Resource Recovery from Abandoned Mine Drainage Galleries via Ion Exchange: A Case Study from Freiberg Mining Area, Germany
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mine Water Chemistry
2.2. Zinc-Adsorption Isotherms
2.3. Pretreatment of the Mine Water
2.4. Recovery of the Zinc via Ion Exchange
2.5. Effect of Regenerant on Metal Recovery
2.6. Characterization of the Physical Status of the Used Resins
2.7. Significant of Metal Recovery from Point Sources
3. Materials and Methods
3.1. Study Area
3.2. Mine Water Sampling and Preservation
3.3. Pretreatment of Mining-Influenced Water Before the Ion-Exchange Process
3.4. Characterization of Ion-Exchange Materials
3.5. Batch Adsorption Studies
3.6. Column Experiments
3.7. Chemical Analyses
3.8. SEM-EDX Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henckens, T. Scarce Mineral Resources: Extraction, Consumption and Limits of Sustainability. Resour. Conserv. Recycl. 2021, 169, 105511. [Google Scholar] [CrossRef]
- Reynolds, S.; Anderson, L. Exploring Economic Adaptations: Qualitative Insights into the Metal Industry Amidst Shifting Towards Renewable Energy. Preprint 2023. [Google Scholar] [CrossRef]
- Aybarç, U.; Seydibeyoğlu, M.Ö. Recycling for a Sustainable World with Metal Matrix Composites. In Recycling of Plastics, Metals, and Their Composites; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-314876-0. [Google Scholar]
- Leal, V.M.; Ribeiro, J.S.; Coelho, E.L.D.; Freitas, M.B.J.G. Recycling of Spent Lithium-Ion Batteries as a Sustainable Solution to Obtain Raw Materials for Different Applications. J. Energy Chem. 2023, 79, 118–134. [Google Scholar] [CrossRef]
- Hudson-Edwards, K.A.; Jamieson, H.E.; Lottermoser, B.G. Mine Wastes: Past, Present, Future. Elements 2011, 7, 375–380. [Google Scholar] [CrossRef]
- Kalisz, S.; Kibort, K.; Mioduska, J.; Lieder, M.; Małachowska, A. Waste Management in the Mining Industry of Metals Ores, Coal, Oil and Natural Gas—A Review. J. Environ. Manag. 2022, 304, 114239. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Recycling, Reuse and Rehabilitation of Mine Wastes. Elements 2011, 7, 405–410. [Google Scholar] [CrossRef]
- Plumlee, G.S.; Morman, S.A. Mine Wastes and Human Health. Elements 2011, 7, 399–404. [Google Scholar] [CrossRef]
- Mensah, M.K.; Drebenstedt, C.; Hoth, N.; Ola, I.M.; Okoroafor, P.U.; Wiafe, E.D. Artisanal Gold Mine Spoil Types within a Common Geological Area and Their Variations in Contaminant Loads and Human Health Risks. Environ. Monit. Assess. 2023, 195, 312. [Google Scholar] [CrossRef]
- Moro, K.; Haubrich, F.; Martin, M.; Grimmer, M.; Hoth, N.; Schippers, A. Reductive Leaching Behaviour of Manganese and Cobalt Phases in Laterite and Manganese Ores. Hydrometallurgy 2023, 220, 106101. [Google Scholar] [CrossRef]
- Wolkersdorfer, C. Water Management at Abandoned Flooded Underground Mines: Fundamentals, Tracer Tests, Modelling, Water Treatment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-77331-3. [Google Scholar]
- Agboola, O.; Babatunde, D.E.; Isaac Fayomi, O.S.; Sadiku, E.R.; Popoola, P.; Moropeng, L.; Yahaya, A.; Mamudu, O.A. A Review on the Impact of Mining Operation: Monitoring, Assessment and Management. Results Eng. 2020, 8, 100181. [Google Scholar] [CrossRef]
- Lu, P.; Zhou, L.; Cheng, S.; Zhu, X.; Yuan, T.; Chen, D.; Feng, Q. Main Challenges of Closed/Abandoned Coal Mine Resource Utilization in China. Energy Sources Part Recovery Util. Environ. Eff. 2020, 42, 2822–2830. [Google Scholar] [CrossRef]
- Wolkersdorfer, C.; Mugova, E. Effects of Mining on Surface Water. In Encyclopedia of Inland Waters; Elsevier: Amsterdam, The Netherlands, 2022; pp. 170–188. ISBN 978-0-12-822041-2. [Google Scholar]
- Bouzek, J.; Koutecký, D.; Simon, K. Tin and Prehistoric Mining in the Erzgebirge (Ore Mountains): Some New Evidence. Oxf. J. Archaeol. 1989, 8, 203–212. [Google Scholar] [CrossRef]
- Kvet, R. Mineral Mining on the Bohemian Side of the Krušné Hory Mountains/Erzgebirge up to the Time of Agricola. GeoJournal 1994, 32, 101–102. [Google Scholar] [CrossRef]
- Jelen, J. Mining Heritage and Mining Landscape Krušnohoří/Erzgebirge as a Part of the UNESCO Heritage. Land 2022, 11, 955. [Google Scholar] [CrossRef]
- Haubrich, F.; Tichomirowa, M. Sulfur and Oxygen Isotope Geochemistry of Acid Mine Drainage—The Polymetallic Sulfide Deposit “Himmelfahrt Fundgrube” in Freiberg (Germany). Isot. Environ. Health Stud. 2002, 38, 121–138. [Google Scholar] [CrossRef]
- Zhiteneva, V.; Brune, J.; Mischo, H.; Weyer, J.; Simon, A. Water Quality of Reiche Zeche Teaching Mine, Freiberg, Saxony, Germany. In Proceedings of the SME Annual Meeting, Phoenix, AZ, USA, 21–24 February 2016. [Google Scholar]
- Junghans, M.; Tichomirowa, M. Using Sulfur and Oxygen Isotope Data for Sulfide Oxidation Assessment in the Freiberg Polymetallic Sulfide Mine. Appl. Geochem. 2009, 24, 2034–2050. [Google Scholar] [CrossRef]
- Tichomirowa, M.; Heidel, C.; Junghans, M.; Haubrich, F.; Matschullat, J. Sulfate and Strontium Water Source Identification by O, S and Sr Isotopes and Their Temporal Changes (1997–2008) in the Region of Freiberg, Central-Eastern Germany. Chem. Geol. 2010, 276, 104–118. [Google Scholar] [CrossRef]
- Stockmann, M.; Hirsh, D.; Lippmann-Pipke, J.; Kupsch, H. Geochemical Study of Different-Aged Mining Dump Materials in the Freiberg Mining District, Germany. Environ. Earth Sci. 2013, 68, 1153–1168. [Google Scholar] [CrossRef]
- Nobahar, A.; Melka, A.B.; Marín-Beltrán, I.; Neves, L.; Costa, M.C.; Carlier, J.D. Zinc Recovery from an Extreme Copper-Free Acid Mine Drainage: Studying the Prior Separation of Ferric Iron by Solvent Extraction Using AliCy and/or Alkalinization. J. Sustain. Metall. 2022, 8, 1509–1531. [Google Scholar] [CrossRef]
- Reichl, C.; Schatz, M. World Mining Data 2020; Federal Ministry of Agriculture Regions and Tourism: Vienna, Austria, 2020; Volume 35. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Vahidi, E.; Rashchi, F.; Moradkhani, D. Recovery of Zinc from an Industrial Zinc Leach Residue by Solvent Extraction Using D2EHPA. Miner. Eng. 2009, 22, 204–206. [Google Scholar] [CrossRef]
- Fernando, W.A.M.; Ilankoon, I.M.S.K.; Syed, T.H.; Yellishetty, M. Challenges and Opportunities in the Removal of Sulphate Ions in Contaminated Mine Water: A Review. Miner. Eng. 2018, 117, 74–90. [Google Scholar] [CrossRef]
- Johnson, D.B. Biomining-Biotechnologies for Extracting and Recovering Metals from Ores and Waste Materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef]
- Doyle, S.; Figueroa, L. Removal of Molybdenum from Mining-Impacted Water by Sorption onto Manganese-Rich Sludge. Mine Water Environ. 2022, 41, 721–730. [Google Scholar] [CrossRef]
- Ryu, S.; Naidu, G.; Hasan Johir, M.A.; Choi, Y.; Jeong, S.; Vigneswaran, S. Acid Mine Drainage Treatment by Integrated Submerged Membrane Distillation–Sorption System. Chemosphere 2019, 218, 955–965. [Google Scholar] [CrossRef]
- Gan, Y.; Ding, C.; Xu, B.; Liu, Z.; Zhang, S.; Cui, Y.; Wu, B.; Huang, W.; Song, X. Antimony (Sb) Pollution Control by Coagulation and Membrane Filtration in Water/Wastewater Treatment: A Comprehensive Review. J. Hazard. Mater. 2023, 442, 130072. [Google Scholar] [CrossRef] [PubMed]
- Timofeev, K.L.; Kurdiumov, V.; Maltsev, G. Sorption and Membrane Technologies for Mine Water Purification. Mater. Sci. Forum 2019, 946, 621–627. [Google Scholar] [CrossRef]
- Abeywickrama, J.; Grimmer, M.; Hoth, N. Recovery of Strategically Important Heavy Metals from Mining Influenced Water. In Hybridized Technologies for the Treatment of Mining Effluents; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 41–78. ISBN 978-1-119-89692-0. [Google Scholar]
- van Deventer, J. Selected Ion Exchange Applications in the Hydrometallurgical Industry. Solvent Extr. Ion Exch. 2011, 29, 695–718. [Google Scholar] [CrossRef]
- Botelho, A.B.; Espinosa, D.C.R.; Dreisinger, D.; Tenório, J.A.S. Effect of pH to Recover Cu(II), Ni(II) AND Co(II) from Nickel Laterite Leach Using Chelating Resins. Tecnol. Metal. Mater. Min. 2019, 16, 135–140. [Google Scholar] [CrossRef]
- Sole, K.C. Present and Future Applications of Ion Exchange in Hydrometallurgy: An Overview. In Proceedings of the IEX 2016, The International Ion Exchange Conference, Cambridge, UK, 6–8 July 2016; pp. 2–33. [Google Scholar]
- Sole, K.C. The Evolution of Cobalt–Nickel Separation and Purification Technologies: Fifty Years of Solvent Extraction and Ion Exchange. In Extraction 2018: Proceedings of the First Global Conference on Extractive Metallurgy, Ottawa, ON, Canada, 26–29 August 2018; Davis, B.R., Moats, M.S., Wang, S., Gregurek, D., Kapusta, J., Battle, T.P., Schlesinger, M.E., Alvear Flores, G.R., Jak, E., Goodall, G., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1167–1191. [Google Scholar]
- Harland, C.E. Ion Exchange; Royal Society of Chemistry: London, UK, 1994; ISBN 978-0-85186-484-6. [Google Scholar]
- Littlejohn, P.; Vaughan, J. Recovery of Nickel and Cobalt from Laterite Leach Tailings through Resin-in-Pulp Scavenging and Selective Ammoniacal Elution. Miner. Eng. 2013, 54, 14–20. [Google Scholar] [CrossRef]
- Pedregal Montes, A.I.; Abeywickrama, J.; Hoth, N.; Grimmer, M.; Drebenstedt, C. Modeling of Ion Exchange Processes to Optimize Metal Removal from Complex Mine Water Matrices. Water 2021, 13, 3109. [Google Scholar] [CrossRef]
- Abeywickrama, J.; Hoth, N.; Ussath, M.; Drebenstedt, C. Selective Extraction of Cobalt and Copper from Chilean Mine Water by Ion Exchange Resin. Gorn. Nauk. Tekhnol. Min. Sci. Technol. Russ. 2020, 5, 25–29. [Google Scholar] [CrossRef]
- Choi, J.-W.; Song, M.-H.; Bediako, J.K.; Yun, Y.-S. Sequential Recovery of Gold and Copper from Bioleached Wastewater Using Ion Exchange Resins. Environ. Pollut. 2020, 266, 115167. [Google Scholar] [CrossRef]
- Aşçı, Y.; Kaya, Ş. Removal of Cobalt Ions from Water by Ion-Exchange Method. Desalin. Water Treat. 2014, 52, 267–273. [Google Scholar] [CrossRef]
- Strauss, M.L.; Diaz, L.A.; McNally, J.; Klaehn, J.; Lister, T.E. Separation of Cobalt, Nickel, and Manganese in Leach Solutions of Waste Lithium-Ion Batteries Using Dowex M4195 Ion Exchange Resin. Hydrometallurgy 2021, 206, 105757. [Google Scholar] [CrossRef]
- Abeywickrama, J.; Hoth, N.; Grimmer, M.; Haubrich, F.; Drebenstedt, C. Optimizing Metal Recovery from Slag Leaching Solutions: Advanced Ion Exchange Techniques for Sustainable Resource Extraction. J. Water Process Eng. 2023, 56, 104482. [Google Scholar] [CrossRef]
- Bezzina, J.P.; Robshaw, T.; Dawson, R.; Ogden, M.D. Single Metal Isotherm Study of the Ion Exchange Removal of Cu(II), Fe(II), Pb(II) and Zn(II) from Synthetic Acetic Acid Leachate. Chem. Eng. J. 2020, 394, 124862. [Google Scholar] [CrossRef]
- Felipe, E.C.B.; Batista, K.A.; Ladeira, A.C.Q. Recovery of Rare Earth Elements from Acid Mine Drainage by Ion Exchange. Environ. Technol. 2021, 42, 2721–2732. [Google Scholar] [CrossRef]
- Hermassi, M.; Granados, M.; Valderrama, C.; Skoglund, N.; Ayora, C.; Cortina, J.L. Impact of Functional Group Types in Ion Exchange Resins on Rare Earth Element Recovery from Treated Acid Mine Waters. J. Clean. Prod. 2022, 379, 134742. [Google Scholar] [CrossRef]
- Debord, J.; Chu, K.H.; Harel, M.; Salvestrini, S.; Bollinger, J.-C. Yesterday, Today, and Tomorrow. Evolution of a Sleeping Beauty: The Freundlich Isotherm. Langmuir 2023, 39, 3062–3071. [Google Scholar] [CrossRef]
- Bao, S.; Hawker, W.; Vaughan, J. Scandium Loading on Chelating and Solvent Impregnated Resin from Sulfate Solution. Solvent Solvent Extr. Ion Exch. 2018, 36, 100–113. [Google Scholar] [CrossRef]
- Liu, Y. Fluoride Removal from Zinc Sulfate Solution. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2017. [Google Scholar]
- Lanxess LEWATIT-MonoPlus-TP-260 2019. Available online: https://lanxess.com/en-us/products-and-brands/products/l/lewatit--monoplus-tp-260 (accessed on 10 May 2024).
- Taute, J.; Sole, K.; Hardwick, E. Zinc Removal from a Base-Metal Solution by Ion Exchange: Process Design to Full-Scale Implementation. In Proceedings of the Southern African Institute of Mining and Metallurgy Base Metals Conference, Mpumalanga, South Africa, 2–4 September 2013. [Google Scholar]
- Baacke, D. Geochemisches Verhalten umweltrelevanter Elemente in stillgelegten Polysulfiderzgruben am Beispiel der Grube “Himmelfahrt” in Freiberg Sachsen. Ph.D. Dissertation, TUBA Freiberg, Freiberg, Germany, 2000. Available online: http://webdoc.sub.gwdg.de/ebook/diss/2003/tu-freiberg/archiv/html/Geowissenschaften_BaackeDelf966689.html (accessed on 4 December 2022).
- Degner, T. Prognose von Geochemischen Auswirkungen der Nachnutzung Stillgelegter Bergbau-Stollen-Systeme am Beispiel des Freiberger Grubenreviers. Ph.D. Thesis, Freiberg University of Mining and Technology, Freiberg, Germany, 2003. [Google Scholar]
- Martin, M.; Beuge, P.; Kluge, A.; Hoppe, T. Grubenwässer des Erzgebirges—Quelle von Schwermetallen in Der Elbe; Spektrum der Wissenschaft: Heidelberg, Germany, 1994; pp. 102–107. [Google Scholar]
- Baumann, L.; Kuschka, E.; Seifert, T. Lagerstätten Des Erzgebirges; Enke im Thieme Verlag: Stuttgart, Germany, 2000; p. 300. [Google Scholar]
- Knittel, U.; Klemm, W.; Greif, A. Heavy Metal Pollution Downstream of Old Mining Camps as a Result of Flood Events: An Example from the Mulde River System, Eastern Part of Germany. TAO Terr. Atmos. Ocean. Sci. 2005, 16, 919–931. [Google Scholar] [CrossRef]
- Kolitsch, S.; Junghans, M.; Klemm, W.; Tichomirova, M. Der Flutungsraum Des Grubenfeldes Freiberg—Hydrochemie, Isotpenchemie Und Hydraulik. Wiss. Mitt. 2001, 18, 14–26. [Google Scholar]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
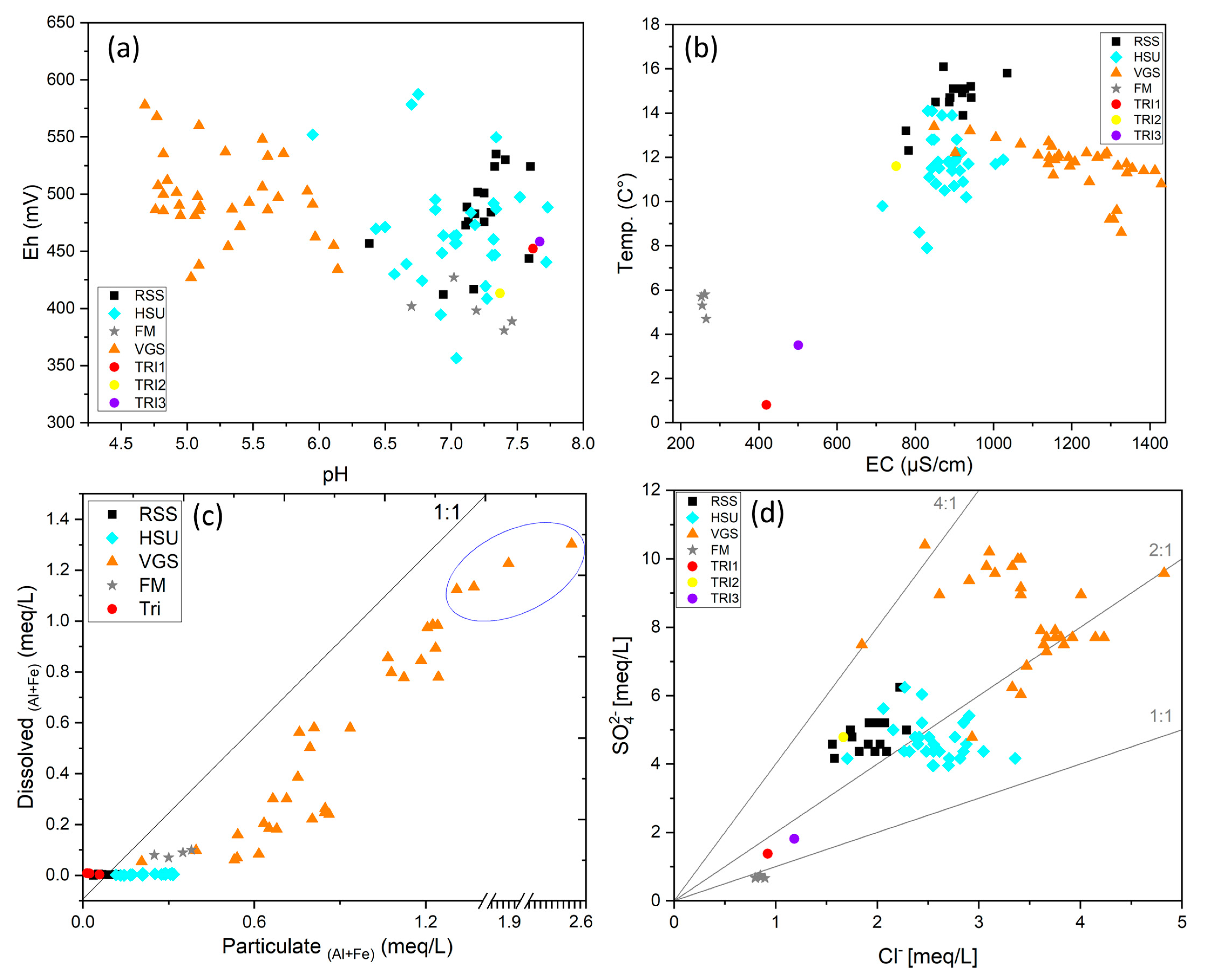
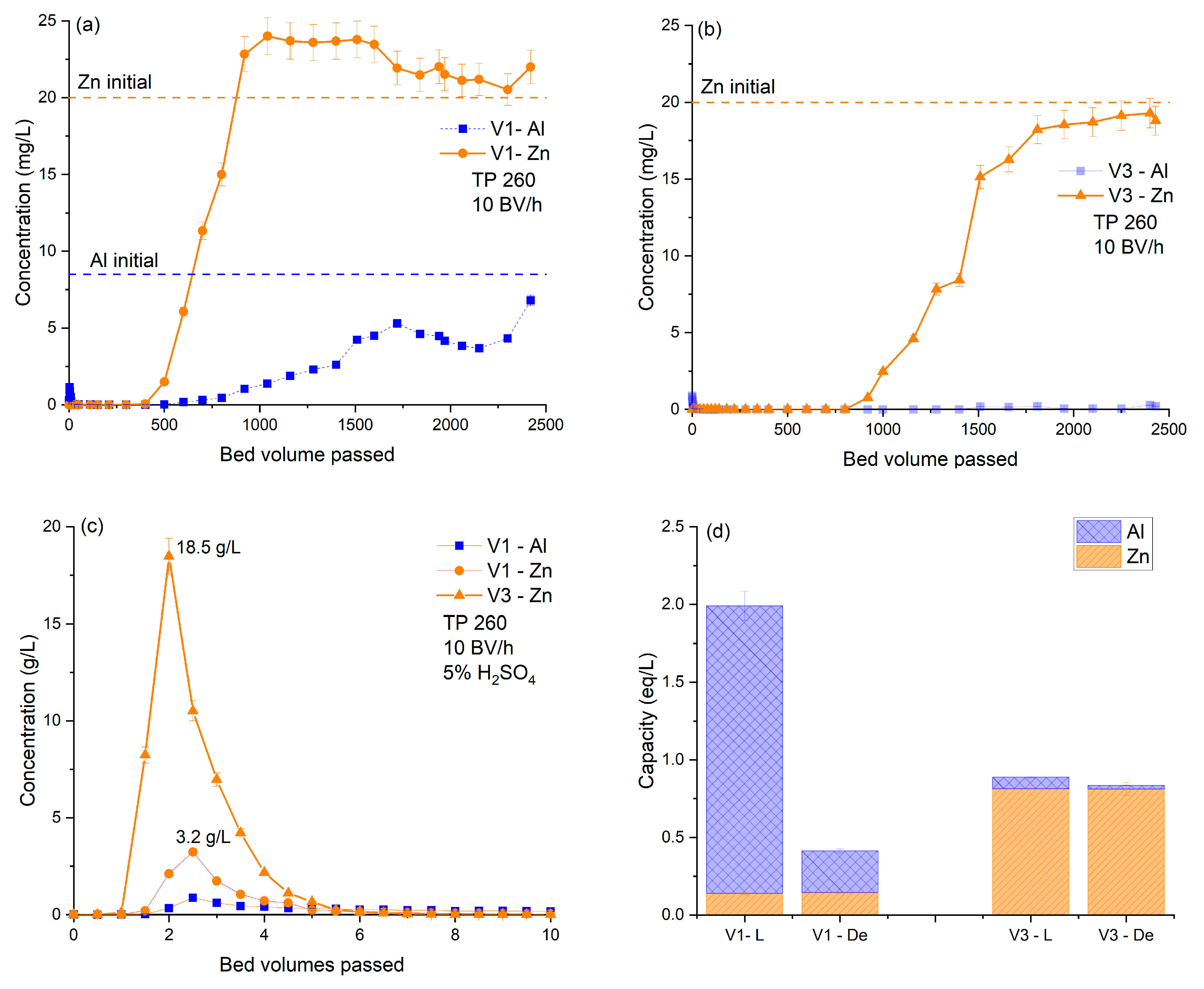


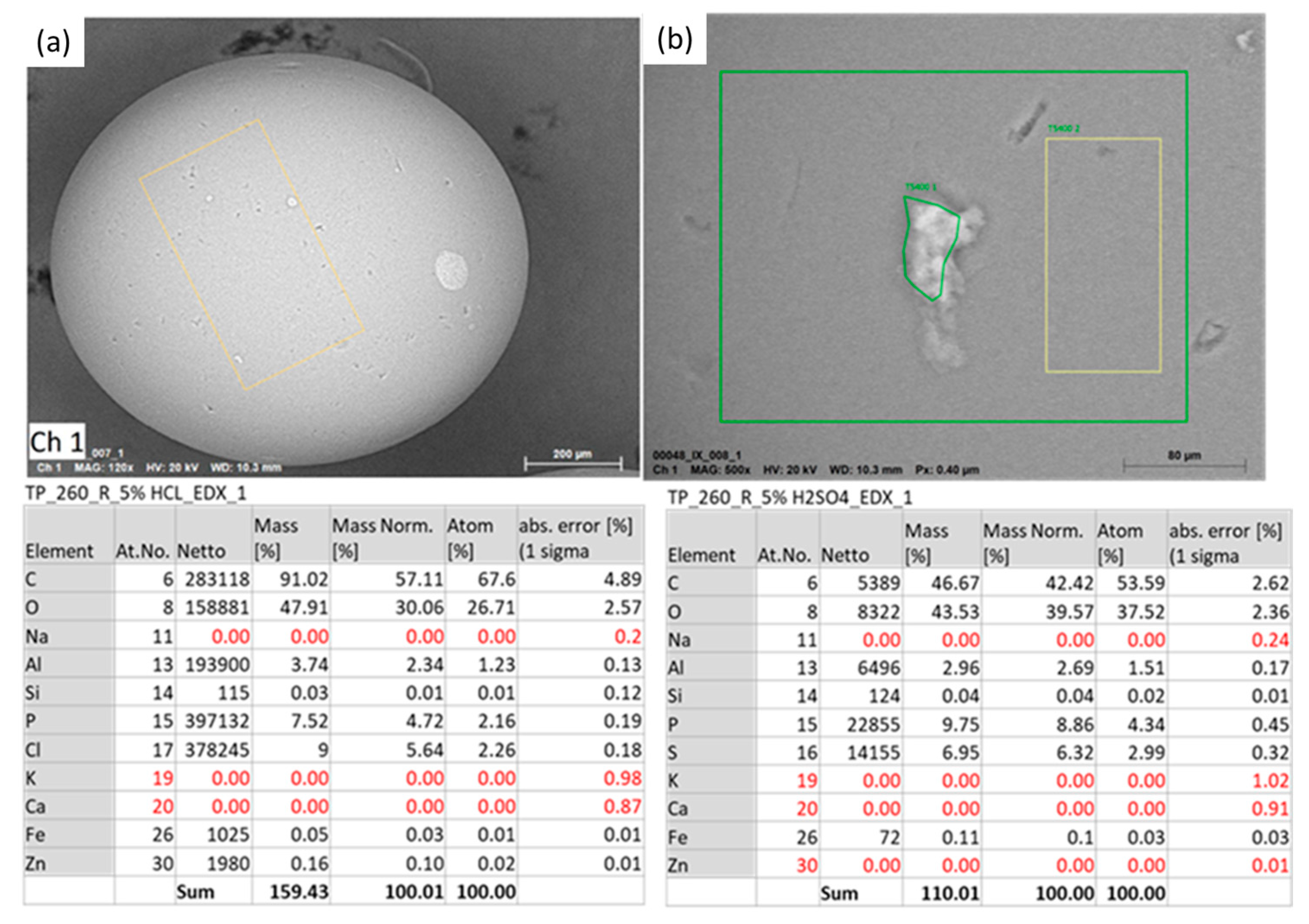

| Gallery Name | HSU Water (n = 23) | VGS Water (n = 26) | RSS Water (n = 12) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Min | Max | Mean | Stdv | Min | Max | Mean | Stdv | Min | Max | Mean | Stdv |
| Flowrate (L/s) | 37.0 | 134 | 69.2 | 30.4 | 25.3 | 48.0 | 33.4 | 6.0 | 262 | 520 | 466 | 93.3 |
| T (°C) | 10.5 | 14.1 | 12.2 | 1.0 | 10.8 | 13.4 | 11.9 | 0.7 | 14.5 | 16.1 | 15.1 | 0.5 |
| EC (µs/cm) | 832 | 1005 | 881 | 40.8 | 848 | 1429 | 1217 | 149.7 | 852 | 1035 | 915 | 48.8 |
| pH | 6.5 | 7.7 | 7.2 | 0.3 | 4.7 | 6 | 5.3 | 0.5 | 7.1 | 7.6 | 7.3 | 0.1 |
| Eh (mV) | 409 | 550 | 466 | 30.5 | 427 | 578 | 488 | 37.2 | 473 | 530 | 496 | 21.2 |
| Na (mg/L) | 23.1 | 49.0 | 30.1 | 5.6 | 20.2 | 48.4 | 33.3 | 7.8 | 27.6 | 40.6 | 34.2 | 3.9 |
| K (mg/L) | 3.4 | 10.8 | 5.3 | 1.6 | 2.7 | 8.4 | 5.2 | 1.1 | 4.4 | 5.8 | 4.9 | 0.5 |
| Mg (mg/L) | 20.9 | 29.8 | 24.7 | 2.4 | 23.0 | 44.4 | 37.8 | 4.9 | 20.5 | 26.2 | 23.6 | 2.0 |
| Ca (mg/L) | 47.0 | 117 | 78.2 | 17.6 | 46.0 | 183 | 118 | 30.0 | 86.4 | 129 | 102 | 12.3 |
| Al (mg/L) | 0.2 | 2.6 | 0.9 | 0.6 | 1.7 | 18.5 | 7.7 | 3.9 | 0.1 | 0.3 | 0.2 | 0.0 |
| Fe (mg/L) | 1.8 | 10.1 | 3.4 | 1.9 | 0.3 | 11.1 | 1.8 | 2.3 | 0.5 | 1.5 | 0.8 | 0.3 |
| Mn (mg/L) | 1.9 | 5.6 | 3.6 | 0.8 | 3.9 | 11.2 | 7.8 | 1.7 | 0.4 | 0.8 | 0.7 | 0.1 |
| Si (mg/L) | 8.3 | 10.6 | 9.8 | 0.6 | 6.6 | 10.4 | 8.9 | 1.0 | 8.7 | 10.1 | 9.3 | 0.4 |
| Zn(mg/L) | 1.5 | 10.3 | 4.6 | 2.3 | 8.0 | 21.5 | 16.0 | 3.7 | 2.5 | 4.2 | 3.6 | 0.5 |
| Cl− (mg/L) | 190 | 300 | 222 | 29.2 | 87.5 | 171 | 122 | 18.8 | 61.6 | 78.8 | 70.1 | 4.7 |
| SO42− (mg/L) | 80.3 | 119 | 94.8 | 9.5 | 230 | 500 | 406 | 73.7 | 210 | 300 | 237 | 27.2 |
| TIC (mg/L) | 0.81 | 1.54 | 1.16 | 0.3 | 1.21 | 3.51 | 2.86 | 1.1 | 1.17 | 3.55 | 2.51 | 1.0 |
| TN (mg/L) | 1.61 | 3.59 | 2.45 | 0.9 | 1.53 | 3.51 | 2.86 | 1.5 | 2.52 | 3.79 | 2.14 | 0.8 |
| Langmuir | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|
| Zn | qm | Km | R2 | Zn | Kf | N | R2 | |
| (mg/L) | (mg/g) | (L/mg) | (mg/L) | (mg/g) | ||||
| Synthetic water | 10 | 37.45 | 133 | 0.99 | 10 | 1.21 | 136.99 | 0.65 |
| 20 | 60.24 | 11.06 | 0.99 | 20 | 1.15 | 156.25 | 0.34 | |
| VGS water | 10 | 20 | 4 | 0.98 | 10 | 1.51 | 10.7 | 0.73 |
| 20 | 24 | 1 | 0.97 | 20 | 1.13 | 25.06 | 0.78 | |
| Column | Loading | De-Loading | Remained |
|---|---|---|---|
| Zinc | |||
| V1 | 14.6 ± 0.7 | 14.5 ± 0.7 | 0.1 ± 0.005 |
| V2 | 16.9 ± 0.8 | 16.6 ± 0.8 | 0.3 ± 0.015 |
| Aluminum | |||
| V1 | 185 ± 9.2 | 26.8 ± 1.3 | 158 ± 7.9 |
| V2 | 211 ± 10.5 | 30.7 ± 1.5 | 181 ± 9.1 |
| Zinc | |||
| V3 | 81.3 ± 4.1 | 81.2 ± 4.1 | 0.04 ± 0.002 |
| V4 | 23.9 ± 1.2 | 28.3 ± 1.4 | - |
| Aluminum | |||
| V3 | 7.30 ± 0.4 | 2.3 ± 0.1 | 4.97 ± 0.2 |
| V4 | 5.04 ± 0.3 | 49.6 * ± 2.4 | - |
| Load (Median) | HSU | VGS | RSS | |||
|---|---|---|---|---|---|---|
| g/min | 2003 [55] | 2022 | 2003 [55] | 2022 | 2003 [55] | 2022 |
| Na | 51 | 96 | 140 | 67 | 1780 | 1021 |
| K | 11 | 16 | 19 | 10 | 250 | 141 |
| Mg | 65 | 80 | 178 | 76 | 970 | 697 |
| Ca | 180 | 261 | 410 | 240 | 3800 | 3097 |
| Al | 2.3 | 3 | 54 | 13 | 7.9 | 6 |
| Fe | 7.4 | 10 | 7 | 3 | 2.3 | 20 |
| Mn | 0.2 | 12 | 2 | 16 | 1.2 | 21 |
| Si | NA | 33 | NA | 18 | NA | 275 |
| Zn | 14 | 18 | 130 | 33 | 170 | 113 |
| Cl | 120 | 693 | 250 | 239 | 2180 | 2116 |
| SO42− | 590 | 316 | 2200 | 851 | 11,700 | 7243 |
| Property | TP 260 |
|---|---|
| Structure | Macroporous weak acidic |
| Matrix | Crosslinked polystyrene |
| Functional group | Amino Methyl Phosphonic |
| Average particle size (mm) | 0.85 ± 0.05 |
| Uniformity coefficient | 1.1 |
| Bulk density (g/L) | 709 |
| Total exchange capacity (H form) | 2.4 eq/L |
| pH range | 2–10 |
| Resin | Column ID | Bed Volume (mL) | Flow Rate (BV/h) | De-Loading | |
|---|---|---|---|---|---|
| Untreated VGS water | TP 260 (new) | V1 | 100 | 10 | 5%H2SO4 |
| TP 260 (new) | V2 | 100 | 10 | 5% HCl | |
| Treated VGS water | TP 260 (new) | V3 | 100 | 10 | 5%H2SO4 |
| TP 260 (used) | V4 | 100 | 10 | 5%H2SO4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeywickrama, J.; Karimi, K.; Grimmer, M.; Hoth, N.; Drebenstedt, C. Resource Recovery from Abandoned Mine Drainage Galleries via Ion Exchange: A Case Study from Freiberg Mining Area, Germany. Recycling 2024, 9, 105. https://doi.org/10.3390/recycling9060105
Abeywickrama J, Karimi K, Grimmer M, Hoth N, Drebenstedt C. Resource Recovery from Abandoned Mine Drainage Galleries via Ion Exchange: A Case Study from Freiberg Mining Area, Germany. Recycling. 2024; 9(6):105. https://doi.org/10.3390/recycling9060105
Chicago/Turabian StyleAbeywickrama, Janith, Katayoun Karimi, Marlies Grimmer, Nils Hoth, and Carsten Drebenstedt. 2024. "Resource Recovery from Abandoned Mine Drainage Galleries via Ion Exchange: A Case Study from Freiberg Mining Area, Germany" Recycling 9, no. 6: 105. https://doi.org/10.3390/recycling9060105
APA StyleAbeywickrama, J., Karimi, K., Grimmer, M., Hoth, N., & Drebenstedt, C. (2024). Resource Recovery from Abandoned Mine Drainage Galleries via Ion Exchange: A Case Study from Freiberg Mining Area, Germany. Recycling, 9(6), 105. https://doi.org/10.3390/recycling9060105






