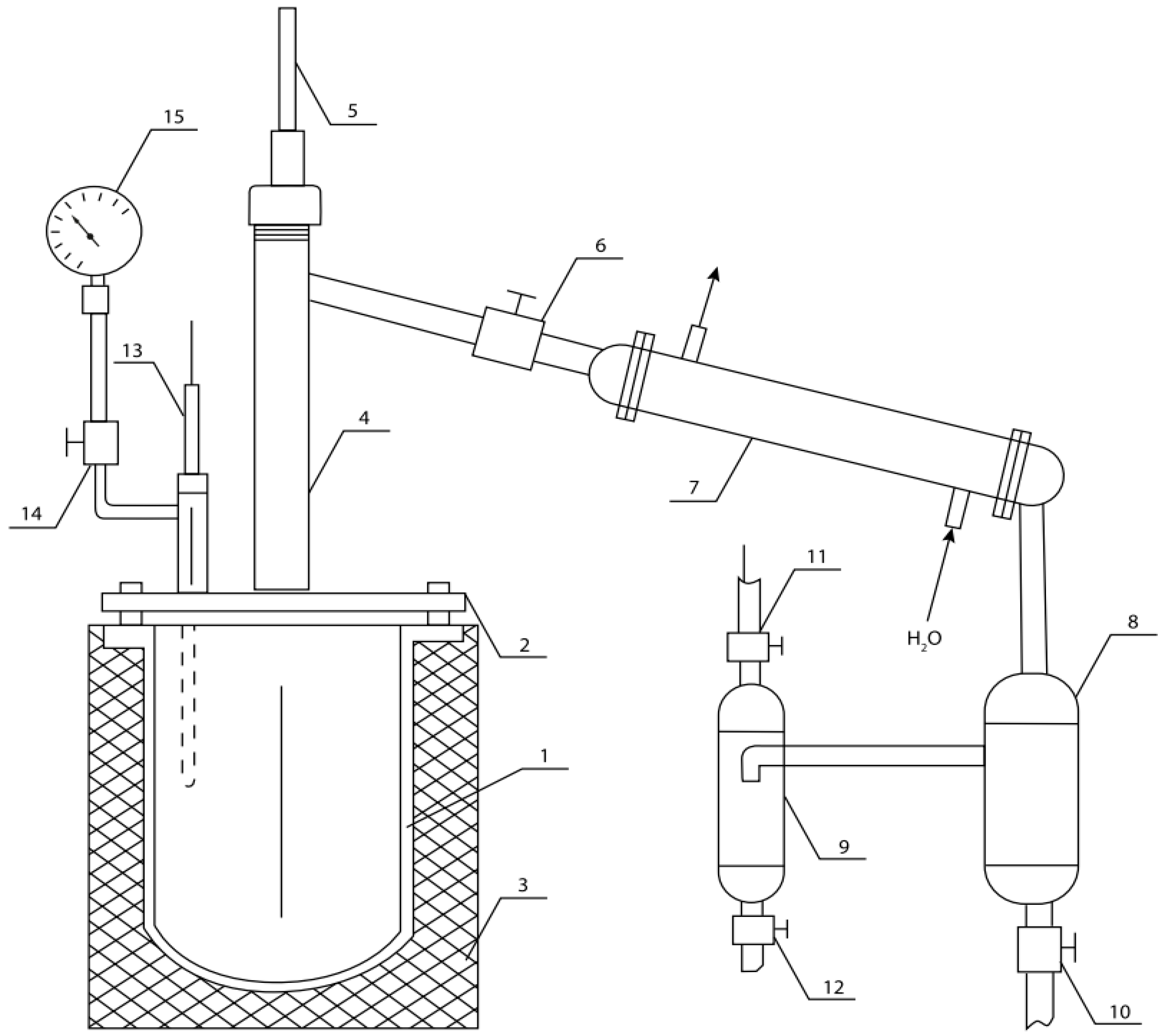
In this study, the mixture of polymer and paper insulators was treated using the equipment for low-temperature pyrolysis (Figure 2).
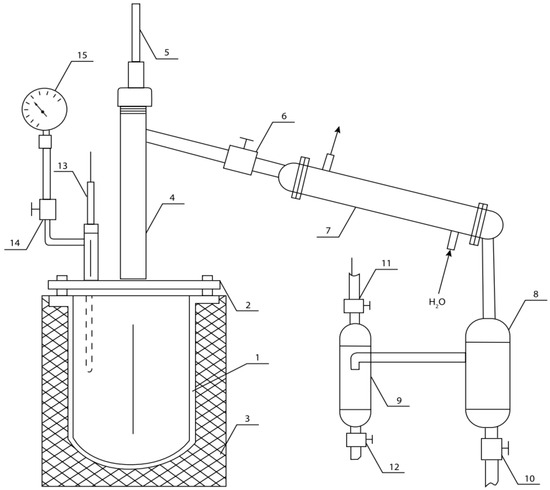
Figure 2.
Equipment for low-temperature pyrolysis: 1—reactor; 2—lid; 3—electric heater with insulating case; 4—separation column; 5, 13—thermocouples; 6, 10, 11, 12 and 14—shut-off valves; 7—condenser; 8—collector–condenser of heavy fraction; 9—collector–condenser of light fraction; 15—manometer.
For this research, two samples were prepared. The first sample consisted of polymer and paper insulators of waste batteries, while the second sample consisted of the same insulators and additional waste polyethylene (Table 4). This second sample was used to verify the efficiency of polypropylene and paper insulator processing along with additional polymer waste generated in high volumes in different processes.

Table 4.
Samples’ contents and parameters of pyrolysis.
Both samples were processed under the same conditions. The samples were placed in reactor 1 (Figure 2). The temperature of the process (Table 4) was regulated by a power transformer. The heating rate was 5 °C per minute. The steam–gas mixture generated during the thermal destruction of raw materials was condensed using condenser 7 and condensate collectors 8 (for heavy fraction) and 9 (for light fraction). The volume of gas mixture generated was measured by a gas meter, and the amount of pyrocarbon was measured by its mechanical extraction and weighing on electronic scales. The results are shown in Table 5.

Table 5.
The material balance of the pyrolysis process.
2.4. Graphite Electrode Recycling
Mechanically undamaged graphite rods can be directly used in the production of new batteries or as graphite electrodes for welding.
The overall results of complex processing of spent zinc–carbon batteries are shown in Table 6. Some parts of waste batteries can be used for new battery manufacture after simple operations (washing, calcination). Other parts of waste batteries can be considered as valuable resources after processing by pyrolysis, electrolysis and metallurgical processing.

Table 6.
Data on complex processing of waste zinc–carbon batteries.
2.5. Discussion on Recycling Process
Taking into account the above-mentioned recycling processes, the scheme of waste zinc–carbon battery recycling was developed (see Figure 3).
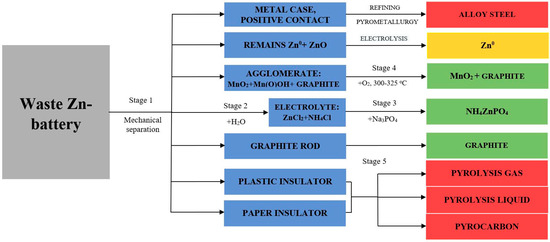
Figure 3.
Technological scheme of complex processing of waste zinc–carbon batteries: 1st stage—mechanical separation, 2nd stage—washing of electrolyte (NH4Cl + ZnCl2 + [Zn(NH3)2]Cl2), 3rd stage—production of double salt NH4ZnPO4, 4th stage—regeneration of Mn(O)OH; 5th stage—pyrolysis.
The data obtained in this study were confirmed by battery operation. The reactions at the anodes and cathodes can be expressed in the following way:
Anode (−): Zn0 − 2e → Zn2+
Cathode (+): 2MnO2 + 2H3O+ + 2e → 2MnO(OH) + 2H2O
During the agglomerate processing, the reactions occurring in the volume (between the anode and cathode) are important. At the graphite electrode, the reduction of H3O+ ions occurs in the absence of MnO2 as a depolarizer [33,34], as shown below:
2H3O+ + 2e → H2 + 2H2O
Then, the hydrogen gas layer is generated near the graphite electrode, and it blocks the battery operation. At the cathode, reaction (5) occurs in the presence of MnO2. The electrolyte NH4Cl dissociates as follows:
and forms a complex salt of white color (zinc diamine chloride) by reacting with zinc ions, as shown below:
2NH4Cl + 2H2O ⇄ 2NH3 + 2H3O+ + 2Cl−,
Zn2+ + 2NH3 + 2Cl− → [Zn(NH3)2]Cl2
Taking into account reactions (4)–(8), the overall reaction in the agglomerate is as follows:
Zn0 + 2MnO2 + 2NH4Cl → 2MnO(OH) + [Zn(NH3)2]Cl2
The complex salt generated is usually treated with an alkali solution according to the scheme, as shown below:
followed by converting the Zn(OH)2 generated to the salt form, as shown below:
[Zn(NH3)2]Cl2 + 2NaOH → Zn(OH)2 + 2NH3 + 2NaCl,
Zn(OH)2 + H2SO4 → ZnSO4 + 2H2O
At the next stage, the electrolysis of zinc sulfate is carried out with a lead anode doped with 1% silver, and high-purity zinc is accumulated at the cathode, as shown below:
Cathode (+): Zn2+ + 2e → Zn0
At the same time, at the anode, oxygen is generated in the following reaction:
Anode (−): 2H2O → O2 + 4H+ + 4e
In reaction (12), the output of zinc is 80–90% [34]. In addition, in the electrolyte solution, sulfuric acid is formed, as follows:
2H+ + SO42− → H2SO4
Reactions (9)–(13) indicate that the recovery of high-purity metallic zinc by the electrochemical method is the most complex stage of spent zinc–carbon batteries recycling, which was not carried out in this study.
The recovery efficiency of waste battery components is estimated as follows. For agglomerates and electrolytes, it is up to 96% (losses up to 4% are declared mainly due to incomplete chemical reactions and mechanical losses during component dismantling). For plastic and paper insulators, the recovery efficiency is estimated at 95% and the losses occur in the pyrolysis chamber. At the same time, the graphite rod and zinc remains are completely extracted from a waste battery and, being in pure form after washing out the electrolyte (as described in this study), these components can be used as a resource in industry (directly or after processing). Similarly, it is suggested that the metal case and positive contact can be used as a raw material. Therefore, the recovery efficiency of the above-mentioned components depends on further processing and cannot be estimated in this study.
In this paper, we studied the complex processing of waste portable batteries, which allows us to completely recycle all the battery’s components. Such a comprehensive method fully complies with circular economy principles and provides environment protection and significant resource conservation.
3. Materials and Methods
3.1. Study Object
In this research, waste zinc–carbon batteries (AA size, R6 type) without visible external damage were studied. The main specifications of the batteries are listed in Table 7.

Table 7.
Specifications of waste zinc–carbon batteries.
An amount of 5 batteries of each producer, in total 10 pcs, was retrieved from a special collection point for waste batteries in Vinnytsia National Technical University, Ukraine. Ahead of the dismantling procedure, the performance of each battery was assessed by a portable multimeter DE-965 TRN (DER EE Electrical Instrument, Taiwan). The residual voltage data (Table 1) indicate that the batteries studied are out of operation and can be effectively recycled.
3.2. Battery Dismantling
For the recycling process, waste batteries were first dismantled. The batteries were fixed by mechanical clamps, a T-shaped cut was made using the angle grinder GWS 1400 (Bosch, Gerlingen, Germany), and the steel case was removed. Afterwards, the following elements were consecutively separated by manual extraction:
- Zinc electrode or its residue after battery discharge;
- Graphite electrode;
- Metal of the positive terminal;
- Agglomerate (black powdered material) (mixture of MnO2, Mn(O)OH, and graphite);
- Electrolyte (mixture of Zn(NH3)2Cl2, NH4Cl, and ZnCl2);
- Polypropylene insulator;
- Paper insulator.
3.3. Waste Battery Recycling Procedure
In this study, five Varta batteries were processed. In waste batteries, residues of electrolyte can be found in many components (Zn electrode, graphite electrode, agglomerate, polymer and paper insulators). Therefore, at the first stage, the electrolyte and its residues in other components were treated with a 1% solution of hydrochloric acid in order to recover ammonium and zinc chlorides. The details of the process are given in the Results section. The chloride solutions were mixed and placed in a flask. Distilled water was added to obtain 1000 mL of the solution used to measure the concentration of NH4+ and Zn2+ cations. Afterwards, other components of the battery were treated. The features of recycling procedures for each component are discussed in the Results section.
4. Conclusions
- The complex processing of waste zinc–carbon batteries was studied. To achieve complete recycling, mechanical dismantling of waste batteries was used. This provides the possibility to recover all the resources comparing to methods traditionally used for battery recycling where many resources are lost.
- Leaching of waste electrolyte (mixture of NH4Cl, ZnCl2, [Zn(NH3)2]Cl2) by double salt NH4ZnPO4 sedimentation was studied. It was shown that adding an equivalent amount of Na3PO4 to an aqueous solution of equivalent amounts of NH4Cl and ZnCl2 results in sparingly soluble salt NH4ZnPO4 generation. This salt was separated by filtration under normal conditions (96.0% yield).
- The regeneration of waste agglomerate (mixture of MnO2, MnO(OH), and graphite) to its original composition (MnO2, graphite) was studied. It was shown that oxidation of manganese (III) hydroxide to manganese (IV) dioxide takes place at a temperature of 300–325 °C over 1 h with a 98.0% yield.
- The organic part of waste batteries (polypropylene and paper insulators) can be recycled by low-temperature pyrolysis (along with other waste plastic). It was shown that the organic components can be completely destroyed at temperatures of 300–410 °C over 1.5–2.5 h. The final products are pyrolysis liquid (67.2% wt.), pyrocarbon (23.1% wt.) and pyrolysis gas (8.1% wt.).
- The feasibility of complex processing of waste zinc–carbon batteries was justified. The practical use of products obtained and compliance with the environmental requirements of the suggested method of waste battery recycling were shown.
Author Contributions
Conceptualization, A.R. and V.I.; methodology, A.R.; formal analysis, O.G.; investigation, A.R.; resources, V.I.; writing—original draft preparation, A.R. and O.G.; writing—review and editing, V.I.; visualization, V.I.; supervision, V.I.; funding acquisition, V.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education and Science of Ukraine, grant number 16D406.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Terazono, A.; Oguchi, M.; Iino, S.; Mogi, S. Battery collection in municipal waste management in Japan: Challenges for hazardous substance control and safety. Waste Manag. 2015, 39, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Hlavatska, L.; Ishchenko, V.; Pohrebennyk, V.; Salamon, I. Material Flow Analysis of Waste Electrical and Electronic Equipment in Ukraine. J. Ecol. Eng. 2021, 22, 199–208. [Google Scholar] [CrossRef]
- Xará, S.; Delgado, J.; Almeida, M.F.; Costa, C. Laboratory study on the leaching potential of spent alkaline batteries using a MSW landfill leachate. J. Mater. Cycles Waste Manag. 2013, 15, 61–72. [Google Scholar] [CrossRef]
- Melchor-Martínez, E.M.; Macias-Garbett, R.; Malacara-Becerra, A.; Iqbal, H.M.; Sosa-Hernández, J.E.; Parra-Saldívar, R. Environmental impact of emerging contaminants from battery waste: A mini review. Case Stud. Chem. Environ. Eng. 2021, 3, 100104. [Google Scholar] [CrossRef]
- Hagelüken, C.; Goldmann, D. Recycling and circular economy—Towards a closed loop for metals in emerging clean technologies. Miner. Econ. 2022, 35, 539–562. [Google Scholar] [CrossRef]
- Ishchenko, V.; Dworak, S.; Fellner, J. Hazardous household waste management in Ukraine and Austria. J. Mater. Cycles Waste Manag. 2024, 26, 635–641. [Google Scholar] [CrossRef]
- Nigl, T.; Schwarz, T.E.; Walch, C.; Baldauf, M.; Rutrecht, B.; Pomberger, R. Characterisation and material flow analysis of end-of-life portable batteries and lithium-based batteries in different waste streams in Austria. Waste Manag. Res. 2020, 38, 649–659. [Google Scholar] [CrossRef]
- Islam, M.T.; Iyer-Raniga, U. Lithium-ion battery recycling in the circular economy: A review. Recycling 2022, 7, 33. [Google Scholar] [CrossRef]
- Kim, H.; Jang, Y.C.; Hwang, Y.; Ko, Y.; Yun, H. End-of-life batteries management and material flow analysis in South Korea. Front. Environ. Sci. Eng. 2018, 12, 3. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Szczepaniak, W.; Marcinkowski, T.; Zamorska-Wojdyła, D. Content of mercury and cadmium in the stream of spent zinc-carbon batteries. Ecol. Chem. Eng. A 2013, 20, 573–583. [Google Scholar] [CrossRef]
- Le, P.A.; Nguyen, N.T.; Nguyen, P.L.; Phung, T.V.B. Minireview on Cathodic and Anodic Exfoliation for Recycling Spent Zinc–Carbon Batteries To Prepare Graphene Material: Advances and Outlook of Interesting Strategies. Energy Fuels 2023, 37, 7062–7070. [Google Scholar] [CrossRef]
- Pattaweepaiboon, S.; Hirunpinyopas, W.; Iamprasertkun, P.; Pimphor, K.; Roddecha, S.; Dirayanti, D.; Boonchun, A.; Sirisaksoontorn, W. Upcycling electrode materials from spent single-use zinc-carbon/alkaline batteries into rechargeable lithium-ion battery application. J. Energy Storage 2024, 76, 109755. [Google Scholar] [CrossRef]
- Silva, R.G.; Silva, C.N.; Afonso, J.C. Recovery of manganese and zinc from spent Zn–C and alkaline batteries in acidic medium. Quim. Nova 2010, 33, 1957–1961. [Google Scholar] [CrossRef]
- Ferella, F.; Michelis, I.; Beolchini, F.; Innocenzi, V. Extraction of Zinc and Manganese from Alkaline and Zinc-Carbon Spent Batteries by Citric-Sulphuric Acid Solution. Int. J. Chem. Eng. 2010, 8, 659434. [Google Scholar] [CrossRef]
- Chen, W.; Liao, C.; Lin, K. Recovery Zinc and Manganese from Spent Battery Powder by Hydrometallurgical Route. Energy Procedia 2017, 107, 167–174. [Google Scholar] [CrossRef]
- Ferella, F.; Michelis, I.; Vegliò, F. Process for the recycling of alkaline and zinc–carbon spent batteries. J. Power Sources 2008, 183, 805–811. [Google Scholar] [CrossRef]
- Baba, A.A.; Adekola, F.A.; Bale, R.B.; Alabi, A.G.; Raji, M.A. Economic metals rescue from spent zinc–carbon batteries for industrial value additions. In Energy Technology 2020: Recycling, Carbon Dioxide Management, and Other Technologies; Chen, X., Zhong, Y., Zhang, L., Howarter, J.A., Baba, A.A., Wang, C., Sun, Z., Zhang, M., Olivetti, E., Luo, A., et al., Eds.; Springer: Cham, Switzerland, 2020; pp. 49–55. [Google Scholar] [CrossRef]
- Maryam Sadeghi, S.; Jesus, J.; Soares, H.M.V.M. A critical updated review of the hydrometallurgical routes for recycling zinc and manganese from spent zinc-based batteries. Waste Manag. 2020, 113, 342–350. [Google Scholar] [CrossRef]
- Shangguan, E.; Wang, L.; Wang, Y.; Li, L.; Chen, M.; Qi, J.; Wu, C.; Wang, M.; Li, Q.; Gao, S.; et al. Recycling of zinc−carbon batteries into MnO/ZnO/C to fabricate sustainable cathodes for rechargeable zinc-ion batteries. ChemSusChem 2022, 15, e202200720. [Google Scholar] [CrossRef]
- Abid Charef, S.; Affoune, A.M.; Caballero, A.; Cruz-Yusta, M.; Morales, J. Simultaneous recovery of Zn and Mn from used batteries in acidic and alkaline mediums: A comparative study. Waste Manag. 2017, 68, 518–526. [Google Scholar] [CrossRef]
- Buzatu, M.; Sǎceanu, S.; Petrescu, M.I.; Ghica, G.V.; Buzatu, T. Recovery of zinc and manganese from spent batteries by reductive leaching in acidic media. J. Power Sources 2014, 247, 612–617. [Google Scholar] [CrossRef]
- Nogueira, C.A.; Margarido, F. Hydrometallurgy Selective process of zinc extraction from spent Zn–MnO2 batteries by ammonium chloride leaching. Hydrometallurgy 2015, 157, 13–21. [Google Scholar] [CrossRef]
- Kang, Z.; Huang, Z.; Peng, Q.; Shi, Z.; Xiao, H.; Yin, R.; Fu, G.; Zhao, J. Recycling technologies, policies, prospects, and challenges for spent batteries. iScience 2023, 26, 108072. [Google Scholar] [CrossRef] [PubMed]
- Petruk, R. A Complex Method of Phosphorus-Containing Pesticides Processing into Environmentally Safe Products and Rehabilitation of Contaminated Soils. Ph.D. Thesis, Vinnytsia National Technical University, Vinnytsia, Ukraine, 2013. (In Ukrainian). [Google Scholar]
- Amtraptseva, N.; Primak, S.; Bila, G. Optimal conditionals of synthesis of biologically active additive on basis of zinc-magnesium phosphate. Mod. Eng. Innov. Technol. 2018, 3, 107–111. (In Ukrainian) [Google Scholar] [CrossRef]
- Kohler, T.; Armbruster, T.; Libowitzky, E. Hydrogen Bonding and Jahn–Teller Distortion in Groutite, α-MnOOH, and Manganite, γ-MnOOH, and Their Relations to the Manganese Dioxides Ramsdellite and Pyrolusite. J. Solid State Chem. 1997, 133, 486–500. [Google Scholar] [CrossRef]
- Reidies, A. Manganese Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000; pp. 495–542. [Google Scholar] [CrossRef]
- Yuan, Z.-Y.; Ren, T.-Z.; Du, G.; Su, B.-L. A facile preparation of single-crystalline α-Mn2O3 nanorods by ammonia-hydrothermal treatment of MnO2. Chem. Phys. Lett. 2004, 389, 83. [Google Scholar] [CrossRef]
- Dolina, L.; Gunko, E.; Mashikhina, P. Water Protection from Radioactive Contamination; Lira: Dnipro, Ukraine, 2016. [Google Scholar]
- Hense, P.; Reh, K.; Franke, M.; Aigner, J.; Hornung, A.; Contin, A. Pyrolysis of waste electrical and electronic equipment (WEEE) for recovering metals and energy: Previous achievements and current approaches. Environ. Eng. Manag. J. 2015, 14, 1637–1647. [Google Scholar] [CrossRef]
- Ranskiy, A.; Gordienko, O.; Korinenko, B.; Ishchenko, V.; Sakalova, H.; Vasylynych, T.; Malovanyy, M.; Kryklyvyi, R. Pyrolysis processing of polymer waste components of electronic products. Chem. Chem. Technol. 2024, 1, 103–108. [Google Scholar] [CrossRef]
- Korinenko, B. Improvement of Technology of Polymer Waste Pyrolytic Processing. Ph.D. Thesis, Vinnytsia National Technical University, Vinnytsia, Ukraine, 2023. (In Ukrainian). [Google Scholar]
- Yavorskyi, V.; Zozulia, H.; Bukliv, R. Recycling of valuable components of spent small batteries. Bull. Natl. Univ. “Lviv. Polytech.” Ser. Chem. Technol. Subst. Their Appl. 2014, 787, 117–121. [Google Scholar]
- Diamant, V.; Simonov, A. Recycling technology of active materials of the zinc-manganese current sources. Ukr. Chem. J. 2021, 87, 128–136. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).