Utilizing Ceramic Factory Waste to Produce Low-Cost Refractory Ceramics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Studied Materials
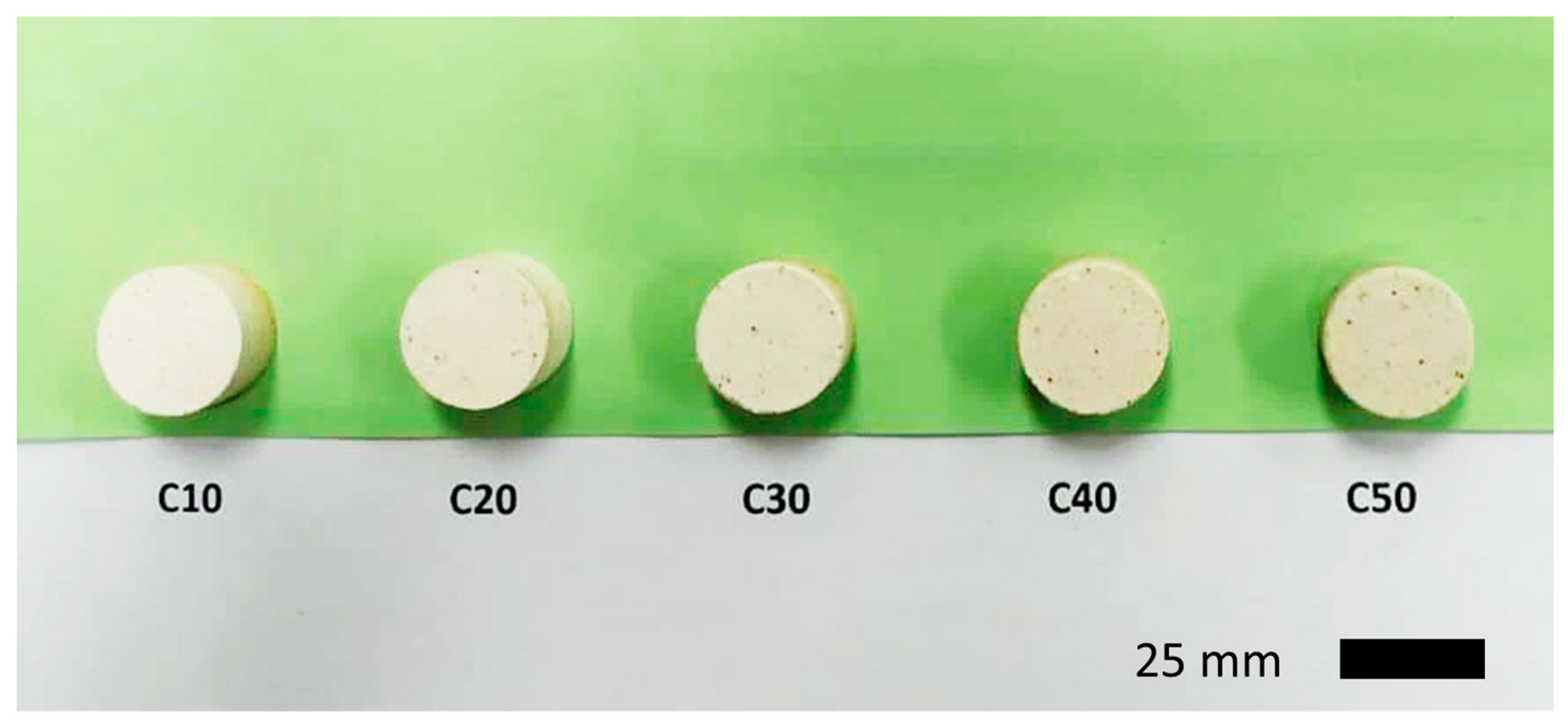
2.2. Physical Properties of the Investigated Samples
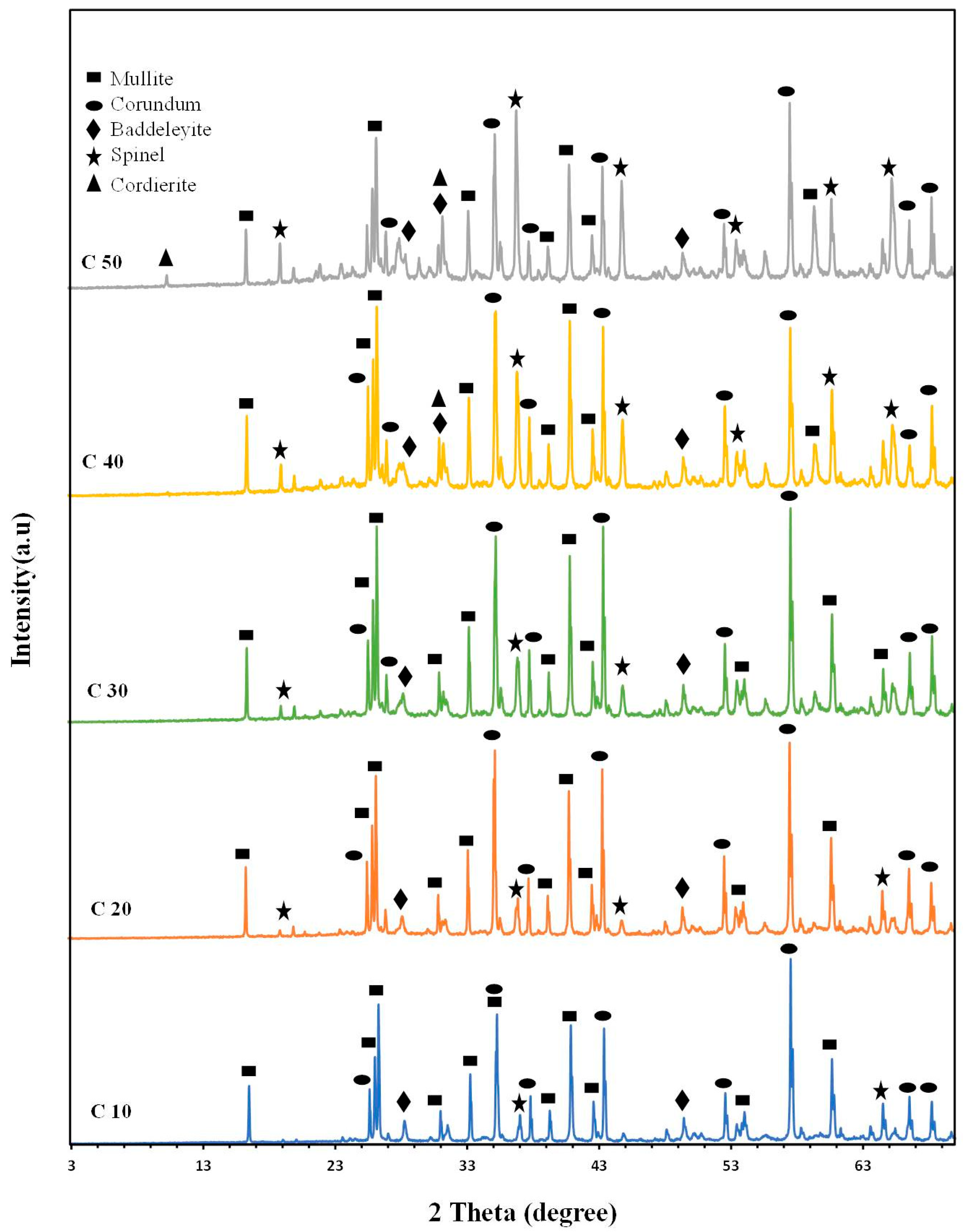
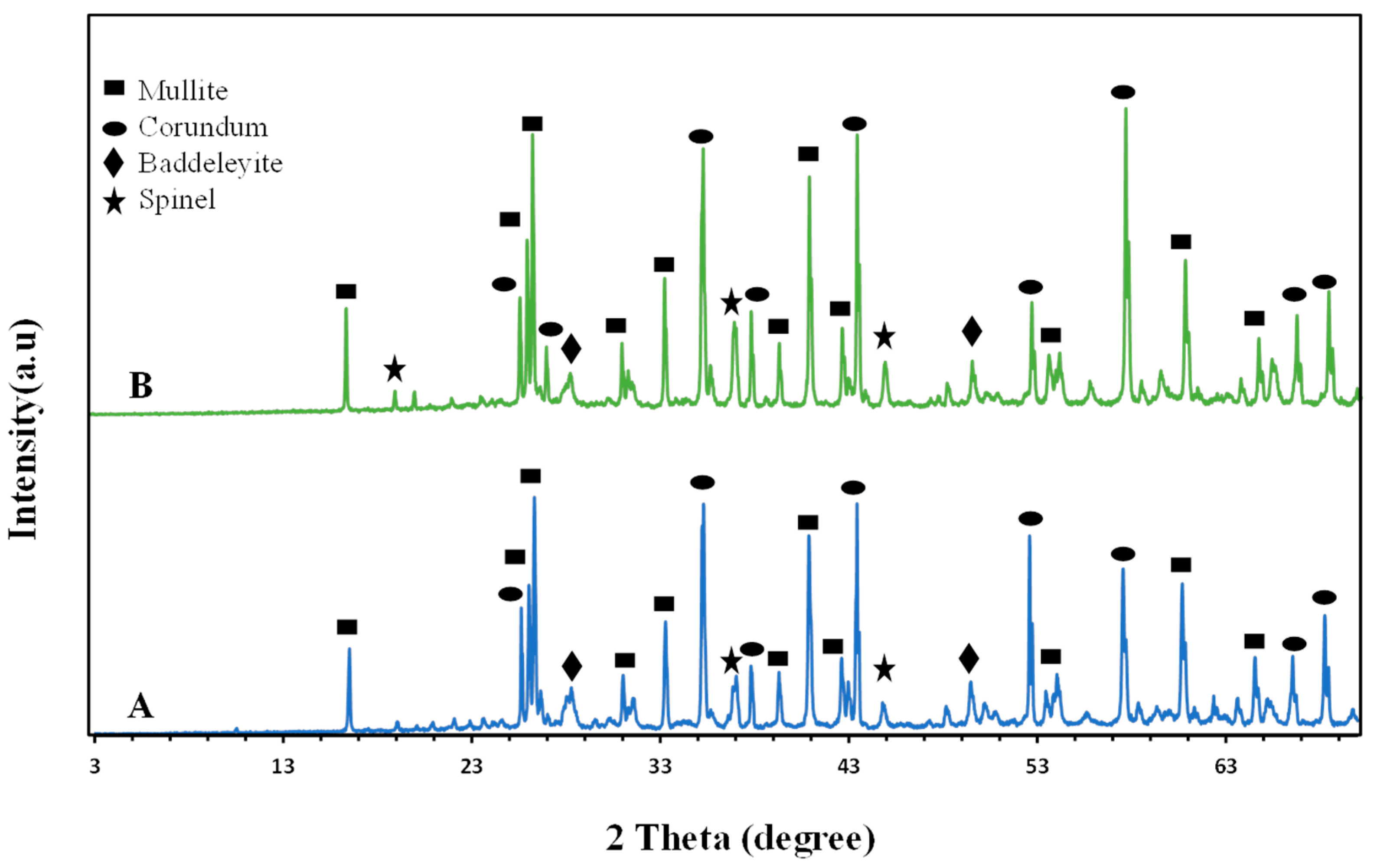
2.3. X-ray Diffraction of the Investigated Samples
2.3.1. X-ray Diffraction (XRD) of All Samples
2.3.2. Influence of Firing Temperature on the Formed Crystalline Phases
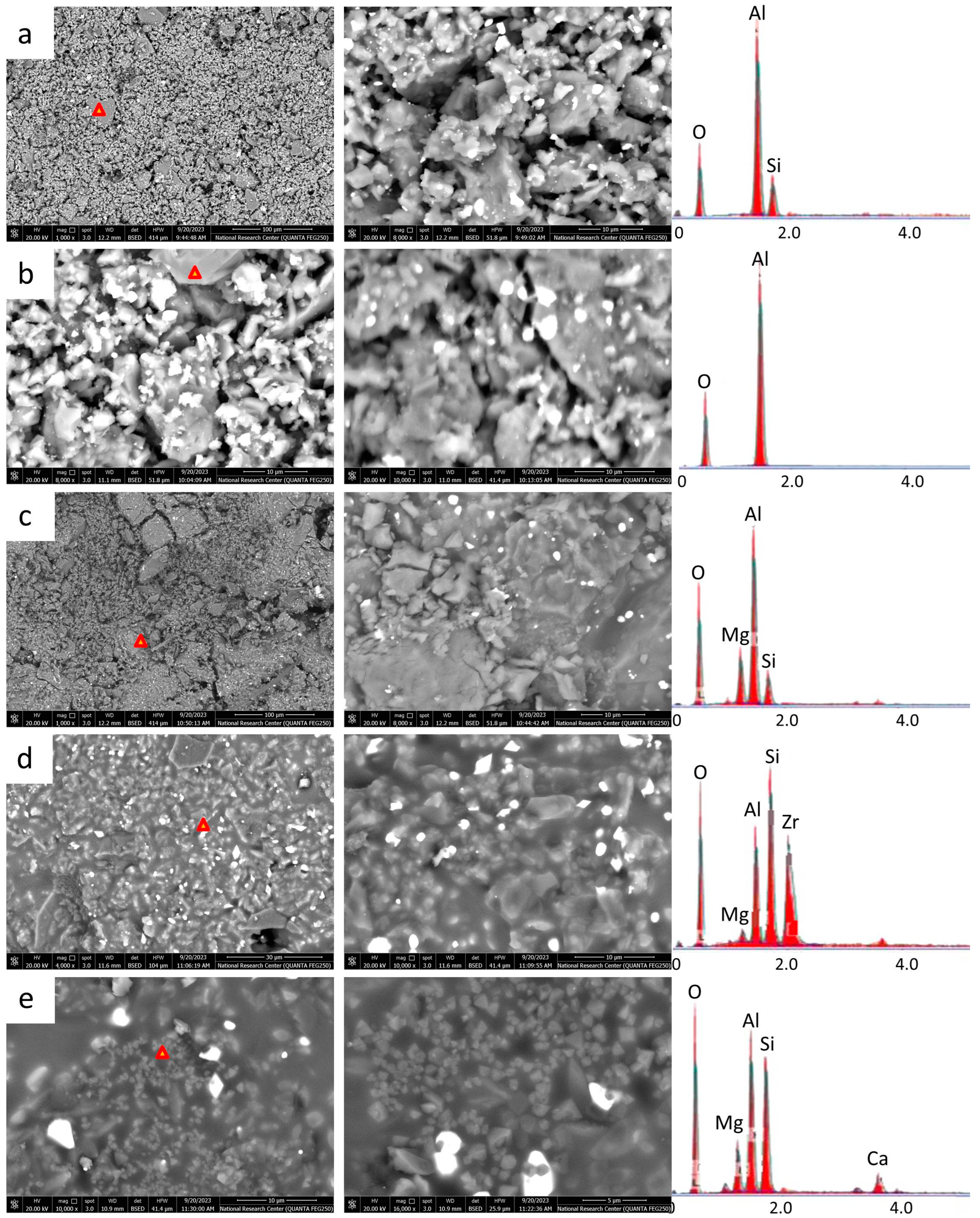
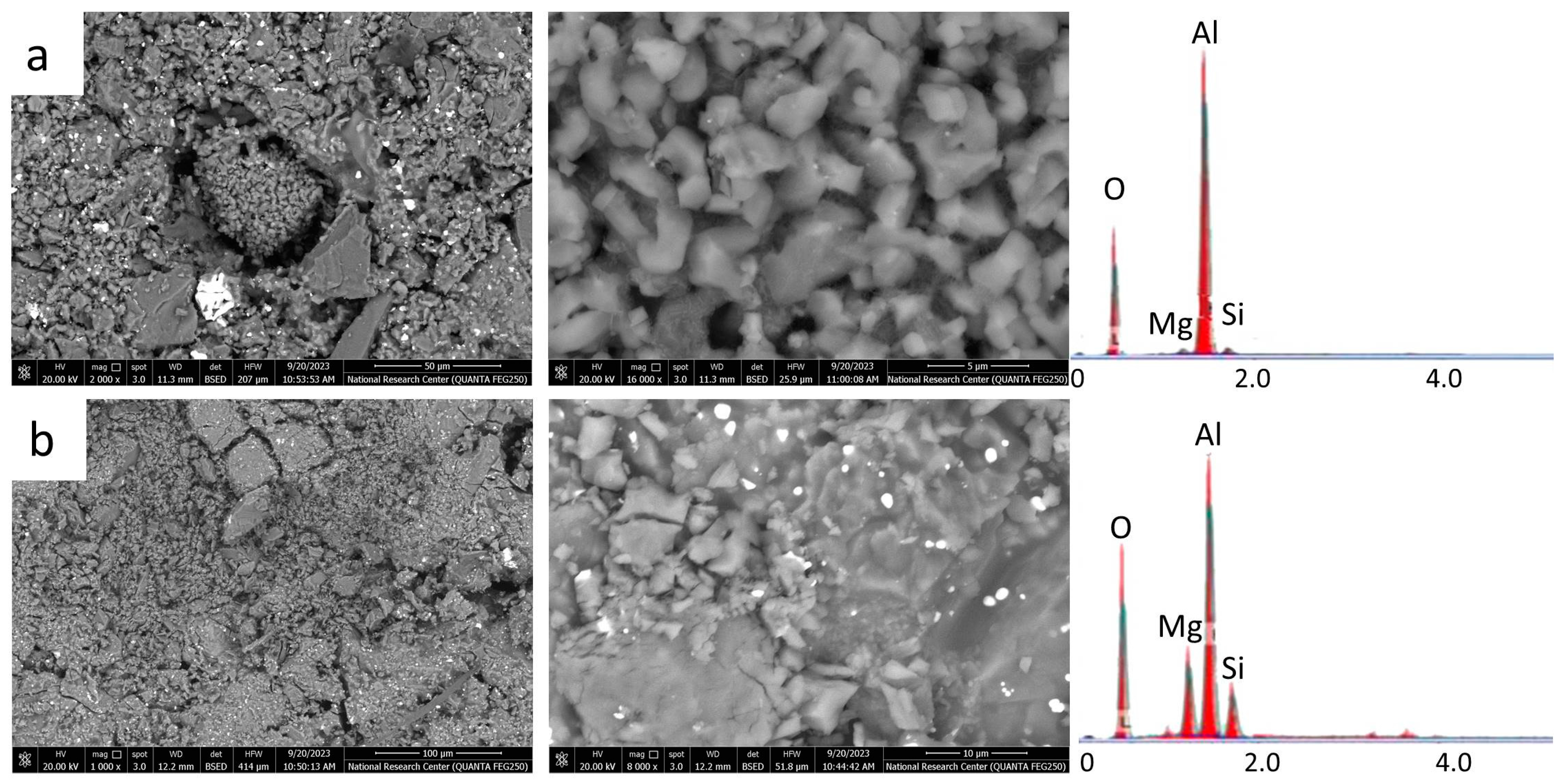
2.4. Microstructures (SEM and EDX)
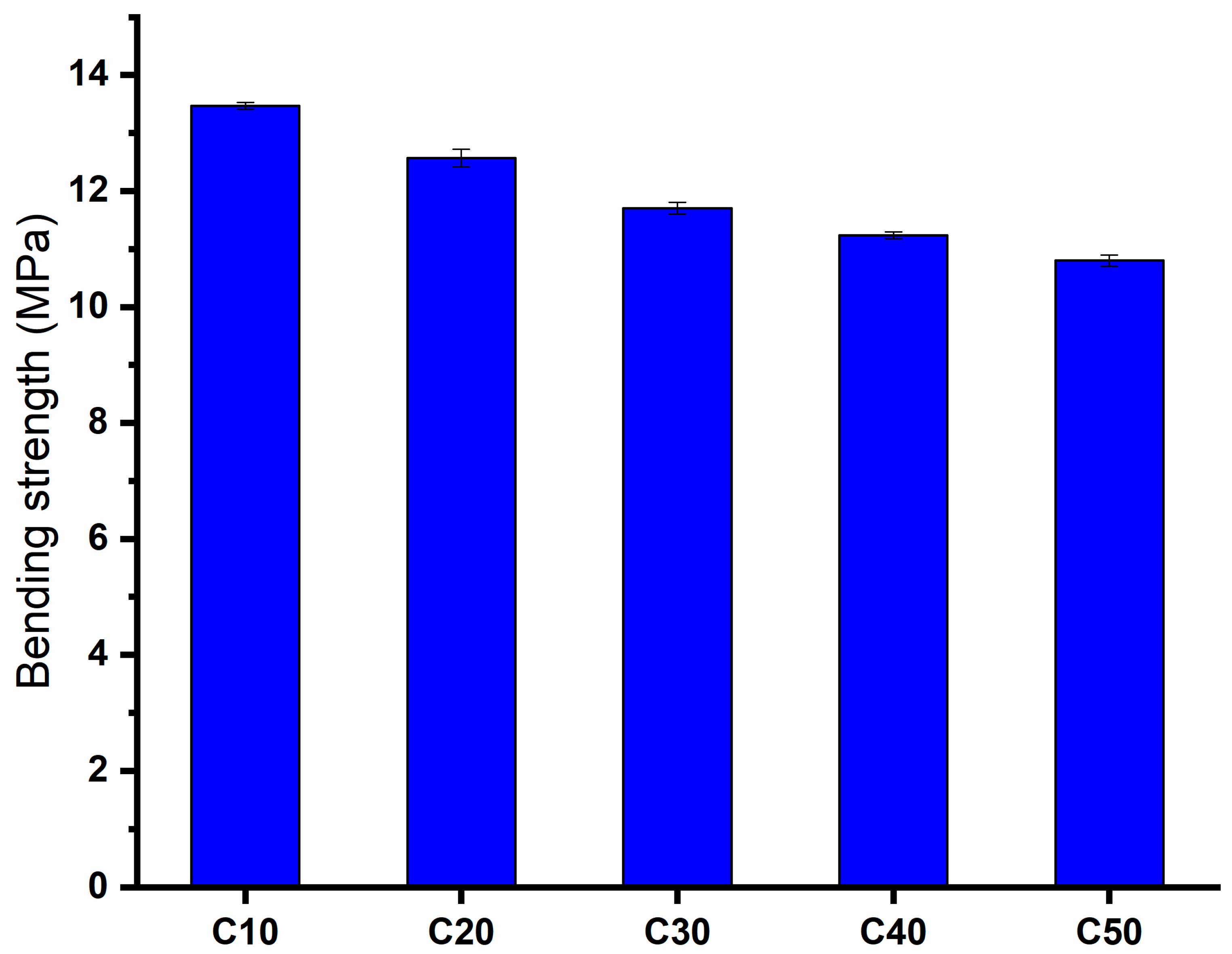
2.5. Bending Strength (BS)
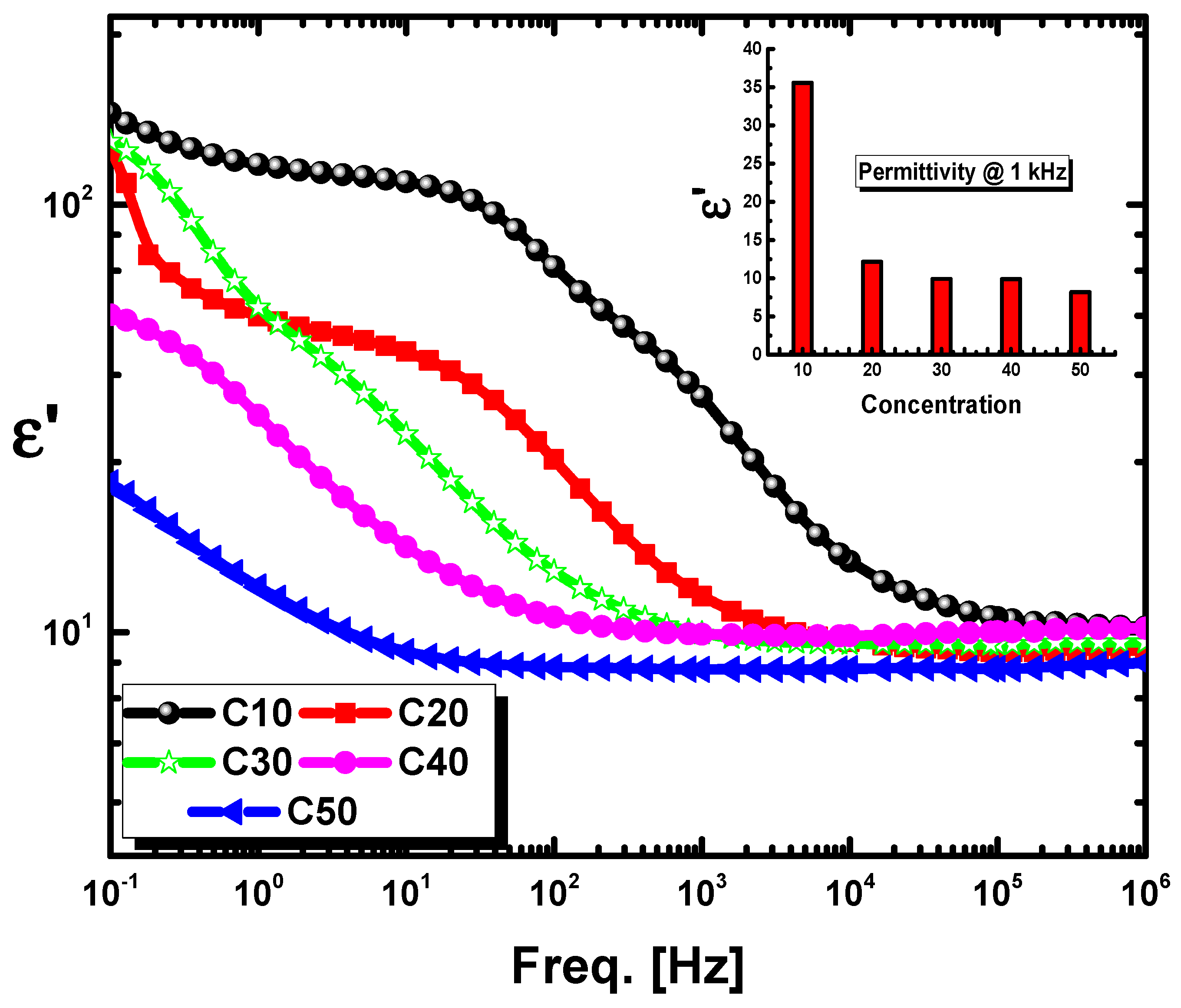
2.6. Dielectric Properties
3. Materials and Experimental Techniques
3.1. Materials
3.2. Chemical Analysis
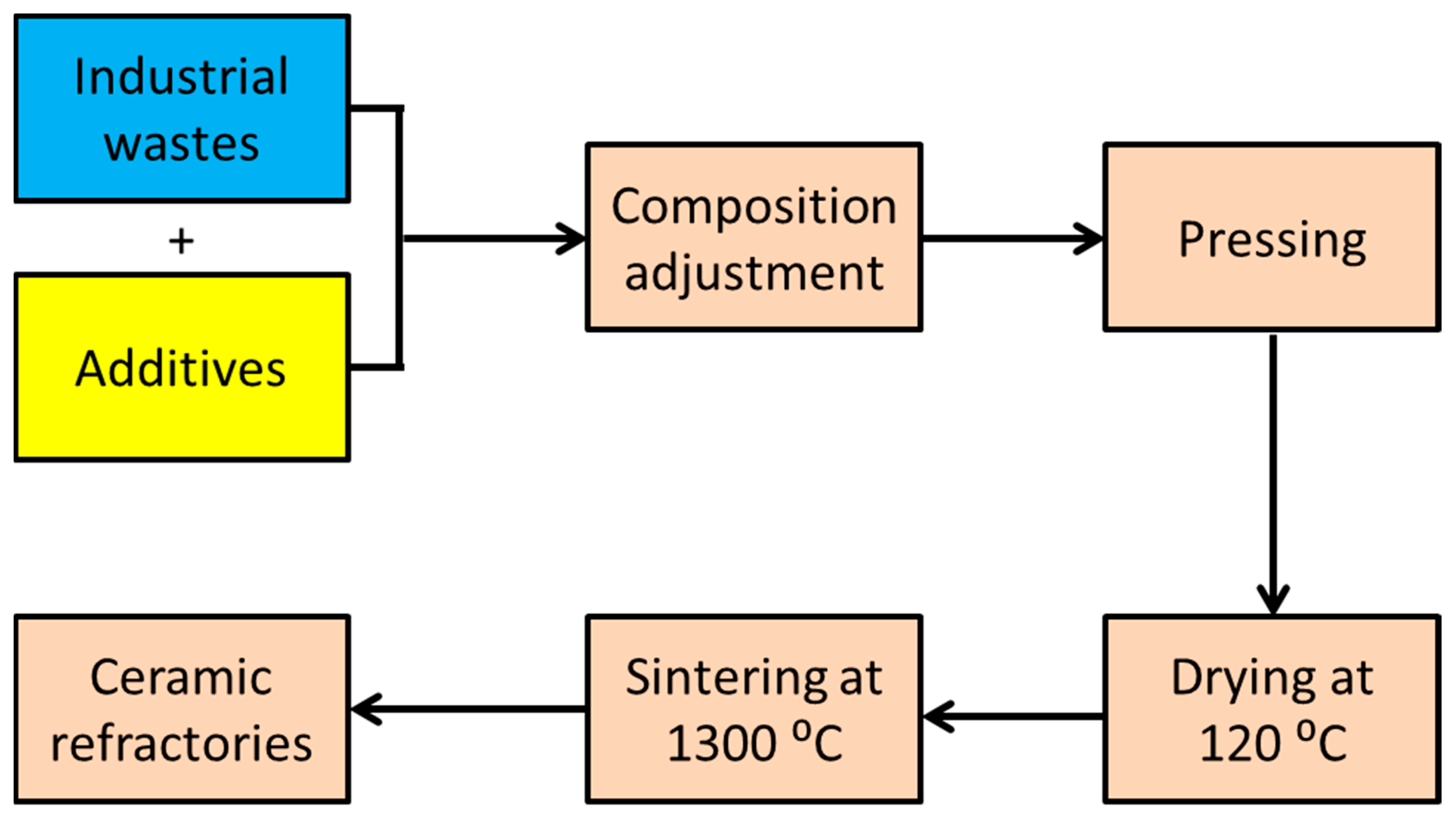
3.3. Batch Calculation and Preparation
3.4. Densification Properties
3.4.1. Bulk Density (BD) and Apparent Porosity (AP)
3.4.2. Water Absorption (WA, %)
3.5. X-ray Diffraction (XRD)
3.6. Scanning Electron Microscope (SEM)
3.7. Bending Strength
3.8. Electrical Properties and Electrical Conductivity
4. Conclusions
- Solid waste in the form of ceramic rollers, ceramic sludge, and magnesite can be used to manufacture heat-resistant ceramics at 1300 °C.
- The main precipitated phases are mullite, corundum, and spinel.
- Bending strength increases with increasing mullite concentration, bulk density, and refined grain microstructures
- By increasing the concentration of cordierite, the density decreases and the porosity and water absorption increase.
- Bulk density increases with increasing sintering temperature, whereas water absorption and porosity decrease due to liquid phase formation.
- With the increase in cordierite concentration, the microstructures vary from fine grained to coarse grained.
- Mullite led to decomposition, inhibited the formation of cordierite, and enhanced spinel formation.
- The increase in cordierite content from C10 to C50 significantly lowered the dielectric properties of the compositions, as the maximum values of permittivity, AC conductivity, and resistivity for the C10 sample were found to be 35.6, 10−8 s/cm, and 108 cm/s, respectively, at room temperature with the lowest values for the C50 sample as its dielectric constant of about 8.2, conductivity of around 3 × 10−11 s/cm, and resistivity of 3 × 1010 cm/s. These values suggest the potential of these compositions as perfect insulation materials.
- We successfully achieved the production of refractory ceramics from industrial waste. Therefore, the application areas for which the product is suitable are furnace lining and refractory bricks
- Based on the physical, mechanical, electrical, crystalline, and economic properties, it appears that the best sample that can be used for industrial purposes is C10, and its chemical composition is SiO2 = 30.48%, Al2O3 = 68.14%, and MgO = 1.38
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhary, J.; Kumar, B.; Gupta, A. Utilization of solid waste materials as alternative fillers in asphalt mixes: A review. Constr. Build. Mater. 2020, 234, 117271. [Google Scholar] [CrossRef]
- Brunner, P.H.; Rechberger, H. Waste to energy–key element for sustainable waste management. Waste Manag. 2015, 37, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Juan, A.; Medina, C.; Guerra, M.I.; Morán, J.M.; Aguado, P.J.; Sánchez de Rojas, M.I.; Frías, M.; Rodríguez, O. Re-use of ceramic wastes in construction. In Ceramic Materials; IntechOpen, 2010; pp. 197–214. Available online: https://www.researchgate.net/profile/Olga-Largo/publication/221909437_Re-Use_of_Ceramic_Wastes_in_Construction/links/54102e480cf2f2b29a3f066c/Re-Use-of-Ceramic-Wastes-in-Construction.pdf (accessed on 3 August 2024).
- Juan, A.; Medina, C.; Morán, J.; Aguado, P.; Guerra, M. Re-use of industrial and construction and demolition wastes for the elaboration of recycled eco-efficient concretes. In Proceedings of the International Conference on Agricultural Engineering—AgEng 2010: Towards Environmental Technologies, Clermont-Ferrand, France, 6–8 September 2010. [Google Scholar]
- Koyuncu, H.; Guney, Y.; Yılmaz, G.; Koyuncu, S.; Bakis, R. Utilization of ceramic wastes in the construction sector. Key Eng. Mater. 2004, 264, 2509–2512. [Google Scholar] [CrossRef]
- Daigo, I.; Kiyohara, S.; Okada, T.; Okamoto, D.; Goto, Y. Element-based optimization of waste ceramic materials and glasses recycling. Resour. Conserv. Recycl. 2018, 133, 375–384. [Google Scholar] [CrossRef]
- Nepomuceno, M.C.; Isidoro, R.A.; Catarino, J.P. Mechanical performance evaluation of concrete made with recycled ceramic coarse aggregates from industrial brick waste. Constr. Build. Mater. 2018, 165, 284–294. [Google Scholar] [CrossRef]
- Abadou, Y.; Mitiche-Kettab, R.; Ghrieb, A. Ceramic waste influence on dune sand mortar performance. Constr. Build. Mater. 2016, 125, 703–713. [Google Scholar] [CrossRef]
- Zhang, G.-Y.; Bae, S.-C.; Lin, R.-S.; Wang, X.-Y. Effect of waste ceramic powder on the properties of alkali–activated slag and fly ash pastes exposed to high temperature. Polymers 2021, 13, 3797. [Google Scholar] [CrossRef]
- Meena, R.V.; Jain, J.K.; Chouhan, H.S.; Beniwal, A.S. Use of waste ceramics to produce sustainable concrete: A review. Clean. Mater. 2022, 4, 100085. [Google Scholar] [CrossRef]
- Coletti, C.; Maritan, L.; Cultrone, G.; Mazzoli, C. Use of industrial ceramic sludge in brick production: Effect on aesthetic quality and physical properties. Constr. Build. Mater. 2016, 124, 219–227. [Google Scholar] [CrossRef]
- Nandi, V.; Raupp-Pereira, F.; Montedo, O.; Oliveira, A. The use of ceramic sludge and recycled glass to obtain engobes for manufacturing ceramic tiles. J. Clean. Prod. 2015, 86, 461–470. [Google Scholar] [CrossRef]
- Khater, G.; Nabawy, B.S.; El-Kheshen, A.A.; Abdel-Baki, M.; Farag, M.; Abd Elsatar, A. Preparation and characterization of low-cost wollastonite and gehlenite ceramics based on industrial wastes. Constr. Build. Mater. 2021, 310, 125214. [Google Scholar] [CrossRef]
- Khater, G.A.; Nabawy, B.S.; El-Kheshen, A.A.; Abdel Latif, M.A.-B.; Farag, M.M. Use of arc furnace slag and ceramic sludge for the production of lightweight and highly porous ceramic materials. Materials 2022, 15, 1112. [Google Scholar] [CrossRef] [PubMed]
- Khater, G.; Nabawy, B.S.; El-Kheshen, A.A.; Abdel-Baki, M.; Farag, M. Utilizing of solid waste materials for producing porous and lightweight ceramics. Mater. Chem. Phys. 2022, 280, 125784. [Google Scholar] [CrossRef]
- Roushdy, M.H. Recycling of the Mixture Resulted from Roller Kiln Waste and Ceramic Tiles Sludge Waste in the Manufacturing of Ceramic Floor Tiles. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 1132–1138. [Google Scholar] [CrossRef]
- Mark. A International Company Catalogue; Mark: Gujarat, India, 2009. [Google Scholar]
- Quah, S.R. International Encyclopedia of Public Health; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Mehta, A.J.; Miedinger, D.; Keidel, D.; Bettschart, R.; Bircher, A.; Bridevaux, P.O.; Curjuric, I.; Kromhout, H.; Rochat, T.; Rothe, T.; et al. Occupational exposure to dusts, gases, and fumes and incidence of chronic obstructive pulmonary disease in the Swiss Cohort Study on Air Pollution and Lung and Heart Diseases in Adults. Am. J. Respir. Crit. Care Med. 2012, 185, 1292–1300. [Google Scholar] [CrossRef]
- Oytac, Z.E.; Tarhan, M.; Yay, B. Investigation of the effects of kiln roller waste addition on porcelain tile matte-opaque glazes. J. Therm. Anal. Calorim. 2024, 149, 2137–2146. [Google Scholar] [CrossRef]
- Yan, W.; Chen, J.; Li, N.; Han, B.; Wei, Y. Lightweight cordierite–mullite refractories with low coefficients of thermal conductivity and high mechanical properties. Bull. Mater. Sci. 2015, 38, 409–415. [Google Scholar] [CrossRef]
- Chotard, T.; Soro, J.; Lemercier, H.; Huger, M.; Gault, C. High temperature characterisation of cordierite–mullite refractory by ultrasonic means. J. Eur. Ceram. Soc. 2008, 28, 2129–2135. [Google Scholar] [CrossRef]
- Chlup, Z.; Dlouhý, I.; Boccaccini, A.R.; Boccaccini, D.N.; Leonelli, C.; Romagnoli, M. Thermal shock resistance of cordierite-mullite refractory composites. Key Eng. Mater. 2005, 290, 260–263. [Google Scholar] [CrossRef]
- Boccaccini, D.N.; Leonelli, C.; Rivasi, M.R.; Romagnoli, M.; Boccaccini, A. Microstructural investigations in cordierite–mullite refractories. Ceram. Int. 2005, 31, 417–432. [Google Scholar] [CrossRef]
- Sittiakkaranon, S.; Phonphuak, N. Microstructural and Physical Characterization of Cordierite-Mullite Ceramics Refractories. GEOMATE J. 2023, 25, 233–240. [Google Scholar] [CrossRef]
- de Brito, I.P.; de Almeida, E.P.; de Araújo Neves, G.; de Lucena Lira, H.; Menezes, R.R.; da Silva, V.J.; de Lima Santana, L.N. Development of cordierite/mullite composites using industrial wastes. Int. J. Appl. Ceram. Technol. 2021, 18, 253–261. [Google Scholar] [CrossRef]
- Ganesh, I. Fabrication of magnesium aluminate (MgAl2O4) spinel foams. Ceram. Int. 2011, 37, 2237–2245. [Google Scholar] [CrossRef]
- Ren, X.; Ma, B.; Fu, G.; Qian, F.; Liu, G.; Yu, J.; Li, Y. Facile synthesis of MgO–Mg2SiO4 composite ceramics with high strength and low thermal conductivity. Ceram. Int. 2021, 47, 19959–19969. [Google Scholar] [CrossRef]
- Marimuthu, S.; Malathi, A.C.; Raghavan, V.; Grace, A.N. Fundamentals of electronic ceramics. In Advanced Ceramics for Energy Storage, Thermoelectrics and Photonics; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Marikkannan, S.K.; Ayyasamy, E.P. Synthesis, characterisation and sintering behaviour influencing the mechanical, thermal and physical properties of cordierite-doped TiO2. J. Mater. Res. Technol. 2013, 2, 269–275. [Google Scholar] [CrossRef]
- Khalil, N.M.; Algamal, Y. Recycling of Ceramic Wastes for the Production of High Performance Mullite Refractories. Silicon 2020, 12, 1557–1565. [Google Scholar] [CrossRef]
- Silva, V.J.; Taveira, S.K.; Silva, K.R.; Neves, G.A.; Lira, H.L.; Santana, L.N. Refractory Ceramics of Clay and Alumina Waste. Mater. Res. 2021, 24, e20200485. [Google Scholar] [CrossRef]
- Abomostafa, H.; Mansour, D.-E.A.; Mahani, R.; Nasralla, N. Investigation of structure, thermal and dielectric study of Dy0.05Ba0.7Sr0.25TiO3/polystyrene nanocomposites. Phys. Scr. 2023, 98, 085919. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Tohamy, H.-A.S.; Elnasharty, M.M.; Abdel-Aziz, M.S.; El-Sakhawy, M.; Turky, G.; Kamel, S. Antibacterial activity and dielectric properties of the PVA/cellulose nanocrystal composite using the synergistic effect of rGO@ CuNPs. Int. J. Biol. Macromol. 2024, 261, 129801. [Google Scholar] [CrossRef]
- Harper, C.A. Handbook of Ceramics, Glasses and Diamonds; McGraw-Hill Companies: New York, NY, USA, 2001. [Google Scholar]
- Mouiya, M.; Bouazizi, A.; Abourriche, A.; El Khessaimi, Y.; Benhammou, A.; Taha, Y.; Oumam, M.; Abouliatim, Y.; Smith, A.; Hannache, H. Effect of sintering temperature on the microstructure and mechanical behavior of porous ceramics made from clay and banana peel powder. Results Mater. 2019, 4, 100028. [Google Scholar] [CrossRef]
- Sembiring, S.; Simanjuntak, W.; Situmeang, R.; Riyanto, A.; Karo-Karo, P. Effect of alumina addition on the phase transformation and crystallisation properties of refractory cordierite prepared from amorphous rice husk silica. J. Asian Ceram. Soc. 2017, 5, 186–192. [Google Scholar] [CrossRef]
- Cao, Z.; Li, P. In-Situ Crystallization Process of Ferrochrome Slag for Preparing Cordierite Material Using Quartz as Nucleating Agent. Available online: https://ssrn.com/abstract=4272274 (accessed on 3 August 2024). [CrossRef]
- Wang, S.; Yan, W.; Yan, J.; Schafföner, S.; Chen, Z.; Sang, S. Microstructures and properties of microporous mullite-corundum aggregates for lightweight refractories. Int. J. Appl. Ceram. Technol. 2022, 19, 3300–3310. [Google Scholar] [CrossRef]
- Manullang, R.J.; Purnawan, M.; Taufik, D.; Noordiningsih, K. The effect of pore former addition and sintering temperature on the characteristic of ceramic membrane. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2022. [Google Scholar]
- Osendi, M.; Baudin, C. Mechanical properties of mullite materials. J. Eur. Ceram. Soc. 1996, 16, 217–224. [Google Scholar] [CrossRef]
- Idbenjadi, F.E.; Benhammou, A.; Abourriche, A.; Abouliatim, Y. Effect of sintering temperature on the thermal and mechanical properties of cordierite-based refractory. Mater. Today Proc. 2024. [Google Scholar] [CrossRef]
- Atef, N.; El Damrawi, G.; Hassan, A.; El-Deen, L.S. Dielectric Studies on CuO-Na2OB2O3 Glasses. New J. Glass Ceram. 2020, 10, 45. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Pappu, A.; Gupta, M.K. Unraveling the role of agro waste-derived graphene quantum dots on dielectric and mechanical property of the fly ash based polymer nanocomposite. J. Alloys Compd. 2022, 903, 163953. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Pappu, A.; Srivastava, A.K.; Gupta, M.K. Synthesis dielectric and mechanical properties of paddy straw derived graphene quantum dots-stone waste nanocomposite. Mater. Lett. 2021, 301, 130323. [Google Scholar] [CrossRef]
- Abouhaswa, A.; Rammah, Y.; Turky, G.M. Characterization of zinc lead-borate glasses doped with Fe3+ ions: Optical, dielectric, and ac-conductivity investigations. J. Mater. Sci. Mater. Electron. 2020, 31, 17044–17054. [Google Scholar] [CrossRef]
- Rehim, M.H.A.; Badawy, A.A.; Turky, G. Electrical properties and heavy ions removal ability of graphitic carbon nitride/polypyrrole composite. J. Phys. Chem. Solids 2022, 167, 110741. [Google Scholar] [CrossRef]
- Şahingöz, R.; Kanbur, H.; Voigt, M.; Soykan, C. The determination of interface states and series resistance profile of Al/polymer/PEDOT-PSS/ITO heterojunction diode by I–V and C–V methods. Synth. Met. 2008, 158, 727–731. [Google Scholar] [CrossRef]
- Yıldız, D.E.; Dökme, İ. Frequency and gate voltage effects on the dielectric properties and electrical conductivity of Al/SiO2/p-Si metal-insulator-semiconductor Schottky diodes. J. Appl. Phys. 2011, 110, 014507. [Google Scholar] [CrossRef] [PubMed]






| Main Constituents (wt%) | Ceramic Roller | Ceramic Sludge | Magnesite | Silica Sand |
|---|---|---|---|---|
| SiO2 | 28.19 | 77.32 | 2.85 | 98.35 |
| Al2O3 | 60 | 7.6 | 1.94 | 1.24 |
| Fe2O3 | 0.76 | 0.35 | 1.28 | 0.26 |
| TiO2 | 0.52 | 0.52 | - | - |
| MgO | 0.3 | 1.03 | 36.92 | - |
| CaO | 0.3 | 7.23 | 8.56 | - |
| ZrO2 | 6.95 | - | - | - |
| BaO | - | 0.296 | - | - |
| P2O5 | 0.12 | 0.28 | - | - |
| Na2O | 0.69 | 2.6 | - | - |
| K2O | 0.53 | 1.13 | - | - |
| L.O.I. | - | - | 48.14 | - |
| Batch Symbol | Densification Parameters | ||
|---|---|---|---|
| Bulk Density gm/cm3 | Apparent Porosity % | Water Absorption | |
| C10 | 1.99 | 29.4 | 12.69 |
| C20 | 1.98 | 34.38 | 12.95 |
| C30 | 1.97 | 36.40 | 13.36 |
| C40 | 1.96 | 37.26 | 14.28 |
| C50 | 1.94 | 38.83 | 14.96 |
| Batch Symbol | Densification Parameters | ||
|---|---|---|---|
| Bulk Density gm/cm3 | Apparent Porosity % | Water Absorption | |
| C10 | 2.32 | 29.54 | 13.12 |
| C20 | 2.28 | 34.82 | 13.94 |
| C30 | 2.19 | 36.93 | 14.21 |
| C40 | 2.15 | 37.45 | 14.87 |
| C50 | 1.98 | 39.67 | 15.02 |
| Batch Symbol | Conductivity (σac) | Resistivity (ρ) |
|---|---|---|
| C10 | 1.19 × 10−8 | 8.38 × 107 |
| C20 | 2.79 × 10−9 | 3.59 × 108 |
| C30 | 8.55 × 10−10 | 1.17 × 109 |
| C40 | 2.03 × 10−10 | 4.91 × 109 |
| C50 | 3.10 × 10−11 | 3.23 × 1010 |
| Batch No. | Nominal Phase | Batch Composition% | Batch Constituents% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cordierite | Mullite | SiO2 | Al2O3 | MgO | Ceramic Roller | Magnesite | Sludge | Silica Sand | |
| C10 | 10 | 90 | 30.48 | 68.14 | 1.38 | 96.81 | 3.19 | - | - |
| C20 | 20 | 80 | 32.81 | 64.43 | 2.76 | 90.23 | 6.30 | 2.61 | 0.86 |
| C30 | 30 | 70 | 35.13 | 60.74 | 4.13 | 82.51 | 9.15 | 6.75 | 1.59 |
| C40 | 40 | 60 | 37.13 | 57.03 | 5.51 | 75.47 | 11.89 | 10.71 | 1.93 |
| C50 | 50 | 50 | 39.76 | 53.35 | 6.89 | 68.63 | 14.50 | 14.40 | 2.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khater, G.A.; Romero, M.; López-Delgado, A.; Padilla, I.; El-Kheshen, A.A.; Farag, M.M.; Elmaghraby, M.S.; Shendy, H.; Nasralla, N.H.S. Utilizing Ceramic Factory Waste to Produce Low-Cost Refractory Ceramics. Recycling 2024, 9, 98. https://doi.org/10.3390/recycling9050098
Khater GA, Romero M, López-Delgado A, Padilla I, El-Kheshen AA, Farag MM, Elmaghraby MS, Shendy H, Nasralla NHS. Utilizing Ceramic Factory Waste to Produce Low-Cost Refractory Ceramics. Recycling. 2024; 9(5):98. https://doi.org/10.3390/recycling9050098
Chicago/Turabian StyleKhater, Gamal A., Maximina Romero, Aurora López-Delgado, Isabel Padilla, Amany A. El-Kheshen, Mohammad M. Farag, Mohammad S. Elmaghraby, Hussain Shendy, and Naglaa H. S. Nasralla. 2024. "Utilizing Ceramic Factory Waste to Produce Low-Cost Refractory Ceramics" Recycling 9, no. 5: 98. https://doi.org/10.3390/recycling9050098
APA StyleKhater, G. A., Romero, M., López-Delgado, A., Padilla, I., El-Kheshen, A. A., Farag, M. M., Elmaghraby, M. S., Shendy, H., & Nasralla, N. H. S. (2024). Utilizing Ceramic Factory Waste to Produce Low-Cost Refractory Ceramics. Recycling, 9(5), 98. https://doi.org/10.3390/recycling9050098









