Mechanical Recycling of PET Multi-Layer Post-Consumer Packaging: Effects of Impurity Content
Abstract
1. Introduction
2. Framework of the Study
3. Results
3.1. Yield of the Mechanical Recycling Process
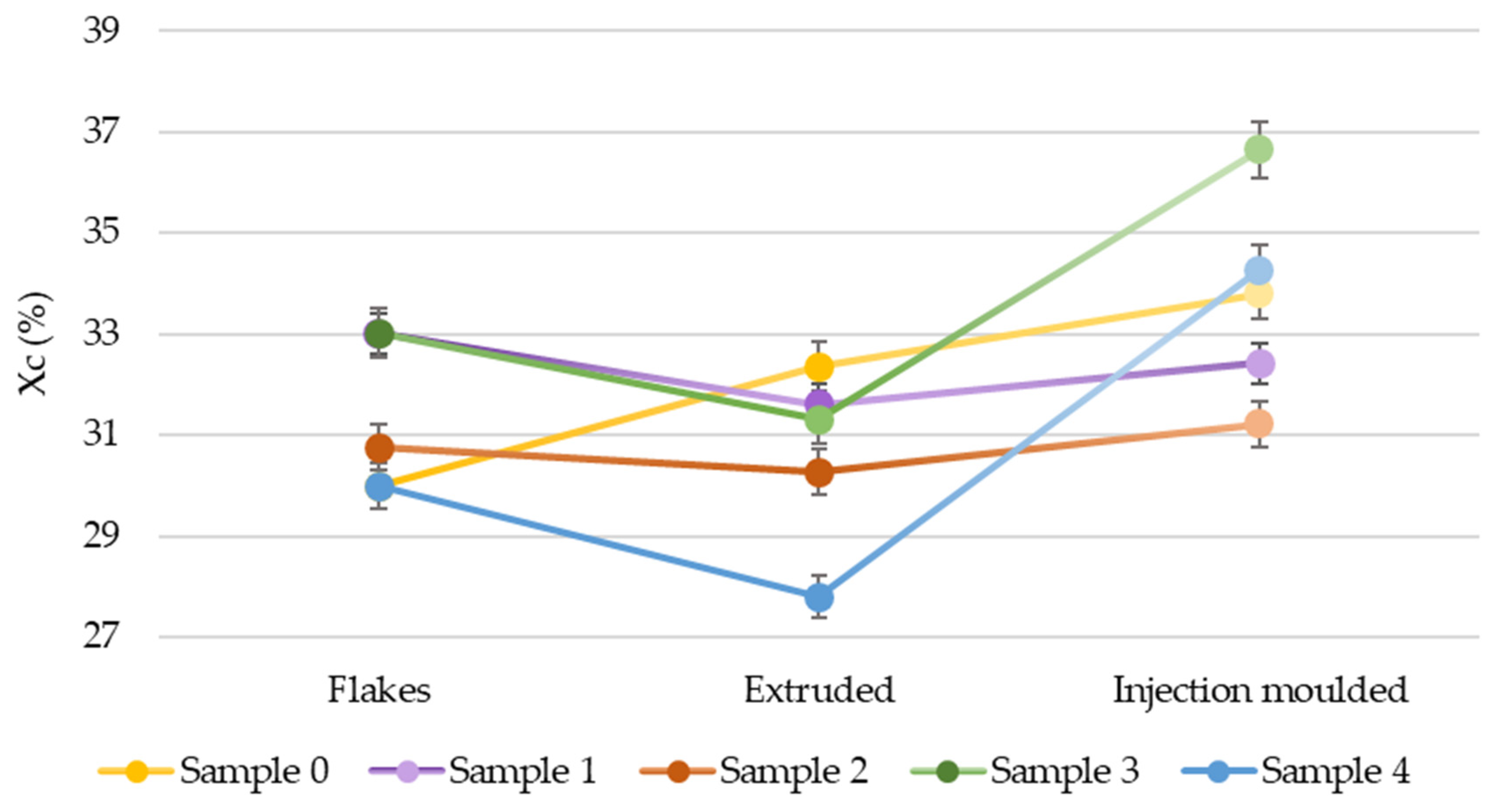
3.2. Crystallinity Evaluation
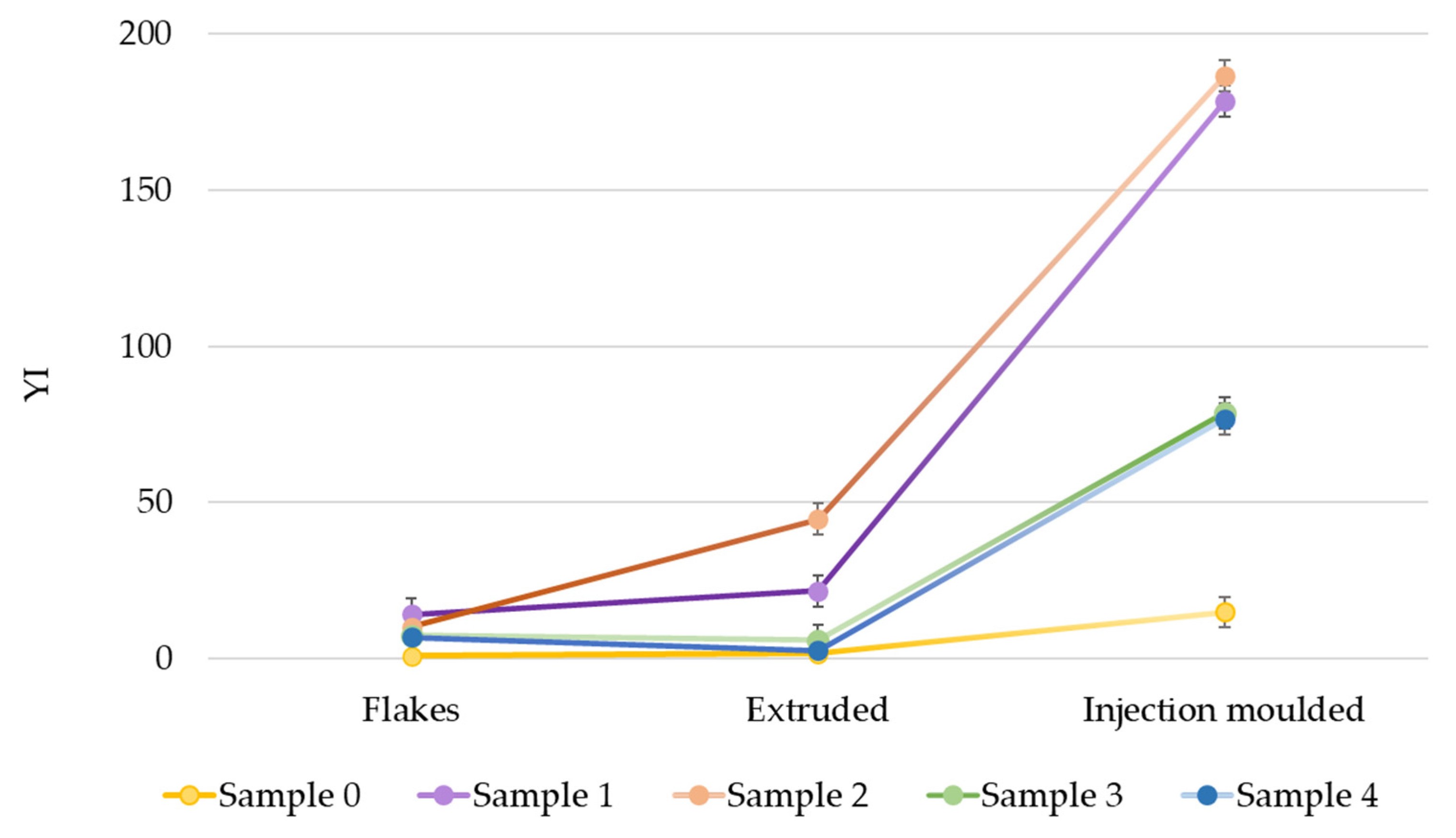
3.3. Optical Properties
4. Materials and Methods
4.1. Materials
4.2. Mechanical Recycling Treatment
4.3. Material Characterisation
4.4. Optical Analyses
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Plastics Europe. The Circular Economy for Plastics—A European Overview. 2022. Available online: https://www.rigk.de/Editors/RIGK/Dateien/Downloads/Studien/PlasticsEurope-CircularityReport-2021_28022022_1.pdf (accessed on 17 August 2023).
- Sharma, N.K.; Govindan, K.; Lai, K.K.; Chen, W.K.; Kumar, V. The Transition from Linear Economy to Circular Economy for Sustainability among SMEs: A Study on Prospects, Impediments, and Prerequisites. Bus Strategy Environ. 2021, 30, 1803–1822. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics the Facts 2022. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 12 August 2023).
- Ferronato, N.; Rada, E.C.; Gorritty Portillo, M.A.; Cioca, L.I.; Ragazzi, M.; Torretta, V. Introduction of the Circular Economy within Developing Regions: A Comparative Analysis of Advantages and Opportunities for Waste Valorization. J. Environ. Manag. 2019, 230, 366–378. [Google Scholar] [CrossRef] [PubMed]
- ICIS; PET. Market in Europe: State of Play, Production, Collection and Recycling Data 2022. Indep. Commod. Intell. Serv. 2024. Available online: https://www.icis.com/explore/resources/pet-market-state-of-play-2022/ (accessed on 10 June 2024).
- European Parliament and Council Directive (EU) 2019/904 on the Reduction of the Impact of Certain Plastic Products on the Environment; Official Journal of the European Union, Brussels: Brussels, Belgium, 2019.
- European Parliament and Council. European Commission Proposal for a Regulation of the European Parliament and of the Council; Joint Research Centre (European Commission): Brussels, Belgium, 2022. [Google Scholar]
- Kahlert, S.; Bening, C.R. Why pledges alone will not get plastics recycled: Comparing recyclate production and anticipated demand. Resour. Conserv. Recycl. 2022, 181, 106279. [Google Scholar] [CrossRef]
- Souder, J.; Kennedy, E.; Xu, C.; Gruber, B.; Paes, C.; Hu, R.; Bustelo, V.; Kamps, J.; Garcia, S.; Björk, M.; et al. The Plastic Product Mass Flow Model 2.0 Modelling Plastic Product Flows and Recycling in the EU; Report; Joint Research Centre (European Commission): Brussels, Belgium, 2024. [Google Scholar] [CrossRef]
- Santomasi, G.; Aquilino, R.; Brouwer, M.; De Gisi, S.; Smeding, I.; Todaro, F.; Notarnicola, M.; Thoden van Velzen, E.U. Strategies to Enhance the Circularity of Non-Bottle PET Packaging Waste Based on a Detailed Material Characterisation. Waste Manag. 2024, 186, 293–306. [Google Scholar] [CrossRef]
- Eriksen, M.K.; Astrup, T.F. Characterisation of Source-Separated, Rigid Plastic Waste and Evaluation of Recycling Initiatives: Effects of Product Design and Source-Separation System. Waste Manag. 2019, 87, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Picuno, C.; Alassali, A.; Chong, Z.K.; Kuchta, K. Flows of Post-Consumer Plastic Packaging in Germany: An MFA-Aided Case Study. Resour. Conserv. Recycl. 2021, 169, 105515. [Google Scholar] [CrossRef]
- Brouwer, M.; Thoden van Velzen, E.U.; Augustinus, A.; Soethoudt, H.; De Meester, S.; Ragaert, K. Predictive Model for the Dutch Post-Consumer Plastic Packaging Recycling System and Implications for the Circular Economy. Waste Manag. 2018, 71, 62–85. [Google Scholar] [CrossRef] [PubMed]
- Kleinhans, K.; Hallemans, M.; Huysveld, S.; Thomassen, G.; Ragaert, K.; Van Geem, K.M.; Roosen, M.; Mys, N.; Dewulf, J.; De Meester, S. Development and Application of a Predictive Modelling Approach for Household Packaging Waste Flows in Sorting Facilities. Waste Manag. 2021, 120, 290–302. [Google Scholar] [CrossRef]
- Faerch Group Cirrec Website. Available online: https://www.cirrec.nl/ (accessed on 5 September 2023).
- B4PET Renewable Plastics B4PET Sito Web. Available online: https://b4pet.com/ (accessed on 10 November 2023).
- Faraca, G.; Astrup, T. Plastic waste from recycling centres: Characterisation and evaluation of plastic recyclability. Waste Manag. 2019, 95, 388–398. [Google Scholar] [CrossRef]
- De Mello Soares, C.T.; Ek, M.; Östmark, E.; Gällstedt, M.; Karlsson, S. Recycling of multi-material multilayer plastic packaging: Current trends and future scenarios. Resour. Conserv. Recycl. 2022, 176, 105905. [Google Scholar] [CrossRef]
- Smith, R.L.; Takkellapati, S.; Riegerix, R.C. Recycling of Plastics in the United States: Plastic Material Flows and Polyethylene Terephthalate (PET) Recycling Processes. ACS Sustain. Chem. Eng. 2022, 10, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Roosen, M.; Mys, N.; Kusenberg, M.; Billen, P.; Dumoulin, A.; Dewulf, J.; Van Geem, K.M.; Ragaert, K.; De Meester, S. Detailed Analysis of the Composition of Selected Plastic Packaging Waste Products and Its Implications for Mechanical and Thermochemical Recycling. Environ. Sci. Technol. 2020, 54, 13282–13293. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, V.H.; Schaffernak, A.; Pfitzner, M.; Fellner, J.; Tacker, M.; Apprich, S. Rigid Polyethylene Terephthalate Packaging Waste: An Investigation of Waste Composition and Its Recycling Potential in Austria. Resources 2023, 12, 128. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Gu, Y.; Wu, Y.; Gong, Y.; Mu, X.; Han, H.; Chang, T. A Systematic Review of the Deposit-Refund System for Beverage Packaging: Operating Mode, Key Parameter and Development Trend. J. Clean. Prod. 2020, 251, 119660. [Google Scholar] [CrossRef]
- Uehara, G.A.; França, M.P.; Canevarolo Junior, S.V. Recycling Assessment of Multilayer Flexible Packaging Films Using Design of Experiments. Polimeros 2015, 25, 371–381. [Google Scholar] [CrossRef]
- Ügdüler, S.; Van Geem, K.M.; Denolf, R.; Roosen, M.; Mys, N.; Ragaert, K.; De Meester, S. Towards Closed-Loop Recycling of Multilayer and Coloured PET Plastic Waste by Alkaline Hydrolysis. Green Chem. 2020, 22, 5376–5394. [Google Scholar] [CrossRef]
- Chen, H.; Wan, K.; Zhang, Y.; Wang, Y. Waste to Wealth: Chemical Recycling and Chemical Upcycling of Waste Plastics for a Great Future. Chem. Sus. Chem. 2021, 14, 4123–4136. [Google Scholar] [CrossRef]
- Tamizhdurai, P.; Mangesh, V.L.; Santhosh, S.; Vedavalli, R.; Kavitha, C.; Bhutto, J.K.; Alreshidi, M.A.; Yadav, K.K.; Kumaran, R. A State-of-the-Art Review of Multilayer Packaging Recycling: Challenges, Alternatives, and Outlook. J. Clean. Prod. 2024, 447, 141403. [Google Scholar] [CrossRef]
- Thoden van Velzen, E.U.; Santomasi, G. Tailor-Made Enzymes Poised to Propel Plastic Recycling into a New Era. Nature 2022, 604, 631–633. [Google Scholar] [CrossRef]
- Lu, H.; Diaz, D.J.; Czarnecki, N.J.; Zhu, C.; Kim, W.; Shroff, R.; Acosta, D.J.; Alexander, B.R.; Cole, H.O.; Zhang, Y.; et al. Machine Learning-Aided Engineering of Hydrolases for PET Depolymerization. Nature 2022, 604, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Amundarain, I.; López-Montenegro, S.; Fulgencio-Medrano, L.; Leivar, J.; Iruskieta, A.; Asueta, A.; Miguel-Fernández, R.; Arnaiz, S.; Pereda-Ayo, B. Improving the Sustainability of Catalytic Glycolysis of Complex PET Waste through Bio-Solvolysis. Polymers 2024, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Naveed, M.; Aayanifard, Z.; Rabnawaz, M. Efficient Chemical Recycling of Waste Polyethylene Terephthalate. Resour. Conserv. Recycl. 2022, 187, 106639. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and Recovery Routes of Plastic Solid Waste (PSW): A Review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- De Moura Giraldi, A.L.F.; De Jesus, R.C.; Mei, L.H.I. The Influence of Extrusion Variables on the Interfacial Adhesion and Mechanical Properties of Recycled PET Composites. J. Mater. Process. Technol. 2005, 162–163, 90–95. [Google Scholar] [CrossRef]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of Plastic Solid Waste: A State of Art Review and Future Applications. Compos. B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Seier, M.; Archodoulaki, V.M.; Koch, T.; Duscher, B.; Gahleitner, M. Polyethylene Terephthalate Based Multilayer Food Packaging: Deterioration Effects during Mechanical Recycling. Food Packag. Shelf Life 2022, 33, 100890. [Google Scholar] [CrossRef]
- Mangold, H.; von Vacano, B. The Frontier of Plastics Recycling: Rethinking Waste as a Resource for High-Value Applications. Macromol. Chem. Phys. 2022, 223, 2100488. [Google Scholar] [CrossRef]
- Ghosh, T.; Avery, G.; Bhatt, A.; Uekert, T.; Walzberg, J.; Carpenter, A. Towards a Circular Economy for PET Bottle Resin Using a System Dynamics Inspired Material Flow Model. J. Clean. Prod. 2023, 383, 135208. [Google Scholar] [CrossRef]
- Trossaert, L.; De Vel, M.; Cardon, L.; Edeleva, M. Lifting the Sustainability of Modified PET-Based Multilayer Packaging Material with Enhanced Mechanical Recycling Potential and Processing. Polymers 2022, 14, 196. [Google Scholar] [CrossRef]
- Gracida-Alvarez, U.R.; Xu, H.; Benavides, P.T.; Wang, M.; Hawkins, T.R. Circular Economy Sustainability Analysis Framework for Plastics: Application for Poly(Ethylene Terephthalate) (PET). ACS Sustain. Chem. Eng. 2023, 11, 514–524. [Google Scholar] [CrossRef]
- Mager, M.; Berghofer, M.; Fischer, J. Polyolefin Recyclates for Rigid Packaging Applications: The Influence of Input Stream Composition on Recyclate Quality. Polymers 2023, 15, 2776. [Google Scholar] [CrossRef]
- Schmidt, J.; Auer, M.; Maletz, R.; Galler, V.; Woidasky, J. Consumer Influence on Lightweight Packaging Waste Generation in Germany. Clean. Responsible Consum. 2024, 12, 100185. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, T.H.; Choo, J.E.; Hwang, S.W. Evaluation of PET Recyclability and Characterization of Modified Reprocessed-PET for Industrial Application. J. Appl. Polym. Sci. 2024, 141. [Google Scholar] [CrossRef]
- Candal, M.V.; Safari, M.; Fernández, M.; Otaegi, I.; Múgica, A.; Zubitur, M.; Gerrica-Echevarria, G.; Sebastián, V.; Irusta, S.; Loaeza, D.; et al. Structure and Properties of Reactively Extruded Opaque Post-Consumer Recycled PET. Polymers 2021, 13, 3531. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Bocz, K.; Molnár, B.; Marosi, G.; Ronkay, F. Preparation of Low-Density Microcellular Foams from Recycled PET Modified by Solid State Polymerization and Chain Extension. J. Polym. Environ. 2019, 27, 343–351. [Google Scholar] [CrossRef]
- Oromiehie, A.; Mamizadeh, A. Recycling PET Beverage Bottles and Improving Properties. Polym. Int. 2004, 53, 728–732. [Google Scholar] [CrossRef]
- Romão, W.; Franco, M.F.; Bueno, M.I.M.S.; De Paoli, M.A. Distinguishing between Virgin and Post-Consumption Bottle-Grade Poly (Ethylene Terephthalate) Using Thermal Properties. Polym. Test. 2010, 29, 879–885. [Google Scholar] [CrossRef]
- Torres, N.; Robin, J.J.; Boutevin, B.; Re, C.E.; Ma, P. Study of Thermal and Mechanical Properties of Virgin and Recycled Poly(Ethylene Terephthalate) before and after Injection Molding. Eur. Polym. J. 1999, 36, 2075–2080. [Google Scholar] [CrossRef]
- Badía, J.D.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Thermal Analysis as a Quality Tool for Assessing the Influence of Thermo-Mechanical Degradation on Recycled Poly(Ethylene Terephthalate). Polym. Test. 2009, 28, 169–175. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D.; Pfaendner, R. Reactive-Extrusion Route for the Closed-Loop Recycling of Poly(Ethylene Terephthalate). J. Appl. Polym. Sci. 2007, 104, 1671–1678. [Google Scholar] [CrossRef]
- Romão, W.; Franco, M.F.; Corilo, Y.E.; Eberlin, M.N.; Spinacé, M.A.S.; De Paoli, M.A. Poly (Ethylene Terephthalate) Thermo-Mechanical and Thermo-Oxidative Degradation Mechanisms. Polym. Degrad. Stab. 2009, 94, 1849–1859. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The Role of Crystalline, Mobile Amorphous and Rigid Amorphous Fractions in the Performance of Recycled Poly (Ethylene Terephthalate) (PET). Polym. Degrad. Stab. 2012, 97, 98–107. [Google Scholar] [CrossRef]
- Cole, K.C.; Ajjii, A.; Pellerin, É. New Insights into the Development of Ordered Structure in Poly(Ethylene Terephthalate). 1. Results from External Reflection Infrared Spectroscopy. Macromolecules 2002, 35, 770–784. [Google Scholar] [CrossRef]
- Peltzer, M.A.; Simoneau, C.; Institute for Health and Consumer Protection. Report of an Inter-Laboratory Comparison from the European Reference Laboratory for Food Contact Materials: ILC 002 2013: Identification of Polymeric Materials; Joint Research Centre (European Commission): Brussels, Belgium, 2013; ISBN 9789279352676. [Google Scholar]
- Sammon, C.; Yarwood, J.; Everall, N. An FTIR Study of the Effect of Hydrolytic Degradation on the Structure of Thin PET films. Polym. Degrad. Stab. 2000, 67, 149–158. [Google Scholar] [CrossRef]
- Edge, M.; Allen, N.S.; Wiles, R.; Mcdonald, W.; Mortlock, S.V. Identification of Luminescent Species Contributing to the Yellowing of Poly(Ethylene Terephthalate) on Degradation. Polymer, 1995; 36, 227–234. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Hussain, S. Perspectives on Yellowing in the Degradation of Polymer Materials: Inter-Relationship of Structure, Mechanisms and Modes of Stabilisation. Polym. Degrad. Stab. 2022, 201, 109977. [Google Scholar] [CrossRef]
- Berg, D.; Schaefer, K.; Koerner, A.; Kaufmann, R.; Tillmann, W.; Moeller, M. Reasons for the Discoloration of Postconsumer Poly(Ethylene Terephthalate) during Reprocessing. Macromol. Mater. Eng. 2016, 301, 1454–1467. [Google Scholar] [CrossRef]
- Fashandi, H.; Zadhoush, A.; Haghighat, M. Effect of Orientation and Crystallinity on the Photodegradation of Poly(Ethylene Terephthalate) Fibers. Polym. Eng. Sci. 2008, 48, 949–956. [Google Scholar] [CrossRef]
- Fechine, G.J.M.; Rabello, M.S.; Souto Maior, R.M.; Catalani, L.H. Surface Characterization of Photodegraded Poly(Ethylene Terephthalate). The Effect of Ultraviolet Absorbers. Polymer 2004, 45, 2303–2308. [Google Scholar] [CrossRef]
- Ciolacu, C.F.L.; Roy Choudhury, N.; Dutta, N.K. Colour Formation in Poly(Ethylene Terephthalate) during Melt Processing. Polym. Degrad. Stab. 2006, 91, 875–885. [Google Scholar] [CrossRef]
- Chilton, T.; Burnley, S.; Nesaratnam, S. A Life Cycle Assessment of the Closed-Loop Recycling and Thermal Recovery of Post-Consumer PET. Resour. Conserv. Recycl. 2010, 54, 1241–1249. [Google Scholar] [CrossRef]
- Masmoudi, F.; Fenouillot, F.; Mehri, A.; Jaziri, M.; Ammar, E. Characterization and Quality Assessment of Recycled Post-Consumption Poly(Ethylene Terephthalate) (PET). Environ. Sci. Pollut. Res. 2018, 25, 23307–23314. [Google Scholar] [CrossRef]
- Gruene Punkt Gruener Punkt—Website. Available online: https://www.gruener-punkt.de/en/downloads (accessed on 5 September 2023).
- Krehula, L.K.; Siročić, A.P.; Dukić, M.; Hrnjak-Murgić, Z. Cleaning Efficiency of Poly(Ethylene Terephthalate) Washing Procedure in Recycling Process. J. Elastom. Plast. 2013, 45, 429–444. [Google Scholar] [CrossRef]
- Bliston Bliston Packaging Producer. Available online: https://bliston.nl/en/ (accessed on 23 October 2023).
- Nisticò, R. Polyethylene Terephthalate (PET) in the Packaging Industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Taheri, S.; Zadhoush, A.; Mehrabani-Zeinabad, A. Hydrolytic Degradation of Poly(Ethylene Terephthalate). J. Appl. Polym. Sci. 2007, 103, 2304–2309. [Google Scholar] [CrossRef]
- Alvarado Chacon, F.; Brouwer, M.T.; Thoden van Velzen, E.U.; Smeding, I.W. A First Assessment of the Impact of Impurities in PP and PE Recycled Plastics; Report; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Spaseska, D.; Civkaroska, M. Alkaline Hydrolysis of Poly(Ethylene Terephthalate) Recycled from the Post-Consumer Soft-Drink Bottles. J. Univ. Chem. Technol. Metall. 2010, 45, 379–384. [Google Scholar]
- Baskaran, S.; Sathiavelu, M. Application of Attenuated Total Reflection—Fourier Transform Infrared Spectroscopy to Characterize the Degradation of Littered Multilayer Food Packaging Plastics. Vib. Spectrosc. 2020, 109, 103105. [Google Scholar] [CrossRef]
- He, J.J.; Gilpatrick, B. Applications of DSC in Conjunction with FTIR in Plastic Identification; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Martin, L.; Brandau, O. PET Preforms. In Bottles, Preforms and Closures; Elsevier: Amsterdam, The Netherlands, 2012; pp. 47–77. [Google Scholar]
- Bertoldo, M.; Labardi, M.; Rotella, C.; Capaccioli, S. Enhanced Crystallization Kinetics in Poly(Ethylene Terephthalate) Thin Films Evidenced by Infrared Spectroscopy. Polymer 2010, 51, 3660–3668. [Google Scholar] [CrossRef]
- Šudomová, L.; Doležalová Weissmannová, H.; Steinmetz, Z.; Řezáčová, V.; Kučerík, J. A Differential Scanning Calorimetry (DSC) Approach for Assessing the Quality of Polyethylene Terephthalate (PET) Waste for Physical Recycling: A Proof-of-Concept Study. J. Therm. Anal. Calorim. 2023, 148, 10843–10855. [Google Scholar] [CrossRef]
- ASTM D4603 03; Standard Test Method for Determining Inherent Viscosity of Poly(Ethylene Terephthalate) (PET) by Glass Capillary Viscometer. ASTM International: West Conshohocken, PA, USA, 2012. [CrossRef]
- Berkowitz, S. Viscosity Molecular Weight Relationships for Poly(Ethylene Terephthalate) in Hexafluoroisopropanol-Pentafluorophenol Using SEC-LALLS. J. Appl. Polym. Sci. 1984, 29, 4353–4361. [Google Scholar] [CrossRef]
- Billmeyer, F.W. Methods for Estimating Intrinsic Viscosity. J. Polym. Sci. 1949, 4, 83–86. [Google Scholar] [CrossRef]
- Sommerhuber, P.F.; Welling, J.; Krause, A. Substitution Potentials of Recycled HDPE and Wood Particles from Post-Consumer Packaging Waste in Wood-Plastic Composites. Waste Manag. 2015, 46, 76–85. [Google Scholar] [CrossRef]
- Adebayo, G.O.; Yahya, R. Characterisation of Heat Modified Mangrove Fibre for Polymer Composite Applications. In Proceedings of the Scholar summit 2017 Conference, Depok, Indonesia, 10–11 October 2017. [Google Scholar]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-Limonene Blends for Food Packaging Applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Saberi, B.; Thakur, R.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Optimization of Physical and Optical Properties of Biodegradable Edible Films Based on Pea Starch and Guar Gum. Ind. Crops Prod. 2016, 86, 342–352. [Google Scholar] [CrossRef]
- Boehme, M.; Charton, C. Properties of ITO on PET Film in Dependence on the Coating Conditions and Thermal Processing. Surf. Coat. Technol. 2005, 200, 932–935. [Google Scholar] [CrossRef]
- Cardi, N.; Po, R.; Giannotta, G.; Occhiello, E.; Garbassi, F.; Messina, G. Chain Extension of Recycled Poly(Ethylene Terephthalate) with 2,2′-Bis(2-Oxazoline). J. Appl. Polym. Sci. 1993, 50, 1501–1509. [Google Scholar] [CrossRef]
- Paci, M.; Mantis, F.P. La Competition between Degradation and Chain Extension during Processing of Reclaimed Poly(Ethylene Terephthalate). Polym. Degrad. Stab. 1998, 61, 417–420. [Google Scholar] [CrossRef]
- Paci, M.; Mantia, F.P. La Influence of Small Amounts of Polyvinylchloride on the Recycling of Polyethyleneterephthalate. Polym. Degrad. Stab. 1999, 63, 11–14. [Google Scholar] [CrossRef]
- Dimitrov, N.; Kratofil Krehula, L.; Ptiček Siročić, A.; Hrnjak-Murgić, Z. Analysis of Recycled PET Bottles Products by Pyrolysis-Gas Chromatography. Polym. Degrad. Stab. 2013, 98, 972–979. [Google Scholar] [CrossRef]
- Szarka, G.; Iván, B. Thermal Properties, Degradation and Stability of Poly(Vinyl Chloride) Predegraded Thermooxidatively in the Presence of Dioctyl Phthalate Plasticizer. J. Macromol. Sci. A 2013, 50, 208–214. [Google Scholar] [CrossRef]
- Elamri, A.; Zdiri, K.; Harzallah, O.; Lallam, A. Progress in Polyethylene Terephthalate Recycling. In Polyethylene Terephthalate: Uses, Properties and Degradation; Nova Science Publishers: Hauppauge, NY, USA, 2020; ISBN 9781536119916. [Google Scholar]
- Park, S.H.; Lee, T.H.; Park, Y., II; Noh, S.M.; Kim, J.C. Effect of the N-Butyl Acrylate/2-Ethylhexyl Acrylate Weight Ratio on the Performances of Waterborne Core–Shell PSAs. J. Ind. Eng. Chem. 2017, 53, 111–118. [Google Scholar] [CrossRef]
- Czech, Z.; Pelech, R. The Thermal Degradation of Acrylic Pressure-Sensitive Adhesives Based on Butyl Acrylate and Acrylic Acid. Prog. Org. Coat. 2009, 65, 84–87. [Google Scholar] [CrossRef]
- Czech, Z.; Kowalczyk, A.; Kabatc, J.; Swiderska, J. Thermal Stability of Poly(2-Ethylhexyl Acrylates) Used as Plasticizers for Medical Application. Polym. Bull. 2013, 70, 1911–1918. [Google Scholar] [CrossRef]
- Navarro, R.; Ferrándiz, S.; López, J.; Seguí, V.J. The Influence of Polyethylene in the Mechanical Recycling of Polyethylene Terephtalate. J. Mater. Process Technol. 2008, 195, 110–116. [Google Scholar] [CrossRef]
- Fitaroni, L.B.; de Oliveira, É.C.; Marcomini, A.L.; Paranhos, C.M.; Freitas, F.L.; Cruz, S.A. Reprocessing and Solid State Polymerization on Contaminated Post-Consumer PET: Thermal and Crystallization Behavior. J. Polym. Environ. 2020, 28, 91–99. [Google Scholar] [CrossRef]
- Welle, F. Simulation of the Decontamination Efficiency of PET Recycling Processes Based on Solid-State Polycondensation. Packag. Technol. Sci. 2014, 27, 141–148. [Google Scholar] [CrossRef]
- Hadi, A.J.; Najmuldeen, G.F.; Ahmed, I. Polyolefins Waste Materials Reconditioning Using Dissolution/Reprecipitation Method. APCBEE Proc. 2012, 3, 281–286. [Google Scholar] [CrossRef]
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic flexible films waste management—A state of art review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef]
- Mostafapoor, F.; Khosravi, A.; Fereidoon, A.; Khalili, R.; Jafari, S.H.; Vahabi, H.; Formela, K.; Saeb, M.K. Chapter 12—Interface analysis of compatibilized polymer blends. In Compatibilization of Polymer Blends; Elsevier: Amsterdam, The Netherlands, 2020; pp. 349–371. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]



| Sample | X | Y |
|---|---|---|
| T.1 | 1 | 717 |
| T.2 | 2 | 950 |
| T.3 | 4.5 | 1473 |
| T.4 | 7 | 1735 |
| E.0 | 7 | 1925 |
| E.1 | 1.5 | 509 |
| E.2 | 1.5 | 589 |
| E.3 | 5 | 1284 |
| E.4 | 6 | 1402 |
| Sample | Feedstocks Composition | Impurity Concentration * | PET Concentration |
|---|---|---|---|
| 0 | Reference sample, mono-material PET tray from producer (not waste), without objects | 0% | 100% |
| 1 | Industrial PET tray SPs from Dutch system, including faulty sorted objects and attached residual waste (only non-coloured waste) | 19.03% | 80.97% |
| 2 | Industrial PET tray SPs from Dutch system, excluding faulty sorted objects and attached residual waste (only non-coloured waste) | 15.52% | 84.48% |
| 3 | Complete PET trays, including packaging components made from non-PET polymers such as labels, caps and closures (only non-coloured waste) | 4.33% | 95.67% |
| 4 | PET trays, excluding packaging components made from non-PET polymers such as labels, caps and closures (only non-coloured waste) | 1.48% | 98.52% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santomasi, G.; Todaro, F.; Petrella, A.; Notarnicola, M.; Thoden van Velzen, E.U. Mechanical Recycling of PET Multi-Layer Post-Consumer Packaging: Effects of Impurity Content. Recycling 2024, 9, 93. https://doi.org/10.3390/recycling9050093
Santomasi G, Todaro F, Petrella A, Notarnicola M, Thoden van Velzen EU. Mechanical Recycling of PET Multi-Layer Post-Consumer Packaging: Effects of Impurity Content. Recycling. 2024; 9(5):93. https://doi.org/10.3390/recycling9050093
Chicago/Turabian StyleSantomasi, Giusy, Francesco Todaro, Andrea Petrella, Michele Notarnicola, and Eggo Ulphard Thoden van Velzen. 2024. "Mechanical Recycling of PET Multi-Layer Post-Consumer Packaging: Effects of Impurity Content" Recycling 9, no. 5: 93. https://doi.org/10.3390/recycling9050093
APA StyleSantomasi, G., Todaro, F., Petrella, A., Notarnicola, M., & Thoden van Velzen, E. U. (2024). Mechanical Recycling of PET Multi-Layer Post-Consumer Packaging: Effects of Impurity Content. Recycling, 9(5), 93. https://doi.org/10.3390/recycling9050093










