Abstract
Excessive use of plastic mulches has triggered a series of environmental problems, primarily due to the large volumes generated and their low or non-existent degradability. For this reason, materials with similar characteristics to synthetic mulches but with a biodegradable character were sought. In this work, mulch films were produced from gelatin/glycerol/cellulose (GelC) and chitosan/glycerol/cellulose (ChiC). Their biodegradation time in soil and photographic analysis using scanning electron microscopy (SEM) were determined. The GelC sample presented a weight loss of 80% at 25 days, compared to 58% for the ChiC sample in the same exposure time. However, the latter was the only sample that could be evaluated up to 70 days, during which it presented its greatest weight loss (97%). The SEM results for both mulch films showed some color changes after 30 days; complete fracturing, growth of mycelium on the surface, and the presence of pores were observed. FTIR spectra revealed a decrease in hydroxyl groups, amides, and carbonyl bands as the number of degradation days increased. Obtaining polymers from waste materials, such as mango, represents an important task to obtain cellulose that can both reinforce and provide biodegradable properties to biobased materials, which can be degraded by microorganisms present in the soil.
1. Introduction
In the agriculture of small fruit and vegetable producers, mulching is a technique that consists of covering the soil with materials such as straw, sawdust, rice husks, and paper or plastic films in order to protect the soil and the root zone of the crop from various environmental factors, as well as pests and weeds [1,2,3]. This technique mainly seeks to protect delicate crops from unfavorable conditions, whether biotic or abiotic, resulting from extreme climatic conditions, with the aim of avoiding production losses that are reflected in crop yields [4,5].
Although it is true that mulches improve production, one of the most significant concerns regarding their use is that most of them are plastic film mulches, which are primarily made of synthetic materials derived from petroleum [2,6]. A major drawback of these synthetic mulches is that once used, they must be removed from the field, and most are either thrown into landfills or burned, which represents a significant threat to the environment [7,8]. However, even after the practice of removing synthetic mulches, there are reports that a considerable percentage (11%) remains in the field, which represents 8 kg/ha [9]. The persistence in the soil of these fragments of synthetic material can last for long periods of time, resulting in both the release of toxins and the adsorption of contaminants, as well as the contamination of water and other organisms [10,11].
A viable strategy to address this accumulation of synthetic material in soil and the associated risks of contamination is the search for materials designed to be degraded by microorganisms present in the soil. Biobased mulch films made from natural biodegradable polymers—which are a special class of environmentally friendly materials obtained from biomass by chemical treatment, microorganisms, and enzymes [12]—are a potential alternative, since they can also provide benefits that reduce production losses of agricultural products and can be degraded by microorganisms present in the soil if traces remain there [2,13].
Various natural materials from biopolymers, which mostly come from renewable resources, are being used to make degradable biobased materials. Among these biopolymers based on natural polysaccharides, we can mention cellulose, the most abundant polymer in the world, which is renewable, biodegradable, and considered a classic example of a reinforcing biopolymer in polymeric matrices [14,15]. Similarly, we find chitosan, a copolymer obtained after a series of deacetylations of chitin, which is considered the second most abundant polymer in nature [16,17]. Another natural polymer, gelatin, a high molecular weight polypeptide, is also of equal importance. This polymer is produced after a series of hydrolyses of collagen from the bones, skin, and muscle membranes of animals [18]. It stands out, among other characteristics, for being low cost and for presenting good properties in the formation of degradable films [19].
However, understanding polymer biodegradation (microbial biological degradation of polymers) in soils remains a major challenge due to its dependence on polymer properties, soil characteristics, environmental conditions [20], and mulch thickness since this determines the durability of mulch films. To address this knowledge gap on biopolymer degradation, the objective of this study was to prepare mulch films from biopolymer formulations and evaluate their biodegradation time in soil.
2. Material and Methods
Micro cellulose from fibrous endocarp of waste mango (Mangifera caesia Jack ex Wall) was employed in this study. This was used as reinforcing material with the following characteristics: 40–400 μm in length and 72.44% crystallinity [21]. The gelatin was of bovine origin, in a granulated form and yellow color, purchased from Gelita Ltd. (Lerma, Mexico). The chitosan was purchased from Sigma-Aldrich Ltd. (Berlin, Germany), with a medium molecular weight, degree of deacetylation of 75–85%, and a viscosity of 200–800 cps. The reagent-grade glycerol was purchased from J.T. Backer, Ltd. (Phillipsburg, NJ, USA).
2.1. Preparation of Liquid Mulch Film
Two mulch films were prepared in the laboratory using gelatin and chitosan, each material reinforced with microcellulose. The gelatin/glycerol/cellulose (GelC) sample used 3% (w/v) hydrated gelatin. When the blend was homogeneous, 0.1% microcellulose and 0.9 g of glycerol were added. The blend was stirred and heated at a temperature of 45 °C. To prepare the chitosan/glycerol/cellulose (ChiC) sample, 1% (w/v) of chitosan was hydrated in an aqueous solution of glacial acetic acid (0.1 M) at 25 °C, 0.1% microcellulose and 0.9 g of glycerol were added. The formulation was degassed in an ultrasonic cleaner (Branson 2510MT ultrasonic cleaner, Marshall Scientific LLC. Hampton, NH, USA) for 15 min [22]. For both solutions, the film-forming solution was poured onto a plate and dried at 40 °C in a continuous flow oven for 12 h [23]. The films obtained were separated from the Petri dishes and stored in a desiccator (25 °C) for later analysis; to prevent the samples from drying out, a relative humidity of 57% was provided by a saturated saline solution of NaBr.
2.2. Film Thickness
The thickness of each sample was determined according to the ASTM D6988-21 standard [24] using a digital micrometer with an accuracy of 0.001 mm (Mitutoyo Corp. Tokyo, Japan) at 6 random positions along the film.
2.3. Soil Composition
The composition of the soil used for the degradation analysis is shown in Table 1; the content of heavy metals (mg kg−1) was determined using the X-ray fluorescence technique [25].

Table 1.
Soil analysis values.
2.4. Biodegradation Studies in Soil
The samples were cut into 3 cm2 pieces and placed on the surface of the Petri dish with the soil sample. The weight loss assessment was determined gravimetrically; the samples were weighed at 5, 10, 15, 20, 30, and 70 days of degradation. For this, four records were taken per sample; using the ANOVA statistical method p < 0.05, the data obtained were analyzed. A transparent mulch LDPE sample (0.1 mm thick) was used as a reference (hereafter PM). The samples were cleaned and dried at 60 °C for 24 h, weighed, and the final weight was recorded. Weight loss was calculated using Equation (1), and photographic analysis was performed [26].
2.5. Surface Analysis of Mulches
Photomicrographs were obtained with Carl Zeiss EVO LS 10 equipment (Woonsocket, RI, USA). Samples (5 × 5 mm) that were undergoing biodegradation tests were taken for analysis and were attached to a dual-adhesion carbon conductive tape. The analyzed sample was not returned to the petri dish. Images were obtained at a voltage of 20.0–30.0 kV, at controlled laboratory temperature (20 °C), with a resolution of 3–10 nm and magnifications at 500× [27].
2.6. Functional Group Analysis
The samples were previously dried (40 °C) for 24 h with the intention of removing traces of adsorbed water. Fourier transform infrared spectroscopy (FTIR) studies were carried out using a Perkin-Elmer-Spectrum 100–100 N FT-IR Infrared Spectrometer (Waltham, MA, USA) following the methodology of Montoya-Escobar et al. [28], with some modifications made specifically to the resolution and number of scans. The infrared region was in the range of 4000–650 cm−1 in transmittance mode, with a resolution of 16 cm−1 and 24 scans.
3. Results
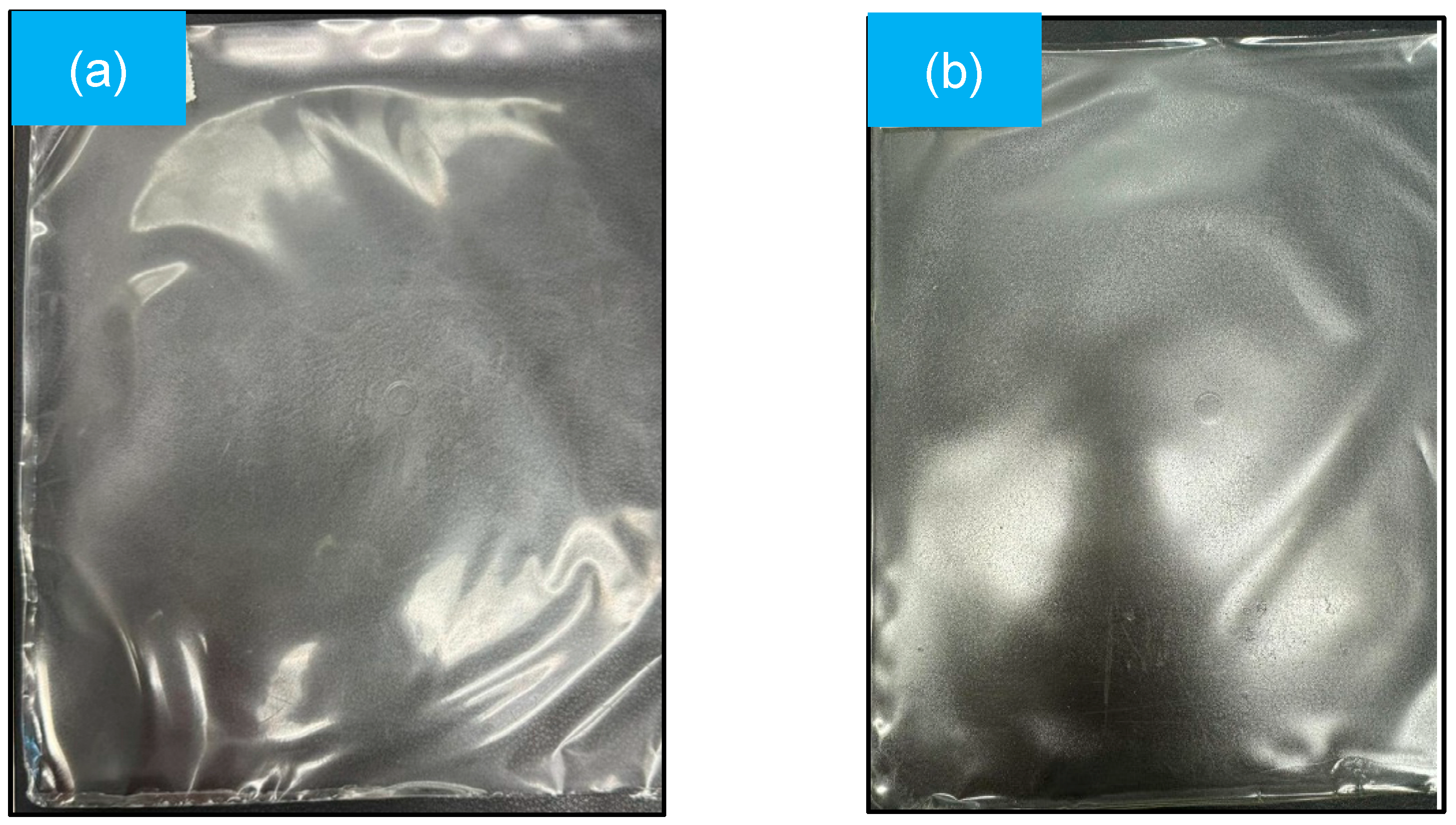
Figure 1 shows the mulch film obtained by the plate casting method, with thicknesses of 0.045 and 0.055 mm for the GelC and ChiC samples, respectively. These values are within the parameters indicated by the ASTM standard [24], whose nominal thickness does not exceed 0.25 mm. The thickness of the mulch is important, as it can determine the durability and positively influence factors such as soil temperature and humidity, as well as the type of agricultural or horticultural crop where it will be used [29,30,31].
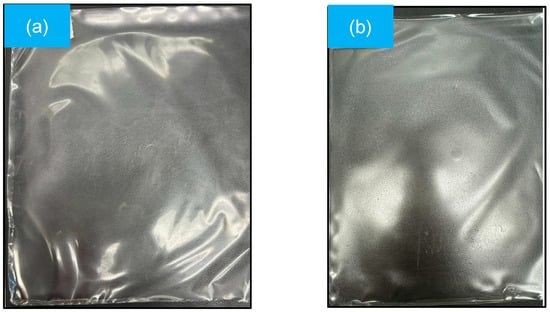
Figure 1.
Mulch films obtained by the plate casting method: (a) GelC and (b) ChiC.
Both mulch films (Figure 1a,b) tend to have a uniform, smooth, transparent, and homogeneous appearance; furthermore, no material that has not dissolved is detected.
3.1. Biodegradation Studies in Soil
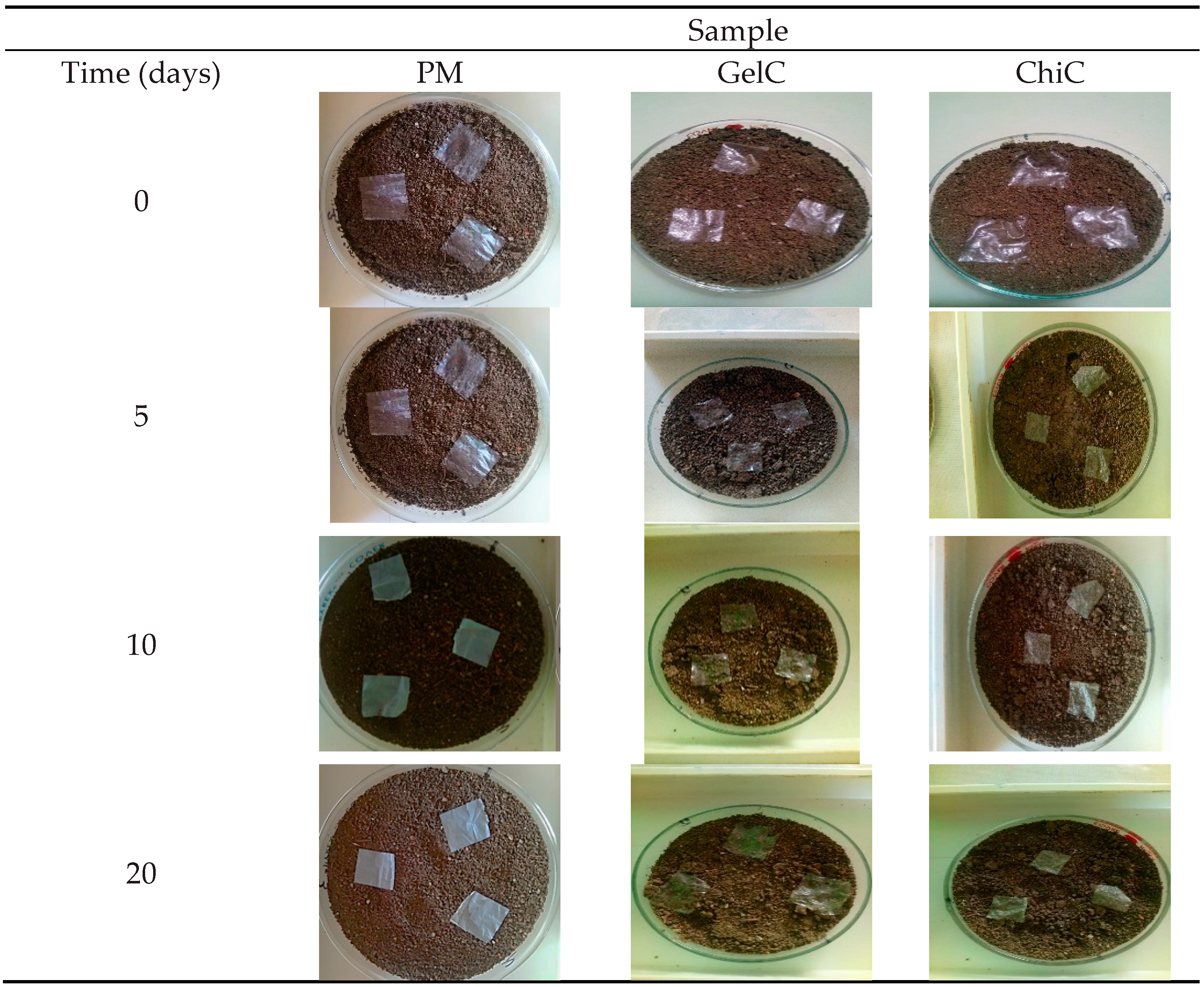
The weight loss values and photographic records of the materials during degradation are shown in Table 2 and Figure 2.

Table 2.
Record of weights and percentages of weight loss of mulch films.
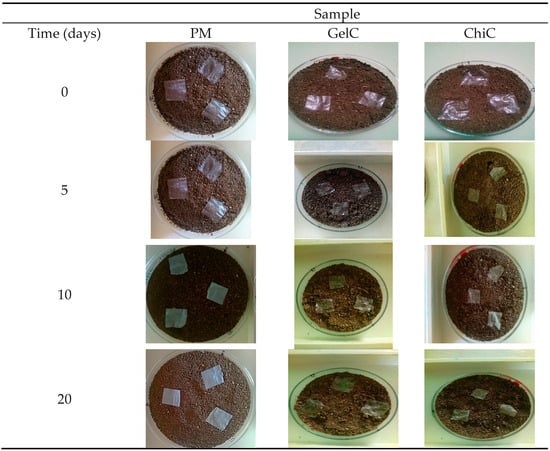

Figure 2.
Photographic record of biodegraded mulch films.
The PM sample was taken as a reference and did not show any visible changes throughout the analysis. No color changes or cracks were observed, which would be an indicator of its degradation. Furthermore, no fragmentation of this material was observed, and its weight remained constant during the 70 days of degradation. This result clearly indicates that the presence of these synthetic mulches in soil and in contact with microorganisms is not sufficient for biodegradation, and they can, therefore, remain for long periods of time in the environment [32].
It can be observed that after five days, the GelC sample exhibited changes in appearance: the material shrank, and yellow marks appeared around it. These changes are attributed to the polymer matrix due to its hydrophilic character; the content of glycerine increases the absorption and activity of water within the film [33].
Ten days into the experiment, GelC became brittle, an occurrence attributed to the leaching of the plasticizer. After 20 days of exposure, GelC and ChiC showed a weight loss of 68% and 55% (Table 2), respectively. At the end of 25 days, GelC showed 80% weight loss, while ChiC only 58%. Observations indicate that GelC degrades faster than ChiC, since at day 70, a weight loss of 97% could be observed for ChiC. This occurrence can be attributed to the crystallinity of cellulose and chitosan. Since they have complex structures, microorganisms take longer to adapt and break the bonds between the glucose units [34], making biodegradation slower. The results showed that microbial activity in the soil for each sample could be easily observed after the first few days, similar to the biodegradation studies with cotton and linen fibers [35], which presented a behavior similar to that found here, this referring to biodegradation times and without forgetting that cellulose is the main component in both cases.
On the other hand, the biodegradation results found here clearly indicate that cellulose-reinforced mulch demonstrates that it is a promising alternative to synthetic materials mulches. While biodegradable mulches have been proposed as alternatives to synthetic mulches to address the ongoing problem of plastic waste accumulation in the environment [36], they are not expected to have any adverse effects on the characteristics of the soil, as this polymer biodegrades into water, carbon dioxide and compost—elements that are assimilated back into nature. The opposite situation occurs with the fragments of synthetic materials, resulting in the release of toxins and the adsorption of contaminants [10,11].
The biobased mulch films will be used as protective covers for soils during the cultivation of horticultural products and, after their useful life, can biodegrade through the action of microorganisms present in the soil. On the other hand, the use of these biodegradable mulches is expected to provide numerous benefits for the growth and productivity of crops, helping to control weeds and insects, reducing the evaporation of irrigation water, and avoiding direct contact between fruits or vegetables and the cultivated soil.
This aligns with the works of Zhang et al. [37] and Bianchini et al. [38], who studied the role that biodegradable films play in crops as a promising replacement for conventional polyethylene-based mulch films. Both authors conclude that biobased mulch films show higher degradation rates than commercial plastic mulches. A situation similar to what was found here, with shorter biodegradation times and even complete biodegradation, will ensure that there is no presence of residual particles in the soil (refer to Figure 2 and Table 2).
3.2. Surface Analysis of Mulches
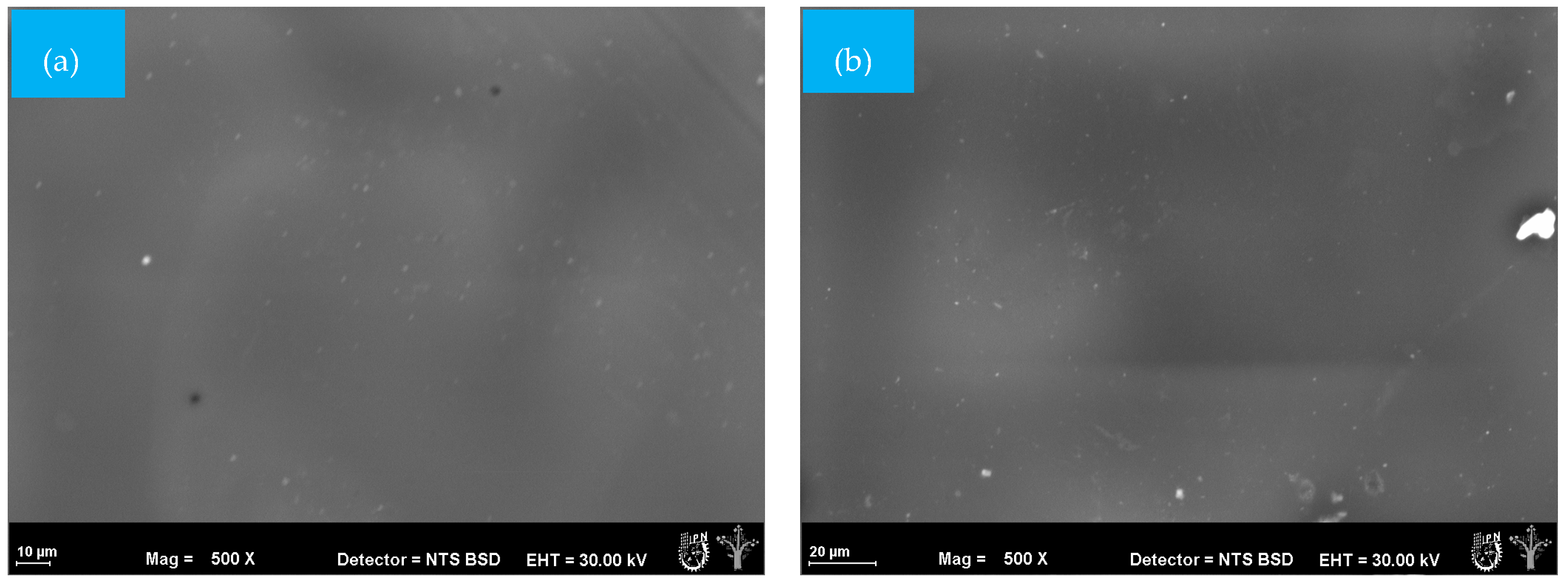
Morphological changes due to exposure to soil at different degradation times were analyzed in mulch films using SEM analysis. Deterioration is a superficial degradation that modifies the mechanical, physical, and chemical properties of a wide variety of materials, such as mulches. It is mainly the result of the activity of microorganisms growing on the surface or inside a material [35]. The surface of the PM sample showed roughness and some irregularities associated with the processing and fabrication of the material (Figure 3a). Some color changes could be observed after 25 days but without the presence of pores or irregularities (Figure 3b).
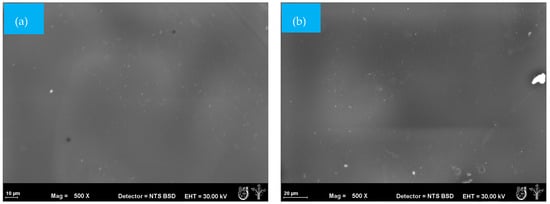
Figure 3.
SEM micrographs (500×) of the surface morphology of PM mulch films at different times during the biodegradation test: (a) = 0 days and (b) 25 days.
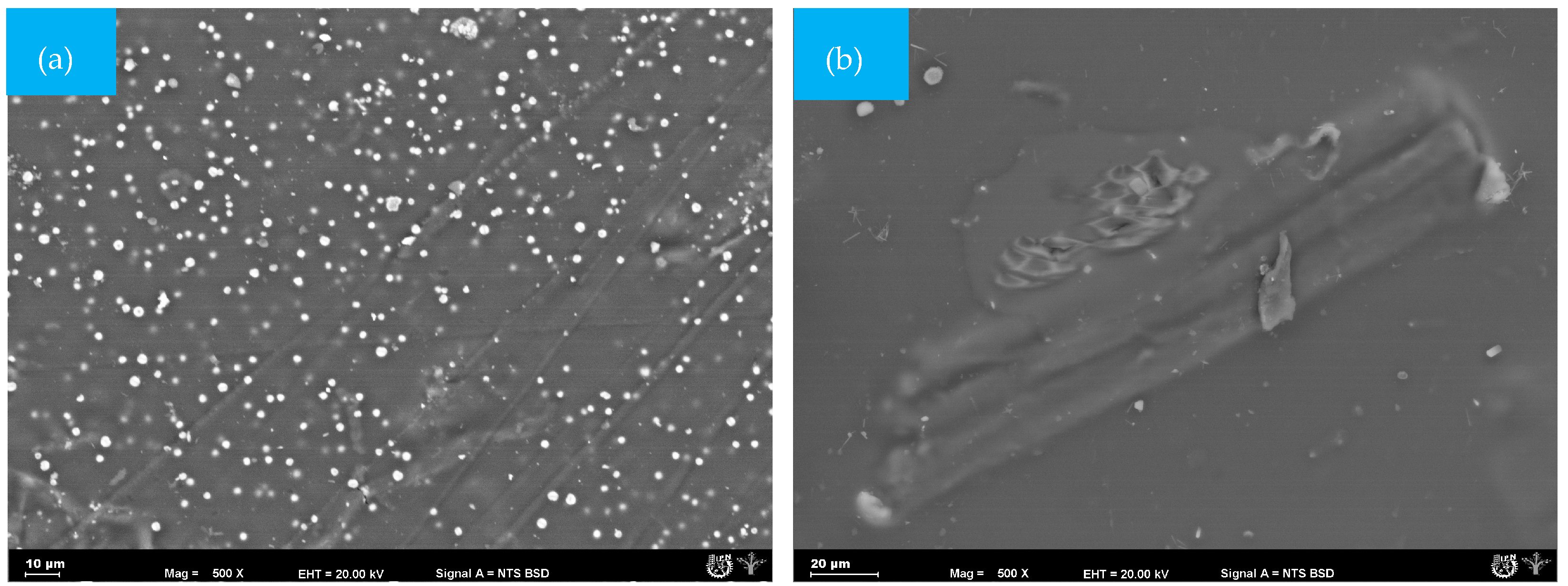
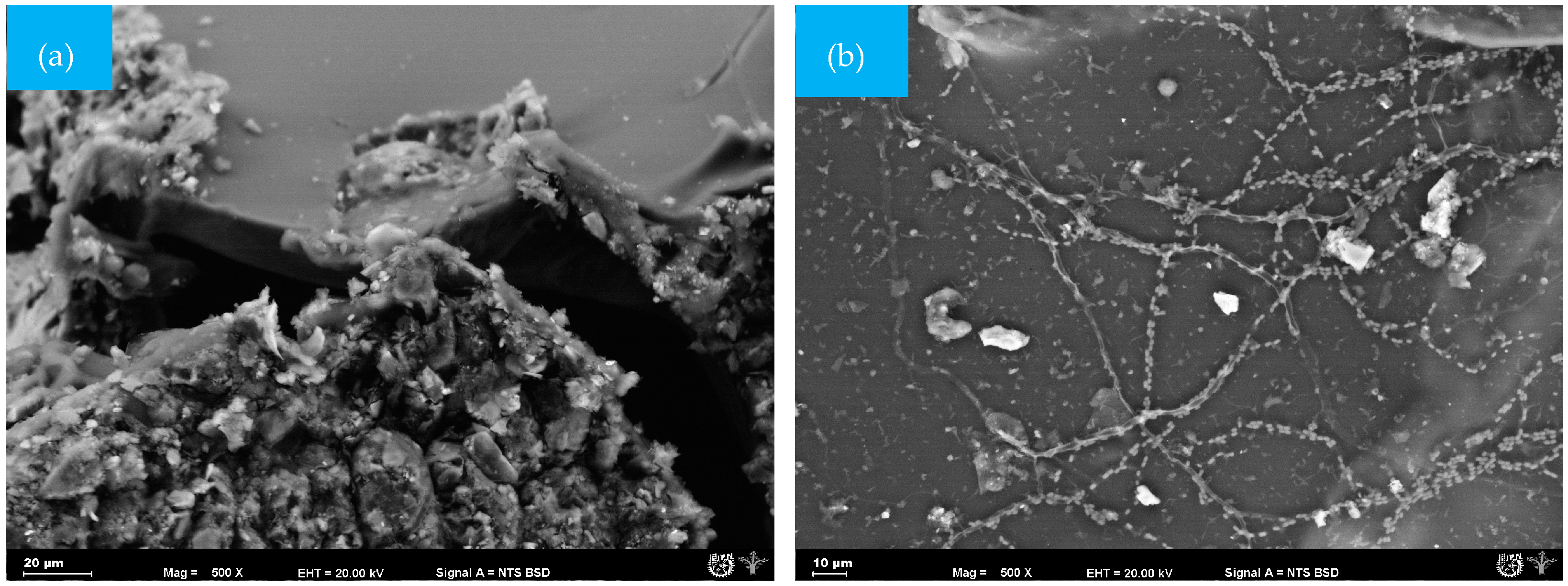
In the GelC film, small spherical structures, similar to gelatin particles, were observed (Figure 4a). However, after 25 days, the sample was completely fractured, and the analyzed fraction that could be recovered showed the growth of mycelia and pores on the surface (Figure 4b). The presence of mycelium is causing damage to the surface of the GelC sample [39]; likewise, weight loss was observed, as detailed in Table 2.
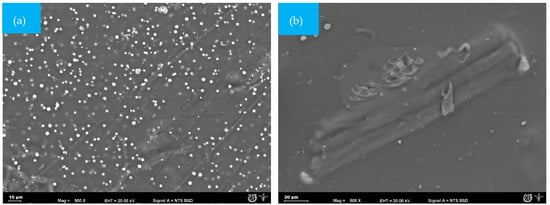
Figure 4.
SEM micrographs (500×) of the surface morphology of CelC mulch films at different times during the biodegradation test: (a) = 0 days and (b) 25 days.
Figure 5a shows the surface of the ChiC sample at the start of the test; this material did not present pores. However, the cellulose microfibres integrated into the mixture can be seen. As degradation progresses, fungi and bacteria become very active, leading to the absolute disappearance of the smooth surface of the composite matrix.
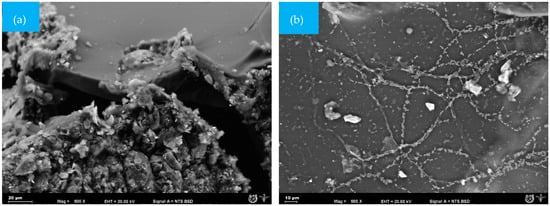
Figure 5.
SEM micrographs (500×) of the surface morphology of ChiC mulch films at different times during the biodegradation test: (a) = 0 days and (b) 25 days.
During the evaluation time, ChiC was the sample that presented the lowest number of mycelia and spores. On day 25 (Figure 5b), the first mycelia appeared on the surface, which was attributed to glycerol and the amorphous regions of the biopolymers present in the matrix. Likewise, the measured attack by microorganisms is due to the antifungal capacity of chitosan, thus avoiding accelerated degradation [40,41,42].
The presence of filaments of microorganisms on the surface of the films aligns well with the fact that fungi are ubiquitous and extremely effective in their biodegradation effect under different moisture regimes [43], as observed during the biodegradation test.
3.3. Functional Group Analysis
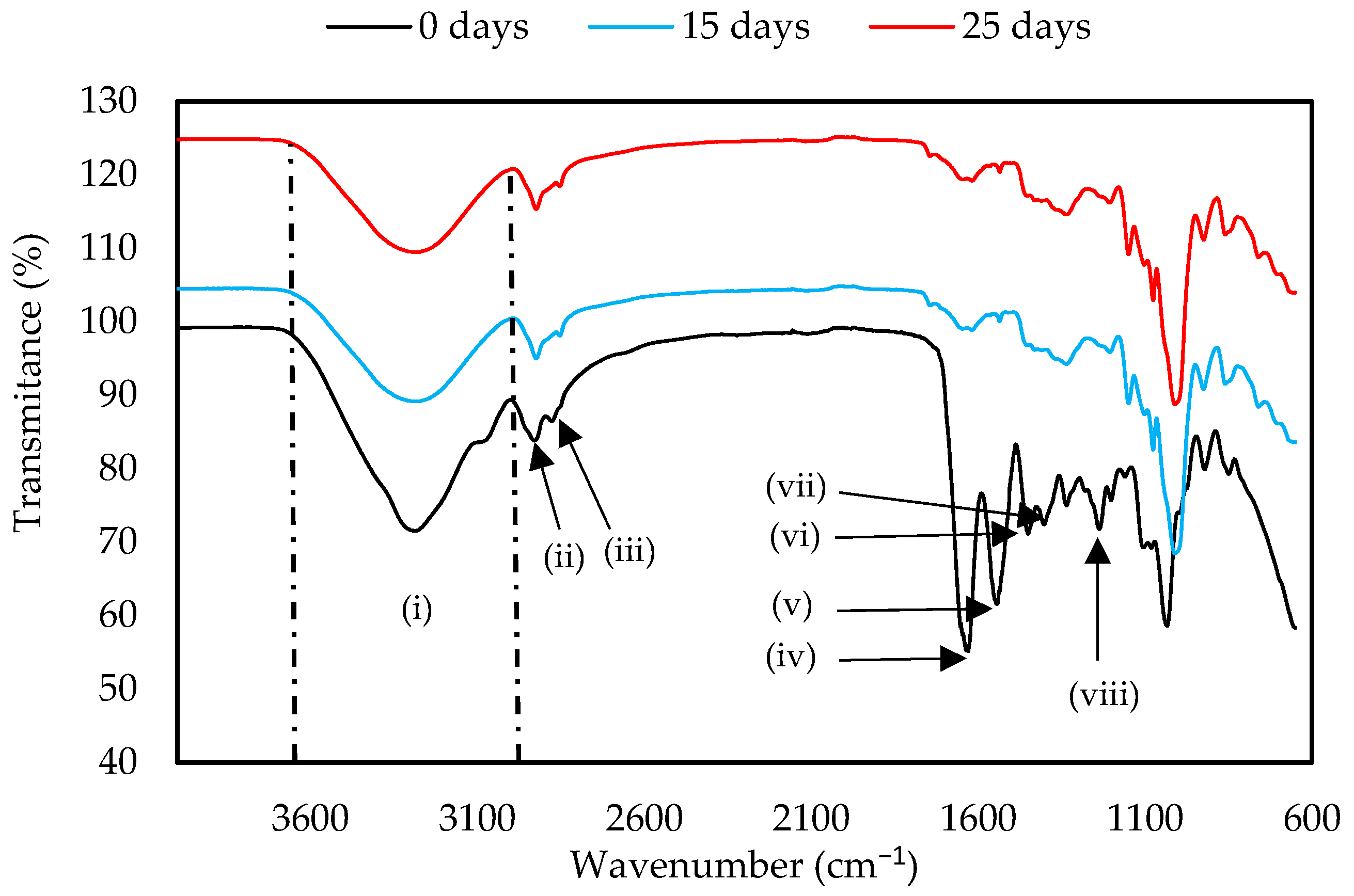
The functional groups of the GelC and ChiC materials were determined before and on day 25 of the test, which has been considered the end of the biodegradation test for this sample, as by day 30, it was not possible to have a sample for analysis due to its almost complete biodegradation (see Figure 2). Both samples showed three intense signals at the beginning of the test (Figure 6 and Figure 7). The first signal corresponds to the stretching of abundant hydroxyl groups (−OH) in polysaccharides between 3600–3000 cm−1 (i), which belong to cellulose, glycerol, and water. It also indicates the presence of H-N-H bonds present in amino acids [44] and chitosan [45]. The intensity of these bands, as well as those of other peaks, decreased due to the loss of moisture in the samples and the partial breakage of intermolecular and intramolecular hydrogen bonds [46]. Additionally, this region can be assigned to the symmetric and asymmetric stretching vibrations of skeletal CH and CH2 in polysaccharides [47]. The second and third peaks occurred in the region of 2920 cm−1 (ii) and 2852 cm−1 (iii), corresponding to the vibration of the H-C-H bond of polysaccharides [48,49]. At the start of the test, the GC sample presented a band at 1630 cm−1 (iv), a signal corresponding to the C=O stretching vibration present in primary amides. A water band appears at this same signal and can shift depending on the strength of interactions [47]. The signal at 1541 cm−1 (v) corresponds to the stretching vibration of the N-H present in secondary amides, while the signal at 1449 cm−1 (vi) is attributed to aliphatic C-H bending vibrations. The intense peaks at 1335 (vii) and 1233 cm−1 (viii) indicate the stretching vibrations of the C-N bond present in amino acids [49,50]. The decrease in the peaks at 1630, 1541, 1337, and 1206 cm−1 indicates the breakage of the amide groups and C-N bonds present in gelatin, as is shown in Figure 6.
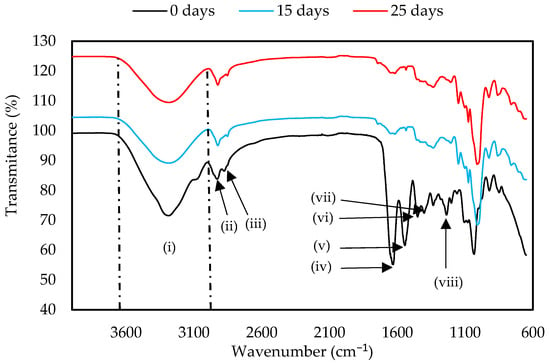
Figure 6.
FTIR spectra of GelC mulch film at 0, 15, and 25 days of the biodegradation test.
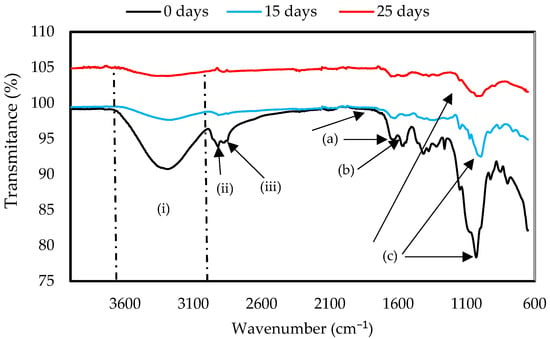
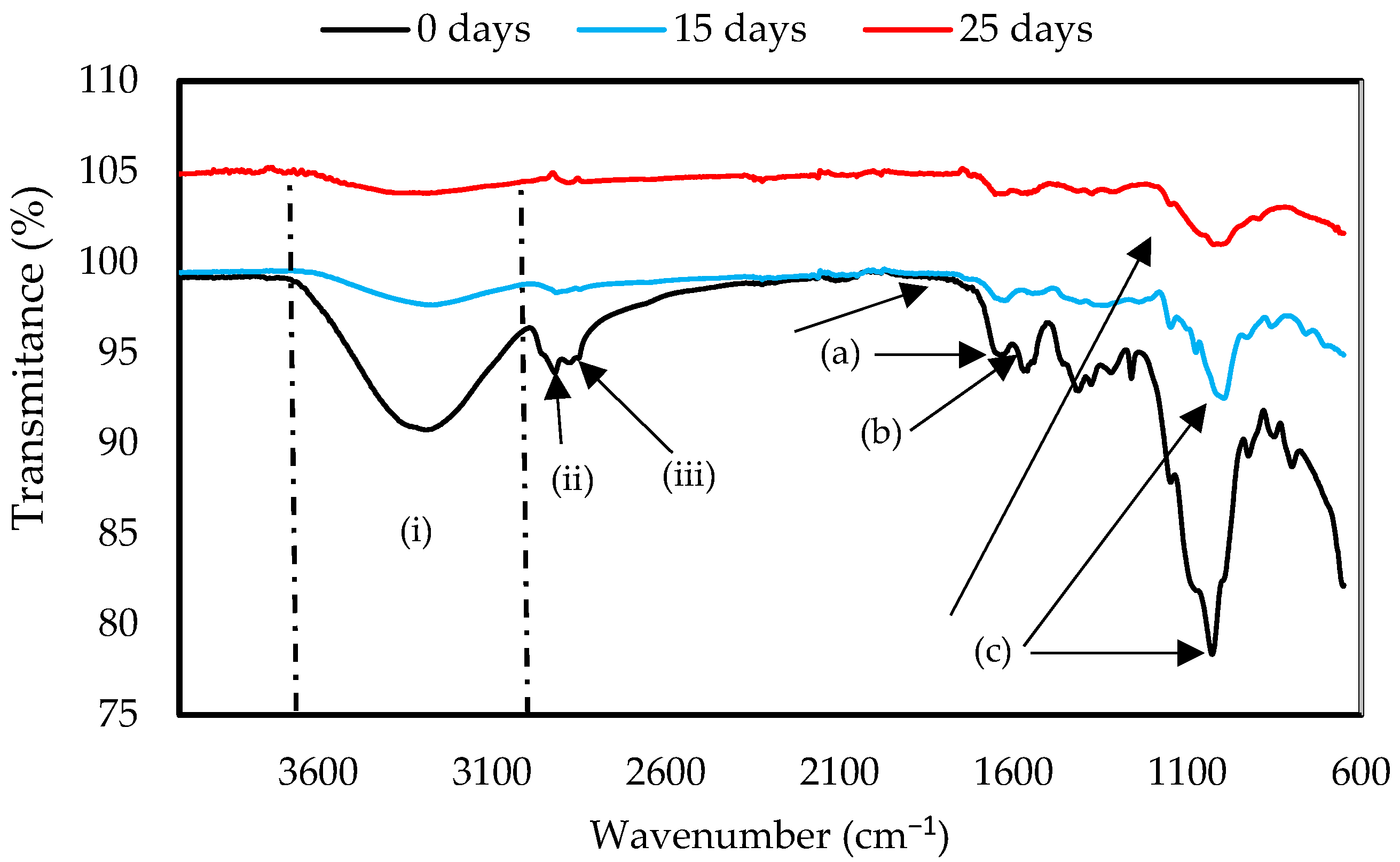
Figure 7.
FTIR spectra of ChiC mulch film at 0, 15, and 25 days of the biodegradation test.
In Figure 7, the spectrum obtained by FTIR for the ChiC sample is shown. Two bands were observed at the amide groups at 1631 cm−1 (a), attributed to the C-O stretching, and at 1593 cm−1 (b), corresponding to the N-H bond present in the chitosan structure [44]. The most intense band was present at 1026 cm−1 (c), a signal corresponding to the glycosidic ring [51] and the interaction between OH groups in glycerol and biopolymers, forming a matrix of chitosan and glycerol. After exposure to soil for 25 days, the ChiC sample showed a decrease in the band at 1026 cm−1, a characteristic signal of chitosan [52], indicating the breakdown of the biopolymer structure. The changes that can be observed in the FTIR spectrum of ChiC at 1631 and 1593 cm−1 indicate the degradation of the material. This is due to the fact that the hydrogen bonds formed between the polymers were broken, which can reduce the crystallinity of polymers.
The FTIR spectra of both samples show the characteristic peaks of the polymers; however, several changes can be observed, which indicate the degradation of the material. This is due to the breaking of the hydrogen bonds formed between the polymers, which can reduce the crystallinity of polymers, making them amorphous and indicating that degradation has occurred, as shown in Figure 6 and Figure 7, where the spectra of GelC and ChiC show a decrease in bands. In this sense, our results are in agreement with the findings of Gonzáles et al. [53] in their studies of starch and chitosan-based films.
4. Conclusions
The ChiC sample presented the greatest resistance to the attack of microorganisms present in the soil, with a maximum degradation time of 70 days, compared to the GelC sample, which presented the highest percentage of weight loss after 25 days. GelC was the sample that showed the greatest weight loss since day 10 of the test. SEM micrographs showed that microorganisms tend to attack chitosan composite matrices with less intensity, which is primarily attributed to their antifungal capacity. FTIR spectra revealed a decrease in hydroxyl, amides, and carbonyl bands during the biodegradation test, which reduced crystallinity and made the biopolymers amorphous, involving their fragmentation or decomposition. Based on the results, the films obtained could be used as biodegradable mulch in short-cycle crops. Additionally, their short biodegradation times will ensure that their presence in the soil will not pose a risk to the environment. While it is true that materials made from biopolymers, including cellulose, are more susceptible to microbial development and growth, they offer certain advantages over materials that do not contain them, especially when looking for biodegradable and environmentally friendly materials. From the perspective of sustainability and environmental protection, biobased mulch films are expected to replace traditional non-degradable mulches due to their natural source and biocompatibility. However, it is important to evaluate its thermal, mechanical, and structural characteristics to have a more comprehensive study of the function of biobased mulch films. In addition, it is necessary to analyze the behavior of these materials in crops to determine their capacity and similarity to commercial plastic mulches, since this work was performed under laboratory-controlled conditions.
Author Contributions
Conceptualization, M.A.L.S., S.M.C.R., J.R.R.V. and E.G.H.; methodology, M.A.L.S., J.R.R.V. and E.G.H.; formal analysis, M.A.L.S., S.M.C.R. and J.R.R.V.; investigation, M.A.L.S. and J.R.R.V.; writing—original draft preparation, M.A.L.S. and J.R.R.V.; writing—review and editing, M.A.L.S., S.M.C.R., J.R.R.V., G.P.V. and E.G.H.; supervision, J.R.R.V.; Project administration, J.R.R.V.; funding acquisition, J.R.R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto Politécnico Nacional, through research projects SIP 20220289, SIP 20230043.
Data Availability Statement
Data are available upon reasonable request.
Acknowledgments
The authors are grateful to the Instituto Politécnico Nacional and the Tecnológico Nacional de México, I.T. Zacatepec for allowing this research to be carried out.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haapala, T.; Palonen, P.; Tamminen, A.; Ahokas, J. Effects of different paper mulches on soil temperature and yield of cucumber (Cucumis sativus L.) in the temperate zone. Agric. Food Sci. 2015, 24, 52–58. [Google Scholar] [CrossRef]
- Tofanelli, M.B.D.; Wortman, S.E. Benchmarking the agronomic performance of biodegradable mulches against polyethylene mulch film: A meta-analysis. Agronomy 2020, 10, 1618. [Google Scholar] [CrossRef]
- Gao, X.; Fu, C.; Li, M.; Qi, X.; Jia, X. Effects of Biodegradation of Corn-Starch–Sodium-Alginate-Based Liquid Mulch Film on Soil Microbial Functions. Int. J. Environ. Res. Public Health 2022, 19, 8631. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Turner, N.C.; Gong, Y.H.; Li, F.M.; Fang, C.; Ge, L.J.; Ye, J.S. Benefits and limitations to straw- and plastic-film mulch on maize yield and water use efficiency, a meta-analysis across hydrothermal gradients. Eur. J. Agron. 2018, 99, 138–147. [Google Scholar] [CrossRef]
- Kader, M.A.; Singha, A.; Begum, M.A.; Jewel, A.; Khan, F.H.; Khan, N.I. Mulching as water-saving technique in dry land agriculture. Bull. Natl. Res. Cent. 2019, 43, 147. [Google Scholar] [CrossRef]
- Samphire, M.; Chadwick, D.R.; Jones, D.L. Biodegradable plastic mulch films increase yield and promote nitrogen use efficiency in organic horticulture. Front. Agron. 2023, 5, 1141608. [Google Scholar] [CrossRef]
- Chen, Y.; Leng, Y.; Liu, X.; Wang, J. Microplastic pollution in vegetable farmlands of suburb Wuhan, Central China. Environ. Pollut. 2020, 257, 113449. [Google Scholar] [CrossRef]
- He, G.; Wang, Z.; Li, S.; Malhi, S.S. Plastic mulch: Tradeoffs between productivity and greenhouse gas emissions. J. Clean. Prod. 2018, 172, 1311–1318. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Briassoulis, D.; Babou, E.; Hiskakis, M.; Kyrikou, I. Analysis of long-term degradation behaviour of polyethylene mulching films with pro-oxidants under real cultivation and soil burial conditions. Environ. Sci. Pollut. Res. Int. 2015, 22, 2584–2598. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Dong, K.; Wu, Z.; Wang, J.; Wang, Z.L. A review on emerging biodegradable polymers for environmentally benign transient electronic skins. J. Mater. Sci. 2021, 56, 16765–16789. [Google Scholar] [CrossRef]
- Kopitar, D.; Marasovic, P.; Jugov, N.; Schwarz, I. Biodegradable Nonwoven Agrotextile and Films—A Review. Polymers 2022, 14, 2272. [Google Scholar] [CrossRef]
- Cerqueira, J.C.; Penha, J.D.S.; Oliveira, R.S.; Guarieiro, L.L.N.; Melo, P.D.S.; Viana, J.D.; Machado, B.A.S. Production of biodegradable starch nanocomposites using cellulose nanocrystals extracted from coconut fibers. Polimeros 2017, 27, 320–329. [Google Scholar] [CrossRef]
- Khenblouche, A.; Bechki, D.; Gouamid, M.; Charradi, K.; Segni, L.; Hadjadj, M.; Boughali, S. Extraction and characterization of cellulose microfibers from Retama raetam stems. Polimeros 2019, 29, e2019011. [Google Scholar] [CrossRef]
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffeys, J.; Manning, L.; Moosavi, S.M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and chitosan derived from crustacean waste valorization streams can support food systems and the UN Sustainable Development Goals. Nat. Food 2022, 3, 822–828. [Google Scholar] [CrossRef]
- Mohan, K.; Ravichandran, S.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Rajeevgandhi, C.; Karthick Rajan, D.; Seedevi, P. Extraction and characterization of chitin from sea snail Conus inscriptus (Reeve, 1843). Int. J. Biol. Macromol. 2019, 126, 555–560. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, P.; Wei, Y.; Guo, X.; Deng, X.; Zhang, J. Properties of allicin–zein composite nanoparticle gelatin film and their effects on the quality of cold, fresh beef during storage. Foods 2023, 12, 3713. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shen, R.; Wang, L.; Yang, X.; Zhang, L.; Ma, X.; He, L.; Li, A.; Kong, X.; Shi, H. Preparation, optimization, and characterization of bovine bone gelatin/sodium carboxymethyl cellulose nanoemulsion containing thymol. Foods 2024, 13, 1506. [Google Scholar] [CrossRef]
- Sander, M. Biodegradation of polymeric mulch films in agricultural soils: Concepts, knowledge gaps, and future research directions. Environ. Sci. Technol. 2019, 53, 2304–2315. [Google Scholar] [CrossRef]
- Lorenzo-Santiago, M.A.; Rendón-Villalobos, R. Isolation and characterization of micro cellulose obtained from waste mango. Polimeros 2020, 30, e2020036. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, J.; Zhang, L. Structure and properties of the nanocomposite films of chitosan reinforced with cellulose whiskers. J. Polym. Sci. Polym. Phys. 2009, 47, 1069–1077. [Google Scholar] [CrossRef]
- Pranoto, Y.; Lee, C.M.; Park, H.J. Characterizations of fish gelatin films added with gellan and κ- carrageenan. Lebensm. Wiss. Technol. 2007, 40, 766–774. [Google Scholar] [CrossRef]
- ASTM D6988-21; Standard Guide for Determination of Thickness of Plastic Film Test Specimens. Book of ASTM Standards. American Society for Testing and Materials (ASTM International): West Conshohocken, PA, USA, 2021.
- Acquafredda, P. XRF technique. Phys. Sci. Rev. 2019, 4, 20180171. [Google Scholar] [CrossRef]
- Rudnik, E.; Briassoulis, D. Degradation behavior of poly (lactic acid) films and fibers in soil under Mediterranean field conditions and laboratory simulations testing. Ind. Crop. Prod. 2011, 33, 648–658. [Google Scholar] [CrossRef]
- Rendón-Villalobos, R.; García-Hernández, E.; Güizado-Rodríguez, M.; Salgado-Delgado, R.; Rangel-Vázquez, N.A. Preparation and characterization of banana starch (Musa paradisiaca L.) acetylated to different degrees of substitution. Afinidad 2010, 67, 294–300. [Google Scholar]
- Montoya-Escobar, N.; Ospina-Acero, D.; Velásquez-Cock, J.A.; Gómez-Hoyos, C.; Serpa Guerra, A.; Gañan Rojo, P.F.; Vélez Acosta, L.M.; Escobar, J.P.; Correa-Hincapié, N.; Triana-Chávez, O.; et al. Use of Fourier Series in X-ray Diffraction (XRD) Analysis and Fourier-Transform Infrared Spectroscopy (FTIR) for Estimation of Crystallinity in Cellulose from Different Sources. Polymers 2022, 14, 5199. [Google Scholar] [CrossRef]
- Suminarti, N.E.; Pamungkas, B.P.A.R.; Fajriani, S.; Fajrin, A.N. Effect of size and thickness of mulch on soil temperature, soil humidity, growth and yield of red beetroot (Beta vulgaris L.) in jatikerto dry land, Indonesia. Asian J. Plant Sci. 2021, 20, 33–43. [Google Scholar] [CrossRef]
- Wang, B.; Niu, J.; Berndtsson, R.; Zhang, L.; Chen, X.; Li, X.; Zhu, Z. Efficient organic mulch thickness for soil and water conservation in urban areas. Sci. Rep. 2021, 11, 6259. [Google Scholar] [CrossRef]
- Shella, R.E.; Wulandari, M.; Wuryan, U.R.T.; Kristianto, S.; Sukian, W.S. Comparison of the thickness of rice straw mulch, rice husk and reeds on the observation of the number of fruit plants tomato (Solanum lycopersicum). J. Nat. Sci. Learn. 2023, 2, 10–17. [Google Scholar]
- Ostadi, H.; Hakimabadi, S.G.; Nabavi, F.; Vossoughi, M.; Alemzadeh, I. Enzymatic and soil burial degradation of corn starch/glycerol/sodium montmorillonite nanocpmposites. Polym. Renew. Resour. 2020, 11, 15–29. [Google Scholar]
- Wang, M.; Liu, K.; Dai, L.; Zhang, J.; Fang, X. The structural and biochemical basis for cellulose biodegradation. J. Chem. Technol. Biotechnol. 2012, 88, 491–733. [Google Scholar] [CrossRef]
- Rodríguez-Soto, K.X.; Piñeros-Castro, N.Y.; Ortega-Toro, R. Laminated composites reinforced with chemically modified sheets-stalk of Musa cavendish. Rev. Mex. Ing. Quím. 2019, 18, 749–758. [Google Scholar] [CrossRef]
- Arshad, K.; Skrifvars, M.; Vivod, V.; Volmajer, V.J.; Vončina, B. Biodegradation of Natural Textile Materials in Soil. Tekstilec 2014, 57, 118–132. [Google Scholar] [CrossRef]
- Miles, C.; DeVetter, L.; Ghimire, S.; Hayes, D.G. Suitability of biodegradable plastic mulches for organic and sustainable agricultural production systems. HortScience 2017, 52, 10–15. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, Y.; Jin, T.; Zhang, K.; Li, Z.; Sun, C.; Mi, Q.; Li, Q. Effect of Long-Term Biodegradable Film Mulch on Soil Physicochemical and Microbial Properties. Toxins 2022, 10, 129. [Google Scholar] [CrossRef]
- Bianchini, M.; Trozzo, L.; D’Ottavio, P.; Giustozzi, M.; Toderi, M.; Ledda, L.; Francioni, M. Soil refinement accelerates in-field degradation rates of soil-biodegradable mulch films. Ital. J. Agron. 2022, 17, 2044. [Google Scholar] [CrossRef]
- Kalka, S.; Huber, T.; Steinberg, J.; Baronian, K.; Müssig, J.; Staiger, M.P. Biodegradability of all-cellulose composite laminates. Compos. Part A 2014, 59, 37–44. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; De La Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Alvarado Hernández, A.M.; Barrera Necha, L.L.; Hernández Lauzardo, A.N.; Velázquez del Valle, M.G. Antifungal activity of chitosan and essential oils on Rhizopus stolonifer (Ehrenb.: Fr.) Vuill causal agent of soft rot of tomato. Rev. Colomb. Biotecnol. 2011, 13, 127–134. [Google Scholar]
- El-Araby, A.; Janati, W.; Ullah, R.; Ercisli, S.; Errachidi, F. Chitosan, chitosan derivatives, and chitosan-based nanocomposites: Eco-friendly materials for advanced applications (a review). Front. Chem. 2024, 11, 1327426. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Degradation behavior of biocomposites based on cassava starch buried under indoor soil conditions. Carbohydr. Polym. 2014, 101, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Santhiya, D. In situ mineralization of bioactive glass in gelatin matrix. Mater. Lett. 2017, 188, 127–129. [Google Scholar] [CrossRef]
- Fardioui, M.; Meftah, K.I.M.; El Kacem, Q.A.; Bouhfid, R. Bio-active nanocomposite films based on nanocrystalline cellulose reinforced styrylquinoxalin-grafted-chitosan: Antibacterial and mechanical properties. Int. J. Biol. Macromol. 2018, 114, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Bajer, D.; Kaczmarek, H. Study of the influence OV UV radiation on biodegradable blends based on chitosan and starch. Prog. Chem. Appl. Chitin Deriv. 2010, 15, 17–24. [Google Scholar]
- Wang, Y.-X.; Xin, Y.; Yin, J.-Y.; Huang, X.-J.; Wang, J.-Q.; Hu, J.-L.; Geng, F.; Nie, S.-P. Revealing the architecture and solution properties of polysaccharide fractions from Macrolepiota albuminosa (Berk.) Pegler. Food Chem. 2022, 368, 130772. [Google Scholar] [CrossRef]
- Tao, H.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar]
- González, S.P.; Medina, C.J.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch-glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar]
- Shi, C.; Tao, F.; Cui, Y. New starch ester/gelatin based films: Developed and physicochemical characterization. Int. J. Biol. Macromol. 2018, 109, 863–871. [Google Scholar] [CrossRef]
- Mauricio-Sánchez, R.A.; Salazar, R.; Luna-Bárcenas, J.G.; Mendoza-Galván, A. FTIR spectroscopy studies on the spontaneous neutralization of chitosan acetate films by moisture conditioning. Vib. Spectrosc. 2018, 94, 1–6. [Google Scholar] [CrossRef]
- Pérez-Cordero, A.; Rojas-Sierra, J.; Rodriguez-Ruiz, J.; Arrieta-Álvarez, I.; Arrieta-Álvarez, Y.; Rodríguez-Carrascal, A. Antibacterial activity of chitosan acid solutions obtained from shrimp exoskeleton. Rev. Colomb. Biotecnol. 2014, 16, 104–110. [Google Scholar]
- Gonzalez-Calderon, J.A.; Vallejo-Montesinos, J.; Martínez-Martínez, H.N.; Cerecero-Enríquez, R.; López-Zamora, L. Effect of chemical modification of titanium dioxide particles Via silanization under properties of chitosan/potato-starch Films. Rev. Mex. Ing. Quím. 2019, 18, 913–927. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).